Abstract
Background
Vector competence describes the efficiency with which vector arthropods become infected with and transmit pathogens and depends on interactions between pathogen and arthropod genetics as well as environmental factors. For arbovirus transmission, the female mosquito ingests viremic blood, the virus infects and replicates in midgut cells, escapes from the midgut, and disseminates to other tissues, including the salivary glands. Virus-laden saliva is then injected into a new host. For transmission to occur, the virus must overcome several “barriers”, including barriers to midgut infection and/or escape and salivary infection and/or escape. By examining the spatial/temporal infection dynamics of Culex tarsalis strains infected with western equine encephalomyelitis virus (WEEV), we identified tissue tropisms and potential tissue barriers, and evaluated the effects of viral dose and time postingestion.
Methods
Using immuno-stained paraffin sections, WEEV antigens were tracked in four Cx. tarsalis strains: two recently colonized California field strains – Coachella Valley, Riverside County (COAV) and Kern National Wildlife Refuge (KNWR); and two laboratory strains selected for WEEV susceptibility (high viremia producer, HVP), and WEEV resistance (WR).
Results and conclusions
Tissues susceptible to WEEV infection included midgut epithelium, neural ganglia, trachea, chorionated eggs, and salivary glands. Neuroendocrine cells in the retrocerebral complex were occasionally infected, indicating the potential for behavioral effects. The HVP and COAV strains vigorously supported viral growth, whereas the WR and KNWR strains were less competent. Consistent with earlier studies, WEEV resistance appeared to be related to a dose-dependent midgut infection barrier, and a midgut escape barrier. The midgut escape barrier was not dependent upon the ingested viral dose. Consistent with midgut infection modulation, disseminated infections were less common in the WR and KNWR strains than in the HVP and COAV strains. Once the virus disseminated from the midgut, all strains were able to develop salivary gland infections. The possible roles of observed pathology will be discussed in a subsequent paper.
Keywords: western equine encephalomyelitis virus, Culex tarsalis, vector competence, viral tropism, mosquito/virus interaction
Introduction
In the field of vector biology, vector competence (ie, the ability of an arthropod to become infected with and successfully transmit a pathogen) is the result of complex interactions between environmental and genetic factors. Because of the multifactorial nature of these interactions, wide variation in vector competence exists among and within mosquito species, and even within the same mosquito populations over time.1–6
When transmission of an arthropod-borne virus (arbovirus) occurs by bite, a sequential series of events takes place: 1) a female mosquito ingests a sufficient dose of infective viral particles with a blood meal; 2) the virus infects and replicates within the midgut cells; 3) viral particles escape from the midgut epithelium and disseminate to cells within the hemocoel, ultimately infecting the salivary glands; 4) infectious virions are injected into a new host when the mosquito blood feeds again.7,8 During this process, an ingested virus must overcome several “barriers” to reach the point where it is transmissible to a new vertebrate host. These barriers may exist at the extracellular level (eg, the basal lamina), at the cell membrane level (eg, absence or low density of viral receptors), or inside the cell (eg, antiviral immune responses, such as RNAi).9,10 Although the molecular mechanisms governing these barriers remain unclear, they appear to be complex and often controlled by multiple genes.11,12
Barriers to virus passage within a mosquito include: 1) midgut infection barrier, ingested virus fails to infect midgut cells; 2) midgut escape barrier, virus infects midgut cells, but fails to disseminate to the hemocoel; 3) salivary gland infection barrier, disseminated virus fails to infect salivary glands; and 4) salivary gland escape barrier, virus infects the salivary glands, but is absent in secreted saliva.6,7
The degree to which these barriers restrict viral passage is often variable and dependent on factors such as the viral dose ingested, time postingestion, temperature during the extrinsic incubation period, nutritional status, strain, and so on. Therefore, these “barriers” represent the interaction between the vector’s genetically determined characteristics and extrinsic or nongenetic factors.6,10
Well-established laboratory strains of Culex tarsalis Coquillett selected for high and low susceptibility to western equine encephalomyelitis virus (WEEV)1,13 as well as recently colonized field strains provide a suitable model for the study of intraspecific differences in vector competence.14,15 Mahmood et al15 described the impact of dose and time postinfection on the patterns of WEEV infection, dissemination, and transmission by these four strains of Cx. tarsalis. Our study extends these observations by providing an immunohistologic evaluation of factors that influence vector competence such as tissue tropisms, infection barriers, and the dynamics of infection over time.
By controlling the ingested viral dose and comparing the temporal dynamics of infection observed in four strains of Cx. tarsalis (HVP, WR, COAV, and KNWR) we aimed: 1) to identify tissues susceptible and refractory to WEEV infection (ie, viral tropisms); 2) to compare the dynamics of infection observed in the different strains; 3) to delineate potential routes of virus dissemination to the hemocoel; and 4) to identify potential barriers to infection, dissemination, and salivary gland infection. The tissue pathology associated with WEEV infection in each of the four strains of Cx. tarsalis will be the topic of a follow-up paper currently in preparation.
Materials and methods
Methods of mosquito rearing, infection, and handling were identical to those described previously.15 Briefly, larvae were reared at 22–24°C, with a 16 hour light:8 hour darkness photoperiod, and fed ground alfalfa pellets and AquaMax® (Purina Mills, LLC; St Louis, MO). Adults were maintained under a similar photoperiod at 26°C, and were provided a 10% sucrose solution ad libitum. All infections were performed using WEEV strain BFS1703, which was isolated from Cx. tarsalis collected in Kern County, California in July 195316 and has been widely used for evaluating the vector competence of Cx. tarsalis.3,13,17 The virus was passaged twice in suckling mice and once in Vero cell culture prior to being used in our study.
Four strains of Cx. tarsalis were used: WEEV resistant (WR), high viremia producer (HVP; derived from the original WEEV susceptible, WS, strain), Coachella Valley (COAV), and Kern National Wildlife Refuge (KNWR). The WR and WS strains were selected in the mid-1970s for refractoriness (WR) or susceptibility (WS) to BFS1703 strain WEEV infection, and have been described elsewhere.13,18 These strains have been maintained at the University of California’s Arbovirus Field Station since the mid 1980s and, prior to experimentation, were reselected for several generations by examining the susceptibility of single families.15 The COAV and KNWR strains (“wildtype” strains) were collected in Riverside and Kern counties, CA, respectively, and had been maintained with no selection as colonies at the aforementioned facility for two years before use in the current study.
Three- to five-day-old mated females were starved for 18 hours and then allowed to engorge on viremic blood via an artificial membrane feeder as previously described.14,15,19 Blood solutions contained ca. 3 or 5 log10 plaque forming units (PFU) of WEEV per 0.1 mL of chicken blood containing 14.3 freeze dried USP units of sodium heparin per mL (Becton-Dickson, Franklin Lakes, NJ). These doses are abbreviated as ‘3-log’ and ‘5-log’ virus groups. The 5-log dose was comparable to viremias produced by competent avian hosts that were able to infect most competent vectors, whereas the 3-log dose was similar to that produced by a less competent host, but still able to infect highly susceptible mosquito hosts such as the HVP strain. Doses below this were insufficient to infect most mosquitoes.20 For uninfected controls, mosquitoes were fed on virus-free chicken blood by the same method. Fully engorged females were transferred to an incubator maintained at 26°C and 16L:8D h photoperiod, and were provided with 10% sucrose solution that was changed daily. Five females from each viral dose group were collected at days 1, 2, 3, 4, 7, 14, and 21 postinfectious blood meal, immobilized on wet ice, injected with 10% buffered formalin, pH 7.5, and stored in 100% ethanol. A total of 70 experimental specimens per strain was thus available for study.
Positive controls consisted of HVP females inoculated intrathoracically (IT) with 0.5 µL of a 103 PFU/0.1 mL virus solution, and maintained on 10% sucrose for at least four days postinfection. Negative controls included: 1) specimens within each post-blood meal time period which were fed on virus-free blood; 2) infected HVP females treated with PBS instead of primary antibody during immunostaining; and 3) HVP females IT inoculated with 1 µL of PBS and killed seven days postinoculation.
Fixed specimens were dehydrated, cleared, and infiltrated with paraffin as previously described21 and then embedded in paraffin blocks, cut into 10 µm thick serial sagittal sections using an American Optical® 820 Spencer™ microtome (American Optical Co, New York, NY), mounted on microscope slides, and stored at 4°C.
Mounted sections were immunostained by the avidinbiotin-peroxidase complex (ABC) technique21 using a 1/1600 dilution of mouse anti-WEEV ascites fluid as the primary antibody, and the horse-anti-mouse Vectastain Elite® ABC kit (Vector laboratories, Burlingame, CA) as the detector antibody, following manufacturer protocols. Stained slides were examined using a Nikon® Optiphot™ compound microscope (Nikon Instruments Inc, Melville, New York, NY) equipped with a digital Spot RT™ camera (Diagnostic Instruments, Sterling Heights, MI). For each experimental specimen, individual tissues were scored as positive or negative for WEEV infection and pathology based on comparison with their corresponding controls.
Throughout this report, the term ‘rate’ denotes the frequency with which an event (ie, infection, dissemination) occurred within a defined group.22 Statistical analyses were performed using the SPSS® software package for windows (v 13.0; SPSS Inc; Chicago, IL). Chi-square tests were used when comparing overall rates (ie, pooled time groups) between 3- and 5-log groups in each strain. If no significant differences between doses were found within a strain, dose groups were pooled for further analysis; otherwise, each dose group was analyzed separately. A two-tailed Fisher’s exact test was used instead of the Chi-square test if the expected frequency of a category was less than five.
Analyses of rate differences among strains were performed using Kruskal–Wallis (K-W) tests. If K-W tests indicated significant (P < 0.05) differences among strains, post hoc analysis was performed by applying Chi-square tests to all pair-wise combinations of strains. To maintain an overall alpha level of 0.05, a Bonferroni correction was applied to these post hoc tests.
Phi coefficients23 were calculated to estimate the strength of associations between cardia infection and dissemination, and between overall rates of neural and salivary gland infection.
Results
Tissue tropisms
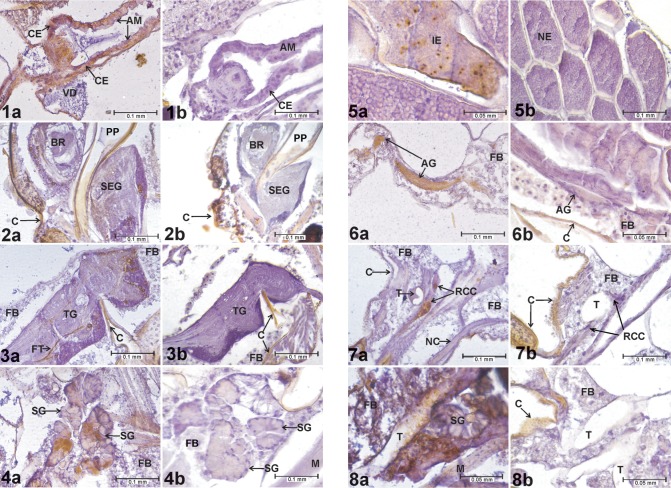
Preliminary tissue analysis identified the midgut epithelium (Figures 1A and B), large neural ganglia (Figures 2A and B; 3A and B), salivary glands (Figures 4A and B), and chorionated eggs (Figures 5A and B) as tissues and organs where WEEV infection was consistently detected (as indicated by positive ABC reactions) in all experimental groups. Subsequent analyses focused on these tissues. A specimen was recorded as having an infected midgut if it exhibited WEEV infection in the cardial epithelium (the outer layer of the proventriculus), anterior midgut, and/or posterior midgut (Figures 1A and B). Due to their size and discrete cell bodies, analysis focused on the three largest ganglia: brain, subesophageal ganglion, and thoracic ganglion (Figures 2A and B; 3A and B). A specimen was recorded as having a positive neural infection if it showed signs of WEEV antigen present in the cell bodies of these ganglia. In the salivary glands, individuals presenting high-intensity staining in at least two consecutive sections were recorded as positive.
Figures 1–8.
Evidence of WEEV infection in Cx. tarsalis tissues. 1a) Infected cardia and anterior midgut, HVP strain, 5-log group, 21 days postinfective blood meal. 1b) Cardia and anterior midgut, negative control. 2a) Infected brain and subesophageal ganglion, HVP strain, 5-log group, 21 days postinfective blood meal. 2b) Brain and subesophageal ganglion, negative control. 3a) Infected thoracic ganglion, HVP strain, 5-log group, 21 days postinfective blood meal. 3b) Thoracic ganglion, negative control. 4a) Infected salivary glands, HVP strain, 5-log group, 21 days postinfective blood meal. 4b) Salivary glands, negative control. 5a) Infected eggs, COAV strain, 5-log group, 21 days postinfective blood meal. 5b) Eggs, negative control. 6a) Infected abdominal ganglion, HVP strain, 3-log group, 7 days postinfective blood meal. 6b) Abdominal ganglion, negative control. 7a) Infected retrocerebral complex, HVP strain, 5-log group, 7 days postinfective blood meal. 7b) Retrocerebral complex, negative control. 8a) Infected trachea, KNWR strain, 5-log group, 14 days postinfective blood meal. 8b) Trachea, negative control. In all figures, rusty-brown staining in internal tissues corresponds to positive ABC reactions, indicating the presence of WEE viral antigen. Notice that the cuticle (exoskeleton) often displays a similar brown coloring (eg, in Figures 2b, 3b, 6b, 7b, and 8b), which is not indicative of positive ABC reactions.
Abbreviations: ABC, avidin-biotin-peroxidase complex; AG, abdominal ganglion; AM, anterior midgut; BR, brain; C, cuticle; CE, cardial epithelium; FB, fat body; FT, neural fiber tract; HVP, high viremia producer strain; IE, infected egg; M, muscle (skeletal); NC, nerve cord; NE, noninfected egg; PP, pharyngeal pump lumen; RCC, retrocerebral complex; SEG, subesophageal ganglion; SG, salivary gland; T, trachea; TG, thoracic ganglion; VD, ventral diverticulum; WEEV, western equine encephalomyelitis virus.
Structures occasionally infected included the abdominal ganglia (Figures 6A and B), tissues of the retrocerebral complex (Figures 7A and B), and tracheae (Figures 8A and B). The Malpighian tubes, foregut, hindgut, and diverticula presented high levels of non-specific staining in negative controls, and therefore were excluded from analysis. No signs of infection were found in either skeletal muscle or fat body.
Dynamics of infection
Passage of WEEV within the mosquito’s body was determined by focusing on three processes: infection (presence of WEEV antigen anywhere in the insect’s body), dissemination (infection of any tissue other than the midgut epithelium), and salivary gland infection (SGI). Figure 9 shows rates for these three variables among all experimental individuals for each strain, time, and infectious dose.
Figure 9.
Infection, dissemination, and salivary gland infection rates among experimental individuals.
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
Infection
In all strains, overall infection rates (Table 1) in the 5-log group were significantly higher than those in the 3-log group (HVP: χ2 = 14.5, df = 1, P < 0.01; WR: χ2 = 18.9; df = 1; P < 0.01; COAV: χ2 = 7.4, df = 1, P = 0.01; KNWR: χ2 = 10.1, df = 1; P < 0.01). Therefore, analyses of each dose-group were carried out separately.
Table 1.
Overall rates of infection, dissemination, and salivary gland infection
| HVP
|
WR
|
COAV
|
KNWR
|
|||||
|---|---|---|---|---|---|---|---|---|
| 3 log (n = 35) | 5 log (n = 35) | 3 log (n = 35) | 5 log (n = 35) | 3 log (n = 35) | 5 log (n = 34) | 3 log (n = 34) | 5 log (n = 35) | |
| Infectiona | 66% | 100% | 31% | 83% | 69% | 94% | 50% | 86% |
| Disseminationb | 31% | 51% | 6% | 11% | 34% | 53% | 6% | 29% |
| Salivary gland infectionc | 29% | 37% | 6% | 9% | 31% | 44% | 6% | 26% |
Notes:
Infection rate = the number of females with midgut infection/total females that ingested an infectious blood meal
Dissemination rate = the number of females with infection beyond the midgut/total number of females infected
Salivary gland infection rate = the number of females with salivary gland infection/total number of females with disseminated infections.
Abbreviations: COAV, Coachella Valley strain; HVP, high viremia producer strain; KNWR, Kern National Wildlife Refuge strain; WEEV, western equine encephalomyelitis virus; WR, WEEV resistant strain.
In the 3-log group, 100% infection rate was reached by day four in the HVP, COAV, and KNWR strains (Figure 9). Although infection rates in the HVP and COAV strains remained above 60% during the rest of the experimental period, these rates tended to decrease over time in the KNWR strain, dropping to 20% by day 14. WR strain infection rates peaked on day two (80%), but then decreased quickly within the next 48 hours, and remained at ≤20% for the rest of the experimental period. The COAV and HVP strains presented the highest overall infection rates (69% and 66%, respectively). The WR strain presented the lowest infection rate (31%), followed by the KNWR strain (50%).
Infection rates in the 5-log group reached 100% by day one in the HVP strain, by day two in both the WR and COAV, and by day three in the KNWR strain (Figure 9). All strains, except WR, maintained high infection rates (80%–100%). In the WR strain, infection rates dropped steadily after the first week, reaching levels comparable to those of the 3-log group (<20%) by day 21. The highest overall infection rate (100%) was seen in the HVP strain (Table 1), followed by COAV (94%). The WR strain presented the lowest overall infection rate (83%) and was very closely followed by the KNWR (86%).
Kruskal–Wallis tests revealed significant differences in overall infection rates in the 3-log group (χ2 = 12.2; df = 3; P = 0.01) but not in the 5-log group (χ2 = 7.6; df = 3; P = 0.06). Post hoc testing in the 3-log group revealed significant differences between the HVP and WR strains (χ2 = 8.2; df = 1; P < 0.01), and between WR and COAV strains (χ2 = 9.7; df = 1; P < 0.01).
Dissemination
Overall dissemination rates were consistently higher in the 5-log group than in the 3-log group (Table 1); however, only in the KNWR strain was this difference statistically significant (χ2 = 6.18; df = 1; P = 0.01). Since at least one strain showed significant differences between doses, analyses were carried separately for the 3- and 5-log groups.
In the 3-log group, the earliest signs of dissemination were detected in the HVP strain during day two post- infection; dissemination was not evident in either field strain (COAV and KNWR) until day four postinfection (Figure 9). Only the COAV and HVP strains developed dissemination rates ≥50% by days 4 and 14, respectively. In contrast, dissemination rates remained ≤20% for the WR and KNWR strains (Figure 9). The COAV and HVP strains presented the highest overall dissemination rates (34% and 31%, respectively), while the WR and KNWR strains presented the lowest dissemination rate (6% in both cases).
In the 5-log group, the earliest signs of dissemination were observed in the COAV and KNWR strains by day two postinfection (Figure 9). No signs of dissemination were observed in the WR strain until 14 days postinfection. Dissemination rates in the 5-log group reached ≥50% in all strains. Although rates increased over time in the HVP and COAV strains, the WR and KNWR strains peaked by day 14 (at 80% and 100%, respectively), and then markedly decreased by day 21, reaching rates of 0% for the WR, and 60% for the KNWR (Figure 9). The highest overall dissemination rate was seen in the COAV (53%), followed by the HVP strain (51%); again, the WR presented the lowest overall rate (11%), followed by the KNWR (29%).
Kruskal–Wallis tests revealed significant differences among strains in overall dissemination rates in both the 3-log group (χ2 = 16.2; df = 3; P < 0.01) and the 5-log group (χ2 = 17.7; df = 3; P < 0.01). Post hoc tests in the 3-log group revealed statistically significant differences between the HVP and WR strains (χ2 = 7.7; df = 1; P < 0.01), HVP and KNWR strains (χ2 = 7.4; df = 1; P = 0.01), COAV and WR strains (χ2 = 8.9; df = 1; P < 0.01), and COAV and KNWR strains (χ2 = 8.6; df = 1; P < 0.01). In the 5-log group, significant differences in overall dissemination rates were found between HVP and WR strains (χ2 = 13.0; df = 1; P < 0.01), and between WR and COAV strains (χ2 = 13.7; df = 1; P < 0.01).
Salivary gland infection
Overall SGI rates were consistently higher in the 5-log group than in the 3-log group (Table 1); however, only in the KNWR strain was this difference statistically significant (χ2 = 5.1; df = 1; P = 0.02). Because at least one strain showed a significant difference between dose-groups, analyses were carried out separately for the 3- and 5-log groups.
SGI rates in the 3-log group closely mirrored dissemination rates (Figure 9): the earliest signs of SGI were displayed by the HVP strain during day two postinfection, whereas neither field strain (COAV and KNWR) showed signs of SGI until day four postinfection. Only the COAV and HVP strains developed SGI rates of ≥50% (by days 4 and 14, respectively), whereas SGI rates remained ≤20% for the WR and KNWR strains. The COAV and HVP strains presented the highest overall SGI rates (31% and 22%, respectively); again, the WR and KNWR strains presented the lowest SGI rate (6%).
In the 5-log group, SGI rates eventually reached ≥50% in all four strains. The earliest signs of SGI were observed in the COAV strain by day two postinfection (Figure 9). The WR and KNWR strains presented similar patterns, displaying the first signs of SGI by day 14 postinfection, and showing a clear reduction in these rates by day 21 postinfection. This contrasts with SGI patterns observed in the other two strains, which displayed steady increases, eventually reaching 100% in the HVP, and a plateau at 80% in the COAV strains. The highest overall SGI rate was seen in the COAV strain (44%), followed by the HVP strain (37%); again, the WR presented the lowest overall rate (10%), followed by the KNWR strain (26%).
Kruskal–Wallis tests revealed statistically significant differences in overall SGI rates in both the 3-log group (χ2 = 13.5; df = 3; P < 0.01) and the 5-log group (χ2 = 12.1; df = 3; P = 0.01). Post hoc tests revealed significant differences in the 3-log group between the COAV and WR strains (χ2 = 7.4; df = 1; P = 0.01) and between the COAV and KNWR strains (χ2 = 7.4; df = 1; P = 0.01). In the 5-log group, significant differences in overall SGI rates were found only between COAV and WR strains (χ2 = 11.3; df = 1; P < 0.01).
Potential dissemination routes
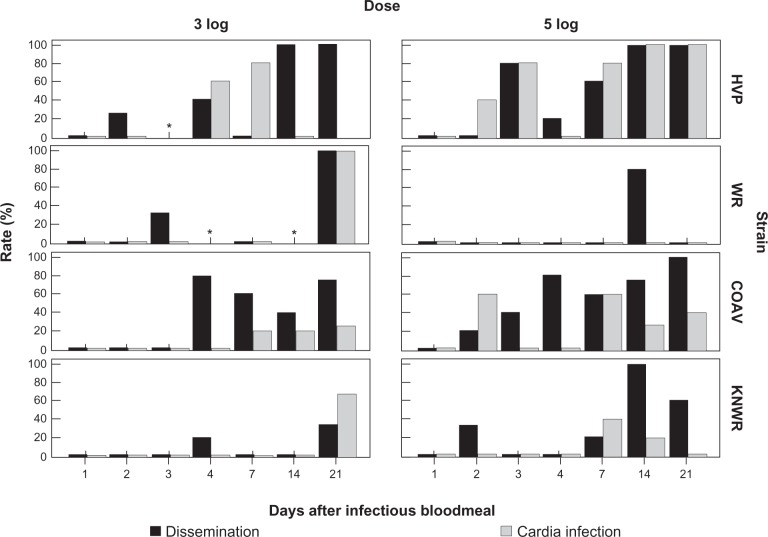
Previous studies by Romoser et al24 suggested that arboviral infection in the region of the foregut/midgut junction and cardia (Figures 1A and B) might facilitate the dissemination of virus into the hemocoel, whereas Hardy et al7 proposed the existence of a neural route by which the virus reached the salivary glands. To determine if the results of our study supported either of these hypotheses, infected individuals were examined for possible time series correlations between rates of cardia infection and dissemination (Figure 10), and between neural and salivary gland infection (Figure 11).
Figure 10.
Rates of dissemination and cardia infection among infected individuals. A significant correlation (P < 0.05) between these variables was found in the HVP strain, 5-log group. No significant correlations were found in the 3-log group. Asterisks mark time points where no infected specimens were found.
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
Figure 11.
Infection rates of neural tissue and salivary glands among infected individuals. A significant correlation (P < 0.05) between these variables was found in the 5-log group in all strains except the HVP. No significant correlations were found in the 3-log group. Asterisks mark time points where no infected specimens were found.
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
No correlation between cardial infection and dissemination was found in the 3-log group. In the 5-log group, only the HVP strain showed a significant correlation between these variables (Phi = 0.55; P < 0.01) However, it is interesting to note that within the 5-log group, cardia infection in the WR strain was not detected at any point (Figure 10) and cardia infection in the KNWR strain occurred late and at low levels in comparison to the HVP and COAV strains. Further, cardial infection preceded dissemination in the 5-log group in the HVP strain.
A significant correlation between neural and salivary gland infection was found in the 5-log group among all strains except the HVP (WR: Phi = 0.85; P < 0.01; COAV: Phi = 0.38; P = 0.03; KNWR: Phi = 0.76; P < 0.01). No significant correlation between these variables was found in any strain in the 3-log group.
Barriers to WEEV infection and dissemination
Midgut infection barrier
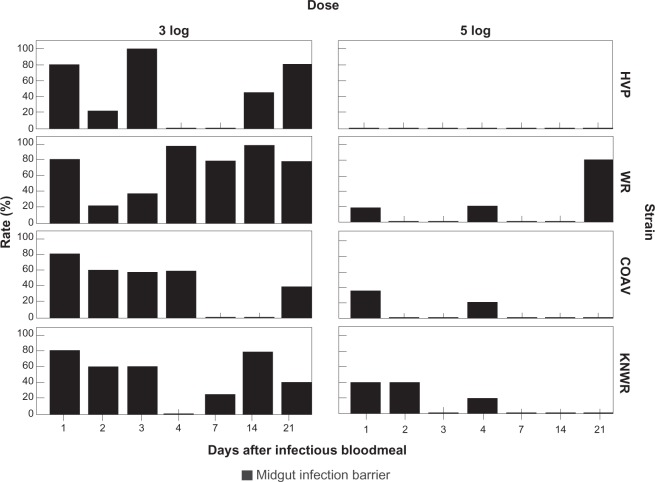
Individuals presenting a midgut infection barrier (MIB) were defined as those that failed to develop a detectable midgut infection despite having ingested infectious virus.7 At each time point, MIB rates (MIBR) were calculated using the formula: MIBR = (N/T)×100, where N is the number of individuals presenting a MIB (ie, experimental specimens showing no sign of midgut infection), and T is the total number of experimental specimens that ingested an infectious blood meal. Figure 12 shows the MIBRs for each experimental group.
Figure 12.
Midgut infection barrier (MIB) rates. Ingestion of 5-log of virus is associated with a significant decrease (P < 0.05) in the percentage of individuals displaying a MIB in all strains. In the HVP strain, MIB was completely overwhelmed in the 5-log group.
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
Significant differences in the overall MIB rates (Table 2) were found in all strains between the 3- and 5-log dose groups (HVP: χ2 = 20.7; df = 1; P < 0.01; WR: χ2 = 18.9; df = 1; P < 0.01; COAV: χ2 = 10.4; df = 1; P < 0.01; KNWR: χ2 = 10.1; df = 1; P < 0.01), supporting the notion that this barrier is dose-dependent.
Table 2.
Overall rates of individuals displaying a midgut infection barrier, midgut escape barrier, and salivary gland infection barrier
| HVP
|
WR
|
COAV
|
KNWR
|
|||||
|---|---|---|---|---|---|---|---|---|
| 3 log | 5 log | 3 log | 5 log | 3 log | 5 log | 3 log | 5 log | |
| MIBR (N/T) | 34% (12/35) | 0% (0/35) | 69% (24/35) | 17% (6/35) | 31% (11/35) | 6% (2/34) | 50% (17/34) | 14% (5/35) |
| MEBR (N/I) | 52% (12/23) | 49% (17/35) | 82% (9/11) | 86% (25/29) | 50% (12/24) | 44% (14/32) | 88% (15/17) | 67% (20/30) |
| SIBR (N/D) | 9% (1/11) | 28% (5/18) | 0% (0/2) | 25% (1/4) | 8% (1/12) | 17% (3/18) | 0% (0/2) | 10% (1/10) |
Abbreviations: COAV, Coachella Valley strain; D, number of specimens with a disseminated infection; HVP, high viremia producer strain; I, number of infected specimens; KNWR, Kern National Wildlife Refuge strain; MEBR, midgut escape barrier; MIBR, midgut infection barrier; N, number of individuals presenting the corresponding barrier; SIBR, salivary gland infection barrier; T, total number of experimental specimens; WEEV, western equine encephalomyelitis virus; WR, WEEV resistant strain.
The WR strain (Table 2) presented the highest overall MIB rates in both the 3- and 5-log groups (69% and 17%, respectively). The lowest MIB rates were observed in the COAV (31%) for the 3-log group, and the HVP (0%) for the 5-log group; the KNWR strain presented MIB rates that were close to the highest (WR) values in the 5-log group (14%), and at mid-range in the 3-log group (50%). There were, however, no significant differences in overall MIB rates among strains.
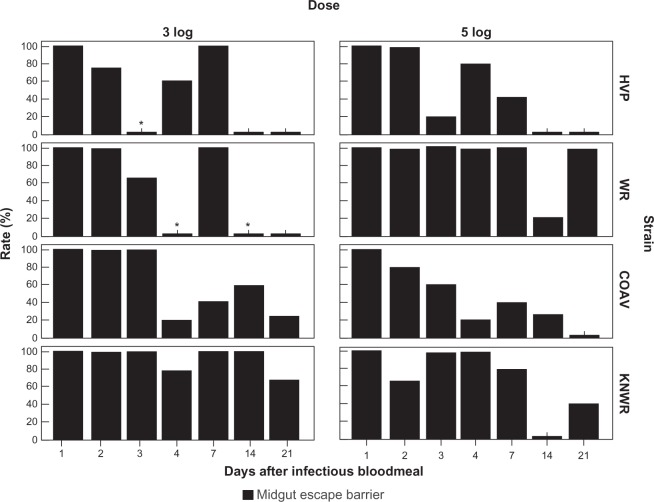
Midgut escape barrier
Individuals presenting a midgut escape barrier (MEB) were defined as those that showed no viral antigen in the hemocoel or tissues outside of the alimentary tract, despite detectable infection in the midgut. MEB rates were calculated using the formula: MEBR = (N/I)×100, where N is the number of individuals presenting a midgut escape barrier (ie, those presenting a detectable infection confined to the midgut), and I is the number of individuals presenting any signs of infection. Figure 13 shows MEBRs for each experimental group.
Figure 13.
Midgut escape barrier (MEB) rates. No significant difference between dose groups was found in overall MEB rates for any strain. Asterisks mark time points where MEB rates could not be estimated because no indication of gut infection was seen (MIB rate = 100%).
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
As shown in Table 2, the COAV strain consistently presented the lowest overall MEB rates (50% and 44% for the 3 and 5-log groups, respectively), closely followed by the HVP (52% and 49% for the 3 and 5-log groups, respectively). The WR strain presented the highest MIB overall rates for the 5-log group (86%); interestingly, it was the KNWR strain that presented the highest rates among in the 3-log group (88%), highlighting this strain’s relative refractoriness as a WEEV vector. Since no significant differences in overall MEB rates were found between dose groups, the data for each strain were pooled for further analysis.
Significant differences in overall MEB rates between strains were found when comparing the HVP vs WR (χ2 = 12.6; df = 1; P < 0.01), WR vs COAV (χ2 = 14.8; df = 1; P < 0.01), and COAV vs KNWR (χ2 = 8.3; df = 1; P < 0.01). It is notable that there were no significant differences between the HVP and COAV or between the WR and KNWR strains.
Salivary gland infection barrier
Individuals presenting a salivary gland infection barrier (SIB) were defined as those that showed no sign of salivary gland infection, despite having disseminated infections. The rate of individuals with a SIB was calculated using the formula: SIBR = (N/D)×100, where N is the number of individuals presenting a SIB (ie, those presenting signs of WEEV antigen in any tissue outside the midgut, except the salivary glands), and D is the total number of individuals presenting a disseminated infection (ie, those with WEEV antigen present in any tissue outside the midgut, including the salivary glands).
Figure 14 and Table 2 show SIB rates for each experimental group. No significant differences in overall SIB rates were found either between dose groups or among strains.
Figure 14.
Salivary gland infection barrier (SIB) rates. No significant differences were found either between dose groups, or between strains. Asterisks mark time points where rates could not be estimated due to the absence of individuals with disseminated infections.
Abbreviations: HVP, high viremia producer strain; WR, WEEV resistant strain; COAV, Coachella Valley strain; KNWR, Kern National Wildlife Refuge strain.
Discussion
Viral tropisms
Highly permissive tissues for WEEV infection in Cx. tarsalis included the midgut epithelium, the salivary glands, the neural ganglia, and the tracheae. Infected vitellogenic eggs were observed at relatively low frequency. The fat body and the skeletal muscle appeared to be refractory to WEEV infection.
Several studies have reported the fat body and skeletal muscle to be a common source for virus amplification in different arbovirus/Culex vector systems, including VEEV, Rift Valley fever, and West Nile viruses – Culex.25–28 However, studies of tissue tropisms of Dengue-2 virus in Aedes albopictus Skuse29 and in Aedes aegypti L.30 revealed few to zero viral particles in the mosquito’s fat body and muscle tissue, respectively. This suggests that not all arbovirus/ vector systems require infection of these tissues to successfully complete the extrinsic incubation cycle.
Dynamics of infection
By all parameters analyzed, the WR mosquito strain emerged as the least competent vector of WEE virus. The low infection and dissemination rates in this strain support the earlier hypothesis that resistance to WEEV infection in Cx. tarsalis is associated with a mesenteronal barrier.1 Although a reduced number of viral receptors in the midgut epithelium could certainly be a component of this barrier, our results suggest the involvement of other mechanisms that act by regulating viral replication in the gut. This is highlighted by the fact that the WR was the only strain in which infection rates showed a tendency to decline over time regardless of the viral dose ingested (Figure 9). These results are consistent with those of Mahmood et al,15 who also reported a reduction in whole-body infection rates over time in the WR strain by plaque assay.
Consistent with the original selection experiments,13,15 the HVP strain presented the highest overall infection rates. Interestingly, dissemination and salivary gland infection rates were slightly higher in the unselected COAV than in the HVP strain. These results were in clear contrast with those of Mahmood et al15 who found the COAV strain to be relatively refractory to WEEV using plaque assays to estimate virus dissemination and transmission. It is possible that these discrepancies are the result of the different methodologies used, and their respective sensitivity levels. While the plaque assay method used by Mahmood et al15 depends on viral replication, the immune-staining technique used in our study detects the presence of viral proteins, regardless of whether or not they are part of intact infectious virions or not. Considering that the concentration of viral components within a mosquito are not necessarily correlated with the concentration of infective virions,30 one may postulate the existence of a mechanism by which mosquitoes of the COAV strain regulate their viral load at the post-translational level. Further research is required to test the existence and nature of this mechanism.
As opposed to the COAV, the KNWR strain presented consistently low infection, dissemination, and SGI rates. Furthermore, statistical analyses failed to reveal any significant differences between the KNWR and WR, suggesting that individuals of the KNWR strain are poor vectors of WEEV, as proposed by Mahmood et al.15 Interestingly, WEEV has recently returned to Kern County, where it was detected repeatedly in Cx. tarsalis collected at the Kern National Wildlife Refuge,31 the source of the KNWR colony. Our standard BFS1703 strain from 1952 was found to be similar in avian and vector competence to newly circulating strains of WEEV.32 Similar findings were recently reported for cell culture studies.33
Potential dissemination routes
Despite the significant correlation between the rates of neural and salivary gland infection, our results do not provide conclusive support for the existence of a neural dissemination pathway. Examination of Figure 9 reveals that the detection of WEEV antigen in the salivary glands often preceded the detection of neural infection. If dissemination occurred via the neural network, one would expect the opposite sequence of events to occur, (that is, infection of the neural tissue before salivary gland infection). Therefore, we cannot rule out the possibility that the observed correlations simply reflect the simultaneous infection of these two tissues, possibly via routes such as the hemolymph or the trachea.8,30
The occasional infection of neuroendocrine cells of the retrocerebral complex and other cells of the stomatogastric nervous system may be a significant finding because this could affect the insect’s behavior and neural physiology. The infection of mosquito neuroendocrine tissues by vector-borne viruses has been previously reported,27 as well as changes in mosquito behavior induced by viral infection.34,35
Our results regarding the role of the cardia as a dissemination route were also inconclusive, although they do not rule out the possibility that this region intermittently serves as a viral escape route from the midgut in some individuals,24 particularly among the more permissive HVP and COAV strains. This dissemination mechanism could explain the early detection of virus in the salivary glands as well as the legs and expectorate reported by Mahmood et al15 in the HVP strain 5-log group.
Barriers to midgut infection, dissemination, and salivary gland infection
The significant differences observed between dose groups in the overall MIB rates are in agreement with the existence of a dose-dependent barrier to midgut infection.1,15,36 In contrast, overall MEB rates displayed no significant differences between dose groups in any strain, suggesting that this barrier is not dependent upon the ingested viral dose but on other factors, among which time postinfection may be of particular relevance.
Kramer et al1 found that upon ingestion of relatively low WEEV loads, viral titers in individuals with disseminated infections reached up to 10-fold higher values than those in individuals displaying a MEB. Based on this evidence, the authors proposed that a ‘threshold’ virus concentration must be reached in the midgut before dissemination can occur. Considering that the viral titers reached in the mosquito midgut are a function of the amount of virus ingested, the permissiveness of the insect’s tissues for viral reproduction, and time postinfection, a mechanism may exist that represents the interaction between ingested dose, time, and the MEB. In a permissive strain (such as the HVP), ingestion of a high viral dose would result in the infection of more cells and, therefore, faster amplification, which in turn generates high dissemination rates over relatively short time periods. On the other hand, if a mosquito strain is either refractory to midgut infection or effectively modulates viral amplification to low levels (such as the WR strain),2 the time required to reach the high viral titers necessary to overcome the MEB may be significantly extended, even after the ingestion of large amounts of infective virus.
The fact that disseminated infections were significantly less common among individuals of the WR strain than among those of the more susceptible HVP and COAV strains supports the notion that it is the WR strain’s ability to contain and modulate infection within the midgut boundaries that defines this strain’s resistance to WEEV. The lack of significant differences in MEBR between the KNWR and WR strains suggests that KNWR mosquitoes are also able to inhibit, at least to some extent, the dissemination of WEE virus.
In agreement with previous reports,2 our data indicate that once the virus escapes from the gut, all strains are equally likely to develop salivary gland infections. It is interesting, however, to point out that transmission rates reported in a study by Mahmood et al15 were consistently lower than the overall salivary gland infection rates reported in our study for all strains. These discrepancies lend further support to the notion that a barrier to salivary gland escape exists, as has already been proposed15 or that there is differential sensitivity between the assays used to detect antigens within the salivary gland and infectious virions in expectorate.
Our findings relative to pathology, specifically in the midgut epithelium and salivary glands, and the possible effects on dissemination and transmission will be reported in a subsequent paper.
Acknowledgments
The authors would like to thank Robert Chiles, Center for Vectorborne Diseases, for providing the antibody used in this study. Emily Butler, Amanda Yant, Ehryn Rose, Carlie Rose, Regan Welch, and Kelley Romoser helped prepare tissue samples for microscopic observation. This project was funded, in part, by Research Grant 1-R01-AI39483 from the National Institutes of Health to WK Reisen.
Footnotes
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- 1.Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30(1):190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 2.Kramer LD, Hardy JL, Houk EJ, Presser SB. Characterization of the mesenteronal infection with western equine encephalomyelitis virus in an incompetent strain of Culex tarsalis. Am J Trop Med Hyg. 1989;41(2):241–250. doi: 10.4269/ajtmh.1989.41.241. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Reeves W. Experimental studies of infection in vectors. In: Reeves W, editor. Epidemiology and Control of Mosquito-borne Arboviruses in California, 1943–1987. Sacramento, CA: Mosquito and Vector Control Association; 1990. pp. 66–127. [Google Scholar]
- 4.Hardy JL, Meyer RP, Presser SB, Milby MM. Temporal variations in the susceptibility of a semi-isolated population of Culex tarsalis to peroral infection with western equine encephalomyelitis and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1990;42(5):500–511. doi: 10.4269/ajtmh.1990.42.500. [DOI] [PubMed] [Google Scholar]
- 5.Reisen WK, Hardy JL, Presser SB, Chiles RE. Seasonal variation in the vector competence of Culex tarsalis (Diptera: Culicidae) from the Coachella valley of California for western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 1996;33(3):433–437. doi: 10.1093/jmedent/33.3.433. [DOI] [PubMed] [Google Scholar]
- 6.Mellor PS. Replication of arboviruses in insect vectors. J Comp Pathol. 2000;123(4):231–247. doi: 10.1053/jcpa.2000.0434. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitos for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 8.Romoser WS, Wasieloski LP, Pushko P, et al. Evidence for arbovirus dissemination conduits from the mosquito (Diptera: Culicidae) midgut. J Med Entomol. 2004;41(3):467–475. doi: 10.1603/0022-2585-41.3.467. [DOI] [PubMed] [Google Scholar]
- 9.Adelman ZN, Sanchez-Vargas I, Travanty EA, et al. RNA silencing of dengue virus type 2 replication in transformed C6/36 mosquito cells transcribing an inverted-repeat RNA derived from the virus genome. J Virol. 2002;76(24):12925–12933. doi: 10.1128/JVI.76.24.12925-12933.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romoser WS, Turell MJ, Lerdthusnee K, et al. Pathogenesis of Rift Valley fever virus in mosquitoes – tracheal conduits and the basal lamina as an extra-cellular barrier. Arch Virol. 2005;19:89–100. doi: 10.1007/3-211-29981-5_8. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J. The Arboviruses: Epidemiology and Ecology. Vol. 1. Boca Raton, FL: CRC Press; 1988. Susceptibility and resistance of mosquito vectors. In: Monath T, editor; pp. 87–126. [Google Scholar]
- 12.Tabachnick WJ. Genetics of insect vector competence for arboviruses. Adv Dis Vector Res. 1994;10:93–108. [Google Scholar]
- 13.Hardy JL, Apperson G, Asman SM, Reeves WC. Selection of a strain of Culex tarsalis highly resistant to infection following ingestion of western equine encephalomyelitis virus. Am J Trop Med Hyg. 1978;27(2):313–321. doi: 10.4269/ajtmh.1978.27.313. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood F, Chiles RE, Fang Y, Reisen WK. Methods for studying the vector competence of Culex tarsalis for western equine encephalomyelitis virus. J Am Mosq Control Assoc. 2004;20(3):277–282. [PubMed] [Google Scholar]
- 15.Mahmood F, Chiles RE, Fang Y, Green EN, Reisen WK. Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. J Am Mosq Control Assoc. 2006;22(2):272–281. doi: 10.2987/8756-971X(2006)22[272:EOTAIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Reeves W, Hammon W. Epidemiology of the arthropod-borne viral encephalitides in Kern County, California, 1943–1952. Publ Public Health Univer Calif. 1962;4:1–257. [PubMed] [Google Scholar]
- 17.Reisen WK, Meyer RP, Presser SB, Hardy JL. Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera, Culicidae) J Med Entomol. 1993;30(1):151–160. doi: 10.1093/jmedent/30.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Hardy J, Reeves W, Bruen J, Presser S. Vector competence of Culex tarsalis and other mosquito species for western equine encephalomyelitis virus. In: Kurstak E, editor. Arctic and Tropical Arboviruses. New York: Academic Press; 1979. pp. 157–171. [Google Scholar]
- 19.Rutledge LC, Ward RA, Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq News. 1964;24:407–419. [Google Scholar]
- 20.Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St Louis encephalitis viruses. J Med Entomol. 2003;40(6):968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- 21.Faran ME, Romoser WS, Routier RG, Bailey CL. Use of the avidinbiotin-peroxidase complex immunocytochemical procedure for detection of Rift-Valley fever virus in paraffin sections of mosquitoes. Am J Trop Med Hyg. 1986;35(5):1061–1067. doi: 10.4269/ajtmh.1986.35.1061. [DOI] [PubMed] [Google Scholar]
- 22.Last J. A Dictionary of Epidemiology. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 23.Sokal RR, Rohlf FJ. Biometry: the principles and practices of statistics in biological research. New York: WH Freeman and Company; 1995. [Google Scholar]
- 24.Romoser WS, Faran ME, Bailey CL. Newly recognized route of arbovirus dissemination from the mosquito (Diptera, Culicidae) midgut. J Med Entomol. 1987;24(4):431–432. doi: 10.1093/jmedent/24.4.431. [DOI] [PubMed] [Google Scholar]
- 25.Léon R. Aedes taeniorrhynchus mosquitoes using nucleic acid hybridization and immunocytochemistry PhD dissertation. Athens: Ohio University; 2000. The localization of Venezuelan equine encephalitis virus. [Google Scholar]
- 26.Weaver SC. Electron-microscopic analysis of infection patterns for Venezuelan equine encephalomyelitis virus in the vector mosquito, Culex (Melanoconion) taeniopus. Am J Trop Med Hyg. 1986;35(3):624–631. doi: 10.4269/ajtmh.1986.35.624. [DOI] [PubMed] [Google Scholar]
- 27.Romoser WS, Faran ME, Bailey CL, Lerdthusnee K. An immunocytochemical study of the distribution of Rift Valley fever virus in the mosquito Culex pipiens. Am J Trop Med Hyg. 1992;46(4):489–501. doi: 10.4269/ajtmh.1992.46.489. [DOI] [PubMed] [Google Scholar]
- 28.Girard YA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004;4(2):109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- 29.Sriurairatna S, Bhamarapravati N. Replication of dengue-2 virus in Aedes albopictus mosquitos – electron-microscopic study. Am J Trop Med Hyg. 1977;26(6 Pt 1):1199–1205. doi: 10.4269/ajtmh.1977.26.1199. [DOI] [PubMed] [Google Scholar]
- 30.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll BD, Takahashi BM, Reisen WK. West Nile virus activity in Kern County during 2006. Proc Mosq Vector Control Assoc Calif. 2007;75:17–22. [PMC free article] [PubMed] [Google Scholar]
- 32.Reisen WK, Fang Y, Brault AC. Limited interdecadal variation in mosquito (Diptera: Culicidae) and avian host competence for Western equine encephalomyelitis virus (Togaviridae: Alphavirus) Am J Trop Med Hyg. 2008;78(4):681–686. [PubMed] [Google Scholar]
- 33.Zhang M, Fang Y, Brault AC, Reisen WK. Variation in western equine encephalomyelitis viral strain growth in mammalian, avian and mosquito cells fails to explain temporal changes in enzootic and epidemic activity in California. Vector-Borne Zoonotic Dis. 2010 doi: 10.1089/vbz.2010.0078. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimstad PR, Ross QE, Craig GB. Aedes triseriatus (Diptera: Culicidae) and Lacrosse virus II. Modification of mosquito feeding behavior by virus infection. J Med Entomol. 1980;17:11–16. doi: 10.1093/jmedent/17.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Turell MJ, Gargan TP, II, Bailey BL. Culex pipiens (Diptera: Culicidae) morbidity and mortality associated with Rift Valley fever virus infection. J Med Entomol. 1985;22(3):332–337. doi: 10.1093/jmedent/22.3.332. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain R, Sudia WD. Mechanism of transmission of viruses by mosquitoes. Annu Rev Entomol. 1961;6:371–390. doi: 10.1146/annurev.en.06.010161.002103. [DOI] [PubMed] [Google Scholar]