Cardiac hypertrophy is a precursor to the development of heart failure and an independent clinical risk factor for sudden death and myocardial infarction.1 Although energetic deficits are associated with clinical heart failure, their relative contribution to the transition from cardiac hypertrophy to heart failure has been challenging to elucidate, in large part because of the unpredictability of the timing of this transition and limitations of studying human cardiac energetics in vivo. Nevertheless, our understating of this pathophysiology is being illuminated using experimental models.
In the normal heart, the oxidation of exogenous fats is the major fuel source, with additional significant contributions from glucose and lactate. The relative contribution of fats diminishes with enhanced reliance on glucose utilization during the development of cardiac hypertrophy. The regulatory programs attenuating fat utilization have been extensively investigated and include regulation at the transcriptional and posttranscriptional levels.2–5 Moreover, this reduction involves coordinate downregulation of proteins controlling fatty acid uptake by the heart and mitochondria as well as of the enzymes controlling mitochondrial fatty acid β-oxidation (FAO).2,6–9 The increased utilization of glucose is similarly regulated at multiple levels and interestingly, at least with robust hypertrophy, the coupling of glycolysis and pyruvate oxidation becomes disrupted with an insufficient increase in glucose oxidation to completely compensate for the reduced FAO.10,11 The mechanisms orchestrating this uncoupling have not been fully delineated, although do not appear to result from disruption in the pyruvate dehydrogenase complex activity.12 With advancing cardiac hypertrophy, perturbations in substrate partitioning and selection become associated with reduced contractile reserve and increased susceptibility to ischemia-reperfusion injury.10,12,13
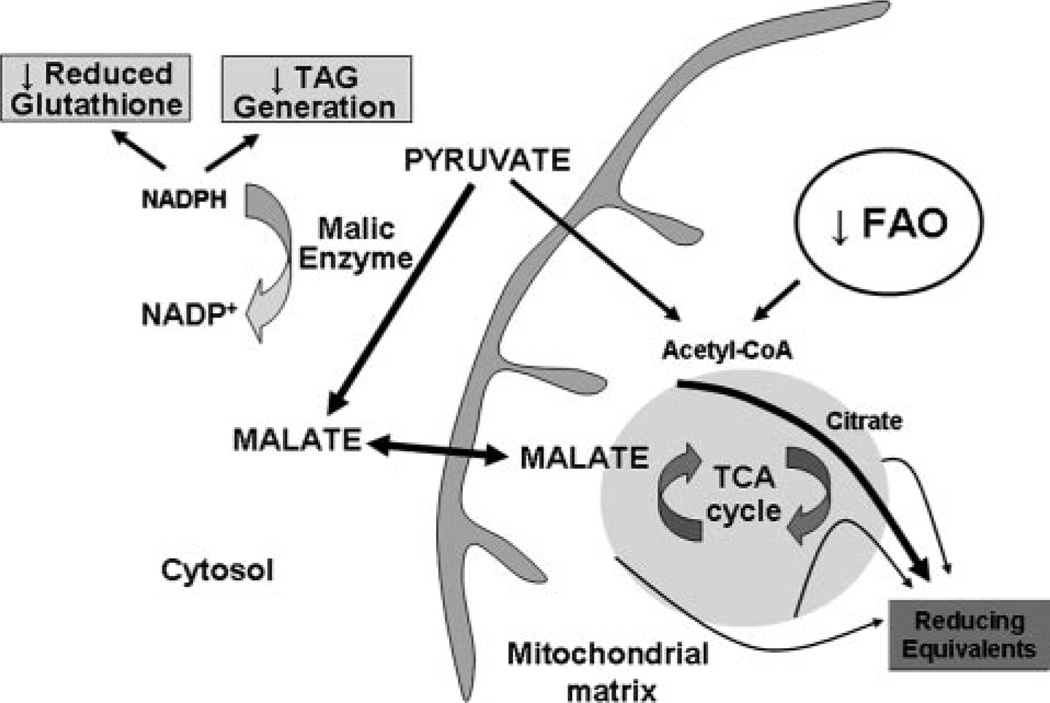
Partial compensation for this energy substrate oxidation deficit has recently been identified to occur via recruitment of alternative intermediary pathways to enhance flux through the tricarboxylic acid (TCA) cycle.8 This intermediary pathway is called anaplerosis, a term etymologically derived from Greek, meaning to “fill up,” and generally describes metabolic pathways that replenish intermediates in metabolic cycles.14 An anaplerotic entry point via malate feeding into the TCA cycle is shown in Figure. Although theoretically, TCA cycle intermediates are maintained by the incorporation of acetyl-coenzyme A and the generation of carbon dioxide and reducing equivalents, there is some physiological/pathological leakage through the mitochondrial membrane.15 Anaplerotic substrates, therefore, “refill” the cycle to maintain TCA cycle flux and ATP production.
Figure.
Schematic of pathway perturbations by increased malic enzyme–driven anaplerosis. The arrow line thickness and text size reflect the hypothetical relative reaction rates and the putative deficits in reducing equivalent generation and reduction in NADPH-dependent pathways associated with malic enzyme carboxylation of pyruvate in cardiac hypertrophy.
The necessity of anaplerosis in the heart is well established, in that the mechanical performance of isolated rat hearts when exclusive precursors of acetyl-coenzyme A are used as substrate shows progressive deterioration with rapid restoration following introduction of anaplerotic substrates.16 The regulation of anaplerosis in the heart is only beginning to be explored. The gene encoding for malic enzyme, which catalyzes the carboxylation of pyruvate to form malate, a TCA cycle intermediate, is shown to be upregulated in concordance with increased cardiac hypertrophy associated anaplerosis.8 Despite this increase in intermediary metabolism, cardiac efficiency is incompletely restored with a persistent deficit in the PCr/ATP ratio compared to control hearts.8
In this issue of Circulation Research, Pound et al add further insight into the consequences of enhanced intermediary metabolism in the hypertrophied heart.17 Here, the authors confirm in isolated perfused rat hearts that pressure overload–induced cardiac hypertrophy results in diminished contractile function in association with a significant increase in malic enzyme linked anaplerosis compared to nonhypertrophied control hearts.17 The authors further note that in association with this increased anaplerosis, cardiac triacylglycerol (TAG) stores are significantly depleted. Because cytosolic NADPH is required to reduce fatty acyl dihydroxyacetone phosphate as a first step in TAG synthesis, and is consumed by malic enzyme during carboxylation of pyruvate, the authors propose that the reduction in TAG is directly linked to accelerated anaplerosis and cytosolic NADPH consumption. To test this, these authors used dichloroacetate to activate pyruvate dehydrogenase complex. This intervention diminished anaplerotic flux and increased TAG levels in association with increased glucose oxidation and reduced FAO without altering cardiac mechanical function.17 These data show that anaplerosis is partially reversible and support the idea that increased malic enzyme orchestrated anaplerosis diminishes cardiac TAG.17 However, whether the dichloroacetate-mediated reduction in FAO under these experimental conditions diverts fat toward lipogenesis has not been investigated.
It has previously been shown in pressure overload–induced heart failure the TAG stores are depleted and that TAG oxidation to facilitate energy transduction is not operational.18 It would be intriguing to surmise that anaplerosis contributes to this phenotype. In contrast, in obese and diabetic subjects and animal models, heart failure is associated with increased intramyocardial triglyceride levels.19 Moreover, excess fat supply to the heart evokes a pathological outcome as shown by the development of lipotoxic cardiomyopathy in numerous genetic models.20–22 The role and consequence of anaplerosis in these “fat excess heart failure syndromes” would also be important to ascertain. Interestingly, the role of anaplerosis and fat-excess has been indirectly evaluated using metabolomic profiling of metabolic intermediates in skeletal muscle in response to high-fat feeding.23 Here, high-fat feeding depletes TCA cycle intermediates,23 suggesting that, at least in skeletal muscle, fat excess may perturb anaplerosis. These data could imply that disrupted anaplerotic metabolism may contribute to cardiac lipotoxicity, although this hypothesis too requires investigation.
An interesting hypothesis proposed by Pound et al is that malic enzyme-mediated consumption of NADPH may additionally limit cytosolic NADPH for the reduction of glutathione. 24 Although this was not directly investigated, a prior study demonstrated an increased ratio of reduced to oxidized glutathione in cardiac hypertrophy. This is thought to contribute to the antioxidant defense program against hypertrophy associated oxidative damage.25 Whether anaplerosis contributes to the depletion of reduced glutathione in the progression to heart failure25 and/or potentially to the enhanced cardiac hypertrophy ischemia susceptibility are interesting hypotheses that warrant exploration. A schematic highlighting the potential consequences of enhanced malic enzyme-mediated anaplerosis in the hypertrophied heart is shown in the Figure.
In conclusion, characterization and delineation of the molecular mechanisms governing modulation in substrate partitioning and pathway activities in cardiac hypertrophy and heart failure should enable the design of rational therapies to modulate cardiac metabolism with potential salutary effects. The study by Pound et al17 not only provides novel insight into the role of anaplerosis in cardiac hypertrophy but additionally generates interesting hypotheses that need to be pursued to further enhance our understanding of the adaptive and/or maladaptive consequences of anaplerosis in cardiac hypertrophy, in heart failure and possibly in fat excess–induced cardiac mechanical dysfunction.
Acknowledgments
Sources of Funding
Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH.
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B, Pfeffer MA, Re RN, Roccella EJ, Savage D, Shub C. The heart in hypertension. N Engl J Med. 1992;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- 2.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid enzyme encoding gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 3.Sack MN, Disch DL, Rockman HA, Kelly DP. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci U S A. 1997;94:6438–6443. doi: 10.1073/pnas.94.12.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 6.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 7.van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- 8.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 9.Allard MF, Schonekess B, Henning SL, English DR, Lopaschuck GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PG, Allard MF, Thomas GD, Bishop SP, Digerness SB. Increased ischemic injury but decreased hypoxic injury in hypertrophied rat hearts. Circ Res. 1990;67:948–959. doi: 10.1161/01.res.67.4.948. [DOI] [PubMed] [Google Scholar]
- 11.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 12.Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, Allard MF. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res. 2002;53:841–851. doi: 10.1016/s0008-6363(01)00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allard MF, Wambolt RB, Longnus SL, Grist M, Lydell CP, Parsons HL, Rodrigues B, Hall JL, Stanley WC, Bondy GP. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. Am J Physiol. 2000;279:E487–E493. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- 14.Gibala MJ, Young ME, Taegtmeyer H. Anaplerosis of the citric acid cycle: role in energy metabolism of heart and skeletal muscle. Acta Physiol Scand. 2000;168:657–665. doi: 10.1046/j.1365-201x.2000.00717.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 16.Russell RR, III, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol. 1991;261:H1756–H1762. doi: 10.1152/ajpheart.1991.261.6.H1756. [DOI] [PubMed] [Google Scholar]
- 17.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, LaNoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate–enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content. Attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–322. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 20.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 25.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol. 1994;266:H1280–H1285. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]