Abstract
Polarized neurites, axons and dendrites, form the functional circuitry of the nervous system. Secreted guidance cues often convert the polarity of neuron migration and neurite outgrowth by regulating ion channels. Here, we show that secreted semaphorin 3A (Sema3A) converts the neurite identity of Xenopus spinal commissural interneurons (xSCINs) by activating CaV2.3 channels (CaV2.3). Sema3A treatment converted the identity of axons of cultured xSCINs to that of dendrites by recruiting functional CaV2.3. Inhibition of Sema3A signalling prevented both the expression of CaV2.3 and acquisition of the dendrite identity, and inhibition of CaV2.3 function resulted in multiple axon-like neurites of xSCINs in the spinal cord. Furthermore, Sema3A-triggered cGMP production and PKG activity induced, respectively, the expression of functional CaV2.3 and the dendrite identity. These results reveal a novel mechanism by which a guidance cue controls the identity of neurites during nervous system development.
Developing neurons become polarized to form axons and dendrites1–3. How immature neurites of developing neurons acquire identities as axons/dendrites is unexplained. It was recently revealed that extracellular signaling molecules induce the axon identity4, 5, but it remains unknown whether they also induce the dendrite identity, and if so, how.
In vivo, acquisition of an identity as either an axon or dendrite occurs at different phases of neuronal polarization; a developing neuron initially acquires an axon extending from the soma and subsequently either a single dendrite as in C. elegans6 or multiple dendrites as in most vertebrates1, 2. Dendrites develop from undifferentiated neurites that emanate either directly from the soma, e.g., of cerebellar granule cells2, or from the tip of the leading process, e.g., of cortical pyramidal neurons2, or from the axonal initial segment (AIS), e.g., of xSCINs7, 8. For example, after xSCIN axons cross the midline of the floor plate and bifurcate into ascending and descending axon branches on the opposite side of the lateral fascicle, multiple neurites extend toward the pial surface and ventrally into the marginal zone from the AIS at tailbud stages 32–34 (ref. 7). How these undifferentiated neurites acquire their dendrite identity is unknown. It is likely that specific, spatiotemporally-regulated extracellular signals activate intracellular signalling pathways to specify the dendrite identity.
Extracellular guidance factors can induce either attraction or repulsion of growth cones9, 10, or reverse the direction of neuron migration11 by causing changes in intracellular Ca2+ levels, suggesting that external factors trigger early signalling events that initiate cellular processes by regulating ion channels. Sema3A, a secreted molecule, functions in several neuronal processes, including the orientation and maturation of dendrites12–15. Sema3A binds to its receptor, neuropilin-1 (Npn-1), in Xenopus spinal neuron growth cones and triggers local production of cGMP by soluble guanylate cyclase (sGC)16. Depending on their intracellular cGMP level ([cGMP]i), growth cones are either repelled from or attracted toward Sema3A; low [cGMP]i induces repulsion16 through the activation of cyclic nucleotide-gated cation channels17 and high [cGMP]i induces attraction16, 18 through the activity of an unidentified channel(s). High level Sema3A expressed at the pial surface of the neocortex contributes to the attraction of dendrites12. Interestingly, a relatively high level of Sema3A is expressed in the marginal zone of the Xenopus spinal cord at tailbud stages 30–32 (ref. 17), immediately before the emergence of putative dendrites from the AIS of spinal cord CINs (ref. 7). Here, we report that, both in vitro and in vivo, Sema3A induces the dendrite identity and simultaneously suppresses the axon identity of xSCINs by regulating cGMP signalling and the expression of functional CaV2.3 channels (CaV2.3).
RESULTS
Sema3A converts the identity of axons to dendrites in vitro
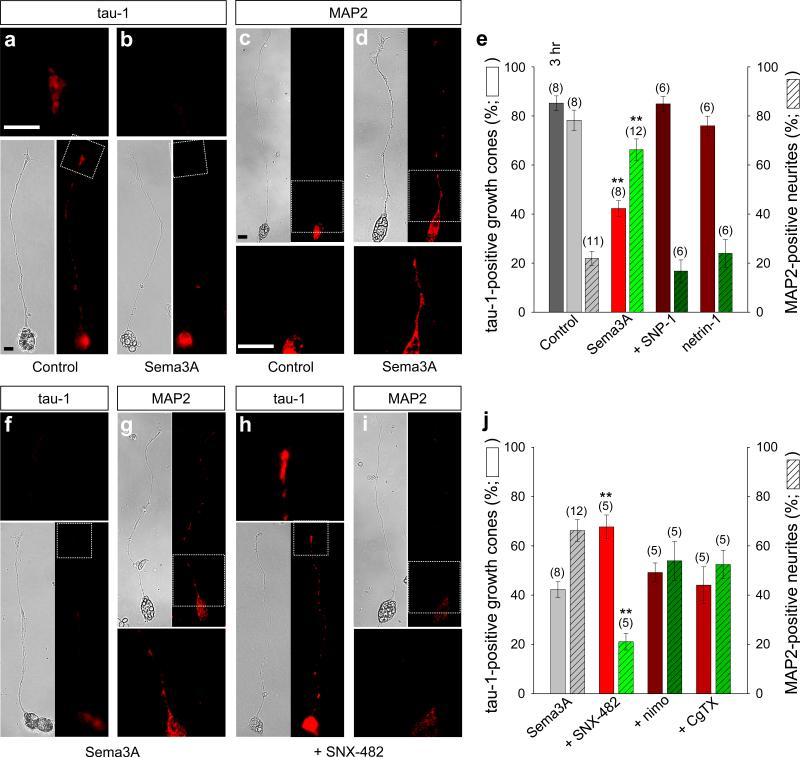
Unlike cultured hippocampal neurons19, cultured xSCINs from stage 26 embryos16, 17 have neurites that are predominantly mono- (Fig. 1a–d, f–i) or bi-polar (Supplementary Fig. S1a, b), without substantial branches (Supplementary Fig. S2) or other distinguishing features of axons and dendrites. When cultured on poly-l-lysine for 16 hours, most neurons were unpolarized, immunonegative for both axonal markers tau-1 and GAP-43 and dendritic marker MAP2 (Supplementary Fig. S3a, b). When cultured on laminin for either three or six hours, most of these unpolarized neurites became tau-1-positive axons20 (Fig. 1a, e). However, when these neurites were treated with Sema3A for three hours, the tau-1 immunoreactivity (IR) was markedly decreased (Fig. 1b, e) while MAP2-IR was significantly increased (Fig. 1d, e). Both changes were prevented by bath-applied SNP-1, the soluble Npn-1 ectodomain peptide that sequesters Sema3A (Fig. 1e)21, indicating the specificity of Sema3A. Sema3A promotes the outgrowth and branching of dendrites of cortical pyramidal neurons13, 14 and axotomy causes conversion of pre-formed axons of cultured hippocampal neurons to dendrites22, 23. We did not observe significant morphological changes in the length or branching of xSCIN neurites after Sema3A treatment (Supplementary Fig. S2). Netrin-1, another secreted factor that effects axon initiation in C. elegans4, had no significant effect on the expression of tau-1- or MAP2-IR (Fig. 1e). We also monitored functional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors, which are present predominantly in dendrites22, 24, by measuring holding current shifts induced by a gradient of AMPA. Significant AMPA currents were detected in almost all growth cones treated with Sema3A, but only in less than 30% of control growth cones (Supplementary Fig. S4). Thus, Sema3A likely initiates conversion of the identity of axons to dendrites before morphological changes occur.
Figure 1.
Sema3A converts the identity of axons to dendrites by CaV2.3 activity. (a–d, f–i) Bright field (left) and immunofluorescence (right) images of xSCINs cultured on a laminin substrate for six hours under various experimental conditions. Insets, magnified fluorescent images of the same sections in either top or bottom. Untreated (Control; a, c), treated with Sema3A (2 U·ml–1; b, d, f, g), or with Sema3A and SNX-482 (1 μM, +SNX-482; h, i), immunostained with tau-1 (a, b, f, h) or MAP2 (c, d, g, i). Scale bars, 10 μm. (e, j) Summaries of the percentage of tau-1-positive growth cones and MAP2-positive neurites that emerged from the soma. Neurons were treated with Sema3A and SNP-1 (20 nM, +SNP-1; e), with netrin-1 (5 μg·ml–1; e), with Sema3A and SNX-482 (j), with Sema3A and nimodipine (20 μM, +nimo; j), or with Sema3A and ω-conotoxin GVIA (1 μM, +CgTX; j). The dark grey bar = tau-1 positive growth cones cultured on a laminin substrate for 3hrs (e). Data are mean ± s.e.m. (n) = number of cultures (ca. 210 to 450 neurons with neurites of ca. 100–250 μm long) examined. Significant differences from Control (e) and Sema3A (j) are indicated (**p < 0.01), respectively.
To determine whether conversion of the neurite identity initiates within the growth cone, we applied a gradient of Sema3A to one growth cone of bipolar neurons (Supplementary Fig. S1a). The majority of those neurites that faced the source of the Sema3A became MAP2-positive dendrites but those that faced away from the source remained MAP2-negative (Supplementary Fig. S1b, c). Downstream signalling events induced by Sema3A in the growth cone, therefore, are likely sufficient to initiate conversion of the neurite identity.
Sema3A-induced conversion of neurite identity requires CaV2.3
Guidance cues induce growth cone membrane potential shifts; attractants cause depolarization and repellents cause hyperpolarization16. A relatively high concentration of Sema3A induced growth cone depolarization of ca. 15 mV from the resting potential of control growth cones, but not in growth cones pre-treated with SNP-1 (data not shown) or that overexpressed a mutant Npn-1 [NP-1 (0111)]16, 17 (Supplementary Fig. S5b, c). This 15 mV depolarization should be sufficient to cause Ca2+ entry through voltage-dependent Ca2+ channels. Functional L-, N-, R (CaV2.3)- and T-type Ca2+channels, but not P/Q types25, have been detected in cultured embryonic Xenopus spinal neurons26–29. We used a computed biophysical growth cone model17 to identify those Ca2+ channels that would be active preferentially during Sema3A-induced membrane depolarization. This model predicted that mainly CaV2.3 would conduct Ca2+, that T-types would undergo voltage-dependent inactivation, and that activation of L-/N-types would require further depolarization (Supplementary Fig. S5d). We then examined the effect of CaV2.3 on the ability of Sema3A to induce the dendrite identity. A specific CaV2.3 inhibitor, SNX-482, in the bath, indeed, prevented conversion of the tau-1-positve axons to the MAP2-positive dendrites (Fig. 1h–j), but inhibitors of neither L-types (nimodipine) nor N-types (ω-conotoxin GVIA) prevented the conversion (Fig. 1j). Thus, in vitro, Sema3A-induced conversion of xSCIN axons to dendrites depends on CaV2.3 activity.
Sema3A recruits functional CaV2.3
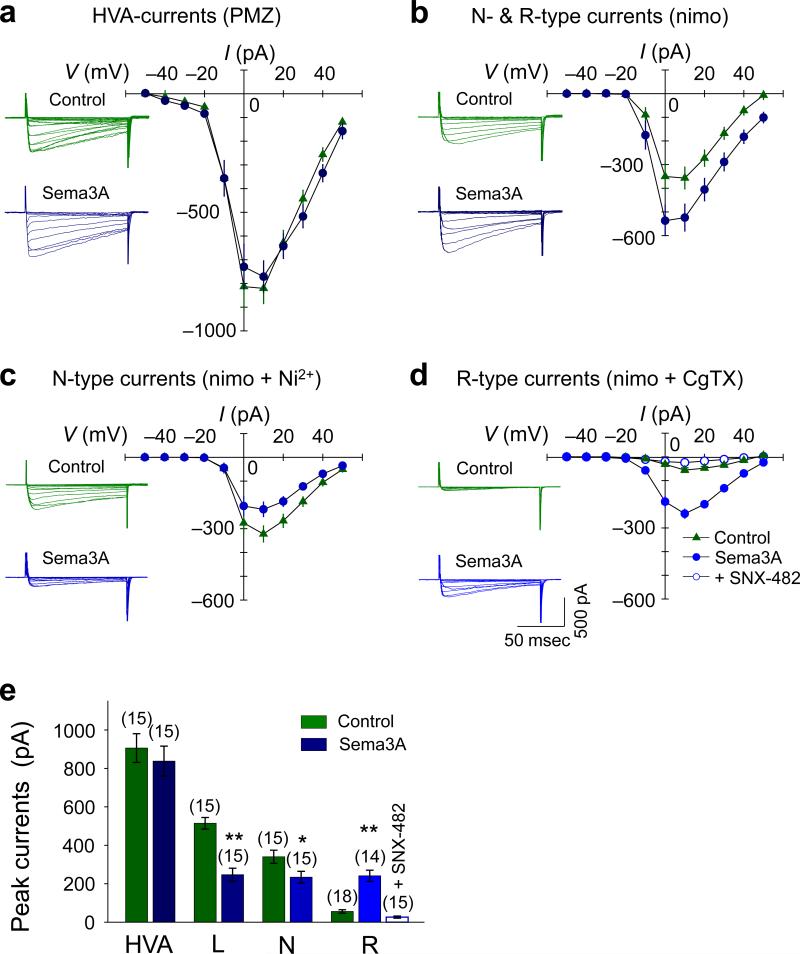
To determine whether Sema3A causes activation of CaV2.3, we measured Ca2+ currents in the growth cone17, 29 in the presence of a gradient of Sema3A. High voltage-activated (HVA) Ca2+ currents were measured in the presence of bath-applied pimozide, a T-type channel blocker. We then isolated N-type and CaV2.3 currents by blocking residual T-and L-type currents with nimodipine29. Sema3A caused an increase in total N-type and CaV2.3 currents (Fig. 2b, e) without affecting the magnitude of total HVA currents (Fig. 2a, e), indicating that Sema3A suppressed L-type currents. We further isolated the Sema3A-induced growth cone Ca2+ currents using Ni2+ (CaV2.3 and T-type channel blocker) and nimodipine and found that N-type currents were significantly decreased (Fig. 2c, e). In contrast, Sema3A caused a 4–5 fold increase in CaV2.3 currents isolated in the presence of nimodipine and ω-conotoxin GVIA (Fig. 2d, e) that were abolished by bath-applied SNX-482 (Fig. 2d, e). Therefore, Sema3A caused an increase in the active pools of CaV2.3 that conduct Ca2+ during the ca. 15 mV membrane depolarization (Supplementary Fig. S5d) and suppressed L-/N-type currents. Since Sema3A did not alter the activation/inactivation time constants of the CaV2.3 currents (Supplementary Table S1), it likely caused an increase in the number of CaV2.3 in the growth cone plasma membrane rather than modulating their gating properties (Supplementary Fig. S5e).
Figure 2.
Sema3A increases growth cone CaV2.3 currents. (a–d) Representative traces of Ca2+ currents (left) and summaries of the current (I)/ voltage (V) relationship (right) in the presence of 500 nM pimozide (PMZ, total HVA currents; a), 20 μM nimodipine (nimo, N- & R-type (Cav2.3) currents; b), 20 μM nimo + 300 μM Ni2+ (N-type currents; c), 20 μM nimo + 1 μM ω-conotoxin GVIA (CgTX, Cav2.3-type currents; d). (e) Average peak Ca2+ currents shown in a–d. Cav2.3 currents were enhanced from 56 ± 4 pA to 246 ± 19 pA. Data are mean ± s.e.m. (n) = number of growth cones examined for each experimental condition. Significant differences from the control (culture medium) are indicated (*p < 0.05; **p < 0.01).
Expression of CaV2.3in vivo requires Sema3A
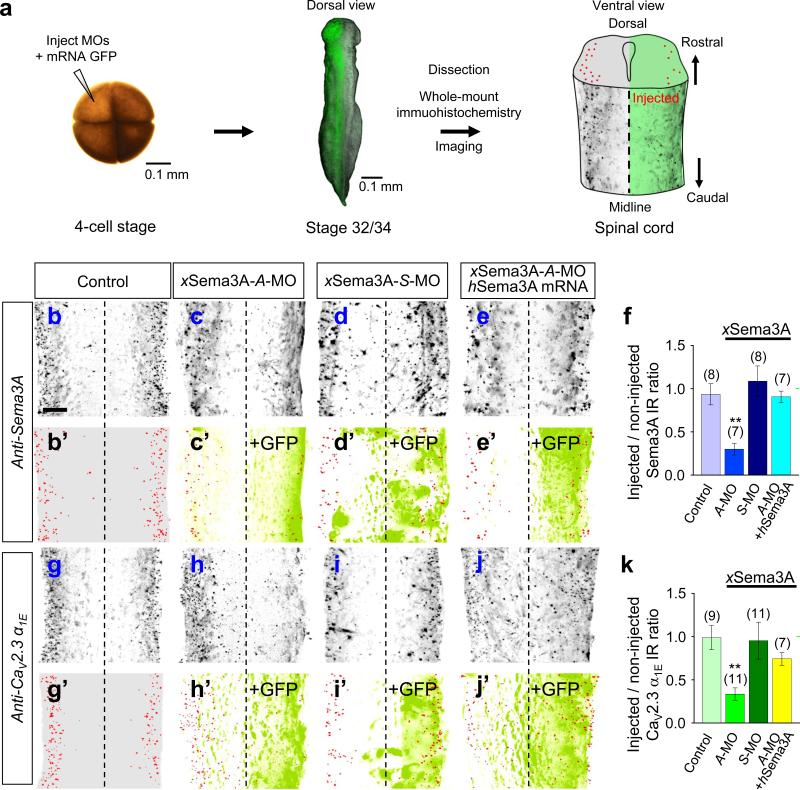
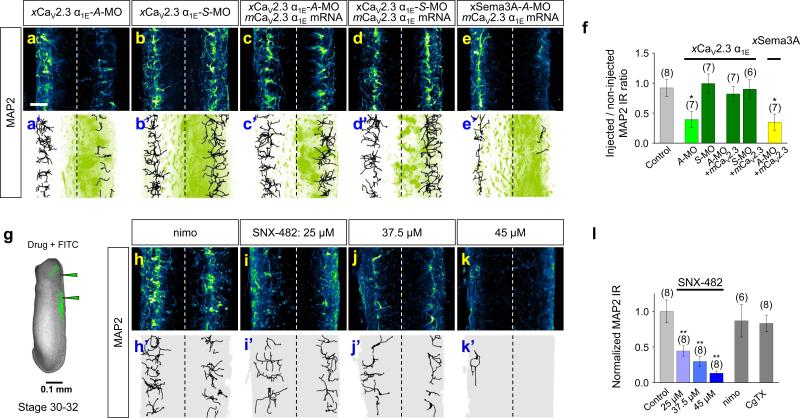
To examine the role of Sema3A in CaV2.3 function in vivo, we first tested the effect of down-regulating endogenous Sema3A by injecting an antisense-morpholino oligonucleotide (A-MO) against Xenopus Sema3A (xSema3A) mRNA [xSema3A-A-MO; a sense-MO (xSema3A-S-MO) served as a control] into one dorsal blastomere of four-cell stage embryos along with GFP mRNA as an injection marker. We then immunostained whole-mounts30 and imaged the immuno- and GFP fluorescence (Fig. 3a) to compare the MO-injected side of the spinal cord with the uninjected side in the same embryo (Fig. 3a) and also with uninjected control embryos. Immunostaining with a polyclonal antibody against human Sema3A (hSema3A) revealed a high level of bilateral expression of xSema3A in stage 30–32 spinal cords (Fig. 3b, b’) that was reduced significantly by xSema3A-A-MO (Fig. 3c, c’, f and Supplementary Movie S1), but not by xSema3A-S-MO (Fig. 3d, d’, f), attesting to the specificity of the A-MO. The reduction of xSema3A-IR was reversed by the injection of hSema3A mRNA along with xSema3A-A-MO (Fig. 3e, e’, f), confirming the specificity of the Sema3A antibody.
Figure 3.
Sema3A is required for CaV2.3 expression in Xenopus spinal cords. (a) Schema depicting experimental paradigms. Injection of morpholino oligonucleotides (MOs) or mRNAs encoding either a green fluorescence protein (GFP) marker or hSema3A into one dorsal blastomere of a 4-cell stage embryo (left) to treat one side of the spinal cord while the contralateral side served as an internal control. Whole mount preparations of stage 32 (for Sema3A) or stage 34 (for CaV2.3) tailbud spinal tissues (centre) were immunostained with antibodies: anti-Sema3A to detect xSema3A and anti-CaV2.3 α1E to detect xCaV2.3 α1E. Spinal cord schema is overlaid with an immunofluorescence image showing the expression of xSema3A in the non-injected side (left), but not in the injected side (right). (b–e, g–j) Immunofluorescence images of Xenopus spinal cords immunostained for xSema3A (b–e) or xCaV2.3 α1E (g–j), without MO injection (Control; b, g) and with injection of antisense (A-MO; c, e, h, j) or sense (S-MO; d, i) MO specific for either xSema3A or xCaV2.3 α1E. Signals in red (b’–e’, g’–j’) indicate the localization of xSema3A (b’–e’) and xCaV2.3 α1E (g’–j’) IRs, which are overlaid with signals from the GFP co-injection marker (+GFP, green; c’–e’, h’–j’). Scale bar, 20 μm. (f, k) Summaries of normalized xSema3A (b–e) and xCaV2.3 α1E (g–j) IRs. Injected sides of spinal cords were normalized to the contralateral, non-injected sides. For non-injected controls, the right sides of spinal cords were normalized against the left sides. Data are mean ± s.e.m. (n) = number of spinal cords examined. Significant differences from control are indicated (**p < 0.01).
We then examined the effect of Sema3A down-regulation on the expression of CaV2.3. Immunostaining with polyclonal antibodies against human CaV2.3 α1E revealed a pattern of expression of Xenopus CaV2.3 α1E (xCaV2.3 α1E) in the lateral marginal zones of the spinal cord (Fig. 3g, g’) similar to that of xSema3A (Fig. 3b, b’). The specificity of the anti-CaV2.3 α1E antibodies was confirmed by their detection of over-expressed mouse CaV2.3 α1E (mCaV2.3 α1E) when endogenous xCaV2.3 α1E was down-regulated by a specific A-MO (xCaV2.3 α1E-A-MO; Supplementary Fig. S6). The endogenous expression of xCaV2.3α1E became prominent at stage 32–34, following the prominent expression of xSema3A at stage 30–32. Strikingly, the expression of xCaV2.3 α1E was reduced markedly by injection of xSema3A-A-MO [Fig. 3h, h’, k and Supplementary Movie S2] but not the control xSema3A-S-MO (Fig. 3i, i’, k). The effect of xSema3A-AMO was specific since over-expression of hSema3A reversed the reduction of xCaV2.3 α1E (Fig. 3j, j’, k). These results demonstrate that Sema3A is required for the expression of CaV2.3 in the Xenopus spinal cord in vivo.
Sema3A-CaV2.3 signalling is required for the dendrite identity in vivo
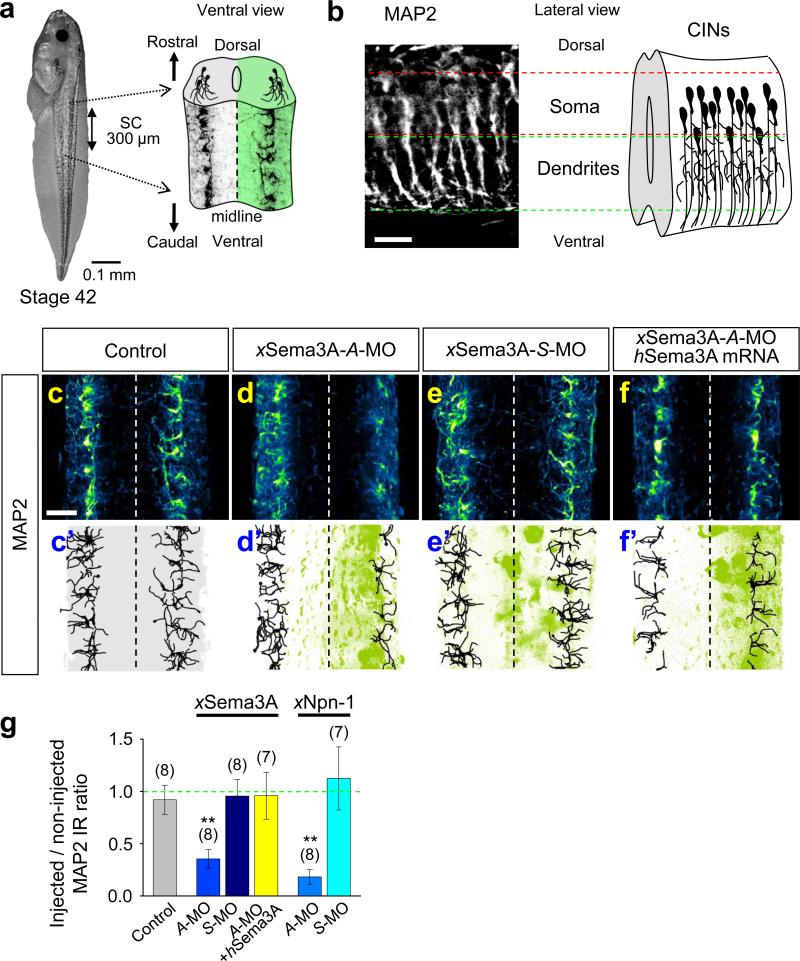
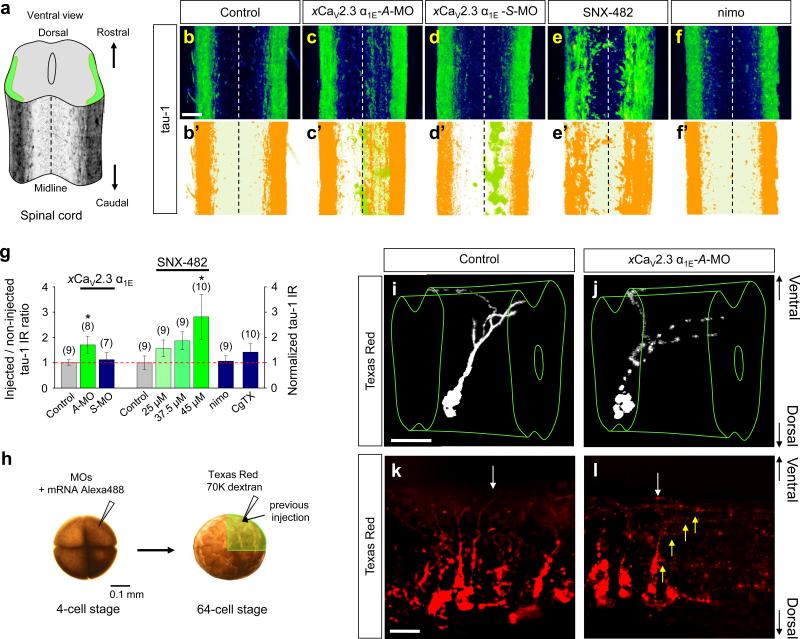
We next confirmed that Sema3A signalling is required to specify the dendrite identity in vivo by analyzing MAP2-IR in whole-mount spinal cords. Dendrites of developing spinal neurons first became prominently MAP2 positive (Fig. 4a, b) at the stage 42 tadpole (Fig. 4a, left). MAP2-IR showed relatively simple dendritic arbors projecting ventrally (Fig. 4b, c, c’). Their morphology and the location of their soma suggest these neurons are highly likely to be either CINs or ascending interneurons31. We found that MAP2-IR in the Sema3A-A-MO injected (Fig. 4d, d’, g and Supplementary Movie S3), but not the xSema3A-S-MO injected (Fig. 4e, e’, g) side of spinal cords was significantly reduced compared to the uninjected side of the same spinal cords, and this effect was reversed by over-expression of hSema3A (Fig. 4f, f’, g). Injection of A-MO against xNpn-1 (Fig. 4g) but not the control xNpn-1-S-MO (Fig. 4g) also significantly reduced the MAP2-IR in the injected side of the spinal cord. Thus, Sema3A is required to specify the dendrite identity.
Figure 4.
Sema3A signalling is required for acquisition of the dendrite identity of putative CINs in vivo. (a) A whole-mount preparation of a Xenopus stage 42 tadpole (left) and spinal cord schema overlaid with immunofluorescence images of MAP2-positive dendrites (right). (b) An immunofluorescence image of a lateral view of MAP2-positive dendrites (control, left) and schematic diagram of CIN neurite trajectories (right). MAP2-IR reveals dendrites projecting ventrally, suggesting that the majority of MAP2-positive dendrites are those of CINs. (c–f) Immunoflluorescence images of spinal cords without MO-injection (Control; c, c'), or injected with antisense (A-MO; d, d’) or sense (S-MO; e, e’) MO specific to xSema3A or xSema3A-A-MO together with hSema3A mRNA (f, f’). Traces in black (c’–f’) indicate the MAP2-positive dendrite trajectories and are overlaid with signals from the GFP marker (green). (g) Summaries of normalized MAP2-positive volumes of samples treated with either xSema3A- or xNpn-1-MOs. Injected sides of spinal cords were normalized against the contralateral, non-injected sides. For the non-injected controls, the right sides of spinal cords were normalized against the left sides. Data are mean ± s.e.m. (n) = number of spinal cords examined. Significant differences from the control are indicated (**p < 0.01). Scale bars, 20 μm.
Because the reduction of MAP2-IR caused by down-regulation of either xSema3A or xNpn-1 could have caused defects in cell migration32, we examined the effects of down-regulation of xCaV2.3 α1E on the dendrite identity. Consistent with the idea that CaV2.3 is a Sema3A downstream effector required to specify the dendrite identity, MAP2-IR was significantly reduced by xCaV2.3 α1E-A-MO, to an extent similar to that caused by Sema3A-A-MO (Fig. 5a, a’, f and Supplementary Movie S4), but not by xCaV2.3 α1E-S-MO (Fig. 5b, b’, f). Injection of the mCaV2.3 α1E mRNA together with xCaV2.3 α1E-A-MO sufficiently reversed the reduction of MAP2-IR (Fig. 5c, c’, f) resulting from injection of xCaV2.3 α1E-A-MO alone. However, restoration of MAP2-IR by mCaV2.3 α1E in the side of the spinal cord treated with xCaV2.3 α1E-A-MO occurred only in the presence of endogenous xSema3A (Fig. 5c, c’, f), not when xSema3A was down-regulated (Fig. 5e, e’, f), suggesting other parallel signal(s) are also required to specify the dendrite identity. We also tested the effects of pharmacological blockade of CaV2.3 on the acquisition of the dendrite identity by administering different doses of SNX-482 into the spinal cords of stage 30–32 tailbuds (Fig. 5g). Administration of 45 μM SNX-482 eliminated almost all dendrites (Fig. 5k, k’, l) while lower doses caused significant reductions (Fig. 5i, i’, j, j’, l). The local administration of nimodipine (Fig. 5h, h’, l) or ω-conotoxin GVIA (Fig. 5l) had no significant effect. These results support the idea that the Sema3A signal propagates through CaV2.3, whose activity is required for the acquisition of the dendrite identity.
Figure 5.
CaV2.3 function is required for the dendrite identity of xSCINs in vivo. (a–e, h–k) Immuofluorescence images of spinal cords injected with antisense (A-MO; a, a’) or sense (S-MO; b, b’) MO specific to xCaV2.3 α1E, or xCaV2.3 α1E-A-MO or xCaV2.3 α1E-SMO together with mCaV2.3 α1E mRNA (c, c’, d, d’) or with xSema3A-A-MO together with mCaV2.3 α1E mRNA (e, e’), or with local administration of either nimodipine (nimo; h, h') or the indicated concentrations of SNX-482 (i, i', j, j', k, k'). Traces in black (a’– e’, h'–k') indicate the MAP2-positive neurite trajectories and are overlaid with the signals from the GFP marker (green). (f, l) Summaries of normalized MAP2-positive volumes of samples treated with xCaV2.3 α1E-MOs, xSema3A-A-MO, or the local administration of Ca2+ channel blockers. For animals treated with MOs, injected sides of spinal cords were normalized against the contralateral, non-injected sides. For animals treated with Ca2+ channel blockers, the MAP2-positive volumes of the bilateral sides of the spinal cord were normalized against the average MAP2-positive volumes in control animals. (g) Administration of Ca2+ channel blockers (FITC as an injection marker) into the hindbrain and spinal cords of stage 30–32 tailbuds. Data are mean ± s.e.m. (n) = number of spinal cords examined. Significant differences from control are indicated (*p < 0.05; **p < 0.01). Scale bars, 20 μm.
CaV2.3 suppresses the axon identity in vivo
Since Sema3A induced the conversion of axons of cultured xSCINs to dendrites in vitro (Fig. 1), we tested whether down-regulation of CaV2.3 expression also affects the axon identity in vivo. Whole mounts of normal stage 42 tadpole spinal cords showed equal intensities of bilateral axon tracts along their longitudinal axis (Fig. 6a, b, b’). Injection of xCaV2.3 α1E-A-MO (Fig. 6c, c’, g), but not the control xCaV2.3 α1E-S-MO (Fig. 6d, d’, g), resulted in a significant increase of tau-1-IR in the injected side of the spinal cords (Fig. 6c’, d’), as compared with control, uninjected animals (Fig. 6b, b’, g). The increased tau-1-IR was particularly noticeable close to the ventral midline (Fig. 6c, c’) where it is normally absent (Fig. 6b, b’), implying that the default neuronal polarization state in the absence of CaV2.3 is that axons. Furthermore, local administration of SNX-482 into the spinal cord caused a significant increase in tau-1-IR throughout the spinal cord (Fig. 6e, e’, g). In contrast, administration of neither nimodipine (Fig. 6f, f’, g) nor ω-conotoxin GVIA (Fig. 6g) had significant effect. Western blot analysis showed that the ratio of MAP2 to tau-1 in total protein extracted from the SNX-482-treated spinal cords was significantly lower than that from untreated, control, spinal cords (Supplementary Fig. S7), corroborating the immunohistochemical observations of spinal cords shown above. Thus, CaV2.3 activity is required not only for acquisition of the dendrite identity but also for suppression of the axon identity.
Figure 6.
CaV2.3 activity suppresses the acquisition of the axon identity of xSCINs in vivo. (a) A schematic diagram of the spinal cord was overlaid with an image of the immunofluorescence of tau-1-positive axons. (b–g) Immunofluorescence images (b–f) and summaries (g) of tau-1-IR of Xenopus spinal cords without MO injection (Control; b, b’) and with injection of antisense (A-MO; c, c’) or sense (S-MO; d, d’) MO specific to xCaV2.3 α1E, or with local administration of SNX-482 (e, e’) or nimodipine (nimo; f, f’). Signals in orange (b’–f’) indicate the localization of tau-1-IR and are overlaid with the signals from the GFP marker (green; c’, d’). The injected side of the spinal cord was normalized against the contralateral, uninjected side. For the uninjected controls, the right sides were normalized against the left sides. For local administration of Ca2+ channel antagonists, tau-1-IR of the bilateral sides of the spinal cord was normalized against the average tau-1-IR in control animals. Data are mean ± s.e.m. (n) = number of spinal cords examined. Significant differences from control are indicated (*p < 0.05). (h) Visualization of individual xSCINs and their neurite projections after the xCaV2.3 α1E MO treatment. After the microinjection of MO together with Alex-488 conjugated with 10K-dextran as an injection marker into one dorsal blastomere of four-cell stage embryos, Texas Red conjugated with 70K dextran was injected into one dorsal blastomere of a 64-cell stage. In this preparation, approximately one sixteenth of the spinal neurons in one side of the spinal cord are visualized. (i–l) Ventrolateral views of a Xenopus spinal cord showing CIN axons crossing the midline (white arrows) in non-treated (i, k) and treated with xCaV2.3-A-MO (j, l). In animals treated with xCaV2.3-AMO, multiple axon-like neurites extending from the initial segment and migrating longitudinally are observed (j, l, yellow arrows).
To exclude off-target effects of A-MO treatment, we examined the neuronal integrity of xCaV2.3 α1E-A-MO-treated animals. We microinjected Texas Red conjugated with 70K-dextran into one dorsal blastomere of 64-cell stage embryos into which xCaV2.3 α1E-A-MO had previously been injected at the four-cell stage (Fig. 6h). We then visualized individual spinal neurons in the MO-injected side of stage 42 tadpole spinal cords by their Texas Red. We imaged isolated, putative CINs whose soma were located ca. two thirds of the distance from the ventral midline and whose single axon and dendrites projected ventrally (Fig. 6i, k, control). Individual putative CINs in the side of spinal cords treated with xCaV2.3-A-MO, indeed, showed multiple axon-like neurites that emanated from the proximal segment and many that projected longitudinally in the ipsilateral axon tract (Fig. 6j, l), but such axon-like neurites were only rarely observed in control animals (Fig. 6i, k). The ipsilateral projection (Fig. 6j, l) of these putative CIN axons may be a result of their reduced sensitivity to netrin-1 (ref. 33). These results clearly demonstrate that the activity of CaV2.3 suppresses the axon identity.
Cyclic GMP-dependent expression of CaV2.3 and PKG-dependent acquisition of the dendrite identity
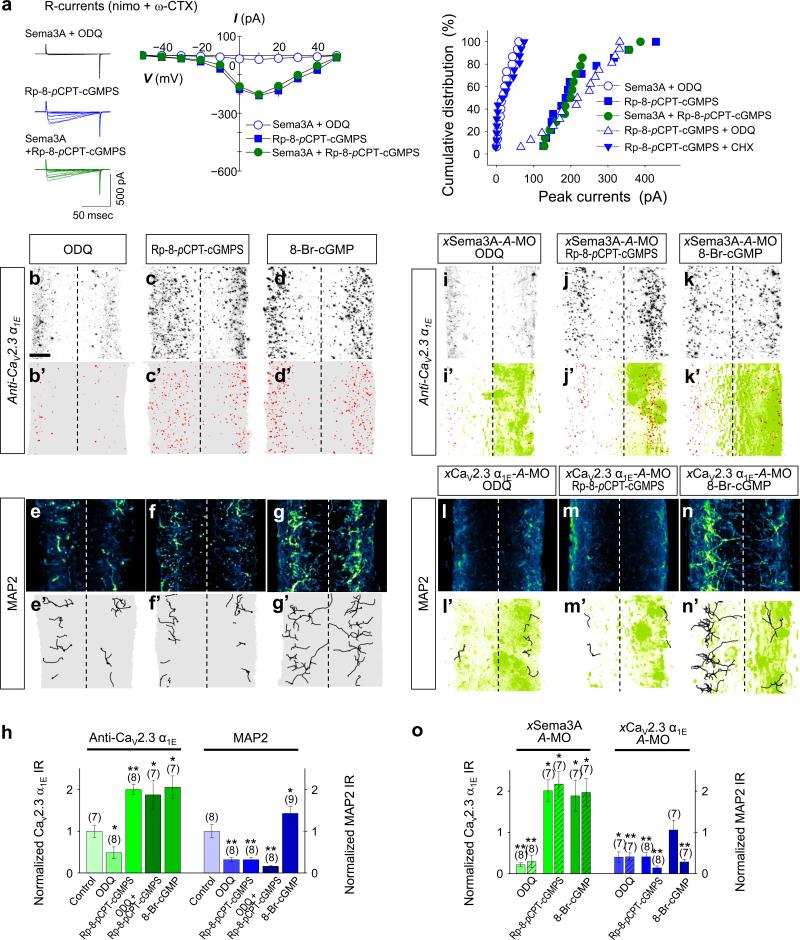
We previously showed that Sema3A triggers the local production of cGMP by sGC (ref. 16). A recent study showed that cGMP-PKG signalling induces initiation of the dendrite identity in undifferentiated neurites of cultured hippocampal neurons34. Interestingly, bath-application of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of sGC, prevented the induction of CaV2.3 currents by a gradient of Sema3A in xSCIN growth cones (Fig. 7a). Moreover, inhibition of sGC by local administration of ODQ abolished not only CaV2.3 α1E expression (Fig. 7b, b’, h), but also dendrite formation (Fig. 7e, e’, h), suggesting that cGMP signalling functions as an upstream regulator of the expression of CaV2.3 as well as acquisition of the dendrite identity. Surprisingly, a gradient of Rp-8-pCPT-cGMPS, a PKG-inhibiting cGMP analogue16 induced CaV2.3 currents (Fig. 7a) and pre-empted their induction by Sema3A (Fig. 7a). Moreover, Rp-8-pCPT-cGMPS-induced CaV2.3 currents were not affected by ODQ, concordant with the notion that cGMP is required to target functional CaV2.3 to the plasma membrane. Importantly, these CaV2.3 currents were eliminated by pretreatment with cycloheximide (CHX), a protein synthesis inhibitor (Fig. 7a), suggesting that cGMP, not PKG, mediates both the Sema3A-induced synthesis of CaV2.3 proteins and their targeting to the plasma membrane in vitro. Rp-8-pCPT-cGMPS also caused a significant increase in the expression of xCaV2.3 α1E (Fig. 7c, c’, h), which extended beyond the marginal zones of the spinal cord. It is noteworthy that pre-treatment with CHX eliminated the Sema3A-induced expression of CaV2.3 both in vitro and in vivo (Supplementary Fig. S8), supporting the idea that cGMP regulates the expression of functional CaV2.3 at the translational level. In contrast, Rp-8-pCPT-cGMPS drastically reduced MAP2-IR (Fig. 7f, f’, h) to an extent similar to that caused by treatment with either ODQ (Fig. 7e, e’, h) or SNX-482 (Fig. 5i–l), demonstrating that PKG activity is required for the acquisition of the dendrite identity. Consistent with these observations, local administration of 8-BrcGMP, a PKG-activating cGMP analogue, enhanced both the expression of xCaV2.3 α1E (Fig. 7d, d’, h) and dendrite formation (Fig. 7g, g’, h).
Figure 7.
Sema3A-induced cGMP causes recruitment and expression of CaV2.3 and PKG is required as a co-activator for acquisition of the dendrite identity. (a) Representative traces of Ca2+ currents (left), summary of the current (I)/ voltage (V) relationship (centre) of CaV2.3 currents and cumulative distribution of peak CaV2.3 currents (right) monitored in cultured xSCIN growth cones. Either ODQ (1 μM), Rp-8-pCPT-cGMPS (2.5 μM) or cycloheximide (CHX, 25 μM) was applied in the bath 30 min before experiments were performed. Sema3A (2,000 U·ml–1 in micropipettes) was applied to the growth cone as a gradient. (b–g, i–n) lmmunofluorescence images of Xenopus spinal cords immunostained for xCaV2.3 α1E (b–d, i–k) or MAP2 (e–g, l–n), without MO injection (b–g) or with injection of A-MO specific for either xSema3A (i–k) or xCaV2.3 α1E (l–n). Signals in red (b’–d’, i’–k’) and traces in black (e'–g', l'–n') indicate the location of the xCaV2.3 α1E and MAP2 IRs, respectively, which are overlaid with signals from the GFP injection marker (green, i’–k’, l’–n’). ODQ (1 mM), Rp-8-pCPT-cGMPS (0.25 mM) and 8-Br-cGMP (1 mM) were injected into the hindbrain and spinal cord of stage 30–32 tailbud animals (see Fig. 5g). Scale bar, 20 μm. (h, o) Summaries of normalized xCaV2.3 α1E IR (b–d, i–k) and MAP2-positive volumes (e–g, l–n). xCaV2.3 α1E IR and MAP2-positive volumes in bilateral sides of the spinal cord treated with ODQ or cGMP analogues were normalized against the average IR and positive volumes in control animals (h). Injected sides of spinal cords were normalized to the average IRs and positive volumes in control animals (o). Data are mean ± s.e.m. (n) = number of growth cones and spinal cords examined. Significant differences from control are indicated (*p < 0.05; **p< 0.01).
Finally, we down-regulated the expression of either xSema3A or xCaV2.3 α1E with specific A-MOs in one side of spinal cords and tested the effects of varying the levels of cGMP on the expression of xCaV2.3 α1E or acquisition of the dendrite identity. Despite the absence of Sema3A, local administration of either Rp-8-pCPT-cGMPS (Fig. 7j, j’, o) or 8-Br-cGMP (Fig. 7k, k’, o) induced the expression of xCaV2.3 α1E, while local administration of ODQ caused their significant reduction (Fig. 7i, i’, o), supporting the idea that cGMP induces the expression of xCaV2.3 α1E. In contrast, ODQ (Fig. 7l, l’, o) or Rp-8-pCPT-cGMPS (Fig. 7m, m’, o) prevented the appearance of dendrites even in the presence of the xCaV2.3. However, local administration of 8-Br-cGMP failed to rescue dendrites in the absence of xCaV2.3 (Fig. 7n, n’, o), indicating that CaV2.3 is required for the acquisition of the dendrite identity. Taken together, our results reveal that Sema3A induced cGMP-PKG signalling regulates the cGMP-dependent expression of functional xCaV2.3 that together with PKG as a co-effector specify the dendrite identity while suppressing axon identity.
DISCUSSION
Extracellular factors signal to regulate the initiation and maintenance of the axon identity4, 5, 35 and dendrite growth and branching13–15. However, the signalling events that regulate the acquisition of an identity as a dendrite in response to extracellular factors are unknown. We demonstrate in vitro and in vivo that a secreted guidance factor, Sema3A, induces the dendrite identity and concomitantly suppresses the axon identity by inducing the expression of functional CaV2.3. We further demonstrate that Sema3A-triggered cGMP production and PKG activity are required, respectively, for the expression of CaV2.3 and acquisition of the dendrite identity.
We found that the relatively high Sema3A concentration required to convert the axons of cultured xSCINs to dendrites caused growth cone depolarization and the recruitment of functional CaV2.3 to the plasma membrane. We also found that increased expression of Sema3A occurs in close spatiotemporal proximity to the expression of CaV2.3 in the spinal cords of tailbud stage animals, when putative dendrites emanate from pre-existing axons. We propose that a high Sema3A concentration is required to induce CaV2.3-mediated Ca2+ signalling in growth cones, which, in turn, causes the up-regulation of MAP2 required to specify the dendrite identity while simultaneously down-regulating the unphosphorylated tau required to specify the axon identity (see Fig. 8). Local protein synthesis and trafficking can occur in cultured growth cones of Xenopus retinal ganglion cells (xRGCs) within 5 minutes of their exposure to Sema3A (ref. 36). Thus, Sema3A signalling may induce cGMP-dependent de novo synthesis of CaV2.3 and their targeting to the plasma membrane. Sema3A has been demonstrated to activate the ERK (extracellular-signal-regulated-kinase)-TOR (target-of-rapamycin) signalling pathway during repulsion/collapse of cultured xRGC growth cones37, 38. CNrasGEF, a cyclic nucleotide-activated guanine exchanging factor that activates GTPase Ras, could couple Sema3A-induced production of cGMP to the ERK-TOR signalling pathway39 to induce de novo synthesis of CaV2.3. A similar mechanism could account for the cGMP-mediated targeting of CaV2.3 to the plasma membrane, since Ras/Rap1 are known to induce vesicle transport that trafficks the ionotropic glutamate receptor40, and Sema3A enhances axonal vesicle transport21.
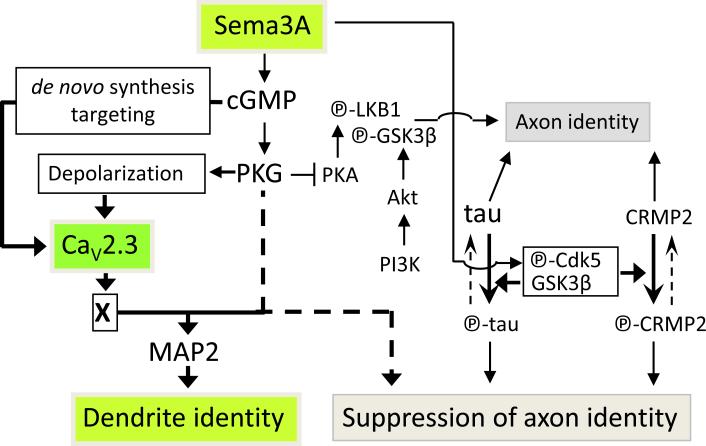
Figure 8.
Model depicting Sema3A-induced acquisition of the dendrite identity. Sema3A induces the cGMP-mediated expression of functional CaV2.3 and PKG-dependent depolarization. Thus, PKG gates Ca2+ signaling through CaV2.3 channels to promote the acquisition of the dendrite identity and suppresses the acquisition of the axon identity. Unidentified signaling molecule(s) “X” likely increases MAP2 and decreases unphosphorylated tau proteins.
The identity of neurites as either axons or dendrites depends on the relative signalling of cAMP-PKA and cGMP-PKG; greater cGMP-PKG signalling causes undifferentiated neurites of hippocampal neurons to become dendrites34. PKG antagonizes acquisition of the axon identity induced by PKA-dependent phosphorylation of LKB1 (serine/threonin kinase 11)35, 41 and glycogen synthase kinase-3β (GSK3β)42. Sema3A-induced PKG activity12 may gate Ca2+ entry through CaV2.3 by inducing membrane depolarization16 and simultaneously opposing the PKA activity required for the axon identity. The details of the mechanism that underlies the activation of CaV2.3 required for the acquisition of the dendrite identity is unknown, but may require an unidentified factor(s) (Factor “X”, Fig. 8). This hypothesized factor is unlikely any of the known signalling molecules that promote the axon identity because disruption of the expression of these molecules does not cause the conversion of axons to dendrites. Sema3A causes the phosphorylation of collapsing-response-mediator-protein-2 (CRMP2) through the action of cyclin-dependent-kinase-5 and GSK3β (ref. 43) that is known to suppress the axon identity44. Whether CaV2.3 functions by modulating the phosphorylation states of either tau-1 (ref. 45) or CRMP2 (ref. 44) requires further study.
Although the localization of CaV2.3 predominantly to dendrites46 and spine heads47 has been demonstrated, their function in vivo has not been elucidated previously. Our study demonstrates the importance of the spatiotemporal and dynamic expression patterns of both guidance factors and ion channels in establishing neurite identity during nervous system development. CaV2.3-induced acquisition of the dendrite identity and the simultaneous suppression of the axon identity may also contribute to the Sema3A-induced failure of axons to regenerate after nerve injury48.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. W. R. Jelinek, N. J. Cowan, M. E. Rice, M-M. Poo, D. Ginty and A. L. Kolodkin for critical comments on the manuscript; S.M. Strittmatter for the NP-1(0111); L.I. Binder and G. Tian for technical assistances. This work was supported by grants from NIH/NINDS (NS042823 and CRCNS program, NS064671, KH).
Footnotes
AUTHOR CONTRIBUTIONS
M.N., T.K., M.J.vS. and K.H. designed, carried out experiments and analyzed the data; C-S.L. helped immunocytochemisty; N.Y. and Y.G. provided proteins; M.N., T.K., S-I.M. and S.I. carried out or supervised in vivo imaging analyses; M.N. and K.H. supervised the project and wrote the manuscript.
Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIA INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 2.Barnes A, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jan Y, Jan L. The control of dendrite development. Neuron. 2003;40:229–242. doi: 10.1016/s0896-6273(03)00631-7. [DOI] [PubMed] [Google Scholar]
- 4.Adler C, Fetter R, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-β signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon V, Klassen M, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts A, Dale N, Ottersen O, Storm-Mathisen J. Development and characterization of commissural interneurones in the spinal cord of Xenopus laevis embryos revealed by antibodies to glycine. Development. 1988;103:447–461. doi: 10.1242/dev.103.3.447. [DOI] [PubMed] [Google Scholar]
- 8.Roberts A, Dale N, Ottersen OP, Storm-Mathisen J. The early development of neurons with GABA immunoreactivity in the CNS of Xenopus laevis embryos. J. Comp. Neurol. 1987;261:435–449. doi: 10.1002/cne.902610308. [DOI] [PubMed] [Google Scholar]
- 9.Henley JR, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–30. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong K, Nishiyama M. From guidance signals to movement: Signaling molecules governing growth cone turning. Neuroscientist. 2010;16:65–78. doi: 10.1177/1073858409340702. [DOI] [PubMed] [Google Scholar]
- 11.Guan C, Xu H, Jin M, Yuan X, Poo M. Long-range Ca2+ signaling from growth cone to soma mediates reversal of neuronal migration induced by slit-2. Cell. 2007;129:385–395. doi: 10.1016/j.cell.2007.01.051. 2007. [DOI] [PubMed] [Google Scholar]
- 12.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 13.Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J. Neurobiol. 2004;58:403–412. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- 14.Morita A, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-fyn signaling. J. Neurosci. 2006;26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran TS, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama M, von Schimmelmann MJ, Togashi K, Findley W, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat. Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- 17.Togashi K, et al. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Song HJ, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 19.Craig AM, Banker G. Neuronal polarity. Annu. Rev. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 20.Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 1999;19:6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goshima Y, et al. A novel action of collapsin: collapsin-1 increases antero- and retrograde axoplasmic transport independently of growth cone collapse. J. Neurobiol. 1997;33:316–328. doi: 10.1002/(sici)1097-4695(199709)33:3<316::aid-neu9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Bradke F, Dotti CG. Differentiated neurons retain the capacity to generate axons from dendrites. Curr. Biol. 2000;10:1467–1470. doi: 10.1016/s0960-9822(00)00807-1. [DOI] [PubMed] [Google Scholar]
- 23.Gomis-Rüth S, Wierenga CJ, Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Song AH, et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-González C, McLaren GJ, Dale N. Development of Ca2+-channel and BK-channel expression in embryos and larvae of Xenopus laevis. Eur. J. Neurosci. 2003;18:2175–2187. doi: 10.1046/j.1460-9568.2003.02955.x. [DOI] [PubMed] [Google Scholar]
- 26.Gu X, Spitzer NC. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J. Neurosci. 1993;13:4936–4948. doi: 10.1523/JNEUROSCI.13-11-04936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Thaler C, Brehm P. Calcium channels in Xenopus spinal neurons differ in somas and presynaptic terminals. J. Neurophysiol. 2001;86:269–279. doi: 10.1152/jn.2001.86.1.269. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 30.Moon MS, Gomez TM. Adjacent pioneer commissural interneuron growth cones switch from contact avoidance to axon fasciculation after midline crossing. Dev. Biol. 2005;288:474–486. doi: 10.1016/j.ydbio.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Li WC, et al. Axon and dendrite geography predict the specificity of synaptic connections in a functioning spinal cord network. Neural Develop. 2007;2:17. doi: 10.1186/1749-8104-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 33.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 34.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 35.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat. Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 38.Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 39.Pham N, et al. The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP. Curr. Biol. 2000;10:555–558. doi: 10.1016/s0960-9822(00)00473-5. [DOI] [PubMed] [Google Scholar]
- 40.Stornetta RL, Zhu JJ. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2011;17:54–78. doi: 10.1177/1073858410365562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes A, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 43.Uchida Y, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3β phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura T, et al. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3β. FEBS Lett. 2002;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- 46.Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J. Physiol. 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabatini BL, Svoboda K. Analysis of calcium channels in single spines using optical fluctuation analysis. Nature. 2000;408:589–593. doi: 10.1038/35046076. [DOI] [PubMed] [Google Scholar]
- 48.Kaneko S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.