Abstract
Endothelial progenitor cells (EPCs) are involved in the maintenance of endothelial homoeostasis and in the process of new vessel formation. Experimental and clinical studies have shown that atherosclerosis is associated with reduced numbers and dysfunction of EPCs; and that medications alone are able to partially reverse the impairment of EPCs in patients with atherosclerosis. Therefore, novel EPC-based therapies may provide enhancement in restoring EPCs’ population and improvement of vascular function. Here, for a better understanding of the molecular mechanisms underlying EPC impairment in atherosclerosis, we provide a comprehensive overview on EPC characteristics, phenotypes, and the signaling pathways underlying EPC impairment in atherosclerosis.
Keywords: Endothelial progenitor cells, Atherosclerosis, Inflammation, Review
2. INTRODUCTION
Myocardial infarction and stroke are the leading causes of death worldwide (1, 2). Current therapies for these cardiovascular diseases (CVD) include life style management, pharmacological control of risk factors, and surgical revascularization, which cannot completely control the diseases. Therefore, new approaches like-cell based therapies are needed (3). Since endothelial progenitor cells (EPCs) have been shown to contribute to tissue vascularization after ischemic events in limbs, retina, and myocardium (4, 5), EPCs therefore hold a great promise as a novel therapeutics in vascular repair and CVD control.
Vascular endothelium plays an essential role in maintaining and regulating vascular tone, structure, growth, fibrinolysis, and homeostasis, and thus protects the vessels from inflammation, immune response, thrombosis and CVD (6). Endothelial cell (EC) activation is associated with the shedding of components of the glycocalyx (7), upregulation of adhesion molecules (8), and endothelial microparticles (0.1–1 microm) (9, 10) into the circulation. As a related process, EC dysfunction is considered to be an early event, which subsequently leads to vascular wall disorders. The loss of EC function could result from the changes in the hemodynamic forces (shear and/or hoop stress), drug-induced cytotoxicity, device implant-induced injury, and immune-mediated mechanisms (11). These processes may result in the detachment of ECs and recruitment of circulating myeloid and progenitor cells, which are involved in vascular remodeling and repair (12). Apparently, inflammation of the endothelium caused by several stimuli such as hyperlipidemia and other CVD risk factors (13) leads to injury of the endothelium. According to the response-to-injury hypothesis (14), injuries to the endothelium trigger the concomitant invasion of macrophages and lipid depositions (15). Therefore, endothelium damage may represent an early, causative event involving arteriogenesis, atherogenesis, and angiogenesis (16, 17), which compromise the capabilities of ECs in maintaining vascular function and homeostasis. Recent studies indicate that EPCs, which were first isolated by Asahara and his colleagues in 1997 (18), play an important role in the recovery and repair of injured endothelium by counteracting the detrimental CVD risk factor-induced damages (19). Thus, EPCs contribute to postnatal physiological and pathological neovascularization. EPCs are precursor cells that express some cell surface markers characteristic of mature endothelium and some from hematopoietic stem cells (NIH stem cell report http://stemcells.nih.gov/staticresources/info/scireport/PDFs/H.percentage20Chapterpercentage206.pdf) (20–23). In fact, evidence suggests that these EPCs are mobilized from the bone marrow (BM) into the peripheral blood in response to a chemical or mechanical injury of the endothelium caused by tissue ischemia or trauma. The mobilized EPCs migrate to sites of injured endothelium and differentiate into mature ECs in situ (24). The identification of EPCs from circulating, blood-derived mononuclear cells further supports this hypothesis (25, 26). Moreover, studies in both animals and humans demonstrated that EPCs contribute to neovascularization and re-endothelialization (24), which supports the possibility that exogenous therapeutic EPCs may provide additional benefits to endogenous repair mechanisms by counteracting ongoing risk factor-induced EC injury and by replacing dysfunctional/damaged endothelium. EPCs display three fundamental activities within the vascular system: I) paracrine, II) healing of endothelial damage (integration) and III) formation of new blood vessels in ischemic tissues(3). In the latter studies, EPCs have been shown to express a variety of EC surface markers (27), incorporate into sites of neovascularization (5, 28, 29), and home to sites of endothelial denudation (30–32), which further shows the potential of EPCs as a novel therapeutic approach for the neovascularization in some diseases. EPCs are of great interest for investigators, who have studied vessel repairing mechanisms in atherosclerosis (33–37), ischemic cardiomyopathy (38), hypercholesterolemia, smoking, aging (39), rheumatoid arthritis (40), inflammation (41), pulmonary hypertension, systemic hypertension (42), chronic kidney disease (43), metabolic syndrome, and diabetes (44–49). Some preclinical or clinical studies have shown that EPC-based treatment alone or in combination with traditional treatments hold promise to cure vessel diseases in patients with atherosclerosis and diabetes, thus providing novel concepts and therapeutic strategies in the treatment of various CVDs (50, 51).
Despite significant progress in demonstrating the pathophysiological roles and therapeutic applications of EPCs, there are still challenges in the characterizations of EPCs. Although multiple pathways have been extensively examined, more in-depth studies are needed to better define the pathways or mechanisms by which EPC function can be rescued in diseases. It also needs to be determined if EPCs provide protection endothelium against acute and chronic inflammation, immune responses, and other CVD risk factor stimuli that deregulate mature ECs (52). It is also uncertain that whether the decreased numbers of EPCs in patients with atherosclerotic risk factors (53, 54) and restenosis (55) are resulted from decreased production and/or increased cell death of EPCs (41). In addition, issues concerning EPC origins, EPC functions, and the significance of diversified cell surface markers of EPCs needs to be clarified. In our opinion, these cell surface markers need to be standardized as well. Furthermore, to the best of our knowledge, the pre- and/or clinical treatment studies have not yet been concluded, and some results to date are controversial. Thus, the characterizations of factors and mechanisms modulating EPC numbers and function are currently under intensive investigation. Although EPCs are extensively studied in tumor metastasis (56), in this review, we focus on reviewing recent results from experimental and clinical studies investigating the phenotypes and characteristics of EPCs, the alterations of EPCs in inflammation and atherosclerosis. In addition, we will also discuss the possible mechanisms underlying the abnormalities of EPCs and therapeutic potential of EPCs in atherosclerosis.
3. ENDOTHELIAL PROGENITOR CELLS (EPCs)
3.1. Introduction of EPCs
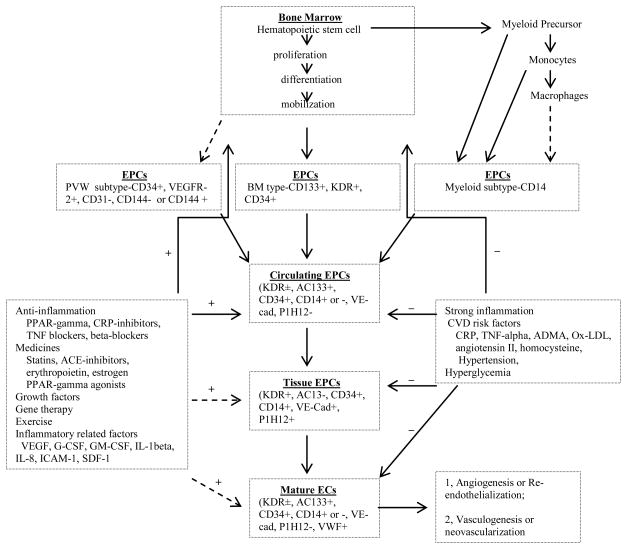
In the accepted paradigm for new blood vessel formation in adults from the 1990s, new capillaries are formed by the local migration and replication of existing ECs, usually from venues, followed by lumen formation and investment with mural cells (57) such as pericytes. However, in 1997 Asahara and colleagues published a landmark paper in Science (18), showing that BM-derived CD34+VEGFR-2+ (vascular endothelial growth factor receptor 2) monocytic cells, isolated from human blood and grown in culture, are able to differentiate into cells with EC characteristics, including expressions of CD31+, E-selectin+, endothelial nitric oxide synthase (eNOS)+, and uptake of modified low density lipoprotein (LDL) (6). These cells were termed as EPCs. Currently, the PubMed lists more than 10,000 publications when searching with the key words of EPCs (58). Adult BM is a rich reservoir of tissue-specific stem and progenitor cells with EPCs constituting 1 – 5 percent of the total BM cells (59). Figure 1 describes the origin and differentiation of EPCs in detail (24). EPCs can be mobilized, by various stimuli, into the circulation and contribute to the neo-angiogenic process or to the repair of the damaged EC layer. Therefore, the updated paradigm suggests that adult angiogenesis results from migration and proliferation of both local ECs and BM-derived EPCs. Many articles have been published in attempting to clarify the definition, origin, and function of EPCs and the roles of EPCs in CVD (60). It has become apparent that many different cell types play a role in vessel repair and regeneration, and that these cells may display a variety of cell surface markers. Thus, one of the greatest challenges in studying human EPC biology is the lack of a specific marker to unequivocally identify this circulating cell subset (61) (also see a list of stem cell markers in the NIH stem cell report website (http://stemcells.nih.gov/info/scireport/appendixe.asp#eii). Considering this situation, the ISHAGE (International Society of Hematotherapy and Graft Engineering) has published a protocol for enumeration of hematopoietic stem cells to enable the comparison of clinical and laboratory data (62–64). Of note, EPCs are different from circulating endothelial cells (CECs). CECs are very rare cells present in the blood at levels in the range of three cells/ml, and are detectable in vascular injury like heart catheterization, sickle cell anemia, bacterial infection, thrombotic thrombocytopenic purpura or acute coronary syndromes. Thus, CECs might be used as a surrogate, non-invasive marker for the study of vascular alterations (65). Of note, based on the proliferative status, EPCs can also be divided into two types, early EPCs and late outgrowth EPCs. Early EPCs are present within 10 days of culture have the characteristics including slow proliferation; CD133 expression; less capable in new vessel formation; and vastly capable in integrating in existing vessels. In contrast, the late outgrowth EPCs are present after 14 days of culture have the characteristics different from early EPCs including elongated “cobblestone morphology”; highly proliferative, CD144 expression, and highly capable in tube formation in vitro (3). In addition, based on the EPC source three major types of EPCs can be identified including EPCs from peripheral blood and bone marrow, vascular wall EPCs, and monocyte/macrophage derived EPCs.
Figure 1.
Schematic representation of endothelial progenitor cell (EPC) generation and the processes by which EPCs differentiate to mature endothelial cells (ECs). Multiple types of EPCs contribute to the accumulation of circulating EPCs, e.g.: bone marrow sub type (BM), vascular wall subtype (VW), and myeloid subtype. Bone marrow is the major reservoir of multiple types of circulating EPCs. Multiple factors stimulate or inhibit the processes of EPCs differentiating to ECs. (+): stimulate; (−): inhibit. (?): uncertain. VEGFR-2: vascular endothelial growth factor receptor-2; KDR: kinases insert domain receptor. vWF: von willebrand Factor. VE-cad: vascular endothelial cadherin. CD146−: cell surface marker. PPAR-gamma: Peroxisome proliferator-activated receptor gamma. CRP-inhibitors: C-reactive protein inhibitor. TNF blockers: tumor necrosis factor blocker. ACE-inhibitors: angiotensin converting enzyme inhibitors. G-CSF: granulocyte colony-stimulating factor. GM-CSF: granulocyte monocyte colony-stimulating factor. IL-1 beta: interlukin-1 beta. IL-8: interleukin-8. ICAM-1: intercellular adhesion molecule-1. SDF-1: stromal cell-derived factor-1. ADMA: asymmetric dimethylarginine. Ox-LDL: oxidized low-density lipoprotein.
3.2. EPCs from peripheral blood and bone marrow
3.2.1. Isolation, characterization, and quantification of EPCs
At present, human EPCs are identified and quantitated by using three general approaches. First, putative EPCs can be isolated using the cell surface marker phenotypes, such as positive staining of CD34+CD133+ KDR+ (kinase insert domain receptor), which display some ability to participate in new blood vessel formation. As noted in the first EPC description, both CD34 and VEGFR2 (KDR for humans or fetal liver kinase-1 (Flk-1) for rodents) expressions on human peripheral blood mononuclear cells (PBMCs) can be used to enrich for putative EPC phenotype and function (18). CD34 has been used for identification of BM-derived hematopoietic progenitor and stem cells (66). The function of CD34 is for cell-to-cell adhesion as a ligand for E-selectin in regulating the tightness of EC adhesion to adjoining cells (67). VEGFR2 is one of three VEGFR family members, including VEGFR1 (Flt1) and VEGFR3 (Flt4). VEGFR2 is a receptor tyrosine kinase for VEGF, which is critical for EC functions such as maturation and migration (68). VEGFR2 is present in cells involved in vasculogenesis and those with potential to differentiate into mature ECs (69). Other investigators have suggested that a circulating population of endothelial precursors could be identified in human subjects by the phenotype of CD34+AC133 (CD133)+KDR+ (27, 70). Of note, CD133 is technically the same as AC133 (71). CD133 is a five-transmembrane protein found on 20–60 percentage of CD34bright cells in BM and blood, but not on mature ECs (70). CD133 has a function in regulating lipid composition within plasma membrane (72). Thus, the emerging consensus is that EPCs identified with cell surface markers CD34 and VEGFR2 contain very few, if any, genuine EC progenitors, and are more accurately described as angiogenic macrophages. Three recent papers have further strengthened this view (73–75). However, another study has most comprehensively demonstrated that so-called EPCs are predominantly of monocytic lineage (76). One estimate suggests that EPCs constitute less than 0.0001–0.01 percent of the peripheral circulating mononuclear cells (59). While the cell surface markers of the endothelial colony-forming cell (ECFC) progeny are nearly indistinguishable from the marker expression in ECs, some progress has been made in enriching this population by depleting monocytes, red blood cells, dead cells, and CD45+ blood cells (77). In addition, other EPC surface marker combinations such as stem cell antigen-1(78) (Sca-1)+/VEGFR2(Flk1)+(79) and c-kit+(78)/VEGFR2+ (56) have also been widely used in mouse EPC studies. The CD34-highly expressed cell population is shown to co-expresses CD146, CD31, and CD105. The CD34highCD45− cells are enriched by 300- to 400-fold for ECFC activity (80). Other cell surface markers used for EPC identification, such as von Willebrand factor (vWF), CD31, and CD144 are actually mature EC markers, whereas another potential EPC marker C-X-C chemokine receptor type 4 (CXCR4) is expressed on numerous cell types(3). Thus, no specific cell surface marker has been identified yet that can unequivocally discriminate ECFC from other circulating blood cells. Second, one may isolate putative EPCs using a colony forming unit-Hill (CFU-Hill) or ECFC assay. In the original EPC description (18), it was noted that plated human peripheral blood CD34+ cells rapidly form cellular clusters in vitro, particularly if re-exposed to CD34− cells during the co-culture. These clusters are comprised of an aggregate of round cells with spindle-shaped cells radiating from beneath the aggregate and away from the cluster. Recent studies indicate that the CFU-Hill assay measures a heterogeneous mixture of hematopoietic cells including monocytes, lymphocytes, and hematopoietic progenitor cells (81–84). The early outgrowth “endothelial” colonies derived in vitro from circulating CD34+ mononuclear cells, express the pan-leukocyte marker CD45 and the monocyte marker CD14, but fail to proliferate (85). By contrast, a very small proportion of the cells in culture form late outgrowth colonies (endothelial colony-forming cells, ECFC), which lack hematopoietic markers and do proliferate (86). These cells are believed to be genuine EPCs but they make up less than one percent of the circulating “EPCs” and an even smaller fraction in the CD34+ BM cell preparations used in clinical trials (87). This assay ultimately permits outgrowth of alternatively activated macrophages, which have significant stimulatory effects on angiogenesis but fail to directly contribute to postnatal vasculogenic activity (81). In contrast, the ECFC assay permits the identification of colonies of endothelium with a hierarchy of levels of proliferative potential that form human blood vessels, when seeded within three-dimensional collagen/fibronectin gels and implanted into immunodeficient mice (76). While both cord blood and adult peripheral blood ECFCs spontaneously form human blood vessels when implanted in matrix scaffolds into immunodeficient mice, cord blood ECFCs displays a greater density of vessels than the ECFCs from adult blood samples (76, 88). The ECFCs emerge as tightly adherent colonies with a typical cobblestone appearance and are rare in adult human blood samples with approximately one colony/108 mononuclear cells plated. The important aspects of the human blood vessel forming ability of ECFCs are reflected in the features of these newly-formed vessels: 1) to be connected to the immunodeficient mouse host vessels; and 2) to become a part of the systemic circulation of the host animals (76, 89).
Third, one may isolate putative EPCs by cell adhesion to fibronectin-coated dishes with display of certain lectin and lipoprotein binding properties. The attached cells, after 7 day culture, display the ability to take up lectin Ulex europeaus agglutinin-1 (UEA-1) and fluorescence-labeled acetylated low density lipoprotein (acLDL) (18). In subsequent studies, EPCs were defined as those cells that attach to fibronectin-coated culture dishes within 4 – 7 days and display the ability to take up UEA-1 and acLDL (5, 90, 91). Use of this definition permits investigators to isolate low-density peripheral blood mononuclear cells or BM cells from rodents, rabbits, or human subjects and to compare their properties to rescue blood flow in ischemic states in animals with induced vascular injuries. However, UEA-1, which recognizes L-fucosylated molecules on the surface of mammalian cells, is not only restricted to binding to ECs, but also bound to many types of epithelial cells (transformed and non-transformed) and various hematopoietic cells including platelets (84, 92–99). In addition, peripheral blood monocytes are known to be highly enriched upon plating on fibronectin-coated dishes (100) and are expected to display many proteins. The scavenger receptors are believed as one kind of these proteins and these receptors are capable of binding with acLDL, which are also expressed by ECs (in the presence of the growth factors and serum used in the “EPC” assay culture medium) (101, 102). In summary, neither acLDL nor UEA-1 binding is restricted in binding of EPCs. Furthermore, platelets (or platelet microparticles) and monocytes bind to the fibronectin-coated dishes used in this assay and have to be depleted from the mononuclear cells prior to defining any putative adherent cell as an EPC. These findings suggest that putative EPCs are identified using UEA-1 and ac-LDL binding to adherent human mononuclear cells as the sole definitive criteria. Of note, a recent report clearly suggests the stem/progenitor differentiation hierarchy of c-Kit+/Sca-1+/Lin− cells (KSL), c-kit+/Lin− cells (KL), Sca-1+/Lin− cells (SL), and CD34+ cells. In the EPC-colony forming assay, small EPC colonies (mostly KSL cells and some KL cells) are capable of differentiating into large colonies (KL cells and SL cells), and large EPC colonies can further differentiate into adherent, more vasculogenic non-colony forming EPCs in culture (CD34+ cells). Small colonies reveal a predominant potential for proliferation, while large colonies demonstrate a predominantly vasculogenic potential including cell adhesion and tube-like structure formation in vitro (103).
3.2.2. Mobilization of EPCs
EPCs in BM are located within a stem cell niche, which is characterized by low oxygen tension and high levels of SDF-1/CXCL12, a chemoattractant for EPCs (3). Physiological factors mobilizing EPCs from BM niches include peripheral tissue hypoxia, trauma, physical exercise, estrogen, age and others. These conditions cause the production and release of EPC activation factors including stromal cell-derived factor-1 (SDF-1) (104, 105), soluble intercellular adhesion molecule (sICAM), VEGF(106), hypoxia-inducible factor-1alpha (HIF-1alpha), erythropoietin (EPO) (107), granulocyte colony-stimulating factor (G-CSF) (108), granulocyte monocyte colony-stimulating factor (GM-CSF), hepatocyte growth factor (109), interleukin-6 (IL-6), estrogen, and matrix metalloproteinase-9 (MMP-9) (110) (3, 107, 111, 112). These EPC activation factors mediate EPC mobilization, proliferation, and migration via activation of the Akt (protein kinase B, PKB) pathway (3, 113, and 114). Moreover, estrogens mobilize EPCs through a direct action on alpha and beta estrogen receptors via matrix MMP-9 and an eNOS-mediated mechanism. The role of estrogen in mobilizing EPCs helps to explain the lower rates of CV events in pre-menopausal women when compared with men (115). Furthermore, MMP-9 functions in releasing soluble kit ligand from EPCs in the BM, allowing EPCs to move out to the peripheral circulation (3, 116). In contrast, several studies have demonstrated that aging has a negative impact on EPCs’ mobilization at different steps (117, 118).
3.2.3. Homing of EPCs
The peripheral blood of healthy adults contains very low concentrations of circulating EPCs. However, as we mentioned above, several EPC activation factors and transducers can boost the mobilization of these progenitor cells from the BM into peripheral blood for accelerated in situ endothelialization. These growth factors, cytokines, and chemokines may also influence the homing of EPCs.
However, the numbers of EPCs can be reduced by pre-activation of these cells with cytokines that upregulate expression of adhesion molecules, which in turn enhances the adhesion of EPCs at sites of vascular injury. Therefore, several groups propose the stimulation of isolated EPCs before application with for e.g. SDF-1 (104), 8-pCPT-2′-O-MecAMP (an analogue of the natural signal molecule cyclic AMP), ephrin-B2-Fc chimera (119), Mn2+ or an activating beta2-integrin antibody (KIM185), and high mobility group box-1 protein (HMGB-1) to increase homing of systemically administered progenitor cells to the sites where vascular repair and neovascularization are needed. The SDF-1/chemokine C-X-C receptor 4 (CXCR4) axis plays a critical role in homing and engraftment of hematopoietic stem/progenitor cells (HSCs) during BM transplantation.
The interaction of EPC surface molecules with their ligands on activated ECs or sub-endothelial matrix proteins plays an important role in EPC homing (57). In general, the recruitment of EPCs involves chemotaxis, tethering, adhesion, and migration of cells into the sub-endothelial tissue (120). The adhesion molecules, P-selectin and E-selectin, appear to play an important role in adhesion and migration of EPCs. Further studies showed that EPCs also express beta2-integrins, the ligand of intercellular adhesion molecule-1 (ICAM-1). These integrins mediate the adhesion of EPCs to mature ECs, recombinant human ICAM-1, fibrinogen and the chemokine-induced transendothelial migration of EPCs. The activation of beta2-integrins on EPCs significantly enhances the homing of these cells to sites of ischemia and EPC-induced neovascularization (121). Furthermore, the tissue cells that are activated by pro-inflammatory cytokines and the necrotic cells in damaged tissue lead to the secretion of the high-mobility group protein B1 (HMGB1) into the extracellular space. EPCs express the HMGB1 receptors, the receptor for advanced glycation end products (RAGE), and Toll-like receptor 2 (TLR2), suggesting that EPCs are equipped to respond to stimulations by HMGB1, advanced glycation end products (AGE), and endogenous/exogenous ligands for TLR2. More findings (122) show that the binding of HMGB1 activates beta1- and beta2-integrins on the surface of EPCs and induces the EPC adhesion to mature ECs, ICAM-1, and fibronectin. Moreover, the increase of EPC recruitment to sites of vascular injury accelerates re-endothelialization and decreases neointima formation in vivo (123, 124).
3.3. Vascular wall EPCs
In addition to EPCs identified in BM and peripheral circulation, progenitor and stem cells also reside in the vascular wall. Traditionally, vascular wall cells include ECs in the intima, vascular smooth muscle cells (VSMCs) in the media, and fibroblasts in the adventitia. The human embryonic aorta is a rich source of CD34+CD31− EPCs (125). This report is correlated well with similar findings in the human fetal aorta, where the immature vascular cells coexpress endothelial and myogenic markers located in the human fetal paraortic membrane or at the periphery of aorta wall parenchyma (126). Further study strongly supports the presence of EPCs in the adult vascular wall (127) by providing evidence of the existence of EPCs and stem cells in a distinct zone of the vascular wall, which are capable of differentiating into mature ECs, hematopoietic, and local immune cells such as macrophages. Evidence in animal models and human vessels have also shown that various types of stem and progenitor cells, such as vascular wall resident vascular stem cells (VW-VSCs)/vascular wall-EPCs (VW-EPCs), VW resident hematopoietic stem/progenitor cells (VW-HSCs/−HPCs), and VW resident mesenchymal stem cells (MSCs) located in fetal and adult arterial and venous vessel walls. This niche-zone acts as a source of progenitors for angiogenesis and postnatal vasculogenesis (128). Some in vivo data have shown that liver-derived c-kit+CD45− progenitors can arrive in the peripheral blood and contribute to the pool of circulating EPCs and then contribute to the neovascularization of ischemic hindlimb, which suggests the existence of EPC sources outside the BM (129). The abundant evidence strongely indicate that EPCs exist not only in the wall of large blood vessels outside the organs, like aorta or internal thoracic artery, but also in the wall of large and middle-sized blood vessels within organs such as liver, prostate, heart, kidney, testes, and lung. In searching for the detailed location of EPCs in vessel walls, a different vasculogenic niche-containing progenitor cells at the medial-adventitial border has been described in murine arteries (130). These cells, in a similar location in human thoracic arteries, could be differentiated in culture to yield cells with smooth muscle markers and EPCs (127, 128). Finally, in single cell clonogenic assays a small proportion of human arterial ECs in culture have a very high proliferative potential (131), suggesting that in situ endothelium has embedded within it a small number of resident progenitor cells (132), which act as a niche for endothelial regeneration and repair.
Further study using immunostaining methods shows the VW-EPCs are CD34+VEGFR-2+Tie-2+CD31−CD144−, whereas the outgoing VW-EPCs become CD144+ (128). The advantage of VW-EPCs might be in their location with easy accessibility in ischemic disorders and tissue regeneration. Therefore, the concept of the non-BM EPCs is an essential prerequisite not only for a better understanding of their role in postnatal vasculogenesis but also for their therapeutic manipulation and diagnostic use in diseases such as cardiovascular disorders, tumor growth, and metastasis.
3.4. Monocyte-/macrophage-derived EPCs
Conventionally, researchers have considered CD34+ or CD133+ cells, which are derived from peripheral blood or BM to be EPCs (27, 133), but these cells are a very small percentage of the PBMCs ranging from 0.02 to 0.1 percent (134), which implies that 12 liters of autologous blood would be needed to perhaps have enough EPCs to increase angiogenesis in patients after intravenous cell infusion (135). Thus, to acquire sufficient EPCs, granulocyte colony-stimulating factor (G-CSF) is used to mobilize BM-derived CD34+ or CD133+ (136, 137).
Being the big subset of PBMCs, monocytes are also recognized to have the potential to reside in the endothelium of vessels in mouse ischemic limbs (138). Recent evidence showed that circulating monocytes have potential to differentiate into a variety of cell types. After 2 – 4 weeks in culture, CD14+/CD34− monocytes, isolated from PBMCs, express EC markers such as von willebrand factor (vWF), VE-cadherin and eNOS (101). In addition, CD14+ monocytes experience phenotypical changes toward EC morphology. The attached cells gradually develop into spindle shape and undergo a functional change, and cord- and tubular-like structures in matrigel are observed after 4 – 7 days in culture. More interestingly, when these cells upregulate the expression of EC markers, the expression of CD14 is decreased without cell proliferation, indicating that the existing cells change their characteristics. These findings are supported by another study (85). A subset of cells termed monocyte-derived multipotential cells (MOMCs) also are able to differentiate into ECs (139, 140). MOMCs, previously termed monocyte-derived mesenchymal progenitors, originate from circulating CD14+ monocytes since they are positive for monocytic markers. In endothelial cell induction medium, these MOMCs can change their morphology from spindle shape to typical caudate appearance. Similar to CD14+/CD34− monocytes, these MOMCs also have some EC characteristics, such as acLDL uptake, releasing of vWF, and the promotion of tubular formation. Different from the monocyte-derived endothelial like cells (ECLs), MOMCs-derived ECLs express higher levels of CD34/CD144, implying that MOMCs correspond to early EPCs. On the other hand, CD14+CD34low cells are also recognized as the source of MOMCs, indicating that those cell-types have the capacity to differentiate into different cell types.
The PBMCs are able to express both CD14 (47percent) and CD34 (16percent). The expression of CD14 can be easily detected using flow cytometry in almost all PBMCs-derived adherent cells in EC culture medium (134). However, the expression of CD34 in these cells can be detected only by using antibody-conjugated magnetofluorescent liposomes since this method can significantly amplify the fluorescence signal intensity up to 100–1000 fold higher than traditional flow cytometry. Further study shows that more than 90percent of KDR positive cells from PBMNCs are CD14+, which strongly supports that this cell population can also differentiate into ECs. Furthermore, these cells are found to express the unique markers of stem cells such as Nanog, which confirms the multi-differentiation capacity of the cells. More interestingly, similar to PBMCs, almost all of the BM-derived CD14+ cells also appear to be CD14+CD34low.
Overall, the up-to-date evidence implies that monocytes consist of a subset of cells termed EPCs which can differentiate into mature ECs. The consequence of their differentiation is shown in Figure 1. Same as BM-derived EPCs, treatment with monocyte-derived EPCs may also provide a potential therapeutical opportunity for CVDs.
3.5. Roles of EPCs in vascular biology
EPCs are differentiated into an endothelial phenotype, express endothelial markers, and incorporated into neovessels at sites of ischemia (18). EPCs provide both instructive (release of pro-angiogenic cytokines) and structural (vessel incorporation and stabilization) functions that contribute to the initiation of neo-angiogenesis (141). EPCs enhance angiogenesis and vascular repair in a variety of experimental models.
3.5.1. EPCs and neovascularization
Recent studies (85) show that EPCs have multiple surface markers and secrete angiogenic factors including VEGF. Herein, the cell markers of progenitor cells in the vessel wall are summarized as shown in the Table. 1 (125, 128, 130, 131, 142–150). An interesting study revealed that both early outgrowth EPCs and late outgrowth ECFCs secrete pro-inflammatory cytokines, though with different profiles, which could be inhibited by statin treatment (151). A recent review (152) summarizes that new adult blood vessel formation is primarily due to sprouting and replication of ECs from existing local blood vessels, but is stimulated and facilitated by the prior arrival of blood-derived monocytic EPCs. Via a plethora of mechanosensors and mechanotransduction signaling pathways, stem cells differentiate into endothelial cells when exposed to fluid shear stress (153). The cited evidence suggest that a fraction of the new ECs may be BM-derived, presumably from the rare CD34+CD14-circulating cells. Though this remains controversial, at least four studies in animals with reconstituted genetically-marked BM have concluded that BM-derived cells are present only as perivascular cells, and not as luminal lining cells in new blood vessels (154–157) (158). Finally, the therapeutic efficacy of EPCs is shown in a myocardial infarction (MI) model (159).
Table.
Summary of cell marker expression on progenitor cells
| Source/species | Cell marker expression | PMID |
|---|---|---|
| mouse aortic | Sca-1+, c-kit(−/low); Lin− CD34(−/low) | 16306431 |
| mouse embryonic/adult arteries | Sca-1+ | 18591670 |
| ApoE−/− mouse | Sca-1+ | 15124016 |
| rat aorta | CD34/Tie-2, NG2, nestin, PDGFR | 16079185 |
| human arteries and veins | CD34+, CD31−, CD34+VEGFR2+, Tie-2 | 2006 16524930; 12384418 |
| human embryonic aorta rings | CD31+/CD31− | 11406648 |
| human | CD34+FGFR1+ | 12411316 |
| human | CD34+CD11b+; CD34+CD45+CD146+ | 15213103; 16411405 |
| human | CD34+CD45+ | 12969964 |
| human HUVEC/HAEC | CD31+, CD141+, CD105+, CD146+, CD144+, vWF+, FlK-1+ | 15585655 |
| human saphenous vein-internal surface | CD13+, CD29+, CD44+, CD54+, CD90+ HLA classl+, HLA-DR | 16018999 |
| human | CD14+CD34+ | 16020753 |
| human | CD34− CD133+VEGFR2+; CD34+CD133+VEGFR2+CD45+ | 16439688; 15191940 |
| human thoracic aorta | CD34+ or c-kit + | 17446560 |
| human | CD34+CD133+; CD34+CD133+VEGFR2+ | 17417992; 10648408 |
| human aorta and mammary arteries | CD34, c-kit, Sca-1 | 16787646 |
| human | ALDHbright | 18061073 |
| human | CD31+CD34+; CD34+CD62L+ | 18063830 |
| human | CD34+CD133+CD45+; CD133+CD45− | 18401642 16455670 |
| human | CD34+CD144+CD3− | 18402976 |
| human blood and transplant atherosclerotic vessels | EC: eNOS, Tie-2, CD31, VECAD Myeloid: CD14, CD68 | 19571578 |
| human saphenous vein | CD34+/vWF−, CD34+/CD31− | 20368523 |
| human arteries | Oct-4, Stro-1, Sca-1, Notch-1 | 17446560 |
| human fetal aorta | CD105+, CD34−, FLK+ | 20404027 |
3.5.2. EPCs and endothelial regeneration
Other parallel lines of evidence have also advanced the idea that BM-derived EPCs are important for large vessel repair after injury or in atherosclerosis. There is evidence that EPCs can enhance vascular repair and reduce neo-intima formation and atherogenesis. Early results (51) show that after wire injury to denude endothelium in murine carotid arteries, BM-derived cells could be detected adhering to the vessel area. Statin treatment increases circulating EPCs and adherent BM-derived cells, and concomitantly reduces neointima formation and increases re-endothelialization. In vivo study shows that delivery of purified circulating CD34+ cells to the site of balloon-induced injury in rabbit carotid arteries enhances re-endothelialization and reduces neointima formation (31, 160). Since more rapid EC regrowth reduces intimal hyperplasia, the desired cell therapy should ideally deliver genuine EPCs to the site of injury, or deliver other cell types that can selectively encourage local regrowth and repair of the denuded endothelium. However, despite of the ability (noted above) of blood-derived cells to maintain an antithrombotic lining in large vascular grafts, it is still uncertain that EPCs contribute directly to the re-endothelialization of injured vessels (161, 162).
Taken together, these results strongly suggest that in most models studied to date, the beneficial effects of EPCs are not only because of their direct incorporation and expansion of the endothelial cells in the repairing tissue, but also predominantly due to paracrine effects caused by the release of angiogenic or other factors from the transplanted cells to stimulate local vessel repair mechanisms.
3.6. EPC interaction with other cells
A specialized microenvironment, termed stem cell niche, influences stem cell decision on self-renewal and differentiation (163), which implicates the importance of cell-cell interaction in maintaining the phenotypes and function of stem cells/progenitor cells. This principle has also been demonstrated in EPCs. To improve the survival, vascular differentiation, and regenerative potential of umbilical cord blood (UCB)-derived hematopoietic stem cells (CD34+ cells), the stem cells are co-cultured with CD34+-derived ECs in a 3D (three dimensional) fibrin gel. ECs differentiated from CD34+ cells appear to have superior angiogenic properties to fully differentiated ECs, such as human umbilical vein endothelial cells (HUVECs). The pro-survival effect of CD34+-derived ECs on CD34+ cells is mediated, at least in part, by bioactive factors released from ECs. This effect likely involves the secretion of novel cytokines, including pro-inflammatory cytokine interleukin-17 (IL-17) and anti-inflammatory cytokine interleukin-10 (IL-10), and the activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathway in CD34+ cells. Thus, the endothelial differentiation of CD34+ cells in co-culture with CD34+-derived ECs is mediated by a combination of soluble and insoluble factors. The regenerative potential of this co-culture system is demonstrated in a chronic wound diabetic animal model. The co-transplantation of CD34+ cells with CD34+-derived ECs improves the wound healing comparing to controls, by decreasing the inflammatory reaction and increasing the neovascularization of the wound (164).
In addition to repairing pulmonary endothelium, EPCs can also migrate into the arterial intima and differentiate into smooth muscle-like (mesenchymal) cells contributing to intimal hyperplasia. The molecular mechanisms by which this process proceeds have not been fully elucidated. Co-culture of EPCs with vascular smooth muscle cells (VSMCs) increases the expression of the mesenchymal cell markers including alpha-smooth muscle actin, sm22-alpha, and myocardin, and decreases the expression of the EC marker CD31. In the same conditions, a concomitant increase is found in the gene expression of the endothelial-to-mesenchymal transition (EnMT)-related transcription factors, such as slug, snail, zeb1, and vessel-constricting protein endothelin-1. This indicates that mesenchymal phenotype acquisition of EPCs occurs through an EnMT-like process. Inhibition of transforming growth factor-beta (TGF-beta) receptor I (TGF-betaRI) downregulates snail gene expression, blocks the EnMT, and facilitates the differentiation of EPCs to the EC lineage. Furthermore, TGF-betaRI inhibition decreases migration of EPCs stimulated by VSMC without affecting their functionality and adhesion capacity. These results indicate that EPCs may differentiate into VSMC-like cells through an EnMT-like process and that TGF-betaI plays an important role in the fate of EPCs (165).
The CD34+ cell is often designated as an EPC, because it contributes to the repair of ischemic injuries through neovascularization. However, incorporation of CD34+ cells into the neovasculature is limited, suggesting the potential paracrine role of EPCs. CD14+ cells can also differentiate into ECs and contribute to neovascularization. However, the low proliferative capacity of CD14+ cell-derived ECs hampers their use as therapeutic cells. To determine whether an interaction between CD34+ and CD14+ cells augments endothelial differentiation of the CD14+ EPCs, endothelial differentiation is analyzed by expression of EC markers CD31, CD144, vWF, and endothelial Nitric Oxide Synthase (eNOS); the proliferative capacity; and EC function of the cells in culture. In monocultures, 63 percent of the CD14+-derived cells adopt an EC phenotype, whereas in CD34+/CD14+ co-cultures 95 percents of the cells show EC differentiation. Proliferation increases up to 12 percent in the CD34+/CD14+ co-cultures compared to both monocultures. CD34− conditioned medium also increases endothelial differentiation of CD14+ cells. This effect is abrogated by hepatocyte growth factor-neutralizing antibodies, but not by neutralizing antibodies to interleukin-8 and monocyte chemoattractant protein-1. Thus, co-culturing of CD34+ and CD14+ cells results in a proliferating population of functional endothelial cells, which may be suitable for treatment of ischaemic diseases such as myocardial infarction (166).
4. EPCs AND ATHEROSCLEROSIS
4.1. The earliest changes of atherosclerosis–EC dysfunction
Seventy-five percent of all deaths from cardiovascular diseases are due to heart attack or stroke caused by atherosclerosis, which remains the leading cause of morbidity and mortality in the U.S. (167). Atherosclerosis, a formation of atherosclerotic plaques in large and medium sized arteries (168), is characterized by focal arterial lesions containing cholesterol, fibrosis, and inflammatory cell infiltrates (169, 170). The endothelial lining is a dynamically mutable interface, locally responsive to various stimuli originating from the circulating blood and/or neighboring cells and tissues, and thus can actively participate in the physiological adaptation or pathophysiological dysfunction of a given region of the vasculature (171). From a structure-function relationship, the endothelium appears ideally suited to function in this capacity given its unique anatomical position between blood and tissues. In the early 1980s, a study (172) confirmed the movement of foam cells through vascular endothelium overlying fatty lesions in the aortic arch of hypercholesterolemic swine.
In recent study, the cellular origin of chondrocyte-like cells in atherosclerotic intimal calcification is observed by using BM transplantation (BMT) in C57BL/6 low density lipoprotein receptor deficient (LDLr−/−) mice, an atherogenic mouse model. The data showed that the majority of chondrocyte-like cells are of BM origin (173). These results suggest that BM-derived myeloid CD34+/CD13+ precursors actively infiltrate the plaque, where they are capable of trans-differentiating into chondrocytes-like cells in the progression of atherosclerosis. Numerous pathophysiological findings in humans and animals (174) lead to the formulation of the response-to-injury hypothesis of atherosclerosis, which initially proposed that endothelial denudation is the first step in atherosclerosis. Endothelial dysfunction leads to compensatory responses that alter the normal homeostatic properties of the endothelium (2, 12). Thus, activation and damage of the endothelial monolayer trigger the development of the lesions. These changes include increased endothelial permeability to lipoproteins and the generation of a long list of biological effectors (e.g. nitric oxide, eicosanoids, cytokines, growth stimulators and inhibitors, vasoactive peptides, pro- and anticoagulants, and fibrinolytic factors). In addition, the EC dysfunctional/activation changes also include up-regulation of leukocyte adhesion molecules, including L-selectin, integrins, and platelet EC adhesion molecule 1, and the up-regulation of endothelial adhesion molecules, which include E-selectin, P-selectin, ICAM-1, and vascular-cell adhesion molecule 1 (VCAM-1). Consequently, recruited leukocytes are migrated across activated ECs and into the artery wall, which is mediated by oxidized low-density lipoprotein (oxLDL), monocyte chemotactic protein-1 (MCP-1), interleukin-8 (IL-8), platelet-derived growth factor, macrophage colony-stimulating factor (M-CSF), and osteopontin. Some important issues remain regarding the roles of EPCs in atherogenic process: 1) how do pro-atherogenic factors affect the homeostasis of EPCs? 2) Can EPCs repair impaired ECs induced by pro-atherogenic factors?
4.2. EPC levels–independent predictive biomarker
Several clinical conditions are characterized by both increased inflammation and oxidative stress (175) such as diabetes mellitus (48), heart failure (19), hypertension (176), rheumatoid arthritis (40), and preeclampsia (177). These diseases are significantly associated with reduced numbers and impaired functionality of EPCs. In addition, the availability and function of EPCs are also adversely affected by the proatherogenic risk factors. The numbers of circulating CD34+/KDR+ precursor cells are significantly reduced in patients with coronary artery disease (CAD) by 48 percent. To determine the influence of atherosclerotic risk factors a risk factor score including age, sex, hypertension, diabetes, smoking, positive family history of CAD and LDL cholesterol levels has been used. The number of risk factors is significantly correlated with a reduction of EPC levels (54). In addition, the study shows that chronic treatment with BM derived progenitor cells from young non-atherosclerotic apolipoprotein E knock-out (ApoE−/−) mice prevents atherosclerosis from progression in ApoE−/− recipients in spite of persistent hypercholesterolemia (39). In contrast, treatment with BM cells from older ApoE−/− mice with atherosclerosis is much less effective. These results suggest that ApoE gene deficiency may not affect EPC repairing efficiency but that the chronic stimulation of EPCs with hypercholesterolemia in older ApoE−/− mice significantly weakens EPC repairing function.
Moreover, a strong correlation between the number of circulating EPCs and the combined Framingham risk factor score for atherosclerosis exists, which suggests that EPCs can be used as a predictive biomarker for cardiovascular risk and vascular function(53). The levels of circulating EPCs are a better predictor of vascular reactivity than conventional risk factors. An inverse correlation between the number of EPCs and the number of risk factors has been documented in patients with known CAD (54). In summary, with regard to pathophysiological stimuli, EPC numbers are proven to be inversely related to the number of cardiovascular risk factors (53, 158) and to the Framingham cardiovascular total risk scores, and directly related to brachial reactivity (89, 178). Interestingly, a decrease in circulating EPCs with subsequently impaired EC repair is shown to contribute to reduced arterial elasticity, a hallmark of aging in healthy humans (179). This observation may explain why the measurement of flow-mediated brachial-artery reactivity could reveal a significant relationship between endothelial function and the number of progenitor cells. In general, the greater the EPC numbers, the better the vasculature health.
Hypercholesterolemia causes a reduction in EPC’s proliferative, migratory, and vasculogenetic properties (46) which is secondary to a rise in senescence/apoptosis ratio, as demonstrated after EPC incubation with OxLDL (180), whereas high density lipoprotein (HDL)-cholesterol could exert a vascular protection by increasing EPC numbers. After an acute myocardial infarction (AMI), progenitor/stem cells are mobilized from BM, released into peripheral blood, and subsequently home in the myocardium (38, 181). Furthermore, atherosclerotic patients, suffering from cardiovascular events such as cardiovascular death, unstable angina, myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass graft (CABG), or ischemic stroke, have significantly lower numbers of EPCs. In a prospective study, a single measurement of CD34+KDR+ EPCs is demonstrated to be a useful tool in predicting cardiovascular outcomes in patients with CAD (37). The association between these reduced EPC levels and increased death from cardiovascular diseases is independent of the severity of CAD diagnosis of an acute coronary syndrome at the time of enrollment, cardiovascular risk factors, and drug therapy known to influence cardiovascular outcomes. EPC numbers can also be used to predict severe endothelial dysfunction in patients with coronary heart disease (182). However, the relation between heart failure and EPC levels is complex. A recent study provides novel evidence of a selective functional exhaustion of the EPCs in patients with post-ischaemic heart failure. Interestingly, BM niches as well as the colony-forming capacity seem to be reduced, whereas BM hematopoietic progenitor cell numbers are preserved (183), implying that EPC dysfunction may follow EPC reduction.
4.3. Endothelial progenitor cells repair injured vessels and prevent atherosclerosis
In the atherogenic process there is a balance between two ongoing events, endothelial damage and repair. Under physiological conditions the integrity of the endothelial monolayer is maintained by replication of adjacent cells; however, in the conditions of increased endothelial injury, regeneration of the injured endothelium may be assisted by EPCs homing into the artery wall (184). A more detailed understanding of EPCs in the pathophysiology of vascular turnover may be helpful for more effectively preventing the clinical consequences. The suppression of BM myeloid proliferation, leukocytosis and monocytosis might represent a previously unidentified anti-atherogenic effect of HDL function in the early stage in the leukocyte life cycle, which is followed by subsequent anti-inflammatory of antioxidant effects occurring in the vessel wall (185). Further findings have provided quantitative data on endothelial turnover and repair by EPCs that are, at least in part, derived from BM during development of atherosclerosis in ApoE−/− mice (186). Furthermore, there is evidence that ECs of neointimal lesions in allografts are derived from circulating EPCs and that BM–derived progenitors are responsible for angiogenesis of the allograft, that is, the formation of microvessels in transplant arteriosclerosis (187). Evidence shows that mature ECs of vein grafts are derived from circulating progenitor cells, of which one-third are derived from BM progenitor cells (188). Hyperlipidemia due to apoE deficiency results in a lower number of EPCs in blood, which is consequently correlated with enhanced atherosclerosis. The wild-type BM transplantation results in a restoration of ApoE expression, normalization of plasma cholesterol, and substantial reduction of atherosclerotic lesions in apoE/− mice with irradiated BM (189). Although the mechanisms involved are still unclear, EPCs seem to contribute to the restoration of the endothelial monolayer (150, 190–192).
4.4. Transfer of progenitor cells is not always beneficial
The transfer of progenitor cells is not always beneficial. ApoE−/− mice, that have received BM mononuclear cells following induced hind limb ischemia, displayed not only increased neovascularization to these oxygen deficient regions, but also accelerated atherosclerotic plaque formation and lesion size compared with control animals (187). A study was designed to define the association between the degree of restenosis following wire injury and their mobilization of monocytic cells from BM in response to the injury. Interestingly, the degree of restenosis and the presence of leukocytes in the vessel wall were greater in the strain with higher cell mobilization (193). In an alternative study, EPC-treated mice also displayed accelerated atherosclerosis along with reduced plaque stability. This may be attributed to the pro-inflammatory properties of these cells since a reduction in suppressive cytokine IL-10 levels in the atherosclerotic aortas was observed (194). Nevertheless, one should bear in mind that the term EPC is loosely defined and used to describe a vastly mixed cell population that consists of different progenitors.
4.5. The mechanisms underlying EPC dysfunction and reduction in atherosclerosis
Oxidized low density lipoprotein (oxLDL) is one of the most important causative factors of cardiovascular disease (195, 196). OxLDL decreases EPC survival and impairs its adhesive, migratory, and tube formation capacities in a dose-dependent manner (197). However, all of the detrimental effects of oxLDL are attenuated by the pre-treatment of EPCs with lectin-like oxidized low density lipoprotein receptor (LOX-1) monoclonal antibody or L-arginine, suggesting that LOX-1 or L-arginine pathways mediates the oxLDL effects. OxLDL decreases protein kinase B/Akt (a serine/threonine kinase) phosphorylation and eNOS protein expression, and increases LOX-1 protein expression. In correlation, OxLDL also causes a decrease in eNOS mRNA expression and an increase in LOX-1 mRNA expression. These data indicate that OxLDL inhibits EPC survival and impairs its function, and this action is attributable to an inhibitory effect on eNOS (197). Additionally, OxLDL induces apoptosis and senescence of EPCs (Figure 2). Senescence is a programmed cellular response, parallel to apoptosis, which is turned on when a cell reaches Hayflick’s limit (198). Once cells enter the senescence stage, they cease to proliferate and undergo a series of morphological and functional changes (199). In addition, oxidative alteration of LDL impairs the angiogenic properties of EPCs at sub-apoptotic levels by the downregulation of E-selectin and integrin alphaVbeta5 expressions, both substantial mediators of EPC-EC interaction (200). Cigarette smoking is associated with the formation of L5, electronegative LDL, which inhibits EPC differentiation by impairing Akt phosphorylation via the LOX-1 receptor pathway (201). Moreover, carbamylated low-density lipoprotein (cLDL) plays a role in atherosclerosis. cLDL is generated after incubation of native LDL (nLDL) with uremic serum from patients with chronic kidney disease (CKD) stages 2–4. The percentage of cLDL converted by uremic serum is related to the severity of CKD. Compared with nLDL, cLDL induces an increase in oxidative stress and mitochondrial depolarization, and a decrease in EPC proliferation and angiogenesis. cLDL also induces accelerated senescence of EPCs as judged by beta-galactosidase activity and telomere length, which is associated with a decrease in the expression of phosphorylated histone H2AX. The degree of injury induced by cLDL is comparable to that observed with oxLDL (200 microg/ml). This study supports the hypotheses that cLDL triggers genomic damage in EPCs, resulting in premature senescence and ultimately an increase in atherosclerotic disease in CKD (202). Similarly, smoking increases oxidative stress and reduces NO bioavailability, resulting in depletion of EPCs and attenuation of vascular repair in a dose-dependent manner (203). The EPC levels experience a rapid amelioration after smoking cessation (176).
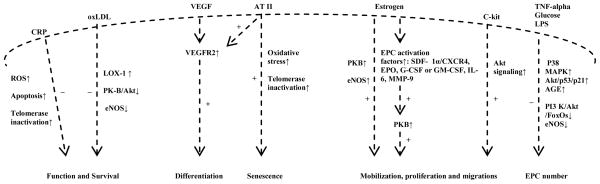
Figure 2.
Schematic summarizes the mechanisms through which the factors influence endothelial progenitor cell (EPC) homeostasis, survival and function. (+): stimulation. (−): inhibition. (↑): up-regulation. (↓): down-regulation. ROS: reactive oxygen species. CRP: C-reactive protein. OxLDL: oxidized low-density lipoprotein. LOX-1: lectin-like oxidized low density lipoprotein receptor. eNOS: endothelial nitric oxide synthase. VEGF: vascular endothelial growth factor. VEGFR2: vascular endothelial growth factor receptor 2. ATII: angiotensin II. SDF-1alpha: stromal cell-derived factor-1alpha. CXCR4: Chemokine receptor type 4. EPO: erythropoietin. G-CSF: granulocyte colony-stimulating factor. GM-CSF: granulocyte monocyte colony-stimulating factor. IL-6: interlukin-6. MMP-9: matrix metalloproteinase-9. TNF-alpha: tumor necrosis factor-alpha. LPS: Lipopolysaccharide. P38 MAPK: P38 mitogen-activated protein kinase. AGE: advanced glycation end products. PI3K/Akt: phosphatidylinositol-3/Akt pathway. FoxOs: forkhead family of transcription factor.
C-reactive protein (CRP) participates in the development of atherosclerosis in part by promoting endothelial dysfunction and impairing EPC survival and differentiation. EPCs, isolated from human peripheral venous blood, are cultured in the absence or presence of native pentameric azide and lipopolysaccharide (LPS)-free CRP (0, 5, 15, and 20 microg/mL), N-acetylcysteine (NAC), hydrogen peroxide (H2O2) or monoclonal anti-CRP antibodies. Incubation of the EPCs with CRP causes a concentration-dependent increase in reactive oxygen species (ROS) production and apoptosis, with an effect quantitatively similar to that of H2O2. This effect is attenuated during coincubation with NAC or anti-CRP antibodies. Furthermore, CRP alters EPC antioxidative enzyme levels, demonstrating a reduced expression of glutathione peroxidase and a significant increase in manganese superoxide dismutase (MnSOD) expression. Transfection of EPCs with MnSOD-RNAi results in a reduction in CRP-induced ROS production, apoptosis, and telomerase inactivation. Thus, CRP may serve to impair EPC antioxidant defenses and promote EPC sensitivity toward oxidant-mediated apoptosis and telomerase inactivation (Figure 2) (204).
Several additional signaling pathways involving in EPC reduction and dysfunction in atherosclerosis have been suggested. In arterial hypertension, angiotensin-II accelerates EPC senescence by reducing telomerase activity and provoking EPC oxidative stress, although it also stimulates VEGF-mediated EPC proliferation, most likely due to VEGFR2 upregulation (Figure 2) (205). The functional New York heart association (NYHA) class is related to different levels of CD34+ cells due to the higher serum levels of tumor necrosis factor-alpha (TNF-alpha) (206), a known myelosuppressive cytokine. gp91phox (Nox2)-containing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase deficiency protects EPCs and neovascularization against hypercholesterolemia-induced impairment. The potential mechanisms involved include reduced ROS formation, preserved activation of angiogenic signals, and improved functional activities of EPCs and mature ECs (207).
5. EPCs AND INFLAMMATION
Apparently, inflammation is involved in the initiation, progression, resolution, and complication of inflammatory cardiovascular diseases. Vascular inflammation is also considered to be a key component in atherogenesis (168, 208). Emerging evidence from in vitro and clinical studies demonstrate that the inflammatory process influences EPC numbers and their functional capacity (40, 41, 209–215). EPCs constitute an important endogenous system to maintain endothelial integrity and vascular homeostasis, thus, enhancement of EPCs by anti-inflammatory therapies could have potential benefit for individuals with inflammatory CVDs. A better understanding of the role of inflammation in the regulation of EPC mobilization and function would provide additional insight into the pathogenesis of CVD and its complications.
5.1. Inflammation, EPC mobilization and EPC recruitment
The effects of acute inflammation on EPC mobilization and recruitment can be classified into two categories: 1) a transient restricted inflammatory response; and 2) persistent or excessive inflammatory stimulation. A transient restricted inflammatory response may contribute a stimulus for EPC mobilization. In vivo study has demonstrated that EPCs are rapidly mobilized after vascular trauma and contribute to the revascularization of injured vessels in response to increased circulating VEGF levels stimulated by pro-inflammatory cytokines (216). IL-1beta is involved in EPC recruitment and homing to ischemic tissue in a VEGF-dependent manner as well as upregulating the expressions of VEGF, VEGFR-2, and adhesion molecules on ECs (217). In addition, further findings indicate that circulating EPCs are mobilized endogenously in response to tissue ischemia or exogenously by cytokine therapy with several other inflammatory molecules such as GM-CSF and SDF-1, which thereby augment the neovascularization of ischemic tissues (5). Moreover, AMI is the most established acute pathological stimulus for EPC mobilization. Indeed, AMI leads to an increase in EPC number, which correlates positively with plasma VEGF levels (218). An positive association of increased CD133+ cells and IL-8, an inflammatory chemokine with angiogenic properties, is also reported in patients with AMI (213). After an AMI, progenitor/stem cells are mobilized from BM, released into peripheral blood, and subsequently homed in the myocardium (38, 181). CD18 and its ligand ICAM-1 play an essential role in mediating EPC recruitment and the subsequent functional effects on the infarcted heart (219).
Recently, in both in vitro and in vivo, microvascular ECs, challenged with inflammatory stimuli, express the membrane-bound form of kit-ligand and recruit EPCs via a c-kit-mediated activation of the Akt signaling pathway. Depletion of endogenous c-kit or inhibition of c-kit enzymatic activity by c-kit/Abl protein tyrosine kinase inhibitor imatinib mesylate prevents adhesion of EPCs to activated ECs in both in vitro and in vivo, thus, indicating that a functional c-kit on EPCs is essential for EPC recruitment (220). Furthermore, a positive association between CRP levels and circulating EPCs has been documented in patients with stable CAD, suggesting that a systemic inflammatory state could potentially stimulate EPC mobilization in these individuals (212). In addition, these pro-inflammatory molecules are elaborated soon after myocardial ischemic injury or vascular trauma, and they stimulate the production of growth factors such as VEGF. Also pro-inflammatory molecules mediate tissue repair and adaptation (221). EPCs stimulated by cytokines will take part in maintaining EC integrity and vascular homeostasis (Figure 1) (222). However, pro-inflammatory cytokines trigger the synthesis of acute phase proteins in the liver while they also stimulate the expression of adhesion molecules on EC surface, and promoting atherogenesis (221). Studies have shown that the functional status of progenitor cells is significantly impaired in the presence of high inflammatory stimulation, such as in heart failure (19, 38). More recently, evidence of selective functional exhaustion of the EPCs is found in patients with post-ischaemic heart failure (183). In line with these laboratory observations, an inverse correlation between EPC levels and TNF-alpha is also detected in patients with heart failure, suggesting an inhibitory effect of this cytokine on EPC mobilization and homeostasis (38).
5.2. Additional molecular mechanisms underlying the effects of inflammation on EPCs mobilization
EPCs originate in the BM where they reside in the local microenvironment of the so-called stem cell niche in a quiescent state or in a more undifferentiated state. Cytokines, released by ischemic or damaged tissues, induce mobilization of these cells by interfering with the interactions between stem cells and BM (5, 223). In the bloodstream EPCs home specifically to sites of ischemic or damaged tissues through interactions between soluble chemokines and their receptors expressed on the surface of EPCs. Studies have demonstrated that the interactions of SDF-1alpha (CXCL12)/CXCR4 and RANTES/CCR5 axis, the IL-8/GRO-alpha CXC system interacting with CXCR2, as well as beta2-integrins and E-selectin, are among the most relevant interactions (121, 224–228). Besides, proteinases such as elastase, cathepsin G, and matrix metalloproteinase (MMPs) also play an important role in stem cell mobilization. A cytokine, such as G-CSF, induces the release of the proteinases elastase and cathepsin G from neutrophils, resulting in the cleavage of adhesive bonds on stromal cells and consequently inducing mobilization of CD34+ cells (223). Moreover, eNOS and CD26, a CD34+ cell-surface peptidase, are also believed to be associated with EPCs mobilization (116, 223).
6. CONCLUSION AND FUTURE DIRECTIONS
We have reviewed and discussed in detail the characteristics of EPCs and their quantitative and functional alterations in atherosclerosis and inflammation. Overall, the emerging data consistently shows that impaired EPC biology and mobilization appear in these disease conditions via diversified mechanisms. Therefore, potential therapeutic approaches are needed to restore the capacity of EPCs and improve neovascularization and re-endothelialization. The indication that medications only lead to partial recovery of EPCs in patients with these diseases, suggests necessary EPC-based approaches as a novel alternative in the near future. Overall, the therapeutic benefit, as well as the optimal cell source, dosage, and infusion method of EPC therapy remain to be determined for each clinical condition.
However, while numerous papers have been published discussing the roles of EPCs in various human disorders and in examining the therapeutic potentials of various EPC preparations in experimentally-induced vascular lesions in rodents or in human clinical trials of EPC therapy for cardiovascular disease, the field continues to struggle in clearly defining EPCs. The inconsistent evidence of clinical efficacy poses further important questions including exactly how EPCs can enhance vascular repair and new vessel growth, and whether there are alternative sources of “genuine” EC progenitors in adults that might be exploited more successfully for clinical use. Because several different types of blood cells are implicated as proangiogenic, future studies will be required to determine the exact roles that each cell type in the process of vascular repair or regeneration, and the mechanisms underlying EPC interactions with the host endothelium.
Acknowledgments
This work was partially supported by the National Institutes of Health Grants HL094451 and HL108910 (XFY), HL67033, HL82774 and HL77288 (HW).
References
- 1.Yang XF. Immunology of stem cells and cancer stem cells. Cell Mol Immunol. 2007;4:161–171. [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond) 2011;120:263–283. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 6.Yang XF, Yin Y, Wang H. VASCULAR INFLAMMATION AND ATHEROGENESIS ARE ACTIVATED VIA RECEPTORS FOR PAMPs AND SUPPRESSED BY REGULATORY T CELLS. Drug Discov Today Ther Strateg. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barakat AI. Dragging along: the glycocalyx and vascular endothelial cell mechanotransduction. Circ Res. 2008;102:747–748. doi: 10.1161/CIRCRESAHA.108.174839. [DOI] [PubMed] [Google Scholar]
- 8.Banks RE, Gearing AJ, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68:122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulanger CM, Tedgui A. Dying for attention: microparticles and angiogenesis. Cardiovasc Res. 2005;67:1–3. doi: 10.1016/j.cardiores.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46:229–237. doi: 10.1016/j.vph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404–414. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Haendeler J, Galle J, Zeiher AM. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases. A mechanistic clue to the ‘response to injury’ hypothesis. Circulation. 1997;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Sukhova G, Lee RT, Liao JK. Molecular biology of atherosclerosis. Int J Cardiol. 1997;62(Suppl 2):S23–29. doi: 10.1016/s0167-5273(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 17.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189:247–254. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 21.Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci U S A. 1997;94:5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 24.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 25.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 26.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 28.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 30.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 31.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 32.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 33.Chen MC, Chen CJ, Yang CH, Liu WH, Fang CY, Hsieh YK, Chang HW. Relationship of the percentage of circulating endothelial progenitor cell to the severity of coronary artery disease. Heart Vessels. 2008;23:47–52. doi: 10.1007/s00380-007-1006-9. [DOI] [PubMed] [Google Scholar]
- 34.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 35.Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27:2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 36.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–116. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 37.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 38.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 39.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 40.Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, Weigel G, Schwarzinger I, Wolozcszuk W, Steiner G, Smolen JS. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 41.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 42.Loomans CJ, Dao HH, van Zonneveld AJ, Rabelink TJ. Is endothelial progenitor cell dysfunction involved in altered angiogenic processes in patients with hypertension? Curr Hypertens Rep. 2004;6:51–54. doi: 10.1007/s11906-004-0011-y. [DOI] [PubMed] [Google Scholar]
- 43.Maruyama S, Taguchi A, Iwashima S, Ozaki T, Yasuda K, Kikuchi-Taura A, Soma T, Ishii H, Murohara T, Takahashi H, Kasuga H, Kumada Y, Toriyama T, Ito Y, Kawahara H, Yuzawa Y, Matsuo S. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 44.Fadini GP, de Kreutzenberg S, Agostini C, Boscaro E, Tiengo A, Dimmeler S, Avogaro A. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–219. doi: 10.1016/j.atherosclerosis.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Chen CH, Dixon RA, Ke LY, Willerson JT. Vascular progenitor cells in diabetes mellitus: roles of Wnt signaling and negatively charged low-density lipoprotein. Circ Res. 2009;104:1038–1040. doi: 10.1161/CIRCRESAHA.109.198051. [DOI] [PubMed] [Google Scholar]
- 46.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 47.Seeger FH, Chen L, Spyridopoulos I, Altschmied J, Aicher A, Haendeler J. Downregulation of ETS rescues diabetes-induced reduction of endothelial progenitor cells. PLoS One. 2009;4:e4529. doi: 10.1371/journal.pone.0004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 49.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 50.Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab. 12:570–583. doi: 10.1111/j.1463-1326.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 51.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 52.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26:257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 53.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 54.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 55.George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, Michowitz Y, Miller H, Keren G. Number and adhesive properties of circulating endothelial progenitor cells in patients with in-stent restenosis. Arterioscler Thromb Vasc Biol. 2003;23:e57–60. doi: 10.1161/01.ATV.0000107029.65274.db. [DOI] [PubMed] [Google Scholar]
- 56.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 57.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 58.Deb A, Patterson C. Hard luck stories: the reality of endothelial progenitor cells continues to fall short of the promise. Circulation. 2010;121:850–852. doi: 10.1161/CIR.0b013e3181d4c360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005;64:1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 60.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 63.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S. Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS One. 2010;5:e13790. doi: 10.1371/journal.pone.0013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dignat-George F, Sampol J, Lip G, Blann AD. Circulating endothelial cells: realities and promises in vascular disorders. Pathophysiol Haemost Thromb. 2003;33:495–499. doi: 10.1159/000083851. [DOI] [PubMed] [Google Scholar]
- 66.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 67.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 69.Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K, Sumitran-Holgersson S. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation. 2004;110:3699–3707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 70.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 71.Wuchter C, Ratei R, Spahn G, Schoch C, Harbott J, Schnittger S, Haferlach T, Creutzig U, Sperling C, Karawajew L, Ludwig WD. Impact of CD133 (AC133) and CD90 expression analysis for acute leukemia immunophenotyping. Haematologica. 2001;86:154–161. [PubMed] [Google Scholar]
- 72.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 73.Piaggio G, Rosti V, Corselli M, Bertolotti F, Bergamaschi G, Pozzi S, Imperiale D, Chiavarina B, Bonetti E, Novara F, Sessarego M, Villani L, Garuti A, Massa M, Ghio R, Campanelli R, Bacigalupo A, Pecci A, Viarengo G, Zuffardi O, Frassoni F, Barosi G. Endothelial colony-forming cells from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood. 2009;114:3127–3130. doi: 10.1182/blood-2008-12-190991. [DOI] [PubMed] [Google Scholar]
- 74.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 75.Raemer PC, Haemmerling S, Giese T, Canaday DH, Katus HA, Dengler TJ, Sivanandam VG. Endothelial progenitor cells possess monocyte-like antigen-presenting and T-cell-co-stimulatory capacity. Transplantation. 2009;87:340–349. doi: 10.1097/TP.0b013e3181957308. [DOI] [PubMed] [Google Scholar]
- 76.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 80.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom. Chapter 9(Unit 9.33):1–11. doi: 10.1002/0471142956.cy0933s52. [DOI] [PubMed] [Google Scholar]
- 81.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 82.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 83.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 84.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 85.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 86.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 87.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 88.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 90.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abdul-Salam F, Mansour MH, Al-Shemary T. The selective expression of distinct fucosylated glycoproteins on murine T and B lymphocyte subsets. Immunobiology. 2005;210:695–708. doi: 10.1016/j.imbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Baldus SE, Thiele J, Charles A, Hanisch FG, Fischer R. Carbohydrate antigens of human megakaryocytes and platelet glycoproteins: a comparative study. Histochemistry. 1994;102:205–211. doi: 10.1007/BF00268897. [DOI] [PubMed] [Google Scholar]
- 94.Chionh YT, Wee JL, Every AL, Ng GZ, Sutton P. M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens. Infect Immun. 2009;77:2962–2970. doi: 10.1128/IAI.01522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, Jiang X. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm. 2007;340:207–215. doi: 10.1016/j.ijpharm.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 96.Georgiou S, Pasmatzi E, Monastirli A, Sakkis T, Alachioti S, Tsambaos D. Age-related alterations in the carbohydrate residue composition of the cell surface in the unexposed normal human epidermis. Gerontology. 2005;51:155–160. doi: 10.1159/000083986. [DOI] [PubMed] [Google Scholar]
- 97.Kobayashi N, Matsuzaki O, Shirai S, Aoki I, Yao M, Nagashima Y. Collecting duct carcinoma of the kidney: an immunohistochemical evaluation of the use of antibodies for differential diagnosis. Hum Pathol. 2008;39:1350–1359. doi: 10.1016/j.humpath.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 98.Schmitz B, Thiele J, Otto F, Theile-Ochel S, Heedt T, Zensen U, Baldus SE, Wickenhauser C, Fischer R. Interactions between endogeneous lectins and fucosylated oligosaccharides in megakaryocyte-dependent fibroblast growth of the normal bone marrow. Leukemia. 1996;10:1604–1614. [PubMed] [Google Scholar]
- 99.Thom I, Schult-Kronefeld O, Burkholder I, Goern M, Andritzky B, Blonski K, Kugler C, Edler L, Bokemeyer C, Schumacher U, Laack E. Lectin histochemistry of metastatic adenocarcinomas of the lung. Lung Cancer. 2007;56:391–397. doi: 10.1016/j.lungcan.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 101.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 102.Schmeisser A, Graffy C, Daniel WG, Strasser RH. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol. 2003;522:59–74. doi: 10.1007/978-1-4615-0169-5_7. [DOI] [PubMed] [Google Scholar]
- 103.Yang J, Li M, Kamei N, Aley C, Kwon S-M, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T. CD34+ cells represent highly functional endothelual cells in murine bone marrow. PLoS ONE. 2011;6:e20219–e20219. doi: 10.1371/journal.pone.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stellos K, Gawaz M. Platelets and stromal cell-derived factor-1 in progenitor cell recruitment. Semin Thromb Hemost. 2007;33:159–164. doi: 10.1055/s-2007-969029. [DOI] [PubMed] [Google Scholar]
- 105.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 106.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 108.Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, Martin H, Haendeler J, Zeiher AM, Dimmeler S. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26:2238–2243. doi: 10.1161/01.ATV.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- 109.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Suzuki T, Mizuno S, Nakamura T, Sasaki H. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun. 2004;324:276–280. doi: 10.1016/j.bbrc.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 110.Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, Lo WY, Wu TC, Sata M, Chen JW, Lin SJ. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1179–1184. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 111.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 112.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 113.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 114.Yang XF, Fang P, Meng S, Jan M, Xiong X, Yin Y, Wang H. The forkhead transcription factors play important roles in vascular pathology and immunology. Adv Exp Med Biol. 2009;665:90–105. doi: 10.1007/978-1-4419-1599-3_7. [DOI] [PubMed] [Google Scholar]
- 115.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 116.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 118.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 119.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Lere-Dean C, Contreres JO, Sulpice E, Levy BI, Plouet J, Tobelem G, Le Ricousse-Roussanne S. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 121.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 123.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 124.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–2646. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 125.Alessandri G, Girelli M, Taccagni G, Colombo A, Nicosia R, Caruso A, Baronio M, Pagano S, Cova L, Parati E. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875–885. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- 126.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, Peschle C, Fascio U, Colombo A, Rizzuti T, Parati E, Alessandri G. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 128.Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, Alviano F, Ricci F, Bonafe M, Orrico C, Bagnara GP, Stella A, Conte R. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 129.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 130.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 132.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 133.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med. 2003;13:201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 134.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 135.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 136.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matsubara H. Risk to the coronary arteries of intracoronary stem cell infusion and G-CSF cytokine therapy. Lancet. 2004;363:746–747. doi: 10.1016/S0140-6736(04)15713-9. [DOI] [PubMed] [Google Scholar]
- 138.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34− blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–312. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 139.Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–2743. doi: 10.1634/stemcells.2006-0026. [DOI] [PubMed] [Google Scholar]
- 140.Seta N, Kuwana M. Derivation of multipotent progenitors from human circulating CD14+ monocytes. Exp Hematol. 38:557–563. doi: 10.1016/j.exphem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 141.Li Calzi S, Neu MB, Shaw LC, Kielczewski JL, Moldovan NI, Grant MB. EPCs and pathological angiogenesis: when good cells go bad. Microvasc Res. 2010;79:207–216. doi: 10.1016/j.mvr.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fang B, Li Y, Song Y, Li N. Isolation and characterization of multipotent progenitor cells from the human fetal aorta wall. Exp Biol Med (Maywood) 235:130–138. doi: 10.1258/ebm.2009.009178. [DOI] [PubMed] [Google Scholar]
- 144.Gutterman DD. Adventitia-dependent influences on vascular function. Am J Physiol. 1999;277:H1265–1272. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- 145.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA, Jr, Proto-Siqueira R, Rizzatti EG, Neder L, Silva AR, Rocha V, Zago MA. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340–344. doi: 10.1016/j.yexcr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 147.Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, Lafont A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26:281–286. doi: 10.1161/01.ATV.0000197793.83391.91. [DOI] [PubMed] [Google Scholar]
- 148.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–1407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 149.Liu C, Wang S, Metharom P, Caplice NM. Myeloid lineage of human endothelial outgrowth cells circulating in blood and vasculogenic endothelial-like cells in the diseased vessel wall. J Vasc Res. 2009;46:581–591. doi: 10.1159/000226226. [DOI] [PubMed] [Google Scholar]
- 150.Torsney E, Mandal K, Halliday A, Jahangiri M, Xu Q. Characterisation of progenitor cells in human atherosclerotic vessels. Atherosclerosis. 2007;191:259–264. doi: 10.1016/j.atherosclerosis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–1682. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krenning G, van Luyn MJ, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med. 2009;15:180–189. doi: 10.1016/j.molmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 153.Tan A, Sumpio BE, Lee S, Seifalian AM. The implications of human stem cell differentiation to endothelial cell via fluid shear stress in cardiovascular regenerative medicine: a review. Curr Pharm Des. 2010;16:3848–3861. doi: 10.2174/138161210794455058. [DOI] [PubMed] [Google Scholar]
- 154.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 155.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125:1771–1777. doi: 10.1002/ijc.24605. [DOI] [PubMed] [Google Scholar]
- 157.Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, Arsic N, Tafuro S, Sinagra G, Giacca M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest. 2008;118:2062–2075. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res. 2009;2:2–15. doi: 10.1016/j.scr.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 160.Ma ZL, Mai XL, Sun JH, Ju SH, Yang X, Ni Y, Teng GJ. Inhibited atherosclerotic plaque formation by local administration of magnetically labeled endothelial progenitor cells (EPCs) in a rabbit model. Atherosclerosis. 2009;205:80–86. doi: 10.1016/j.atherosclerosis.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 161.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 162.Tsuzuki M. Bone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol. 2009;104:601–611. doi: 10.1007/s00395-009-0021-7. [DOI] [PubMed] [Google Scholar]
- 163.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 164.Pedroso DC, Tellechea A, Moura L, Fidalgo-Carvalho I, Duarte J, Carvalho E, Ferreira L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS One. 2011;6:e16114. doi: 10.1371/journal.pone.0016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diez M, Musri MM, Ferrer E, Barbera JA, Peinado VI. Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res. 2010;88:502–511. doi: 10.1093/cvr/cvq236. [DOI] [PubMed] [Google Scholar]
- 166.Krenning G, van der Strate BW, Schipper M, van Seijen XJ, Fernandes BC, van Luyn MJ, Harmsen MC. CD34+ cells augment endothelial cell differentiation of CD14+ endothelial progenitor cells in vitro. J Cell Mol Med. 2009;13:2521–2533. doi: 10.1111/j.1582-4934.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 169.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 170.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 171.Gimbrone MA, Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;100:S61–65. [PubMed] [Google Scholar]
- 172.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 173.Doehring LC, Heeger C, Aherrahrou Z, Kaczmarek PM, Erdmann J, Schunkert H, Ehlers EM. Myeloid CD34+CD13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. Am J Pathol. 177:473–480. doi: 10.2353/ajpath.2010.090758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 175.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 176.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 177.Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, Yaegashi N, Okamura K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90:5329–5332. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- 178.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 179.Tso C, Martinic G, Fan WH, Rogers C, Rye KA, Barter PJ. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler Thromb Vasc Biol. 2006;26:1144–1149. doi: 10.1161/01.ATV.0000216600.37436.cf. [DOI] [PubMed] [Google Scholar]
- 180.Tie G, Yan J, Yang Y, Park BD, Messina JA, Raffai RL, Nowicki PT, Messina LM. Oxidized low-density lipoprotein induces apoptosis in endothelial progenitor cells by inactivating the phosphoinositide 3-kinase/Akt pathway. J Vasc Res. 2010;47:519–530. doi: 10.1159/000313879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 182.Werner N, Nickenig G. Endothelial progenitor cells in health and atherosclerotic disease. Ann Med. 2007;39:82–90. doi: 10.1080/07853890601073429. [DOI] [PubMed] [Google Scholar]
- 183.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–209. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 184.Xu Q. The impact of progenitor cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2006;3:94–101. doi: 10.1038/ncpcardio0396. [DOI] [PubMed] [Google Scholar]
- 185.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation. 2008;117:1856–1863. doi: 10.1161/CIRCULATIONAHA.107.746008. [DOI] [PubMed] [Google Scholar]
- 187.Silvestre JS, Gojova A, Brun V, Potteaux S, Esposito B, Duriez M, Clergue M, Le Ricousse-Roussanne S, Barateau V, Merval R, Groux H, Tobelem G, Levy B, Tedgui A, Mallat Z. Transplantation of bone marrow-derived mononuclear cells in ischemic apolipoprotein E-knockout mice accelerates atherosclerosis without altering plaque composition. Circulation. 2003;108:2839–2842. doi: 10.1161/01.CIR.0000106161.43954.DF. [DOI] [PubMed] [Google Scholar]
- 188.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76–86. doi: 10.1161/01.RES.0000097864.24725.60. [DOI] [PubMed] [Google Scholar]
- 189.Sakai Y, Kim DK, Iwasa S, Liang J, Watanabe T, Onodera M, Nakauchi H. Bone marrow chimerism prevents atherosclerosis in arterial walls of mice deficient in apolipoprotein E. Atherosclerosis. 2002;161:27–34. doi: 10.1016/s0021-9150(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 190.Lesnik P, Chapman MJ. A new dimension in the vasculoprotective function of HDL: progenitor-mediated endothelium repair. Arterioscler Thromb Vasc Biol. 2006;26:965–967. doi: 10.1161/01.ATV.0000219613.90372.c1. [DOI] [PubMed] [Google Scholar]
- 191.Tao J, Wang Y, Yang Z, Tu C, Xu MG, Wang JM. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens. 2006;20:490–495. doi: 10.1038/sj.jhh.1001996. [DOI] [PubMed] [Google Scholar]
- 192.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med. 2006;10:318–332. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Langwieser N, Schwarz JB, Reichenbacher C, Stemmer B, Massberg S, Langwieser NN, Zohlnhofer D. Role of bone marrow-derived cells in the genetic control of restenosis. Arterioscler Thromb Vasc Biol. 2009;29:1551–1557. doi: 10.1161/ATVBAHA.109.188326. [DOI] [PubMed] [Google Scholar]
- 194.George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- 195.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jan M, Meng S, Chen NC, Mai J, Wang H, Yang XF. Inflammatory and autoimmune reactions in atherosclerosis and vaccine design informatics. J Biomed Biotechnol. 2010;2010:459798. doi: 10.1155/2010/459798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma FX, Zhou B, Chen Z, Ren Q, Lu SH, Sawamura T, Han ZC. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res. 2006;47:1227–1237. doi: 10.1194/jlr.M500507-JLR200. [DOI] [PubMed] [Google Scholar]
- 198.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 199.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh’s dilemma. Am J Physiol Heart Circ Physiol. 2006;290:H1729–1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 200.Di Santo S, Diehm N, Ortmann J, Volzmann J, Yang Z, Keo HH, Baumgartner I, Kalka C. Oxidized low density lipoprotein impairs endothelial progenitor cell function by downregulation of E-selectin and integrin alpha(v)beta5. Biochem Biophys Res Commun. 2008;373:528–532. doi: 10.1016/j.bbrc.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 201.Tang D, Lu J, Walterscheid JP, Chen HH, Engler DA, Sawamura T, Chang PY, Safi HJ, Yang CY, Chen CH. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J Lipid Res. 2008;49:33–47. doi: 10.1194/jlr.M700305-JLR200. [DOI] [PubMed] [Google Scholar]
- 202.Carracedo J, Merino A, Briceno C, Soriano S, Buendia P, Calleros L, Rodriguez M, Martin-Malo A, Aljama P, Ramirez R. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. Faseb J. 2011;25:1314–1322. doi: 10.1096/fj.10-173377. [DOI] [PubMed] [Google Scholar]
- 203.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 204.Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:2476–2482. doi: 10.1161/01.ATV.0000242794.65541.02. [DOI] [PubMed] [Google Scholar]
- 205.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 206.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 207.Haddad P, Dussault S, Groleau J, Turgeon J, Maingrette F, Rivard A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: Effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 208.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 209.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 210.Desouza CV, Hamel FG, Bidasee K, O’Connell K. Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes. 2011;60:1286–1294. doi: 10.2337/db10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dong C, Crawford LE, Goldschmidt-Clermont PJ. Endothelial progenitor obsolescence and atherosclerotic inflammation. J Am Coll Cardiol. 2005;45:1458–1460. doi: 10.1016/j.jacc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 212.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 213.Schomig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schomig A, Ott I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–1037. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 214.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 215.Tousoulis D, Andreou I, Antoniades C, Tentolouris C, Stefanadis C. Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: therapeutic implications for cardiovascular diseases. Atherosclerosis. 2008;201:236–247. doi: 10.1016/j.atherosclerosis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 216.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 217.Amano K, Okigaki M, Adachi Y, Fujiyama S, Mori Y, Kosaki A, Iwasaka T, Matsubara H. Mechanism for IL-1 beta-mediated neovascularization unmasked by IL-1 beta knock-out mice. J Mol Cell Cardiol. 2004;36:469–480. doi: 10.1016/j.yjmcc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 218.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 219.Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 220.Dentelli P, Rosso A, Balsamo A, Colmenares Benedetto S, Zeoli A, Pegoraro M, Camussi G, Pegoraro L, Brizzi MF. C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–4271. doi: 10.1182/blood-2006-06-029603. [DOI] [PubMed] [Google Scholar]
- 221.Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17:225–233. doi: 10.1016/j.cytogfr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 222.Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ. Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol. 2004;24:834–838. doi: 10.1161/01.ATV.0000124891.57581.9f. [DOI] [PubMed] [Google Scholar]
- 223.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 224.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, Kim HS. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891–3899. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 225.Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–464. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 226.Rookmaaker MB, Verhaar MC, de Boer HC, Goldschmeding R, Joles JA, Koomans HA, Grone HJ, Rabelink TJ. Met-RANTES reduces endothelial progenitor cell homing to activated (glomerular) endothelium in vitro and in vivo. Am J Physiol Renal Physiol. 2007;293:F624–630. doi: 10.1152/ajprenal.00398.2006. [DOI] [PubMed] [Google Scholar]
- 227.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 228.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]