Abstract
During Mycobacterium tuberculosis infection, a population of bacteria likely becomes refractory to antibiotic killing in the absence of genotypic resistance, making treatment challenging. We describe an in vitro model capable of yielding a phenotypically antibiotic-tolerant subpopulation of cells, often called persisters, within populations of Mycobacterium smegmatis and M. tuberculosis. We find that persisters are distinct from the larger antibiotic-susceptible population, as a small drop in dissolved oxygen (DO) saturation (20%) allows for their survival in the face of bactericidal antibiotics. In contrast, if high levels of DO are maintained, all cells succumb, sterilizing the culture. With increasing evidence that bactericidal antibiotics induce cell death through the production of reactive oxygen species (ROS), we hypothesized that the drop in DO decreases the concentration of ROS, thereby facilitating persister survival, and maintenance of high DO yields sufficient ROS to kill persisters. Consistent with this hypothesis, the hydroxyl-radical scavenger thiourea, when added to M. smegmatis cultures maintained at high DO levels, rescues the persister population. Conversely, the antibiotic clofazimine, which increases ROS via an NADH-dependent redox cycling pathway, successfully eradicates the persister population. Recent work suggests that environmentally induced antibiotic tolerance of bulk populations may result from enhanced antioxidant capabilities. We now show that the small persister subpopulation within a larger antibiotic-susceptible population also shows differential susceptibility to antibiotic-induced hydroxyl radicals. Furthermore, we show that stimulating ROS production can eradicate persisters, thus providing a potential strategy to managing persistent infections.
Infection with the bacterial pathogen Mycobacterium tuberculosis remains a leading infectious cause of death globally (1). In 2009, 1.7 million people died from tuberculosis (TB), with the majority of deaths occurring in the developing world (2). Successful treatment of active, symptomatic TB requires a minimum of 6 mo of combination therapy (typically four drugs) and is frequently complicated by drug toxicities (3). Treating clinically asymptomatic, latent infections, during which bacteria evade the host immune system and persist in infected individuals for decades, requires an even longer treatment course, typically 9 mo. Many patients infected with M. tuberculosis, both in the United States and globally, are thus unable to comply with the complex currently recommended therapeutic regimens (4). The rise of drug-resistant infection around the world further complicates successful TB treatment. It is estimated that 3.3% of new cases of TB are resistant to multiple standard TB drugs (2).
During both active and latent infection, it has been proposed that a subpopulation of bacteria enters a reversible nonreplicating state, refractory to traditional antibiotics (5). This phenomenon is characterized by the reduced efficacy of antibiotics against nonreplicating or slowly replicating bacteria in the absence of genotypic resistance (5–7). In vivo it has been observed when mice are infected with M. tuberculosis, and subsequently treated with antibiotics. In this infection model, antibiotics reduce bacterial cell numbers but do not sterilize the mouse (8). A plateau is typically reached during which numbers of viable bacteria stabilize. In addition to the mouse infection model, the inability to sterilize has been observed in the zebra fish (Mycobacterium marinum), guinea pig (M. tuberculosis), and macrophage (M. tuberculosis) infection models (9–11). In vitro, the survival of a similar small subpopulation can also be observed when a culture is exposed to high doses of antibiotics (12, 13). The surviving cells, often called persisters (14), retain genetic susceptibility to the antibiotic. The ability of M. tuberculosis to enter this physiological state, where intact cells lie dormant and survive despite exposure to bactericidal concentrations of antibiotics, may contribute to the need for long and complex treatment regimens to eradicate TB infection.
Many other human pathogens, including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, are similarly able to enter a drug-refractory state (14–17). Work in E. coli mutants has suggested that, in a bacterial population, persisters exist before antibiotic exposure (18). In contrast, there is also evidence to suggest that antibiotic tolerance can be induced in response to cellular stresses, including antibiotic treatment. For example, a small fraction of a population of E. coli, when faced with increasing levels of antibiotic, secretes the signaling molecule indole to induce transcriptional changes in neighboring cells, most notably in the upregulation of efflux pumps and oxidative stress protective mechanisms, resulting in antibiotic tolerance (19). Additionally, induction of the SOS response in E. coli has been shown to induce both β-lactam and fluoroquinolone antibiotic tolerance (20–22). Recent transcriptome profiling of persisters also revealed that several stress response regulons, including the SOS response, as well as several toxin–antitoxin (TA) genes, are upregulated in persister populations in E. coli (15) and M. tuberculosis (12). The mechanism by which TA loci may induce persistence in E. coli was recently described. Many toxin genes, activated by degradation of the cognate antitoxin, encode mRNases that rapidly degrade mRNA, stopping translation and inducing antibiotic tolerance (23).
Recent work has identified reactive oxygen species as an important antibiotic-induced cellular stress. These studies demonstrate that bactericidal antibiotics with a variety of different mechanisms of action increase ROS production within cells via the Fenton reaction (24–27). Numerous ROS, and in particular hydroxyl radicals, are toxic to cells and can result in cell death. Tolerance to antibiotics may therefore depend on the ability of the cell to defend itself against ROS, as suggested by several recent studies (28–30). For example, the coordinated stringent response to nutrient limitation in P. aeruginosa and E. coli was shown to increase antioxidant enzyme expression and decrease production of prooxidant molecules, resulting in antibiotic tolerance (28). Bacteria also produce nitric oxide (NO) as well as hydrogen sulfide (H2S), both of which result in antibiotic tolerance via suppression of the Fenton reaction as well as increased antioxidant enzyme expression in both Gram-positive and Gram-negative bacteria (29, 30).
One challenge in studying antibiotic-tolerant persister cell physiology has been the difficulty in reproducibly generating and isolating this small subpopulation from the much larger antibiotic-susceptible population. In this work, we describe a model capable of reliably producing a subpopulation of persisters in both M. tuberculosis and the nonpathogenic model organism Mycobacterium smegmatis. We used this model to examine factors important for the formation and survival of the persister population. We demonstrate that persister cells constitute a distinct subpopulation within the larger culture population. We found that the survival of persisters requires a small (20%) drop in dissolved-oxygen (DO) saturation. If the DO concentration is maintained at high levels, this population is killed over time. We demonstrate that the drop in oxygen saturation does not affect the survival of the dominant antibiotic-susceptible population, nor does the drop in DO signal persister cell formation. We hypothesize that high DO conditions may increase levels of ROS generated during antibiotic treatment, leading to killing of persisters. Supporting this theory we found that the hydroxyl-radical scavenger thiourea protects the persister subpopulation even when DO is maintained at high levels. Conversely, we used the antibiotic clofazimine (CFZ) to induce ROS generation and found that CFZ can successfully eradicate the persister population. Taken together these data suggest that the persister subpopulation has differential susceptibility to antibiotic-induced hydroxyl radicals compared with the larger, antibiotic-susceptible population. There is growing evidence for the critical role of ROS and oxidative damage in antibiotic tolerance in other populations that are refractory to antibiotic killing, and in this work we demonstrate the important role of ROS in a stochastic persister population model.
Results
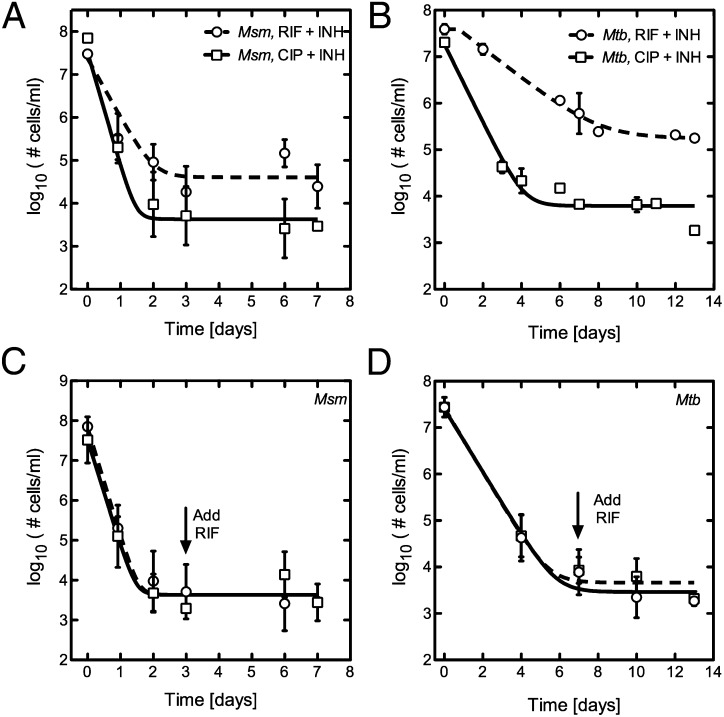
We initially sought to detect a subpopulation of persisters in a larger population of susceptible, logarithmically growing mycobacteria. Cultures of M. smegmatis were grown to stationary phase and then diluted in fresh media roughly 15-fold to an optical density (OD600) of 0.2. Cells were next exposed to pairs of bactericidal antibiotics with different modes of action to prevent the emergence of genetically resistant clones. For example, the DNA gyrase inhibitor ciprofloxacin (CIP) at a concentration well above the minimum inhibitory concentration (MIC) was paired with a bacteriostatic concentration of the cell-wall biosynthesis inhibitor isoniazid (INH). M. smegmatis bacilli treated in this manner show approximately four logs of killing over the first 24 h but exhibit no further reduction in cell number during the following seven days (Fig. 1A and Fig. S1A). We did not observe significant differences in the mean number of persisters identified when CIP was used alone versus in combination with low-dose INH (Fig. S1B). Similarly, treated M. tuberculosis bacilli show three logs of killing over the first four days before stabilization in cell number is achieved (Fig. 1B). Along with surviving in the antibiotic in which they were initially challenged, M. smegmatis and M. tuberculosis persisters also demonstrated tolerance to subsequent challenges with bactericidal levels of rifampicin (RIF) or streptomycin (STM), antibiotics with completely unrelated mechanisms of action (Fig. 1 C and D and Fig. S1C).
Fig. 1.
A system for identifying persister cells. (A) Kill kinetics for M. smegmatis cultures treated with CIP and INH or RIF and INH. (B) Similar killing followed by a stabilization of bacterial numbers is observed in M. tuberculosis cultures treated with CIP and INH or RIF and INH. (C) The M. smegmatis persister population generated with CIP and INH (dashed line, squares) is cross-tolerant to the addition of RIF added at a bactericidal concentration of 30 μg/mL (solid line, circles). (D) The M. tuberculosis persister population generated with CIP and INH (dashed line, squares) is cross-tolerant to the addition of RIF, added at day 7 to a bactericidal concentration of 0.1 μg /mL (solid line, circles).
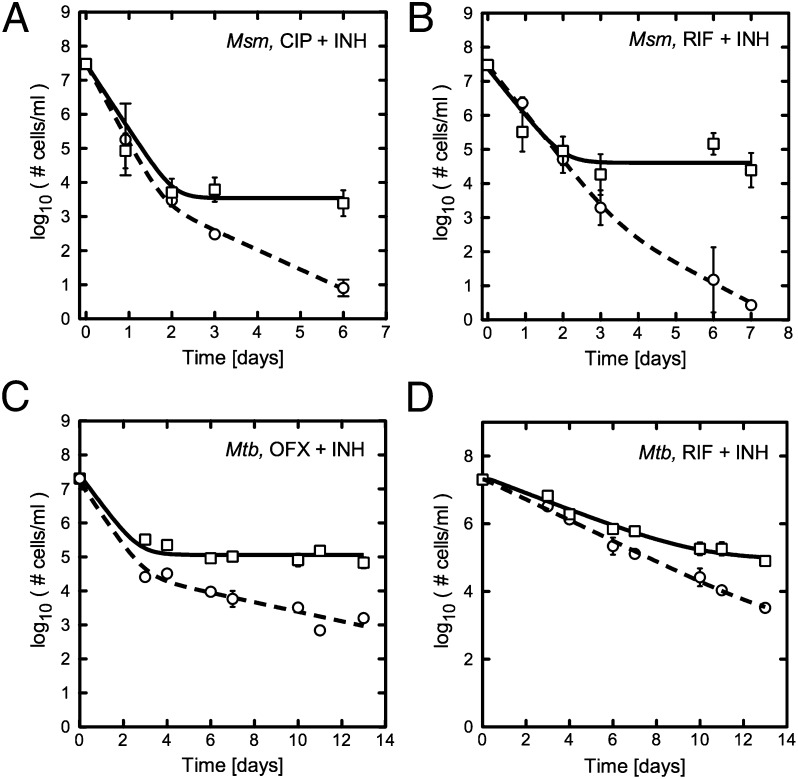
During these initial experiments to characterize the persister subpopulation, we observed substantial experiment-to-experiment variability in the size of the final persister population. In particular we noted that perturbation of the culture during sampling reduced the size of the persister population compared with undisturbed cultures (Fig. S2). This observation led us to hypothesize that the sampling process, which required removing a foil cap, thus introducing ambient air into the flask headspace, may increase the DO saturation of the culture, thereby affecting the number of persisters identified. To test this hypothesis, we characterized the M. smegmatis persister population in two controlled conditions in which oxygen saturations would not be affected by sampling. In the first system, a highly aerobic condition that we will refer to as the “open system,” an oxygen-permeable membrane was used to cover a shaking baffled Erlenmeyer flask, increasing the oxygen transfer rate into the media and resulting in an equilibrium state of high DO. [Note: At 37 °C, the maximum dissolved oxygen in water is ∼6.7 mg/L (31)]. In the second system, the sealed or “closed system” condition, a rubber septum was used to seal a flat-bottom Erlenmeyer flask (headspace/media ratio 5:1), thus preventing ongoing oxygen diffusion from the atmosphere. Consequently, oxygen levels within the flask drop as cell metabolism consumes oxygen within the media and headspace. In both models, a needle and syringe were used to sample the cultures for bacterial enumeration to minimize perturbation of the systems. In the closed system model, we found that for RIF/INH, CIP/INH, and STM/INH, the number of viable M. smegmatis cells stabilized two days after the initial kill in a biphasic manner, as we had previously observed. In contrast, bacilli in the continuously aerated system continued to die (Fig. 2 A and B and Fig. S3).
Fig. 2.
M. smegmatis and M. tuberculosis persister populations are not observed in maximally aerobic conditions. (A) M. smegmatis cells treated with CIP and INH are steadily killed in open, aerated flasks (dashed line) and those in closed, septum-sealed flasks stabilize at 0.01% of the population (solid line). (B) Similar behavior is observed for M. smegmatis exposed to RIF and INH. (C) M. tuberculosis cells treated with OFX and INH exhibit biphasic killing with continued death of cells in aerated flasks (dashed line) and those in septum sealed flasks stabilize (solid line). (D) Similar behavior is observed for M. tuberculosis exposed to RIF and INH.
To understand whether M. tuberculosis cultures were similarly sensitive to the DO system, we treated cells in tightly capped square media bottles with either a headspace ratio of 4:1, designed to approximate septum-sealed conditions, or a comparatively aerated 60:1 ratio. Similar to what we had observed with M. smegmatis, in M. tuberculosis the reduced oxygen condition exhibited slower killing and a stable persister population, in contrast to the more aerated condition where the number of survivors steadily declined (Fig. 2 C and D). To determine whether the functional relationship between DO and antibiotic susceptibility of persisters is specific to mycobacteria we tested the Gram-negative pathogen P. aeruginosa as well as E. coli using our model system (SI Materials and Methods). We found, similar to what was observed in mycobacteria, that fewer cells survive antibiotic treatment in the maximally aerated conditions compared with the septum-sealed conditions (Fig. S4). These results demonstrate that in vitro models used to identify persister populations are extremely sensitive to changes in oxygen tension. In addition, these observations suggest that high levels of DO may either prevent the formation or interfere with the maintenance of the persister population.
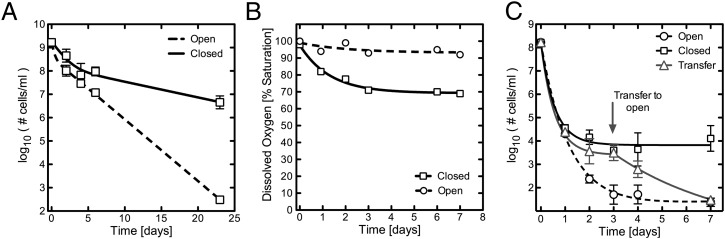
In stationary phase a subpopulation of bacteria also exhibit phenotypic antibiotic tolerance, thought to be due to nutrient exhaustion and changes in the pH of the media (5, 32). Given our finding that persisters identified within a freshly diluted culture are sensitive to antibiotic killing in the presence of sufficiently high DO levels, we next sought to determine if the antibiotic-tolerant population in a fully stationary phase, undiluted culture also becomes susceptible to antibiotic treatment when placed in highly aerated conditions. A culture of M. smegmatis was grown to stationary phase, transferred into either closed or open conditions, and then treated with bactericidal concentrations of CIP and INH. Over three weeks of treatment, stationary phase cultures in the closed system exhibited only a modest two-log drop in census, similar to what has been reported previously for stationary phase cultures (33). In contrast, the treatment of stationary phase cultures in the open system resulted in a reduction in census of more than five orders of magnitude to the lower limit of detection. Based on the observation that in a stationary phase culture the antibiotic-tolerant subpopulation loses antibiotic tolerance when oxygen levels are sufficiently increased, we conclude that tolerance is not due solely to nutrient limitation or changes in pH (Fig. 3A).
Fig. 3.
Increased availability to oxygen allows killing of persister cells. (A) M. smegmatis stationary phase cultures treated in open, aerobic conditions (dashed line) lose more than five logs of viability over three weeks, while cultures maintained in closed, septum-sealed flasks (solid line) during antibiotic treatment lose only two logs of viability. (B) Dissolved-oxygen saturation remains greater than 90% in open, aerated flasks (dashed line) while closed, septum-sealed flasks (solid line) become moderately depleted in oxygen, with DO saturation dropping to 75%. (C) An established M. smegmatis persister population in a closed, septum-sealed flask is transferred to open, aerated conditions at day 3. After transfer the persister population immediately begins losing viability.
Hypoxia has previously been shown to play a role in inducing antibiotic tolerance in M. tuberculosis (34). In the most-studied hypoxia model, commonly referred to as the Wayne model, antibiotic tolerance in the bulk population appears as cells adapt to microaerophilic (DO saturation ∼ 1%) and anaerobic (DO saturation ≤ 0.06%) conditions. To determine the degree of oxygen depletion in the experiments described above (Fig. 2) we measured the dissolved-oxygen saturation in both the open and closed models. To our surprise we found only a 20% difference in DO saturation between the two conditions. In the closed system, the oxygen saturation fell over three days before stabilizing at 75%, but in the open system, oxygen saturations equilibrated around 95% (Fig. 3B). Thus, in contrast to the previously described Wayne model, our data demonstrate that very small decreases in DO are sufficient to promote bacterial survival, albeit of a small persister subpopulation, in the presence of bactericidal antibiotic concentrations. We next tested whether decreasing the inoculum, which should decrease the rate of oxygen consumption and therefore lead to higher DO saturation, would decrease the size of the persister subpopulation. Indeed, we found that with a 10-fold lower starting inoculum, we did not identify a stable persister population above the level of detection (Fig. S5), perhaps in part explaining the “inoculum effect” (35). To further confirm the effect that increasing oxygen levels has on the ability of the antibiotics to kill the persister population, we transferred M. smegmatis persisters generated in the closed system to the open system. This transfer into aerated conditions led to the subsequent death of persisters (Fig. 3C), thus demonstrating that persister cells are in fact vulnerable or can become vulnerable to antibiotics under conditions of higher DO saturation.
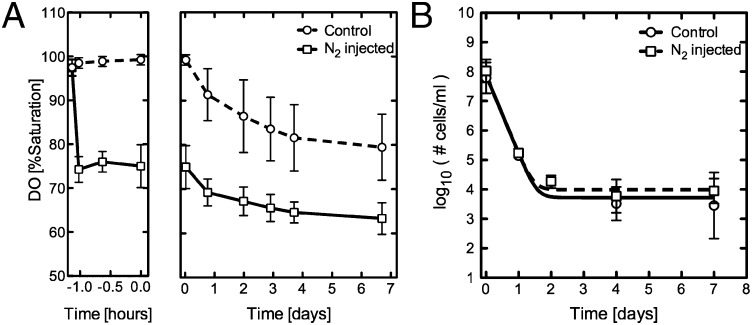
The observation that a 20% drop in DO saturation, the result of metabolic oxygen consumption, results in a stable persister population suggests that the reduction in oxygen saturation either stimulates persister formation or facilitates the survival of a preexisting bacterial population alive at the time of oxygen depletion. To differentiate between these scenarios, we tested whether preadapting a population of cells to lower DO levels might result in a higher percentage of persisters or even induce antibiotic tolerance in the entire population. We used gas displacement with nitrogen gas to preadapt cultures to 75% DO saturation for one hour before treating with high concentrations of CIP and INH (Fig. 4A). Following antibiotic treatment the oxygen-displaced flasks fell from 75% DO saturation to 60% DO saturation over the next four days, while the control flasks dropped from 100 to 80% oxygen saturation (Fig. 4A). Despite these differences in DO, the kill kinetics and mean size of the persister populations for the oxygen-displaced flasks were statistically indistinguishable from the control flasks seven days after treatment (Fig. 4B, t test P value ∼ 0.67). These data suggest that reduced oxygen levels are not driving increased persister cell formation before antibiotic exposure but instead are facilitating the survival of persisters. Furthermore, because the reduction of the DO level by nitrogen displacement did not change the initial first phase of killing compared with nondisplaced cultures, the 20% drop in DO saturation appears to have no effect on the survival of the dominant antibiotic-susceptible population. We therefore conclude that within the bulk culture there is a bacterial subpopulation (which forms the second-phase plateau) whose sensitivity to subtle changes in oxygen when challenged with antibiotics differs from the majority of the population.
Fig. 4.
Preadaptation to reduced DO levels does not enhance persister cell formation or survival. (A) The DO saturation of control septum-sealed flasks (dashed line) or nitrogen-injected septum-sealed flasks (solid line) containing M. smegmatis cells treated with CIP and INH. Data points represent the average of eight replicates. Error bars represent SDs. (B) M. smegmatis cells in control septum-sealed flasks (dashed line, circles) or nitrogen-injected septum-sealed flasks (solid line, squares) are treated with CIP and INH. Similar kill kinetics and persister population size are observed.
This observation, combined with recent work demonstrating both the role of ROS in antibiotic killing and cellular antioxidant responses in antibiotic tolerance, led us to speculate that the sensitivity of the persister population to changes in oxygen levels may be linked to changes in the number of toxic free radicals generated with antibiotic treatment. Exposure to bactericidal antibiotics has been found to result in the intracellular generation of toxic free radicals, resulting in bacterial cell death. Oxygen diffuses freely across cell membranes (36), resulting in an intracellular concentration in equilibrium with that of the surrounding media, with previous measurements demonstrating that reactive oxygen species are generated in direct proportion to oxygen concentration (36, 37). The intracellular free-radical concentration following antibiotic exposure is therefore predicted to be higher in the open model compared with the closed model. To confirm this hypothesis we used the fluorescent reporter dye 3′-(p-hydroxy-phenyl) fluorescein (HPF) to measure relative hydroxyl-radical levels in norfloxacin-treated E. coli cells in maximally aerated media compared with media with a DO level of 75%. As expected, we found higher levels of hydroxyl radicals in maximally aerated media (P value below 0.0002, Fig. S6, SI Materials and Methods).
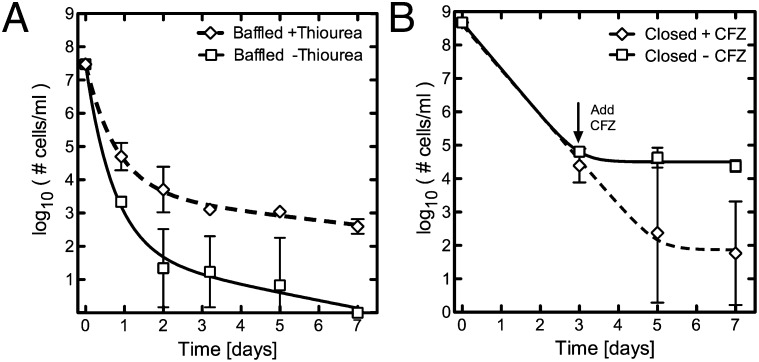
To test the role of free radicals in the killing of both the predominant antibiotic-susceptible population and in the smaller persister subpopulation, we blocked the effects of hydroxyl radicals after CIP treatment using the free-radical quencher thiourea (24) in the aerated, open system. We found that in high DO conditions, thiourea successfully restored the biphasic kill kinetic and an antibiotic-tolerant population was again identified (Fig. 5A). We also observed a reduced kill kinetic for the larger antibiotic-susceptible population, consistent with earlier work demonstrating the role of ROS in antibiotic killing in E. coli (24). From these data we conclude that under high DO conditions, the persister population is killed following antibiotic treatment as a consequence of an increased number of toxic hydroxyl radicals generated with antibiotic treatment.
Fig. 5.
Modulating the free-radical concentration affects the number of persisters identified. (A) M. smegmatis cells treated with CIP and INH in open, aerated conditions in the presence (dashed line) or absence (solid line) of the free-radical quencher thiourea at a concentration of 150 mM. The presence of thiourea restores a biphasic kill kinetic under open aerobic conditions and a persister population is again identified. (B) The M. smegmatis persister population identified after antibiotic treatment with CIP and INH is killed with the addition of CFZ at day 3.
We conversely hypothesized that increasing hydroxyl radicals within cells could successfully eradicate the persister population in the closed system. We used the antibiotic clofazimine, which results in increased intracellular ROS production due to redox cycling (38). CFZ, recently identified as a competing substrate for the enzyme NDH-2 within the electron transport chain, is reduced by NDH-2 and subsequently oxidized by O2, resulting in increased ROS (38). When we challenged persisters with CFZ, the number of surviving persisters decreased by three orders of magnitude, contrasting with the tolerance observed for rifampin and streptomycin (Fig. 5B). In the presence of thiourea the addition of CFZ did not change the number of persisters in a statistically significant manner, suggesting that CFZ is killing the persister population through increased intracellular ROS generation (Fig. S7, t test P value ∼0.42). These data, combined with the nitrogen displacement data demonstrating that subtle changes in oxygen saturation do not affect the survival of the dominant antibiotic-susceptible population, suggest a model in which persisters respond differently to antibiotic-induced hydroxyl radicals compared with the larger antibiotic-susceptible population, except under maximal DO conditions.
Discussion
The important role that oxygen plays in antibiotic killing of bacteria has also long been recognized, bolstered by observations that in abundance it can improve antibiotic efficacy, and when absent can result in complete antibiotic tolerance (24, 34). We now find that much smaller changes in oxygen tension than previously described affect the antibiotic killing of bacterial persisters. Although these small reductions in oxygen tension do not alter the kill kinetic of the larger antibiotic-susceptible population, these changes dramatically affect the size and survival ability of the smaller persister subpopulation. In an environment where dissolved oxygen saturations are high, this subpopulation rapidly dies and can no longer be identified. We observe the same relationship between oxygen and antibiotic sensitivity in Gram-negative bacteria (P. aeruginosa and E. coli), which demonstrates that this phenomenon is not unique to mycobacteria. These results suggest that the persister subpopulation differs from the larger population in its ability to withstand hydroxyl radicals generated by antibiotic challenge. Previous work in E. coli has demonstrated that antibiotic killing in susceptible populations is mediated by hydroxyl radicals (24), in part through oxidation of the guanine nucleotide pool (39). With the addition of thiourea we find that the kill kinetic of the bulk population is reduced, suggesting that hydroxyl radicals may similarly mediate antibiotic killing in mycobacteria and that persisters may survive antibiotic challenge due to a unique capacity to withstand the generation of toxic free radicals. This ability of persisters to survive when the DO level is reduced is in contrast to the larger, susceptible population. When DO levels are near saturation, however, the protective mechanisms in persisters are insufficient and they succumb to antibiotic challenge. When decreased free-radical concentrations are achieved either through a reduction in oxygen saturation or the addition of thiourea, a free-radical quencher, the drug-tolerant persister subpopulation is able to survive in the presence of otherwise bactericidal antibiotic concentrations. In contrast, strategies to increase cellular ROS, such as the addition of the antibiotic CFZ, results in killing of the drug-tolerant population. Antibiotic-generated hydroxyl-radical–mediated killing may therefore represent an Achilles’ heel of persistence.
The impact small changes in oxygen tension have on the maintenance and survival of persisters may explain inconsistencies within the M. tuberculosis persister literature. Recently, two studies were published with conflicting results concerning the enrichment of drug-tolerant persisters in late exponential and stationary phase cultures (12, 13), with one identifying an enrichment of persisters in these cultures while the other did not. Similarly, there are discrepancies regarding the cross-tolerance of M. tuberculosis persisters to heterologous drugs. Based on the data presented here, the origin of these divergent observations may lie in differing culture densities, which impacts the rate of oxygen depletion and oxygen saturation at the time of treatment, thereby changing antibiotic efficacy.
Within its human host, M. tuberculosis sometimes survives within low-oxygen physical niches, which may further protect M. tuberculosis from hydroxyl-radical-mediated antibiotic killing. The most extreme example is the granuloma, a collection of M. tuberculosis-infected macrophages and immune cells, which was recently demonstrated to possess a hypoxic core with an oxygen tension of 3 mm Hg (40). Because phenotypic drug tolerance is observed in vitro under hypoxic conditions, it has been suggested that standard TB therapy is likely ineffective in the granuloma. This work suggests that much more subtle changes in oxygen tension may dramatically reduce antibiotic efficacy with the goal of sterilization. During in vivo infections, M. tuberculosis likely encounters a gradient of oxygen tensions in tissue that range from 100 mm Hg in the alveolus to 60 mm Hg in normal lung tissue to 3 mm Hg in the center of a granuloma (40, 41). The data presented here suggest that effective sterilization of M. tuberculosis may be limited by the gradual decline in oxygen tension even before hypoxic conditions are obtained.
There is a growing body of evidence demonstrating the important role that ROS play in bactericidal antibiotic–mediated killing (24–26). This understanding of how bactericidal antibiotics result in cell death raises the hypothesis that drug tolerance may be mediated by increased abilities within a cell to detoxify ROS. Recent work has suggested that antibiotic tolerance of the bulk population, induced by changes in the environment, specifically starvation, may be the result of enhanced antioxidant strategies (28). Our work focuses on the small persister subpopulation within a larger culture, and we demonstrate that this subpopulation is differentiated from the larger antibiotic-susceptible population by different sensitivities to hydroxyl-radical-mediated damage from antibiotic exposure. Our findings are supported by recent work demonstrating that E. coli persisters produce fewer hydroxyl radicals following antibiotic challenge, and a possible mechanism is suggested by work demonstrating that E. coli secretes the signaling molecule indole that activates oxidative stress responses and increased drug tolerance (19, 42, 43). Although the lack of oxygen in anaerobic or microaerophilic conditions has long been known to affect the efficacy of antibiotics, we demonstrate that just a 20% change in oxygen saturation significantly affects antibiotic efficacy by allowing the survival of a subpopulation of cells. At the same time, we show that this subpopulation remains vulnerable to the common antibiotic-induced hydroxyl-radical–mediated death pathway if sufficiently high free-radical concentrations can be maintained, induced for example by the antibiotic clofazimine. Previous efforts toward eradicating persister populations have focused on identifying small molecules that specifically kill this population, or which potentiate antibiotic efficacy in this population (44, 45). Next-generation approaches to antibiotic discovery may lie in identifying small molecules that potentiate hydroxyl-radical formation or subvert cellular mechanisms that protect against hydroxyl-radical damage (38, 46). This work now argues that such approaches may have the important added benefit of eliminating persister populations, thus sterilizing an infection (46).
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions.
M. smegmatis was grown at 37 °C in Middlebrook 7H9 broth supplemented with 10% ADS (albumin-dextrose-saline, vol/vol), 0.2% glycerol, and 0.05% Tween-80. The M. smegmatis mc2155 strain expressing GFP contains an episomal pUV15tetORm derivative in which the repressor tetR was removed, allowing constitutive GFP expression (47). M. tuberculosis H37Rv was grown at 37 °C in Middlebrook 7H9 broth supplemented with 10% OADC (oleic acid-albumin-dextrose complex, vol/vol), 0.2% glycerol, and 0.05% Tween-80, or on Middlebrook 7H10 plates supplemented with 10% OADC enrichment. All drugs and antibiotics for this study were from Sigma-Aldrich.
Antibiotic Killing Experiments in M. smegmatis.
Freezer stocks of mc2155 were diluted 1:2,000 in 7H9 medium and cultured for three days unless otherwise specified. Cultures were then diluted ∼15-fold in prewarmed media to an OD600 of 0.2 and antibiotics added. Experiments were conducted in 125-mL flat-bottom Erlenmeyer flasks covered with either a rubber septum (Sigma-Aldrich) or 125-mL baffled Erlenmeyer flasks covered with an oxygen-permeable membrane (AeraSeal; Excel Scientific). At various time points 1 mL of cells were collected using a 1-mL syringe connected to a 16-gauge needle. The viability of cells is measured using a most probable number (MPN) assay (48) when possible or plating for colony-forming units otherwise. In some experiments, to increase sensitivity at low cell densities, cells were washed in fresh media. For the MPN assay, cells were aliquoted into Costar 96-well black clear-bottom plates and then serially diluted 10-fold. After 10 d, plates were read in M5 SpectroMax plate reader using GFP fluorescence as the readout. The MPN was calculated using 4–8 replicates (49). Unless otherwise specified, antibiotics were used at the following concentrations: ciprofloxacin 5 μg/mL, isoniazid 40 μg/mL, rifampin 30 μg/mL, streptomycin 2.5 μg/mL, and clofazimine 40 μg/mL. For heterologous drug challenge, the second antibiotic was diluted in 0.5 mL fresh media and injected on day 3 through the septum. For the purposes of calculating the geometric mean and SD, samples with measurements below the threshold of detection (30 cells/mL) were assumed to contain 1 cell/mL All graphed data points in figures represent the average of three biologic replicates and the error bars represent the SDs, except for the initial time point in MPN experiments, which reflects a single measurement. Data in figures were fitted to single or double exponential decays plus an additive constant in cases where cell populations plateaued. In some cases, fits were constrained to a constant before decaying exponentially to mimic the delay in action of some drug combinations or the delayed physical addition of a drug. When multiple conditions came from a common starter culture, line fits were constrained to begin with the same initial number of cells. Confidence intervals of fit parameters were typically quite large, limiting our ability to compare kinetic parameters between conditions.
Antibiotic Killing Experiments in M. tuberculosis.
Freezer stocks of H37Rv were diluted 1:50 in fresh 7H9 media and cultured until late log phase, OD600 between 0.6 and 1.0. Cultures were then diluted to an OD600 of 0.2 and either 12 or 0.75 mL (for maximally aerobic conditions) aliquoted into square media bottles (Nalgene, 30 mL). Three duplicate media bottles were set up for each time point measured. Antibiotics were added at the desired concentration. At indicated time points 300 μL of cells were removed from each media bottle being assayed, and because unsealing of the cap can affect oxygenation, cultures were then discarded. Collected cells were washed once in fresh media, serially diluted, and plated on 7H10 plates. Colonies were counted at day 20 and 25. For heterologous drug challenge, square media bottles were covered with rubber septa (Sigma) and rifampin at a concentration of 0.1 μg /mL in 0.5 mL of fresh media was injected through the septum. Unless otherwise specified, antibiotics were used at the following concentrations: ciprofloxacin 8 μg /mL (10 × MIC), ofloxacin 1 μg /mL (10 × MIC), rifampin 0.1 μg /mL (10 × MIC), and INH 0.1 μg /mL (1 × MIC).
Dissolved-Oxygen Measurements.
Dissolved oxygen levels within flasks were determined using the Fluorometrix Cellphase Dissolved Oxygen Sensors (DO-1). The oxygen-sensing patches were attached to the bottoms of 125-mL Erlenmeyer flasks before autoclaving and setting up the culture. The oxygen-sensing patches were calibrated to media at 37 °C. All measurements were obtained at 37 °C.
Nitrogen Displacement.
Experiments were conducted in 125-mL flat-bottom Erlenmeyer flasks covered with rubber septa (Sigma). After adding cells to each flask, a 16-gauge needle was inserted into each septum to prevent pressurization. After 30 min at 37 °C, 40 mL of nitrogen gas or mock room air was injected into the flask via a second needle. Both needles were then removed.
Supplementary Material
Acknowledgments
We thank T. Kawate and J. Gomez for helpful discussions. B.B.K. thanks the Massachusetts General Hospital Executive Committee on Research and New York Community Trust Heiser Postdoctoral Fellowships for support. S.S.G. is supported by National Institutes of Health Grant K08 AI085033. N.H. is supported by National Human Genome Research Institute Grant T32 HG002295.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203735109/-/DCSupplemental.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . TB Fact Sheet. Geneva: WHO; 2010. [Google Scholar]
- 3.Sahbazian B, Weis SE. Treatment of active tuberculosis: Challenges and prospects. Clin Chest Med. 2005;26:273–282, vi. doi: 10.1016/j.ccm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Espinal MA. The global situation of MDR-TB. Tuberculosis. 2003;83:44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 5.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Warner DF, Mizrahi V. Tuberculosis chemotherapy: The influence of bacillary stress and damage response pathways on drug efficacy. Clin Microbiol Rev. 2006;19:558–570. doi: 10.1128/CMR.00060-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar N, McKinney J. Microbial phenotypic heterogeneity and antibiotic tolerance. Tuberculosis. 2007;84:228–238. doi: 10.1016/j.mib.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 8.McCune RM, Jr, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 10.Adams KN, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:1–15. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad Z, et al. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis. 2009;200:1136–1143. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 12.Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of M. tuberculosis persisters. mBio. 2011;2:e00100–e00111. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Barry CE, 3rd, Boshoff HI. The three RelE homologs of Mycobacterium tuberculosis have individual, drug-specific effects on bacterial antibiotic tolerance. J Bacteriol. 2010;192:1279–1291. doi: 10.1128/JB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigger JW. Treatment of staphylococcal infections with penicillin. Lancet. 1944;244:497–500. [Google Scholar]
- 15.Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol. 2004;186:8172–8180. doi: 10.1128/JB.186.24.8172-8180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood DN, Chaussee MA, Chaussee MS, Buttaro BA. Persistence of Streptococcus pyogenes in stationary-phase cultures. J Bacteriol. 2005;187:3319–3328. doi: 10.1128/JB.187.10.3319-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2006 doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 18.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 19.Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller C, et al. SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science. 2004;305:1629–1631. doi: 10.1126/science.1101630. [DOI] [PubMed] [Google Scholar]
- 21.Dörr T, Vulić M, Lewis K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010;8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dörr T, Lewis K, Vulić M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maisonneuve E, Shakespeare LJ, Jørgensen MG, Gerdes K. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci USA. 2011;108:13206–13211. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolodkin-Gal I, Sat B, Keshet A, Engelberg-Kulka H. The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics. PLoS Biol. 2008;6:e319. doi: 10.1371/journal.pbio.0060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen D, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: A universal defense against antibiotics in bacteria. Science. 2011;334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 30.Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United States Geological Survey Dotables Available at http://water.usgs.gov/software/DOTABLES/
- 32.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- 33.Herbert D, et al. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2296–2299. doi: 10.1128/aac.40.10.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook I. Inoculum effect. Rev Infect Dis. 1989;11:361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- 36.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2007;77:755–766. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassett DJ, Imlay JA. Bactericidal antibiotics and oxidative stress: A radical proposal. ACS Chem Biol. 2007;2:708–710. doi: 10.1021/cb700232k. [DOI] [PubMed] [Google Scholar]
- 38.Yano T, et al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: A pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West JB. Respiratory Physiology. 5th Ed. Baltimore: Williams & Wilkins; 1998. p. 62. [Google Scholar]
- 42.Kim JS, et al. Bacterial persisters tolerate antibiotics by not producing hydroxyl radicals. Biochem Biophys Res Commun. 2011;413:105–110. doi: 10.1016/j.bbrc.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 43.Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat Chem Biol. 2012;8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JS, et al. Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic-sensitive cells. Antimicrob Agents Chemother. 2011;55:5380–5383. doi: 10.1128/AAC.00708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allison KR, Brynildsen MP, Collins JJ. Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr Opin Microbiol. 2011;14:593–598. doi: 10.1016/j.mib.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrt S, et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005;33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cochran WG. Estimation of bacterial densities by means of the “most probable number”. Biometrics. 1950;6:105–116. [PubMed] [Google Scholar]
- 49.Blodgett R. Most probable number from serial dilutions. Bacteriological Analytical Manual. 2010. Available at http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm109656.htm. Accessed June 26, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.