Abstract
Neurobiochemical marker levels in blood after traumatic brain injury (TBI) may reflect structural changes detected by neuroimaging. This study evaluates whether correlations between neuronal (ubiquitin carboxy-terminal hydrolase-L1 [UCH-L1]) and glial (glial fibrillary acidic protein [GFAP]) biomarkers may be used as an indicator for differing intracranial pathologies after brain trauma. In 59 patients with severe TBI (Glasgow Coma Scale [GCS] score≤8) serum samples were obtained at the time of hospital admission and analyzed for UCH-L1 and GFAP. Glial neuronal ratio (GNR) was evaluated as the ratio between GFAP and UCH-L1 concentrations. A logistic regression analysis was used to identify variables associated with type of injury. GNR had a median of 0.85 and was positively correlated with age (R=0.45, p=0.003). Twenty-nine patients presented with diffuse injury and 30 with focal mass lesions as assessed by CT scan at admission and classified according to the Marshall Classification. GNR was significantly higher in the focal mass lesion group compared with the diffuse injury group (1.77 versus 0.48, respectively; p=0.003). Receiver operating characteristic curve analysis showed that GNR discriminated between types of injury (area under the curve [AUC]=0.72; p=0.003). GNR was more accurate earlier (≤12 h after injury) than later (AUC=0.80; p=0.002). Increased GNR was independently associated with type of injury, but not age, gender, GCS score, or mechanism of injury. GNR was significantly higher in patients who died, but was not an independent predictor of death. The data from the present study indicate that GNR provides valuable information about different injury pathways, which may be of diagnostic significance. In addition, GNR may help to identify different pathophysiological mechanisms following different types of brain trauma, with implications for therapeutic interventions.
Key words: biomarkers, computed tomography, diagnostic, glial neuronal ratio, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is often described as a complex spectrum of pathologies (Maas et al., 2007) rather than a single disease. The heterogeneity of TBI is one of the most significant barriers to finding effective therapeutic interventions (Saatman et al., 2008). Indeed, although it is common to refer to distinct focal and diffuse lesions in patients with TBI, these two types of pathologies and their different pathophysiologic mechanisms can overlap and coexist in the same patient, particularly in cases of severe TBI (Povlishock and Katz, 2005). Thus potential therapies are not always tested in the distinct patient populations most likely to benefit.
Because of the link between specific pathoanatomic types and pathophysiologic mechanisms, there has been considerable interest in the development and use of a biochemical measure to determine the specific patterns of brain injury, and to accurately classify various forms of TBI (Dash et al., 2010; Mondello et al., 2011a; Saatman et al., 2008).
Dramatic advances have been made with regard to how injuries occur at the cellular level, resulting in cell damage and death and the release of cell type-specific proteins such as glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1). GFAP is a structural protein expressed almost exclusively in astrocytes and released upon cellular disintegration and degradation of the cytoskeleton (Rosengren et al., 1994). UCH-L1 is a neuron-specific protein which is highly abundant in neuronal cytoplasm (Jackson and Thompson, 1981), and is involved in either the addition or removal of ubiquitin from proteins that are destined to be metabolized via the ATP-dependent proteasome pathway (Tongaonkar et al., 2000). Alterations in the functions of UCH-L1 have also been reported to be involved in the pathogenesis of several neurodegenerative diseases, including Parkinson's disease and Alzheimer's disease (Gong and Leznik, 2007; Setsuie and Wada, 2007). These markers of brain damage have their own unique features and cellular origins that represent diverse cellular vulnerability patterns, as well as a spectrum of injury processes following different types of primary injuries. Previously we showed that GFAP levels were higher in patients with mass lesions than in those with diffuse injury (p=0.003), while UCH-L1 levels were higher in patients with diffuse injury (p=0.01) (Mondello et al., 2011b), indicating that distinct neuronal and glial patterns of biomarker release are associated with distinctly different types of structural damage as detected by neuroimaging.
Therefore, the current study sought to examine the correlations between glial and neuronal damage using a glial neuronal ratio (GNR). We investigated the relationship of this ratio to neuroimaging profiles in patients with severe brain trauma. We compared the GNR with the demographic and clinical indices, and we also investigated the correlations between GNR and the severity of injury and outcome. Finally, implications for the pathogenesis of brain injury are discussed.
Methods
Study sites, design, and population
This study is part of the BANDITS (Biomarker Assessment for Neurotrauma Diagnosis and Improved Triage System) Feasibility Study, a multicenter project involving the clinical, biochemical, and neuroimaging evaluations of patients with severe TBI. In this study we focused on a cohort of 59 patients with severe TBI in whom data about brain damage markers in serum and neuroimaging results were available. For simple mathematical reasons, samples from patients in whom biomarker values were below the limit of detection (previously reported as 0) were excluded from the analysis. Other analyses from this project have been reported elsewhere (Mondello et al., 2011b). Venous blood samples were taken at hospital admission (mean±standard error of the mean [SEM], 9±1 h after injury). Approval of this study was obtained from the local ethics committees of all the sites involved (Pecs, Szeged, Maryland, and University of California–Davis), and from the Western Institutional Review Board (WIRB) and Human Research Protection Office (HRPO). Written informed consent was obtained from next of kin because all eligible patients were in coma within 24 h of admission. Severe TBI was defined as a Glasgow Coma Scale (GCS) score of 8 or less on admission as determined by appropriately qualified personnel. Patients who were sedated or paralyzed for transportation were excluded. Further exclusion criteria were patients younger than 18 years of age, no informed consent, known history of neurological and/or autoimmune disease, female patients that were or may be pregnant (documented proof of negative pregnancy test, menopause, or sterilization), and multiple injuries (Injury Severity Score [ISS] greater than 15). Initial computed tomography (CT) scans obtained on admission (mean±SEM, 9±3 h after injury) were classified by a qualified neuroradiologist at a central location according to the classification of Marshall and associates (Marshall et al., 1991), which has four categories indicating diffuse injury of increasing severity, whereas two categories indicate the presence of a mass lesion. For the purpose of our analysis, the Marshall score was further classified into two groups using dichotomized categories (diffuse injury versus focal mass lesion), as previously described (Raabe et al., 1998).
Patient demographic (age, sex, and race) and clinical variables, including mechanism of injury, GCS score, the occurrence of hypoxia (defined as a Pao2<60 mm Hg or O2 saturation <90%), hypotension (defined as systolic blood pressure <90 mm Hg), and CT findings, were recorded. Because a limited number of pupillary reaction data were available for the study patients (n=14), and ISS was used only as an exclusion criterion without recording individual values, these variables were not included in the statistical analyses. Patient characteristics are shown in Table 1.
Table 1.
Characteristics of 59 Patients with Severe Traumatic Brain Injury (TBI)
| |
Severe TBI |
|---|---|
| (n=59) | |
| Age, y, mean | 46.66 |
| Range | 19–89 |
| Gender, n (%) | |
| Female | 13 (22.03) |
| Male | 46 (77.97) |
| Race, n (%) | |
| Caucasian | 52 (88.14) |
| Black or African-American | 4 (6.78) |
| Asian | 3 (5.08) |
| Injury mechanism, n (%) | |
| Motor vehicle accident | 24 (40.68) |
| Motorcycle accident | 2 (3.39) |
| Gunshot wound | 2 (3.39) |
| Fall | 21 (35.59) |
| Assault | 2 (3.39) |
| Other | 8 (13.56) |
| Glasgow Coma Scale score, median (range) | 5 (3–8) |
| Marshall score, n (%) | |
| Diffuse injury I | 1 (1.69) |
| Diffuse injury II | 16 (27.12) |
| Diffuse injury III | 8 (13.56) |
| Diffuse injury IV | 4 (6.78) |
| Evacuated focal mass lesion V | 2 (3.39) |
| Focal mass lesion VI | 28 (47.46) |
| Survival, 6 months, n (%) | |
| Deceased | 33 (55.92) |
| Alive | 22 (37.30) |
| NA | 4 (6.78) |
| aHypotension, n (%) | |
| No | 31 (52.55) |
| Present | 19 (32.20) |
| NA | 9 (15.25) |
| bHypoxia, n (%) | |
| No | 44 (74.58) |
| Present | 6 (10.17) |
| NA | 9 (15.25) |
Hypotension was defined as follows: no, none; present, systolic blood pressure <90 mm Hg; NA, not available.
Hypoxia was defined as follows: no, no Pao2>60 mm Hg or O2 saturation>90%; present, any Pao2<60 mm Hg or O2 saturation<90%; NA, not available.
(Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care. See Bratton et al., 2007.)
GNR was defined as the GFAP concentration (ng/mL) divided by UCH-L1 concentration (ng/mL). Outcome was assessed at 6 months post-injury.
Measurement of brain damage markers: UCH-L1 and GFAP
Approximately 5 mL of serum was collected from each subject at each sample point. Samples were centrifuged for 10 min at 4000 rpm and immediately frozen and stored at −70°C until the time of analysis. All samples were analyzed in duplicate. Samples were measured using a standard UCH-L1 sandwich ELISA protocol as described below. Reaction wells were coated with capture antibody (500 ng/well purified anti-rabbit UCHL1, made in-house) in 0.1 M sodium bicarbonate (pH 9), and incubated overnight at 4°C. The plates were then emptied out and 300 μL/well blocking buffer was added and incubated for 30 min at ambient temperature with gentle shaking. This was followed by the addition of antigen standard (UCH-L1 standard curve: 0.05–50 ng/well) unknown samples (3–10 μL CSF), or assay internal control samples. The plate was incubated for 2 h at room temperature, then washed using an automatic plate washer (each well was rinsed 5×300 μL with wash buffer [TBST]). Detection antibody (anti-rabbit UCH-L1-HRP conjugate, made in-house at 50 μg/mL) in blocking buffer was then added to the wells at 100 μL/well, and the plates were further incubated for 1.5 h at room temperature. After additional automatic washing, biotinyl-tyramide solution (Perkin Elmer Elast Amplification Kit; Perkin Elmer, Waltham, MA) was added and the plate was incubated for 15 min at room temperature, followed by automatic washing. The addition of streptavidin-HRP (1:500, 100 μL/well) in PBS with 0.02% Tween 20 and 1% BSA for 30 min incubation at room temperature was followed by automatic washing. Lastly, the wells were developed with substrate solution (Ultra-TMB ELISA 100 μL/well) with incubation for 5–30 min, and read at 652 nm with a 96-well spectrophotometer. GFAP protein was analyzed using a commercially available (BioVendor catalog no. rd192072200; BioVendor, Brno, Czech Republic) polyclonal two-sided immunoluminometric assay according to the manufacturer's instructions. A standard curve was constructed by plotting absorbance values versus GFAP concentrations of calibrators.
Statistical analysis
All statistical analyses were performed using SAS software (SAS version 9.2; SAS Institute Inc., Cary, NC). Exploratory analysis was carried out to determine the distribution of the data. Continuous variables are presented as mean (standard deviation [SD]) or median (interquartile range), as appropriate. Distributions of categorical variables are presented as frequencies and percentages. Categorical variables were compared using the chi-square or Fisher's exact test, as indicated. Continuous variables were compared using the Mann-Whitney U test. The relation between GNR and continuous variables was assessed by bivariate correlations (Spearman's ρ). Associations between type of injury, GNR, and demographic and clinical variables were determined using univariate and multivariate logistic regression analyses. Cut-off values were constructed using receiver operating characteristic (ROC) curves under the condition of equal costs of misclassification (i.e., t, the sum of the sensitivity and the specificity, was maximal). Logistic regression analysis was also performed to determine factors that could be considered independent predictors of death. All hypothesis tests conducted were two-tailed. A p value <0.05 was considered significant.
Results
GNR in relation to patient characteristics
A total of 59 patients were included. Clinical and demographic data are summarized in Table 1. The mean age was 46.66 years (range 19–89 years), with 13 females (22.03%) and 46 males (77.97%). The median GNRs for patients with severe TBI are shown in Table 2. GNR did not correlate with GCS (R=0.03, p=0.79) but was positively correlated with age (R=0.45, p=0.003).
Table 2.
Glial Neuronal Ratio at Hospital Admission of 59 Patients with Severe Traumatic Brain Injury (TBI) by Demographic and Clinical Variables and Outcome
| n | TBI (n=59) | |
|---|---|---|
| TBI | ||
| Admission | 59 | 0.85 (0.28–2.09) |
| Gender | ||
| Male | 46 | 0.94 (0.29–2.01) |
| Female | 13 | 0.63 (0.23–2.72) |
| Hypoxia | ||
| Yes | 6 | 0.54 (0.14–2.16) |
| No | 44 | 0.70 (0.27–1.95) |
| Hypotension | ||
| Yes | 19 | 1.35 (0.28–5.24) |
| No | 31 | 0.49 (0.20–1.81) |
| Marshall | ||
| Diffuse injury | 29 | 0.48 (0.27–1.22) |
| Focal mass lesion | 30 | 1.77 (0.56–4.73)† |
| 6 Months | ||
| Survivors | 22 | 0.49 (0.27–1.43) |
| Non-survivors | 33 | 1.67 (0.58–3.02)* |
Data are expressed as median (interquartile range).
p<0.05; †p<0.005 by the Mann-Whitney U test for differences between the groups.
GNR was not affected by gender, whereas it was higher in patients with hypotension and lower in patients with hypoxia, but the differences were not significant (Table 2). The correlation between time to sample withdrawal and GNR was less than 0.2.
GNR in relation to type of injury
Twenty-nine patients (49.15%) were diagnosed with diffuse injury (Marshall class I–IV), and 30 patients (50.85%) with focal mass lesions (Marshall class V and VI) on initial CT scan. Among 29 patients with diffuse injury, 1 (1.69%), 16 (27.12%), 8 (13.56%), and 4 (6.78%) had diffuse injury I, II, III, and IV, respectively. Among 30 patients with focal mass lesions, 2 (3.39%) and 28 (47.46%) had evacuated focal mass lesions and focal mass lesions, respectively. Table 3 shows the characteristics of both groups. Patients with diffuse injury and focal mass lesion were well matched with regard to demographic characteristics, and no differences between the groups were seen regarding clinical variables. Patients with focal mass lesions tended to be older compared to patients with diffuse injuries, although the difference was not significant (median 53 versus 35 years, respectively; p=0.15 by Mann-Whitney U test).
Table 3.
Characteristics of the Diffuse Injury and Focal Mass Lesion Groups
| Diffuse injury | Focal mass lesion | p | |
|---|---|---|---|
| Age, y, mean (SD) | 42.17 (17.93) | 51 (21.73) | 0.15 |
| Range | 19–75 | 20–75 | |
| Gender, n (%) | |||
| Female/male | 7/22 | 6/24 | 0.94 |
| Glasgow Coma Scale score, mean (SD) | 5 (1.86) | 5 (1.77) | 0.73 |
| Median (range) | 5 (3–8) | 5 (3–8) | |
| Survival | |||
| Dead/alive | 13/7 | 20/8 | 0.75 |
| Hypotension, n | |||
| No/present | 17/9 | 14/10 | 0.82 |
| Hypoxia, n (%) | |||
| No/present | 23/3 | 21/3 | 1 |
Mann-Whitney U test for continuous variables and chi-square test or Fisher's exact test for categorical variables.
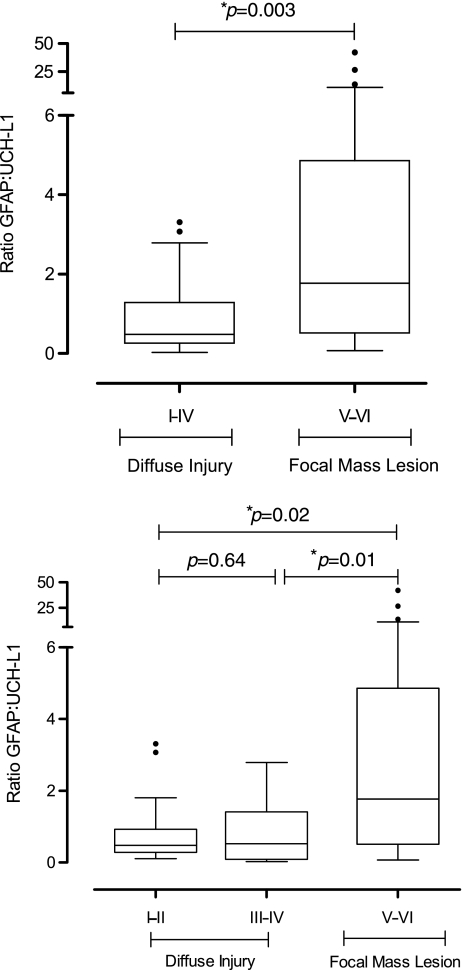
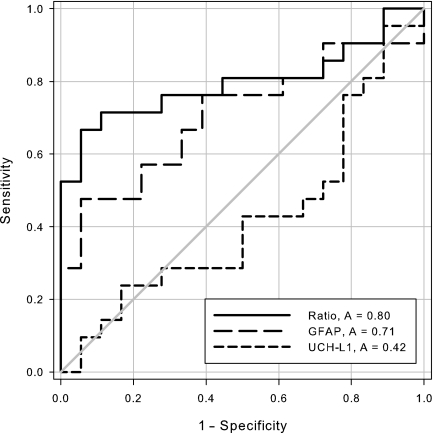
The GNR was significantly higher in patients with focal mass lesions than in those with diffuse injuries (p=0.003 by Mann-Whitney U test; Table 2 and Fig. 1). Marshall Classification was also categorized into three groups: diffuse injury I–II, diffuse injury III–IV, and focal mass lesion. Patients with mild (I–II) to severe (III–IV) diffuse injuries had significantly lower GNRs than patients with focal mass lesions (median 0.48 versus 0.53 versus 1.77, respectively; p=0.01 by Kruskal-Wallis test; Fig. 1). A ROC curve analysis of GNR to predict focal mass lesions showed an area under the curve (AUC) of 0.72 (95% CI 0.59,0.87), with high specificity (83%), although the sensitivity was low (60%), using a cut-off of >1.43. Consistent with the biomarker kinetics within the first 24 h, a ROC curve analysis including only the samples taken within 12 h after TBI (n=39) showed an increased AUC (0.80 [95% CI 0.65,0.95]), with 94% specificity and 67% specificity, using a cut-off of >1.32 (Fig. 2). Figure 2 shows the ROC curves of GNR compared to GFAP and UCH-L1. The higher AUC of GNR indicates a better ability to discriminate between focal mass lesions and diffuse injuries. The differences in AUC were significant when compared to UCH-L1, and nearly significant when compared to GFAP (p=0.004 and p=0.06 based on the Mann-Whitney U test; DeLong et al., 1988). Analysis of the cases in which the blood sample was drawn more than 12 h after injury (n=20) weakened the diagnostic accuracy (AUC 0.63), but the difference was not significant compared to samples collected within12 h (p=0.28).
FIG. 1.
Box-and-whisker plots demonstrating glial neuronal ratio (GNR). Top: GNR in patients with diffuse injury (n=29), and in patients with focal mass lesion (n=30). Bottom : GNR in patients with diffuse injury I–II (n=17), diffuse injury III–IV(n=12), and focal mass lesion (n=30). The black horizontal line in each box represents the median, with the boxes representing the interquartile range. Outliers are plotted with circles. Significant differences are indicated with *p<0.05).
FIG. 2.
Receiver operating characteristic curves for glial neuronal ratio (GNR; solid line), and glial fibrillary acidic protein (GFAP; long dashes), and ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1; short dashes) concentrations in serum on admission to discriminate between focal mass lesions and diffuse injuries. The area under the curve was 80 for GNR, 71 for GFAP, and 42 for UCH-L1.
Stepwise logistic regression analysis using an inclusion criterion of P.10 (i.e., the variable was included only if the p value was below 0.10), and including patient characteristics (age and gender) and TBI characteristics (GCS score on admission and injury mechanism) as covariates, identified GNR as only independent predictor of mass lesions.
GNR in relation to long-term outcome
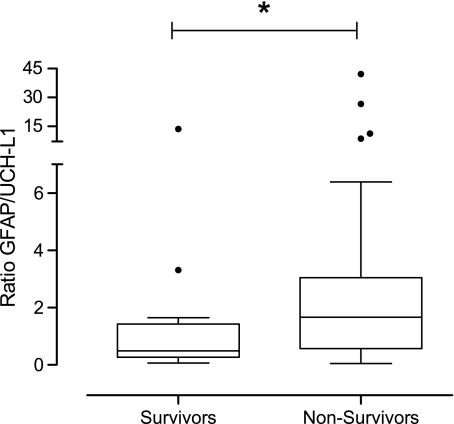
At 6 months of follow-up, the mortality rate was 60%. GNR was significantly higher in patients who died than in patients who survived (p=0.034; Fig. 3 and Table 2). For 6-month mortality, a multivariate logistic regression analysis revealed that age was the most significant independent predictor (OR 1.07 [95% CI 1.02,1.12]; p=0.006), followed by GCS score (OR 0.65 [95% CI 0.43,0.99]; p=0.05, c=0.80). Although GNR was significantly increased in patients who died compared to those who survived, it did not independently predict death. This result may not be surprising, because GNR significantly correlated with age.
FIG. 3.
Box-and-whisker plots demonstrating glial neuronal ratio in patients who died (n=33) and in patients who survived (n=22). The black horizontal line in each box represents the median, with the boxes representing the interquartile range. Significant differences are indicated by *p<0.05 (GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxy-terminal hydrolase-L1).
Discussion
This study demonstrated differences in patterns of cell damage/death, as measured by the GNR between patients with focal mass lesions after TBI and those with diffuse injuries. Measurements of GFAP and UCH-L1 give information about the individual contributions of glial and neuronal cell damage to brain injury, with higher concentrations indicating more severe injuries (Brophy et al., 2011; Papa et al., 2010; Vos et al., 2010). The GNR reflects comparative cellular damage and thus can provide information about the relative predominance of neuronal versus glial injury occurring within brain tissue. An elevated GNR (>1), with massive glial involvement, was observed in patients with focal mass lesions. On the other hand, diffuse injury resulted in a selective and predominant neuronal cell death, with higher levels of UCH-L1 release compared to GFAP (GNR<1).
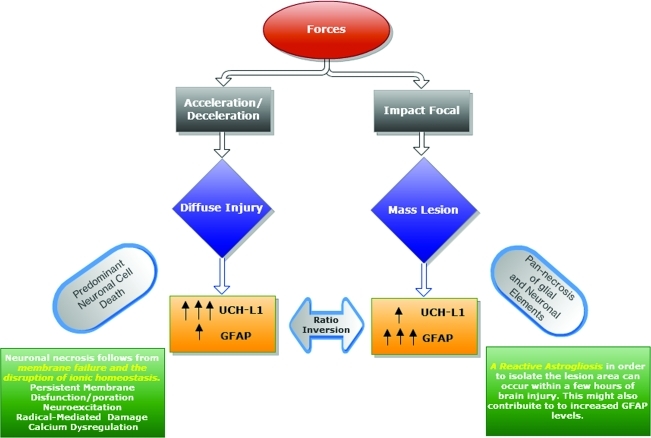
These data confirm the pathoanatomical model that different brain cell types are differently affected by and play a role in the development of different types of injury after TBI (Fig. 4). Several authors described elevated levels of circulating GFAP and UCH-L1 in patients following TBI resulting from cell damage and death in the brain (Brophy et al., 2011; Papa et al., 2010; Vos et al., 2010). The GNR hypothesis of TBI proposes that their relationship, expressed as a ratio, is associated with specific types of structural brain damage, and therefore with the type of injury.
FIG. 4.
The glial neuronal ratio (GNR) hypothesis of traumatic brain injury (TBI). According to the GNR hypothesis of TBI, biomarkers (UCH-L1 and GFAP) are released/produced by the neuronal and glial damaged cells. Their relationship expressed as a ratio is related to the type of injury that occurs after TBI. Diffuse injury, as a consequence of acceleration/deceleration forces, results in a selective and predominant neuronal cell death, with higher levels of UCH-L1 release compared to GFAP (GNR ratio<1; left side). Focal mass lesion, as a consequence of impact forces, results in pan-necrosis. Biomarkers from glial and neuronal sources are released into the bloodstream, with an increased level of GFAP compared to UCH-L1, and therefore a ratio inversion (GNR ratio>1; right side; GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxy-terminal hydrolase-L1).
Further support for this hypothesis comes from two previously reported intrinsic characteristics of the brain (Fig. 4; Mondello et al., 2011b). First, there is a different representation of glial and neuronal cells in the brain, with an astrocyte-to-neuron ratio that can reach 10:1 in most brain regions (Tsacopoulos and Magistretti, 1996). Second, glia and neurons are differently affected by different insults (Bramlett and Dietrich, 2004; Lee et al., 2003), specifically, neurons are much more susceptible than astrocytes to a number of primary and secondary injury processes (Berger et al., 2002). Acceleration-deceleration insults cause shear forces, resulting in primary membrane damage to neuronal cell bodies, and in disruption of ionic homeostasis with rapid degradation of the neuronal cytoskeleton and cytoplasmic constituents (Bramlett and Dietrich, 2004; Povlishock and Katz, 2005). Brief periods of severe ischemia may lead to selective neuronal damage, with minor cellular changes observed in glia (Kirino, 1982; Pulsinelli et al., 1982). On the other hand, severe injuries can lead to massive damage to glial cells. The nature, severity, and duration of these forces and processes determine the extent and pattern of cell damage, and lead to a spectrum of histopathological changes.
The present study showed that GNR ratio may be useful for the identification of TBI patients with different types of injury, specifically diffuse injury versus focal lesions. A ROC analysis including the ratio within 12 h after TBI showed an increased AUC, indicating the immediate impact of biomarker release into the circulation on diagnostic accuracy, which was also confirmed by the decreased diagnostic accuracy seen after 12 h. Previous reports consistently demonstrated a correlation between the time course of the release of biomarkers and diagnostic utility (Berger et al., 2002; Herrmann et al., 2000). It is reasonable to speculate that the half-life of the protein and blood sampling may influence the accuracy of the test. Biokinetic analyses performed on UCH-L1 and GFAP have demonstrated that both are acute injury biomarkers (Brophy et al., 2011). Accordingly, GNR determined at initial assessment within 12 h of injury gave the best prediction of type of injury. However, definitive conclusions cannot be drawn, as studies including larger populations with early time points are needed.
The high specificity in predicting type of injury at early stages after TBI is clinically significant, because GNR could provide a diagnosis when other methods (CT or MRI) are unavailable. Specifically, GNR can aid at the acute phase, to identify diffuse brain/neuronal injury when the initial CT scan reveals no traumatic lesions, and intracranial pressure is normal, despite the severely altered consciousness of the patient. Alternatively, GNR could be used as a screening method for classification of intracranial injury prior to imaging. Of note, if the assay is ported to a point-of-care (POC) system, the results of this test can be available in 30 min or less (Mondello et al., 2011a). Of note, GNR was the only independent predictor of type of injury. The predictive value of GNR was not affected by other factors, including patient (age and gender) and TBI characteristics (GCS score on admission and injury mechanism).
The ability of GNR to specifically identify and quantify the most seriously injured brain cell population might ultimately lead to more effective targeted interventions based on the characteristics of individual patients, as suggested by previous studies (Saatman et al., 2008). In addition, GNR provides insights into the pathophysiological processes of the underlying progressive injury cascade that may lead to better strategies for neuroprotection and reparative processes.
GNR did not reveal any obvious differences between subjects with or without episodes of hypoxia or hypotension in the first 24 h. We found that GNR was higher in patients with hypotension compared to those without hypotension, and lower in patients with hypoxia compared to normoxic subjects, suggesting that different damage patterns might be related to the mechanisms involved in secondary injury. These findings are consistent with those of previous studies of cells in culture, that found a greater susceptibility of neurons than astrocytes to ischemia-like conditions (Chen et al., 1993; Li et al., 1995). Furthermore, astroglial dysfunction might play a critical role in determining neuronal survival following acute hypoxic-ischemic injury, by compromising neuronal-glial interactions (Liu et al., 1999). Therefore a clear conclusion could not be drawn. GNR's relationships to different types of secondary insults warrant further evaluation in larger patient groups.
GNR was not an independent predictor of survival. This finding is attributable to the fact that GNR primarily reflects structural damage, not injury severity. The ratio represents the relationship between glial and neuronal damage, providing information on the cell population primarily injured. It is therefore conceivable that GNR does not indicate the magnitude of injury, which is more reliably monitored by absolute biomarker concentrations. Indeed, biomarker levels and GNR have a complementary significance. Specifically, initial biomarker concentrations assess the acute injury magnitude (staging of the injury), while GNR evaluates the characteristics of the injury (grading of the injury).
Some limitations of this study should be acknowledged. Because of the pathoanatomic significance of GNR, and serum biomarker concentrations below the limits of detection in healthy controls, a comparative reference group was not included in the study. Our study was performed only on patients with severe TBI. Therefore, the results cannot be generalized to patients with mild and moderate injuries. Future studies by our group will focus on patients with mild and moderate TBI. Furthermore, our study focused on analysis of GNR extrapolated from the initial blood samples, because our intention was to study the diagnostic value of this index on admission. Further studies are needed to evaluate this index longitudinally. Future studies should also address if GNR can predict the evolution of a lesion (Servadei et al., 2000). Furthermore, a high mortality rate (55.92%) was observed, presumably reflecting the fact that the patients were more severely injured, and subjects with no detectable serum biomarker concentrations on admission were excluded from the analysis. A similar mortality rate (49%) was previously reported in the full International Data Bank (Foulkes et al., 1991).
Finally, GNR does not evaluate other components such as diffuse axonal injury, an important and consistent feature of TBI (Gentleman et al., 1993; Povlishock, 1992), and microvascular damage. A panel of different biomarkers integrating this information might be a valuable and complementary addition to the GNR approach.
Conclusions
In the near future the care of the head-injured patient should be based on pathophysiology-driven, individualized treatment strategies. These interventions will rely on patient-specific data gained by a panel of different biomarkers, and sophisticated automated calculations, as well as results of traditional imaging. Such a holistic approach might lead to better diagnoses and more effective care of TBI patients.
Acknowledgments
We want to thank Dr. Paul Muizelaar of the University of California–Davis, and Dr. Richard Dutton of the Maryland Shock Trauma Center, for referring patients and for help collecting samples. We also acknowledge Dr. Komaromy as an independent CT reviewer. We are very grateful to the medical and nursing staff of the neurointensive care unit for their valuable cooperation.
This study was primarily sponsored by Department of Defense Award numbers DAMD17-03-1-0772 and DAMD17-03-1-0066, but we also acknowledge additional fund support from National Institutes of Health grants R01 NS049175-01, R01-NS052831-01, and R01 NS051431-01, Navy grant N00014-06-1-1029 (University of Florida), and Developing Competitiveness of Universities in the South Transdanubian Region grant SROP-4.2.1.B-10/2/KONV-2010-0002.
Author Disclosure Statement
Drs. Mondello, Buki, Bullock, Czeiter, and Barzo, are consultants of and received consulting fees from Banyan Biomarkers, Inc. Dr. Jeromin is an employee and received salary from Banyan Biomarkers, Inc. Drs. Wang and Hayes own stock and receive royalties and salaries from, and are officers of, Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research. Banyan Biomarkers, Inc. filed patent applications based upon the disclosure of this publication. Drs. Tortella, Kovacs, and Schmid report no disclosures.
This material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting the views of the Department of the Army or Department of Defense.
References
- Berger R.P. Pierce M.C. Wisniewski S.R. Adelson P.D. Clark R.S. Ruppel R.A. Kochanek P.M. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:E31. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Bratton S.L. Chestnut R.M. Ghajar J. McConnell Hammond F.F. Harris O.A. Hartl R. Manley G.T. Nemecek A. Newell D.W. Rosenthal G. Schouten J. Shutter L. Timmons S.D. Ullman J.S. Videtta W. Wilberger J.E. Wright D.W. J. Neurotrauma. 2007;24(Suppl. 1):S7–S13. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- Brophy G.M. Mondello S. Papa L. Robicsek S.A. Gabrielli A. Tepas J. Buki A. Robertson C. Tortella F.C. Hayes R.L. Wang K.K. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma. 2011;28:861–870. doi: 10.1089/neu.2010.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Chopp M. Schultz L. Bodzin G. Garcia J.H. Sequential neuronal and astrocytic changes after transient middle cerebral artery occlusion in the rat. J. Neurol. Sci. 1993;118:109–116. doi: 10.1016/0022-510x(93)90099-k. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Zhao J. Hergenroeder G. Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E.R. DeLong D.M. Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Foulkes A.M. Eisenberg M.H. Jane A.J. The Traumatic Coma Data Bank; design, methods and baseline characteristics. J. Neurosurg. 1991;75:S1–S15. [Google Scholar]
- Gentleman S.M. Nash M.J. Sweeting C.J. Graham D.I. Roberts G.W. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci. Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- Gong B. Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- Herrmann M. Vos P. Wunderlich M.T. de Bruijn C.H. Lamers K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- Jackson P. Thompson R.J. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J. Neurol. Sci. 1981;49:429–438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Lee D.R. Helps S.C. Gibbins I.L. Nilsson M. Sims N.R. Losses of NG2 and NeuN immunoreactivity but not astrocytic markers during early reperfusion following severe focal cerebral ischemia. Brain Res. 2003;989:221–230. doi: 10.1016/s0006-8993(03)03373-0. [DOI] [PubMed] [Google Scholar]
- Liu D. Smith C.L. Barone F.C. Ellison J.A. Lysko P.G. Li K. Simpson I.A. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res. Mol. Brain Res. 1999;7:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Li Y. Chopp M. Zhang Z.G. Zhang R.L. Expression of glial fibrillary acidic protein in areas of focal cerebral ischemia accompanies neuronal expression of 72-kDa heat shock protein. J. Neurol. Sci. 1995;128:134–142. doi: 10.1016/0022-510x(94)00228-g. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Marmarou A. Murray G.D. Teasdale S.G. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. Van Berkum Clark M. Eisenber H.M. Jane J.A. Luersse T.J. Marmarou A. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75:14–20. [Google Scholar]
- Mondello S. Muller U. Jeromin A. Streeter J. Hayes R.L. Wang K.K. Blood-based diagnostics of traumatic brain injuries. Expert Rev. Mol. Diagn. 2011a;11:65–78. doi: 10.1586/erm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S. Papa L. Buki A. Bullock R. Czeiter E. Tortella F. Wang K.K. Hayes R.L. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care. 2011b;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L. Akinyi L. Liu M.C. Pineda J.A. Tepas J.J., 3rd Oli M.W. Zheng W. Robinson G. Robicsek S.A. Gabrielli A. Heaton S.C. Hannay H.J. Demery J.A. Brophy G.M. Layon J. Robertson C.S. Hayes R.L. Wang K.K. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Traumatically induced axonal injury: Pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Pulsinelli W.A. Brierley J.B. Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Keller M. Döhnert J. Sorge O. Seifert V. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir. 1998;140:787–791. doi: 10.1007/s007010050180. [DOI] [PubMed] [Google Scholar]
- Rosengren L.E. Wikkelso C. Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J. Neurosci. Methods. 1994;51:197–204. doi: 10.1016/0165-0270(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servadei F. Murray G.D. Penny K. The value of the ‘worst’ computed tomographic scan in clinical studies of moderate and severe head injury. Neurosurgery. 2000;46:70–77. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Setsuie R. Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem. Int. 2007;51:105–111. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Tongaonkar P. Chen L. Lambertson D. Ko B. Madura K. Evidence for an interaction between ubiquitin-conjugating enzymes and the 26S proteasome. Mol. Cell Biol. 2000;20:4691–4698. doi: 10.1128/mcb.20.13.4691-4698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M. Magistretti P.J. Metabolic coupling between glia and neurons. J. Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P.E. Jacobs B. Andriessen T.M. Lamers K.J. Borm G.F. Beems T. Edwards M. Rosmalen C.F. Vissers J.L. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]