The purpose of this study was to determine the detection threshold of two clotting tests to minute quantities of factor (F) IX in the canine hemophilia B system: the whole blood clotting time (WBCT) and the activated partial thromboplastin time (aPTT). To our knowledge, the relative sensitivity of these tests for minute amounts of canine FIX has not been previously published. As these tests are relatively easy and quick to perform, they are useful and important for determining when infused FIX has been cleared, correctly identifying onset and estimating level of transgene expression in gene transfer experiments, and detecting inhibitory antibodies to FIX.
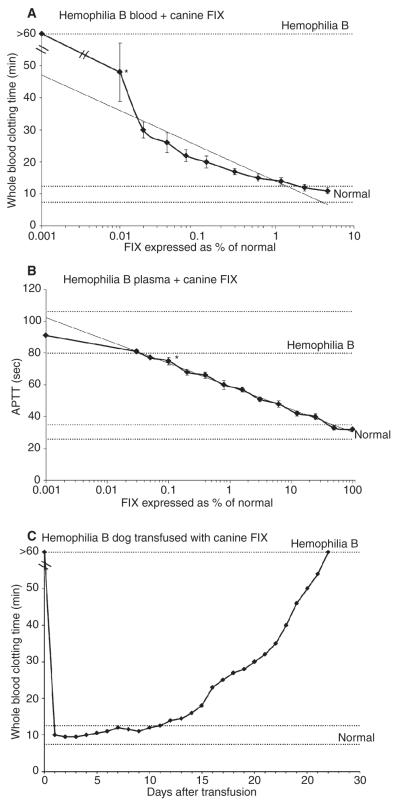
The WBCT was sensitive to as little as 0.01% FIX (Fig. 1A). The estimated level of canine FIX in normal dogs is 5 μg mL−1, thus 0.01% is estimated to be 0.5 ng FIX per mL plasma [1]. The sensitivity of the aPTT to canine FIX was 0.1%, estimated to be 5 ng mL−1 (Fig. 1B). As no activator is used with the WBCT, these data suggest coagulation assays are more sensitive to canine FIX with less activation of the coagulation system. Transfused canine FIX shortened the aPTT for an average of 12 days (range 8–17 days) and the WBCT for an average of 22 days (range 14–27 days). This difference is most likely due to the differences in sensitivity of these two assays. These data provide a reference time-point after which transfused FIX that has not yet been cleared can be distinguished from expressed FIX resulting from successful gene transfer experiments.
Fig. 1.
(A). Effect of canine factor (F) IX on the WBCT in hemophilia B dog blood. The addition of 0.01% canine FIX significantly shortened the WBCT (heavy line) from baseline (*P = 0.039). (B) Effect of canine FIX on the activated partial thromboplastin time in hemophilia B dog plasma. The addition of 0.1% canine FIX significantly shortened the aPTT (heavy line) from baseline (*P = 0.0116). (C) Effect of transfused canine FIX on WBCT in a hemophilia B dog. This dog received 160 U FIX per kg over 3 days to provide hemostasis for a partial hepatectomy performed as a control for a gene therapy protocol (i.e. no gene therapy was given) [21]. The WBCT takes approximately 27 days to return to baseline > 60 min. (Range of values for hemophilia B and normal dogs is indicated by the dotted lines. Linear regression lines [light lines] are included on Panels A and B.)
A test for the delayed clotting of whole blood in severe hemophilia was first described by Wright [2] over a century ago. The original directions of Lee and White [3] in 1913 for the WBCT are now obsolete, and many modifications have been made, including the use of siliconized test tubes to prevent activation of the clotting system [4]. Careful collection of blood samples is essential if consistency in the WBCT values is to be obtained with replicate testing [5]. In contrast to other tests, the WBCT is relatively insensitive to plasma FIX levels above about 3-5% of normal (Fig. 1A). Hence patients with moderate or mild hemophilia may have a normal WBCT even during bleeding episodes [6]. While quite useful in preclinical animal studies, the WBCT tests are infrequently used in diagnosis and monitoring of therapy in humans. The advent of gene therapy in canine hemophilia B has indicated the need for readily available clotting tests that are sensitive to low levels of plasma FIX activity and can be easily repeated over years of follow-up [7]. Additionally, sudden prolongation of the WBCT has been a harbinger of inhibitory antibodies to canine FIX [8].
The hemophilia B dogs require frequent plasma FIX therapy to treat spontaneous bleeding [9,10] and, following invasive procedures, to prevent hemorrhage and facilitate wound healing [11]. Residual plasma FIX post-transfusion was detectable by aPTT for 8–17 days and the WBCT for 14–27 days (Fig. 1C). This late phase of clearance may indicate a change in kinetics of elimination of FIX, influenced perhaps by a slow return to the blood stream of procoagulants. This is especially relevant to FIX that is known to bind to type IV collagen via lysine at position 5 or valine at position 10 [12-15]. Notably, FIX variants mutated at these sites have increased circulating levels of FIX [16]. The animal that required the longest period of time (27 days, Fig. 1C) for the WBCT to return to basal values had a partial hepatectomy and received the largest dose of FIX replacement therapy, suggesting but not proving that dose of FIX may be a factor in the late phase of elimination [17].
Other canine FIX activity assays include FIX bioassay by two-stage [18] and chromogenic methods [19]. In general, these assays are used to detect FIX levels of 1% or greater and are difficult to reliably standardize for levels lower than 1%. A canine FIX ELISA has also been established for determining protein levels [1,20,21]. All three of these assays are quantitative and require considerable laboratory time and expertise. In contrast, the WBCT and the aPTT, while qualitative, can be performed rapidly and are quite sensitive to small amounts of canine FIX that can give an early readout on how to interpret gene transfer and protein replacement experiments for inhibitors.
Supplementary Material
Acknowledgements
Supported by HL063098 (T.C. Nichols)
Footnotes
To cite this article: Nichols TC, Franck HWG, Franck CT, de Friess N, Raymer RA, Merricks EP. Sensitivity of whole blood clotting time and activated partial thromboplastin time for factor IX: relevance to gene therapy and determination of post-transfusion elimination time of canine factor IX in hemophilia B dogs. J Thromb Haemost 2012; 10: 474–6.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Supporting information includes details of methods, analyses, and additional results not included in print.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Herzog R, Arruda VR, Fischer TH, Read MS, Nichols TC, High KA. Absence of circulating factor IX antigen in hemophilia B dogs of the UNC-Chapel Hill Colony. Thromb Haemost. 2000;84:352–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Wright AE. On a method of determining the conditions of blood coagulability for clinical and experimental purposes, and on the effect of the administration of calcium salts in haemohpilia and actual or threatened haemorrhage. Br Med J. 1893;2:223–5. doi: 10.1136/bmj.2.1700.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RI, White PD. A clinical study of the coagulation time of blood. Am J Med Sci. 1913;145:495–503. [Google Scholar]
- 4.Jaques LB, Fidlar E, Feldsted ET, MacDonald AG. Silicones and blood coagulation. Can Med Assoc J. 1946;55:26–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Quick AJ, Honorato CR, Stefanini M. The value and the limitations of the coagulation time in the study of the hemorrhagic diseases. Blood. 1948;3:1120–9. [PubMed] [Google Scholar]
- 6.Brinkhous KM, Langdell RD, Penick GD, Graham JB, Wagner RH. Newer approaches to the study of hemophilia and hemophilioid states. J Am Med Assoc. 1954;154:481–6. doi: 10.1001/jama.1954.02940400019005. [DOI] [PubMed] [Google Scholar]
- 7.Nichols TC, Dillow AM, Franck HWG, Merricks EP, Raymer RA, Bellinger DA, Arruda VR, High KA. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von willebrand disease, and factor VII deficiency. ILAR J. 2009;50:144–67. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–91. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 9.Russell KE, Olsen EH, Raymer RA, Merricks EP, Bellinger DA, Read MS, Rup BJ, Keith JC, Jr, McCarthy KP, Schaub RG, Nichols TC. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune-tolerant hemophilia B dogs. Blood. 2003;102:4393–8. doi: 10.1182/blood-2003-05-1498. [DOI] [PubMed] [Google Scholar]
- 10.Nichols TC, Raymer RA, Franck HW, Merricks EP, Bellinger DA, DeFriess N, Margaritis P, Arruda VR, Kay MA, High KA. Prevention of spontaneous bleeding in dogs with haemophilia A and haemophilia B. Haemophilia. 2010;16(Suppl. 3):19–23. doi: 10.1111/j.1365-2516.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman M, Harger A, Lenkowski A, Hedner U, Roberts HR, Monroe DM. Cutaneous wound healing is impaired in hemophilia B. Blood. 2006;108:3053–60. doi: 10.1182/blood-2006-05-020495. [DOI] [PubMed] [Google Scholar]
- 12.Heimark RL, Schwartz SM. Binding of coagulation factors IX and X to the endothelial cell surface. Biochem Biophys Res Commun. 1983;111:723–31. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- 13.Stern DM, Knitter G, Kisiel W, Nawroth PP. In vivo evidence of intravascular binding sites for coagulation factor IX. Br J Haematol. 1987;66:227–32. doi: 10.1111/j.1365-2141.1987.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 14.Wolberg AS, Stafford DW, Erie DA. Human factor IX binds to specific sites on the collagenous domain of collagen IV. J Biol Chem. 1997;272:16717–20. doi: 10.1074/jbc.272.27.16717. [DOI] [PubMed] [Google Scholar]
- 15.Cheung WF, van den Born J, Kuhn K, Kjellen L, Hudson BG, Stafford DW. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci U S A. 1996;93:11068–73. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuettrumpf J, Herzog RW, Schlachterman A, Kaufhold A, Stafford DW, Arruda VR. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105:2316–23. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
- 17.Brinkhous KM, Sigman JL, Read MS, Stewart PF, McCarthy KP, Timony GA, Leppanen SD, Rup BJ, Keith JC, Jr, Garzone PD, Schaub RG. Recombinant human factor IX: replacement therapy, prophylaxis, and pharmacokinetics in canine hemophilia B. Blood. 1996;88:2603–10. [PubMed] [Google Scholar]
- 18.Goldsmith JC, Chung KS, Roberts HR. A simple assay for human factor IX: use of canine hemophilia B plasma as substrate. Thromb Res. 1978;12:497–502. doi: 10.1016/0049-3848(78)90320-1. [DOI] [PubMed] [Google Scholar]
- 19.Wagenvoord R, Hendrix H, Tran T, Hemker HC. Development of a sensitive and rapid chromogenic factor IX assay for clinical use. Haemostasis. 1990;20:276–88. doi: 10.1159/000216139. [DOI] [PubMed] [Google Scholar]
- 20.Sugahara Y, Catalfamo J, Brooks M, Hitomi E, Bajaj SP, Kurachi K. Isolation and characterization of canine factor IX. Thromb Haemost. 1996;75:450–5. [PubMed] [Google Scholar]
- 21.Kay MA, Rothenberg S, Landen CN, Bellinger DA, Leland F, Toman C, Finegold M, Thompson AR, Read MS, Brinkhous KM. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–9. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.