Abstract
Background
Systemic lupus erythematosus (SLE) patients exhibit T-cell dysfunction which can be regulated through the mitochondrial transmembrane potential (Δψm) and mammalian target of rapamycin (mTOR) by glutathione. Therefore, the safety, tolerance, and efficacy of glutathione-precursor N-acetylcysteine (NAC) were examined in this randomized double-blind placebo-controlled study.
Methods
36 SLE patients received daily placebo or 1.2 g, 2.4 g or 4.8 g of NAC. Disease activity was monthly evaluated by BILAG, SLEDAI and fatigue assessment scale (FAS) before, during, and after 3-month treatment. Δψm and mTOR were assessed by flow cytometry. 42 healthy subjects matched for patients’ age, gender, and ethnicity were studied as controls.
Results
NAC was tolerated by all patients up to 2.4 g/day while 33% of those receiving 4.8 g/day had reversible nausea. Placebo or 1.2 g/day NAC did not influence disease activity. Considered together, 2.4 g and 4.8 g NAC reduced: 1) SLEDAI after 1 month (p=0.0007), 2 months (p=0.0009), 3 months (p=0.0030) and 4 months (p=0.0046); 2) BILAG after 1 month (p=0.029) and 3 months (p=0.0009); and 3) FAS after 2 months (p=0.002) and 3 months (p=0.004). NAC increased Δψm (p=0.0001) in all T cells, it profoundly reduced mTOR activity (p=0.0001), enhanced apoptosis (p=0.0004) and reversed expansion of CD4−/CD8− T cells (1.35 ± 0.12-fold; p=0.008), stimulated Foxp3 expression in CD4+/CD25+ T cells (p=0.045), and reduced anti-DNA production (p=0.049).
Conclusions
This pilot study suggests that NAC safely improves lupus disease activity by blocking mTOR in T lymphocytes.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that often has debilitating and potentially life-threatening consequences. Although current therapies afford significant clinical improvement, they are increasingly costly, not curative, and carry significant side-effects (1–3). While the cause of SLE is unknown, its pathogenesis clearly involves cellular dysfunction of the immune system and the production of anti-nuclear auto-antibodies (4). Existing data show that a natural antioxidant, glutathione (GSH), is depleted in peripheral blood lymphocytes (PBL) of patients with SLE (5–7). GSH regulates the elevation of mitochondrial transmembrane potential (Δψm) or mitochondrial hyperpolarization (MHP), which in turn activates the mammalian target of rapamycin (mTOR) in lupus T cells (6). mTOR skews cell death signal processing, modulates T-cell differentiation (8;9), and, in particular, inhibits the development of CD4+/CD25+/Foxp3+ regulatory T cells (10) which are deficient in patients with active SLE (11;12). Blockade of mTOR with rapamycin, a potent and expensive immunosuppressant, improved disease activity in murine lupus (13) and in patients with SLE (14). N-acetylcysteine (NAC), which serves as a precursor of GSH and an antioxidant in and of itself, inhibited mTOR in vitro (15) and improved the outcome of murine lupus in vivo (16).
GSH is a tripeptide composed of cysteine, glutamic acid, and glycine. The availability of cysteine is rate-limiting for GSH synthesis (17). NAC is the acetylated form of L-cysteine that has the advantages of resistance to oxidation and permeability through cell membrane over other forms of cysteine supplementation (17). NAC can effectively raise intracellular GSH of lymphocytes both in vitro (18) and in vivo (19).Although NAC is relatively inexpensive (~$150/kg), it is currently unavailable as oral medication by prescription or over-the-counter. However, NAC is used as an antioxidant, and it is widely accessible in health food stores. In a European study of idiopathic pulmonary fibrosis patients, “high-dose” oral NAC (1.8g/day) diminished disease severity and reduced the toxicity of pro-oxidant and immunosuppressant medications (20) commonly used in patients with SLE (1). Similar doses of NAC improve muscle fatigue (21), which is reported to be the most disabling symptom in 53% of SLE patients (22). Therefore, we initiated this double-blind placebo-controlled phase I/II study to evaluate the metabolic, immunological, and therapeutic impact of NAC in 36 SLE patients. Importantly, NAC was safe and it improved disease activity and fatigue through profoundly blocking mTOR and expanding CD4+/CD25+/FoxP3+ T cells.
METHODS
Clinical Study Design
36 patients with stable disease were enrolled in double-blind placebo-controlled treatment trial with N-acetylcysteine (NAC; FDA approval, IND No: 101,320; clinicaltrials.gov identifier: NCT00775476). The mean (± SEM) age of patients was 44.6 (± 1.8) years, ranging between 25–64 years (Table 1 in Supplemental Materials). 34 patients were females including 30 Caucasians, two African-Americans, and two Hispanic. 2 patients were Caucasian males. 42 healthy subjects were individually matched for each patient blood donation for age within ten years, gender, and ethnic background and freshly isolated cells were studied in parallel as controls for immunological studies. The mean (± SEM) age of controls was 44.4 (± 1.7) years, ranging between 22–63 years. 39 controls were females including 36 Caucasians, two African-Americans, and one Hispanic. 3 controls were Caucasian males.
SLE patients were randomized to receive either placebo or NAC in one of three treatment arms of increasing doses: 600 mg, 1,200 mg, or 2,400 mg twice daily for three months. 12 patients were enrolled per dosing group, 9 received NAC while 3 received placebo. We employed the following dose-progression rule: 6 of 8 active patients needed to tolerate each dose and show no worsening of SLE as defined in the Data Safety and Monitoring Plan (DSMP) to proceed to the next higher dose.
Inclusion criteria
age > 18 yr, male or female, SLE with ≥ 4 of eleven diagnostic criteria approved by the American College of Rheumatology (23), clinically stable disease on prednisone (≤ 10 mg/day), anti-malarials, azathioprine or mycophenylate mofetil as allowable immunosuppressant medications.
Exclusion criteria
Patients who were pregnant or lactating, had moderately serious or serious co-morbidities (e.g., diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease, chronic renal insufficiency), history of chronic infections (e.g., HIV, hepatitis B virus, hepatitis C virus, mycobacteria, bronchiectasis), infections in the past month, history of severe or recurrent infections, and smokers were excluded. Patients taking over-the-counter antioxidants that can enhance the effect of NAC were excluded. Alternatively, patients taking acetaminophen (Tylenol) which is metabolized by hepatic cytochrome P450 enzymes, primarily CYP2E1, to a toxic intermediate compound (N-acetyl-para benzoquine imide), requiring detoxification by hepatic GSH (24), were also excluded. One daily dose of multivitamin, containing ≤ 500 mg of vitamin C and ≤ 30 IU of vitamin E was allowed for each patient. Patients with acute flare of SLE threatening vital organs and requiring intravenous cyclophosphamide treatment, were excluded. Patients receiving biologicals (rituximab, abatacept) and enrolled in other clinical trials were also excluded.
Study materials
Identical appearing capsules containing NAC or placebo (dextrose) were manufactured by the compounding pharmacy within the Department of Pharmacy at SUNY Upstate Medical Center. Both NAC and dextrose were obtained from Spectrum Chemical Manufacturing Corporation (New Brunswick, NJ). Each capsule contained 600 mg NAC or placebo. All capsules were rolled in NAC to equalize smell. Each bottle contained capsules needed for 32 days. The pills were counted when the bottles were returned to ascertain compliance. Our study biostatistician worked closely with the Department of Pharmacy so that PI and research staff remained blinded to participants’ randomized condition.
Study visits
Routine and lupus-specific clinical and laboratory data were acquired during five monthly visits. Visit 1: baseline assessment before first NAC or placebo capsule with additional blood samples drawn 3h and 6h later for GSH assay; provision of first monthly capsules. Visit 2: after one-month treatment, before morning capsule; provision of second monthly capsules. Visit 3: after two-month treatment, before morning capsule; provision of third monthly capsules. Visit 4: after three-month treatment. Visit 5: four-month visit (end of one month washout).
For each patient visit, we obtained blood from healthy donors matched for age (within one decade), gender, and ethnicity, to be used as control for flow cytometry measurement of mitochondrial function, T-cell activation and death pathway selection, Ca2+ flux, production of nitric oxide (NO) and reactive oxygen intermediates (ROI), activation of mTOR and expression of Foxp3 in subsets of T cells and B cells. GSH was measured in whole blood and isolated peripheral blood lymphocytes (PBL) by HPLC. Each patient provided seven blood samples (visit 1/0 h, visit 1/3 h, visit 1/6 h, visit 2 in 1 month, visit 3 in 2 months, visit 4 in 3 months, visit 5 in 4 months (after one month washout). 42 healthy controls have also donated blood to use as control for HPLC analysis of GSH, flow cytometry of live cells as well as for the gene expression and signaling studies. We have recorded ~384 flow cytometry data points for each of the five patient visits, both for the patients and the matching controls. DNA, RNA, and protein lysates have been saved and catalogued for each visit. Individual controls gave blood on multiple occasions.
Clinical outcomes and assessments
Tolerance: common side effects (nausea, bloating, bad taste) seen in prior trials were specifically asked for at each visit and reviewed by our Data Safety and Monitoring Board (DSMB) bi-annually. Tolerance and safety were primary clinical outcomes.
Blinding: smell or taste was specifically asked for.
Clinical assessments: A complete physical examination was performed before enrollment. A directed physical examination of the cardiovascular, respiratory, gastrointestinal, musculoskeletal, neurological systems, skin, head, neck, sinuses, nasal and oral cavities were performed at each visit. SLE disease activity was assessed by using the British Isles Lupus Assessment Group (BILAG) (25) and SLE Disease Activity Index (SLEDAI) (23). We documented concurrent use and dosage of other medications. Improvement in SLEDAI or BILAG disease score were secondary clinical outcomes.
Fatigue was assessed by using a validated Fatigue Assessment Scale (FAS), a self-questionnaire that provides a subjective measurement of fatigue severity and has shown to have a high degree of internal consistency, validity, and sensitivity to changes in clinical condition (26). Improvement in FAS score was a secondary clinical outcome.
Routine blood tests included complete blood count, liver and kidney function test, urinalysis and lupus-relevant laboratory tests, such as anti-double-stranded DNA, C3, and C4.
Compliance with study procedures is described in the Supplemental Materials section.
Immunobiological outcomes and assessments
The primary outcome was a measurable increase of GSH in PBL by HPLC (5). Secondary outcomes, modulation of Δψm, ROI production (oxidative stress), apoptosis (5), mTOR activity (6) and FoxP3 expression (10), were assessed by flow cytometry.
Statistics
Power and sample size requirements for this study were based on a type I error rate of 0.05, two-tailed testing, and a minimal power level of .80, using Sample Power v2 software (SPSS Chicago, Ill). Estimates of effect size were based on our preliminary data (5) and the relevant literature to compare mean values of GSH across treatment groups (placebo, lowest NAC, medium NAC, highest NAC dose). Our analysis suggested that administration of NAC to a minimum of 8 patients per treatment arm should have 83.7% power to detect a 42% elevation of intracellular GSH in SLE patients (3.60 ± 0.30 ng/µg protein) to reach those in normal donors (5.11 ± 0.50 ng/µg protein) (5). This study compared the longitudinal effects of three different doses of NAC and a placebo control condition, before (visit 1), during (visit 2, after 1 month; visit 3, after 2 months; visit 4, after 3 months) and following a 3-month intervention (visit 5, after 1 month washout). Thus, we were employing a double-blinded longitudinal trial design comparing 4 groups on observations collected at intervals pre, during and post intervention.
Overall clinical effectiveness of NAC relative to placebo was analyzed with multilevel modeling as implemented in the STATA routine XTMIXED (from StataCorp, College Station, TX), with the three nested levels being drug group, subject within drug group and study visit within subject. All models included fixed effects for drug group, study visit and the drug group by study visit interaction along with random intercepts at each design level. Our test for efficacy was the fixed effect for the drug group by study visit interaction which, if significant indicated that the change in outcome scores over time was significantly different about drug groups. Two-tailed paired t-test was used to assess the effects of placebo and of each and all NAC doses on clinical indices and biomarkers recorded on visits 2–5 relative to visit 1; p < 0.05 was considered significant. Patients and controls were compared with two-tailed unpaired t-test.
RESULTS
Patient enrollment and tolerance of NAC
None of the 12 patients in Dosing Group 1 (1.2 g/day) or 2 (2.4 g/day NAC) reported unpleasant smell or taste and all 24 patients completed the treatment. Three of 12 patients in Dosing Group 3 (4.8 g/day) dropped out (Table 1 in Supplemental Materials) due to 1) heartburn after 26 days, which resolved after discontinuing the capsules; 2) recurrent nausea and one-time vomiting after 46 days, with resolution after discontinuing the capsules; 3) headaches, which were eliminated by halving the dose to 2.4 g/day. Since all three patients reporting intolerance received NAC, in accordance with the DSMP, no higher dose was initiated. Following the last Dosing Group 3 visit, the study was un-blinded on 11/30/2010.
Effect of NAC on disease activity
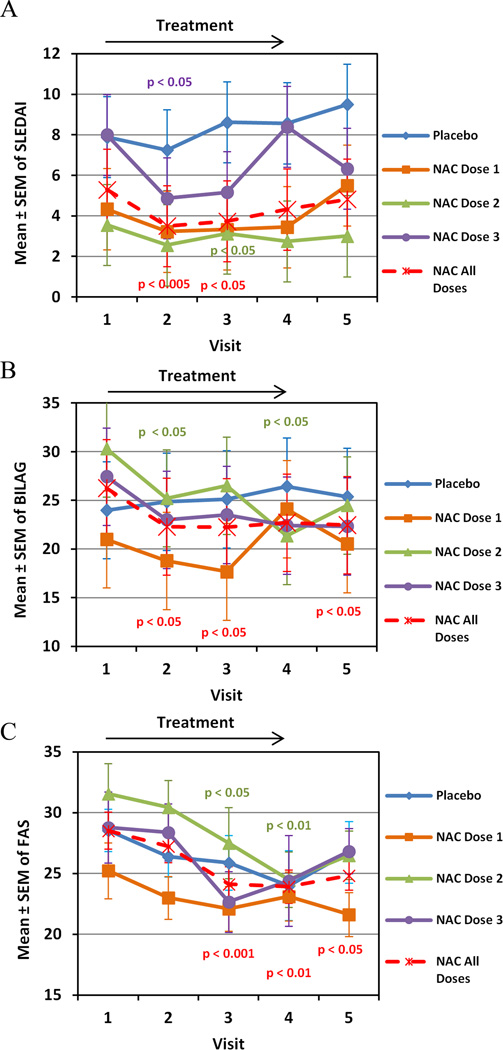
Lupus disease activity was measured by SLEDAI and BILAG and fatigue was evaluated by FAS at baseline (visit 1) as well as monthly during a 3-month intervention (visits 2–4) and after a 1-month washout period (visit 5; Fig. 1). Placebo or NAC dose 1 did not influence SLEDAI, BILAG, or FAS (Fig. 1). NAC doses 2 and 3 reduced SLEDAI (Fig. 1A). We observed a significant improvement in the number of patients who achieved improvements of SLEDAI scores of 3 or more in the 3rd NAC dosing group; placebo group: 2/9; NAC Dose 1: 3/9; NAC Dose 2: 4/9; NAC Dose 3: 5/6 (p=0.0406 relative to placebo, using Fischer= exact test). In all patients treated with NAC, SLEDAI was improved from 5.3 at baseline (visit 1) to 3.5 after 1-month (visit 2; p = 0.0013) and to 3.7 after 2-month treatment (visit 3; p = 0.048; Fig. 1A). In patients treated with NAC doses 2 and 3 combined, SLEDAI improved from 5.78 at baseline on all follow-up visits (visit 2: 3.6, p = 0.0007; visit 3: 4.0, p=0.0009; visit 4: 4.9, p = 0.0030; visit 5: 4.4, p = 0.0046). Using multilevel modeling, the reduction in SLEDAI was greater in the NAC than placebo group as indicated by a significant visit by drug interaction (XTMIXED z = −2.14, p = 0.033). Among biomarkers of SLEDAI, anti-DNA was reduced in patients exposed to all NAC doses considered together from 78.9 ± 45.2 IU/ml at baseline to 19.5 ± 6.0 IU/ml (p=0.049) after 1 month. C3 and C4 were not affected. In all patients treated with NAC, BILAG improved from 26.2 at baseline (visit 1) to 22.3 after 1 month (visit 2; p = 0.0158) and to 22.2 after 2 months (visit 3; p = 0.0223; Fig. 1B). Among the BILAG components reflecting organ system involvement, swollen joint count was reduced after 3-month NAC treatment with dose 3 (XTMIXED z = −2.0; p = 0.046) or all doses combined (XTMIXED z = −2.2; p = 0.028). The reduction in BILAG was also greater for NAC groups relative to the placebo group as indicated by a significant visit by drug interaction in mixed model analysis (XTMIXED z = −2.62, p = 0.009). This analysis also showed a significant reduction of BILAG by NAC dosing group 3 (4.8 g/day; XTMIXED z = −2.19, p = 0.029). In SLE patients treated with all NAC doses combined, FAS was improved from 28.5 at visit 1 to 24.1 at visit 3 (p = 0.0006), 23.9 at visit 4 (p = 0.005), and 24.8 at visit 5 (p = 0.034; Fig. 1C). Mixed model analysis showed a reduction of FAS in NAC dosing group 2 relative to the placebo group (XTMIXED z = −2.08, p = 0.038).
Fig. 1.
Effect of NAC and placebo on disease activity, as measured by SLEDAI (panel A), BILAG (panel B), and FAS scores (panel C), in 36 SLE patients exposed to placebo (n=9), 1.2 g/day NAC (NAC Dose1, n=9), 2.4 g/day NAC (NAC Dose 2, n=9), 4.8 g/day NAC (NAC Dose 3, n=9), or all doses of NAC considered together (n=27). Data represent mean ± SEM. p values reflect comparison of pretreatment values (visit 1) to values after treatment for 1 month (visit 2), 2 months (visit 3), 3 months (visit 4), or 4 months (visit 5, 3 months of treatment followed by 1 month washout) using two-tailed paired t-test.
Effect on NAC on GSH in whole blood and PBL
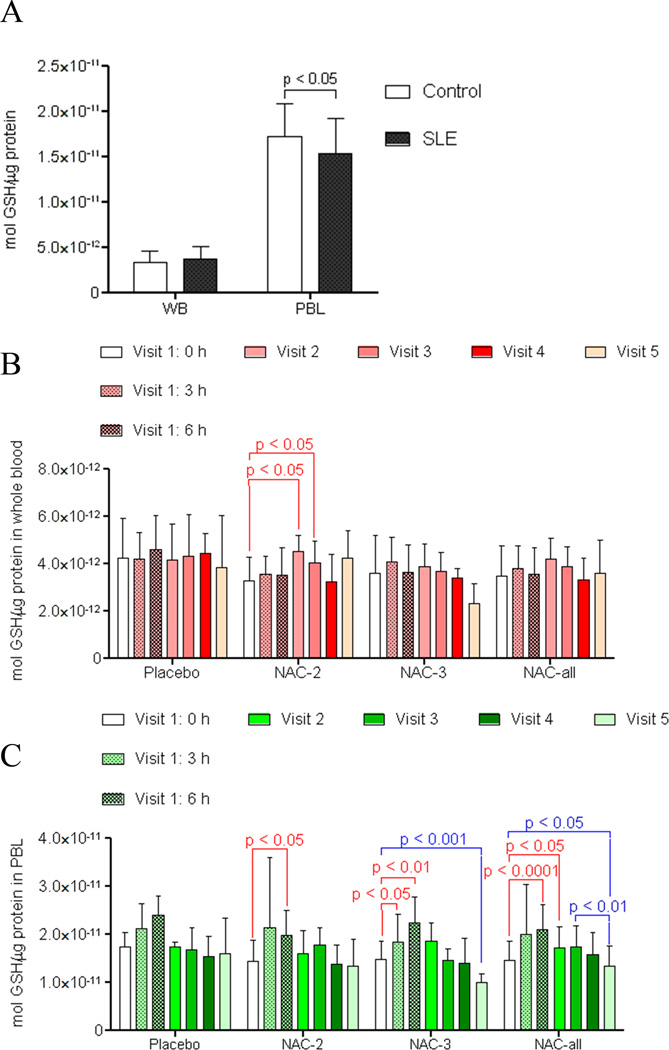
At baseline (visit 1, 0h), GSH was similar in whole blood of lupus and healthy donors. In contrast, GSH was reduced in lupus PBL (Fig. 2A). NAC treatment increased GSH in whole blood of SLE patients after 1 and 2 months (Fig. 2B). In SLE patients= PBL, GSH was increased by a single NAC dose of 1.2 g after 6h (p=0.022; Fig. 2C) and 2.4 g after 3h (p = 0.032) and 6h (p = 0.003; Fig. 2C). Although GSH levels between the 0 h and 6 h time points in the placebo group were statistically not significant (p = 0.053), we appreciated an upward trend that was attributed to the fact that the 0 h sample was obtained after fasting (~between 8 am and 9 am) while the 6 h post-NAC sample was obtained after 1 or 2 meals (between 2 pm and 3 pm). These changes were consistent with diurnal variation and peaking of GSH levels in the early afternoon hours due to nutritional factors (27). GSH was increased in SLE patients= PBL after 3-month treatment with NAC doses 2 and 3 considered together (visit 4; p = 0.027). After one month washout, GSH dropped below baseline in lupus PBL exposed to NAC dose 3 or doses 2 and 3 combined (Fig. 2C). Placebo did not influence GSH in whole blood or PBL (Fig. 2).
Fig. 2.
Effect of NAC on GSH of whole blood and PBL in patients with SLE. A) HPLC analysis of GSH in whole blood (WB) and peripheral blood lymphocytes (PBL) of untreated SLE patients (n=36) and healthy controls matched for age, gender, and ethnicity (n=42). p value reflects comparison with two-tailed unpaired t-test. B) Effect of NAC and placebo on GSH levels in whole blood of lupus patients. p values reflect comparison with two-tailed paired t-test. C) Effect of NAC and placebo on GSH levels in PBL of lupus patients. p values reflect comparison with two-tailed paired t-test.
NAC increases MHP, mitochondrial mass, and apoptosis of CD4−/CD8− double-negative (DN) T cells in SLE patients
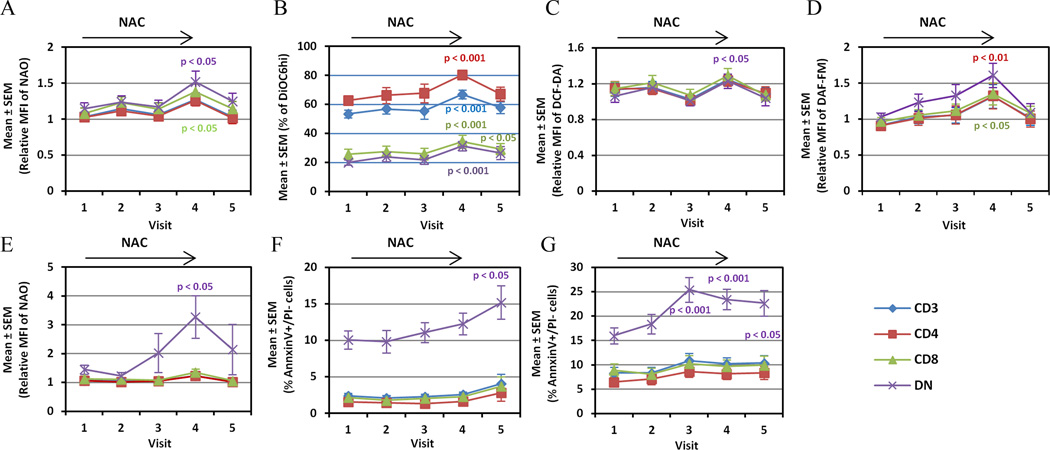
Similar to previous findings (5;28), MHP was detected in lupus T cells. Interestingly, NAC treatment progressively increased MHP of T cells during treatment (Fig. 3A). Mitochondrial mass (Fig. 3B) and H2O2 levels were increased in DN T cells after 3-month NAC treatment and declined after washout (Fig. 3C). These changes were attributed to enhanced production of NO (1.61 ± 0.17-fold after 3 months, p=0.002; Fig. 3D). Mitochondrial mass was also robustly increased in DN T cells following CD3/CD28 co-stimulation (Fig. 3E). Spontaneous apoptosis rate of DN T cells was progressively increased in NAC-treated patients from 10.1 ± 1.3% at baseline to 15.2 ± 2.3 % after 4 months (p = 0.035; Fig. 3F). CD3/CD28-induced apoptosis was increased in NAC-treated patients from 16.0 ± 1.6% at baseline to 25.4 ± 2.6 % after 2 months (p = 0.0006), 23.5 ± 2.1 % after 3 months (p = 0.0004), and 22.7 ± 2.6 % after 4 months (p = 0.0313; Fig. 3G). NAC moderated the expansion of DN T cells from 6.2 ± 0.5% at baseline to 5.3 ± 0.5 % after 3 months (p = 0.043). Mitochondrial homeostasis, oxidative stress, and apoptosis in T cell subsets of lupus patients were not affected by placebo (data not shown).
Fig. 3.
Mitochondrial homeostasis, oxidative stress, and apoptosis in T cell subsets of lupus patients exposed to all NAC doses considered together. Effect of NAC on Δψm (panel A, DiOC6 fluorescence), mitochondrial mass (panel B, NAO fluorescence), and H2O2 levels were measured in T cells rested in culture for 16 h (panel C, DCF fluorescence). NO production (panel D, DAF-FM fluorescence), and mitochondrial mass were measured in T cell subsets following CD3/CD28 stimulation for 16 h (panel E, NAO fluorescence). Panel F) Spontaneous apoptosis rate was enumerated by the percentage of Ann V+/PI− T cells after culture for 16 h. Panel G) Activation-induced apoptosis was assessed following CD3/CD28 co-stimulation for 16 h. Visits: visit 1, before 1st NAC dose; visit 2, after 1-month treatment; visit 3, after 2-month treatment; visit 4, after 3-month treatment; visit 5, after 1-month washout. p values reflect comparison to visit 1 using two-tailed paired t-test.
NAC blocks mTOR activation and stimulates FoxP3 expression in lupus T cells
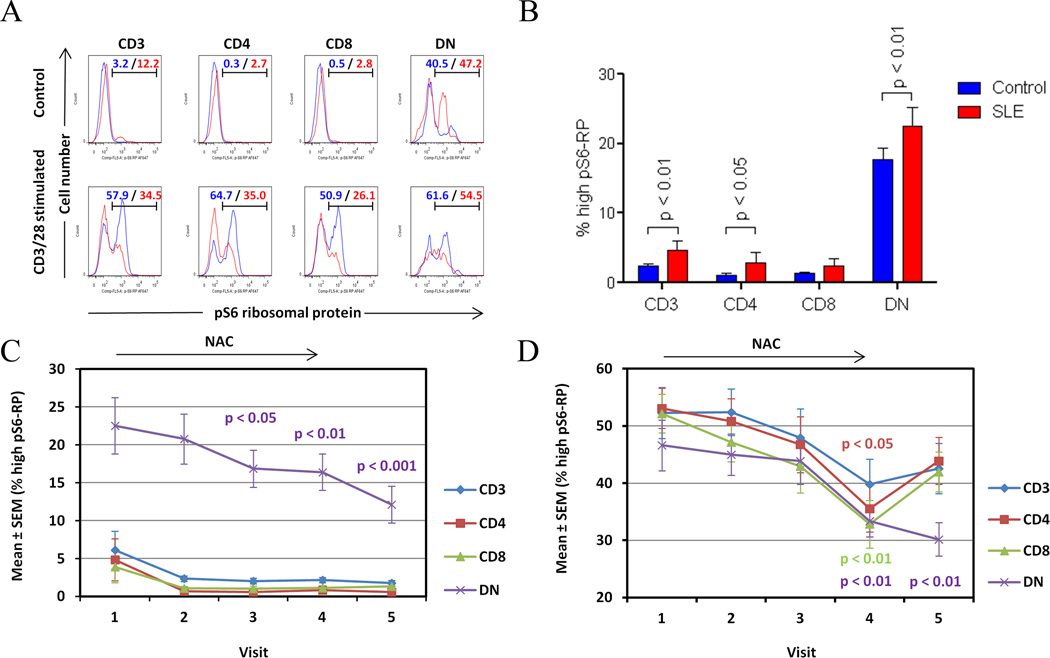
Suppression of mTOR by rapamycin (6) was associated with improved disease activity in SLE (14). Here, increased mTOR activity was evidenced by 2.2 ± 0.45-fold greater prevalence of pS6-RPhi T cells in SLE patients (p=0.007). The absolute frequency of pS6-RPhi T cells was greatest in the DN compartment (Figs. 4A and B). NAC depleted pS6-RPhi cells in the DN T-cell compartment from 22.5 ± 3.7% at baseline to 16.9 ± 2.5% after 2 months (p=0.0104), 16.4 ± 2.4% after 3 months (p=0.0095), and 12.1 ± 2.4% after 4 months (p=0.0009; Fig. 4C). NAC diminished CD3/CD28-induced mTOR activation in all T cells after 2 months and 3 months, which rebounded after washout (Fig. 4D). mTOR activity was not affected by placebo (data not shown). CD3/CD28-stimulated mTOR activity declined in DN T cells at visit 2 in the placebo group (data not shown), however, this effect did not show a sustained or progressive time course and, therefore, it was not considered biologically significant.
Fig. 4.
Detection of increased mTOR activity via phosphorylation of S6 ribosomal protein (pS6-RP) in T-cell subsets from lupus and matched controls. A) Assessment of pS6-RP in CD3+, CD4+, CD8+, and DN T cells from control (blue histograms) and lupus donors (red histograms). Blue/red values show the percentage of cell populations with increased mTOR activity in control and lupus T-cell subsets, respectively. B) Cumulative analysis of mTOR activity in T-cell subsets of all lupus patients relative to all healthy controls. Values represent mean ± SEM of cell populations with increased mTOR activity. p values reflect comparison of lupus and healthy donors with unpaired two-tailed t-test before treatment. C) Effect of NAC on mTOR activity measured by the prevalence of pS6-RPhi T cells in lupus patients exposed to all doses considered together. p values reflect comparison to pre-treatment visit 1 using two-tailed paired t-test. D) Effect of NAC on CD3/CD28-induced mTOR activity in T cell subsets of lupus patients exposed to all doses considered together. p values reflect comparison to pre-treatment visit 1 using two-tailed paired t-test.
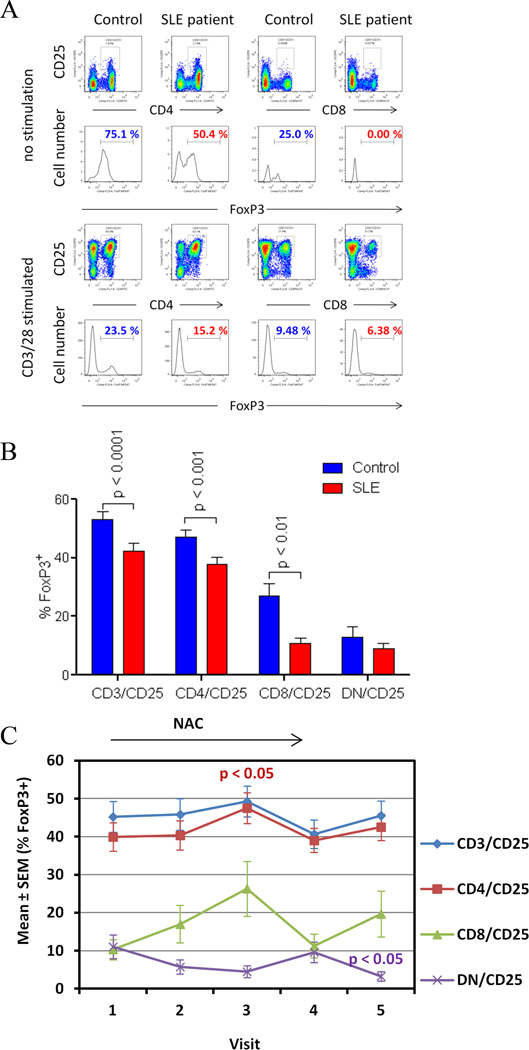
Before treatment, FoxP3+ cells were reduced within the CD25+ T-cell compartment of SLE patients relative to healthy controls (p = 6.0 × 10−5; Fig. 5A and B). FoxP3+ cells within the CD4+/CD25+ T-cell compartment were reduced at 37.8 ± 2.4% in SLE patients relative to 47.2 ± 2.3% in controls (p = 9.1 × 10−5; Fig. 5B). FoxP3+ cells were also reduced within the CD8+/CD25+ T-cell compartment at 10.7 ± 2.0% in SLE patients relative to 26.7 ± 4.4% in controls (p = 0.002; Fig. 5B). Since rapamycin expanded CD4+/CD25+/FoxP3+ T cells in patients with SLE (31), it was important to determine whether mTOR blockade by NAC affected FoxP3 expression. Indeed, the percentage of FoxP3+ cells within the CD4+/CD25+ T-cell compartment was increased in all NAC-treated patients (p=0.045; Fig. 5C). FoxP3 expression was not affected in patients exposed to placebo (data not shown). CD4+/CD25+/FoxP3+ T cells were expanded in patients exposed to 4.8 g/day NAC from 3.2 ± 0.6% at baseline to 5.2 ± 0.9% after 2 months (p=0.018). FoxP3 expression was also induced in CD25+ DN T cells from 1.29 ± 0.23% at baseline to 2.24 ± 0.38% after 2-month NAC treatment (p=0.0160).
Fig. 5.
Simulation of FoxP3 expression by NAC in lupus T cells. A) FoxP3 expression in CD4+/CD25+ and CD8+/CD25+ T cell subsets of lupus and control donors matched for age, gender, and ethnicity by flow cytometry. Red and blue values indicate percentage of FoxP3+ cells in lupus and control donors, respectively. B) Cumulative analysis of FoxP3 expression in CD25+ T-cell subsets in lupus subjects and matched controls. p values reflect comparison with two-tailed unpaired t-test. C) Effect of NAC on Foxp3 expression in CD25+ T cell subsets of lupus patients exposed to all doses considered together. p values reflect comparison with two-tailed paired t-test.
DISCUSSION
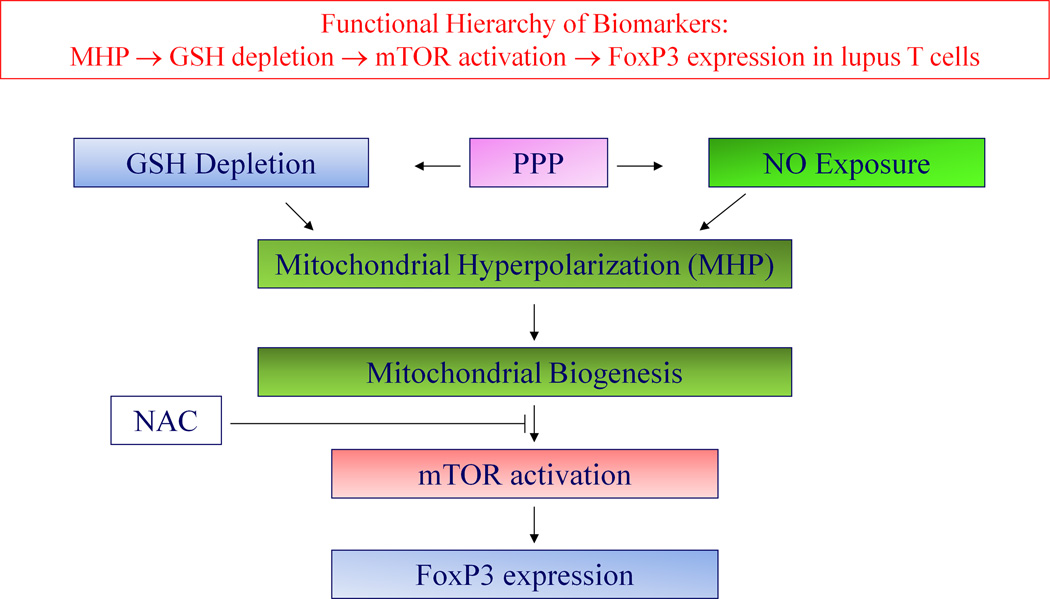
This double-blind placebo-controlled phase I/pilot study provides evidence that NAC is safe and tolerated by all SLE patients up to 2.4 g/day with reversible nausea in 33% of patients receiving 4.8 g/day. The low GSH in PBL, but not in whole blood, suggests that the metabolic dysfunction in lupus (9) is confined to the immune system. NAC increased GSH in PBL and, most importantly, it improved disease activity in SLE patients through a newly identified molecular mechanism: disruption of the MHP-mTOR pathway in T cells (Fig. 6).
Fig. 6.
Schematic functional hierarchy of metabolic biomarkers of T-cell dysfunction in patients with SLE, depicting the proposed site of impact by NAC. MHP is caused by exposure to nitric oxide (NO). De novo synthesis of NO and maintenance of GSH in reduced form are both dependent on the production of NADPH by the pentose phosphate pathway (PPP). MHP causes mTOR activation which in turn controls the expression of the transcription factor FoxP3.
Δψm is subject to regulation by an oxidation-reduction equilibrium of ROI, pyridine nucleotides (NADH/NAD + NADPH/NADP) and GSH (9). NAC may modulate MHP, directly, via neutralizing ROI or, indirectly, via sparing NADPH and promoting de novo GSH production. Interestingly, Δψm and mitochondrial mass were increased in T cells by NAC, particularly in the DN compartment, with reversal of these changes after washout. NAC-induced MHP occurred with a marked increase in NO production, which is required for mitochondrial biogenesis (28). In turn, NO production depends on the availability of NADPH; thus, increased NO production may have resulted from sparing of NADPH by NAC (9). Since mTOR is a sensor of Δψm and oxidative stress in lupus T cells (6) and its blockade by rapamycin was associated with improvement of disease activity in SLE patients (14), blocking of mTOR may be critical for the mechanism of action by NAC. Similar to the effect of rapamycin (31), suppression of mTOR by NAC was accompanied by increased FoxP3 expression in CD4+/CD25+ T cells. These results suggest that the effect of NAC on the immune system is 1) cell type-specific and 2) it occurs through disconnecting the activation of mTOR from the elevation of Δψm in lupus T cells, similar to the effect of rapamycin (14). Such direct inhibitory effect of NAC was confirmed by blocking of CD3/CD28 stimulation-induced mTOR activity in normal PBL upon pretreatment by NAC in vitro (data not shown).
MHP of lupus T cells, which most prominently affects DN T cells, was associated with resistance to activation-induced apoptosis (5). In 27 SLE patients receiving daily NAC doses of 1.2 g, 2.4 g, and 4.8 g considered together, both spontaneous and CD3/CD28-induced apoptosis of DN T cells were markedly increased and the expansion of these cells was effectively reversed. The elimination of DN T cells, which are known promote anti-DNA autoantibody production by B cells (29), is likely to contribute to reduced anti-DNA titers and to the efficacy of NAC.
The therapeutic importance of NAC for SLE is reflected by: 1) achieving clinical improvement in two validated disease activity scores within 3 months; 2) diminishing fatigue (21), which is considered the most disabling symptom in a majority of SLE patients (22); 3) absence of significant side-effects; and 4) affordability of this medication. A monthly supply of 600-mg NAC capsules (120–240 capsules) costs $15–$30 on the retail market. This sharply contrasts with average annual direct medical costs estimated to be ~$22,580 per patient in 2009 (32).Thus, the cost of NAC at $180–$360/year would be negligible in comparison to the overall expenditures to society and the expected benefit in reducing the need for vastly more expensive medications burdened with potentially serious side-effects. While this is a successful proof-of-concept study, it has limitations and warrants follow-up investigations in larger cohorts, including patients with severe lupus and extending the duration of treatment. In summary, the selectivity of NAC for mTOR activation in T cells provides a safe, inexpensive, alternative, mechanism-driven and potentially synergistic approach to B-cell blockade in SLE (1–3).
Supplementary Material
Acknowledgments
We would like to thank Drs. Fatme Allam and Hom Neupane for referring patients for our study and Dr. Travis Boevin and Melissa Reale for study drug packaging and excellent record keeping.
This work was supported in part by grants AT 004332 and AI 072648 from the National Institutes of Health, the Alliance for Lupus Research, and the Central New York Community Foundation.
REFERENCES
- 1.Francis L, Perl A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin Pharmacother. 2009;10:1481–1494. doi: 10.1517/14656560902971003. [DOI] [PubMed] [Google Scholar]
- 2.Lateef A, Petri M. Biologics in the treatment of systemic lupus erythematosus. Curr Opin Rheumatol. 2010;22:504–509. doi: 10.1097/BOR.0b013e32833b475e. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH. BLISS! Lupus learns its lessons. Lancet. 2011;377:693–694. doi: 10.1016/S0140-6736(10)61546-2. [DOI] [PubMed] [Google Scholar]
- 4.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–522. doi: 10.1097/01.bor.0000170479.01451.ab. [DOI] [PubMed] [Google Scholar]
- 5.Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arth Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, et al. Activation of mTOR controls the loss of TCRæ in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–20673. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah D, Aggarwal A, Bhatnagar A, Kiran R, Wanchu A. Association between T lymphocyte sub-sets apoptosis and peripheral blood mononuclear cells oxidative stress in systemic lupus erythematosus. Free Rad Res. 2011;45:559–567. doi: 10.3109/10715762.2011.555765. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez DR, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med. 2010;9:173–178. [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez D, Perl A. Metabolic control of T cell activation and death in SLE. Autoimmun Rev. 2009;8:184–189. doi: 10.1016/j.autrev.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 11.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 12.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T Regulatory Cell Function in Patients with Active Systemic Lupus Erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 13.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arth Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez D, Bonilla E, Mirza N, Perl A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arth Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18:21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 17.Wernerman J, Hammarqvist F. Modulation of endogenous glutathione availability. Curr Opin Clin Nutr Metab Care. 1999;2:487–492. doi: 10.1097/00075197-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione Levels and Sensitivity to Apoptosis Are Regulated by changes in Transaldolase expression. J Biol Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 19.Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 21.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Resp Crit Care Med. 1997;156:1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 22.Krupp LB, LaRocca NG, Muir J, Steinberg AD. A study of fatigue in systemic lupus erythematosus. J Rheumatol. 1990;17:1450–1452. [PubMed] [Google Scholar]
- 23.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, the committee on prognosis studies in SLE Derivation of the SLEDAI. A disease activity index for lupus patients. Arth Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 24.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Therapeut. 2005;12:133–141. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 25.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce JN, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology. 2005;44:902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 26.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 27.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, et al. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 28.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric Oxide-Dependent Mitochondrial Biogenesis Generates Ca2+ Signaling Profile of Lupus T Cells. J Immunol. 2004;173:3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- 30.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai Z, Telarico T, Bartos A, Miklossy G, Hanczko R, Jimah J, et al. Reversal of CD3/CD4/CD25/Foxp3 Treg Depletion in Active SLE Patients with Rapamycin. Arthritis & Rheumatism. 2010;62(Suppl 10):1185. [Google Scholar]
- 32.Zhu TY, Tam Ls, Lee VWY, Lee KKC, Li EK. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arth Care Res. 2009;61:1159–1167. doi: 10.1002/art.24725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.