Abstract
Adequate activation of CD4+ T lymphocytes is essential for host defense against invading pathogens; however, exaggerated activity of effector CD4+ T cells induces tissue damage, leading to inflammatory disorders such as inflammatory bowel diseases. Several unique subsets of intestinal innate immune cells have been identified. However, the direct involvement of innate immune cell subsets in the suppression of T-cell-dependent intestinal inflammation is poorly understood. Here, we report that intestinal CX3C chemokine receptor 1high (CX3CR1high) CD11b+ CD11c+ cells are responsible for prevention of intestinal inflammation through inhibition of T-cell responses. These cells inhibit CD4+ T-cell proliferation in a cell contact-dependent manner and prevent T-cell-dependent colitis. The suppressive activity is abrogated in the absence of the IL-10/Stat3 pathway. These cells inhibit T-cell proliferation by two steps. Initially, CX3CR1high CD11b+ CD11c+ cells preferentially interact with T cells through highly expressed intercellular adhesion molecule-1/vascular cell adhesion molecule-1; then, they fail to activate T cells because of defective expression of CD80/CD86. The IL-10/Stat3 pathway mediates the reduction of CD80/CD86 expression. Transfer of wild-type CX3CR1high CD11b+ CD11c+ cells prevents development of colitis in myeloid-specific Stat3-deficient mice. Thus, these cells are regulatory myeloid cells that are responsible for maintaining intestinal homeostasis.
Keywords: mucosal immunology, innate immunity
Inflammatory bowel diseases (IBDs), represented by Crohn disease and ulcerative colitis in humans, result from genetic abnormalities, as well as uncontrolled intestinal immune responses toward commensal microflora and dietary antigens (1–3). Activation of appropriate mucosal immune responses is responsible for protection against pathogenic microorganisms, whereas excessive immune responses, especially unbalanced T-cell-mediated adaptive immune responses, to commensal microflora and dietary antigens lead to development of intestinal inflammation. Therefore, T-cell-mediated responses are tightly regulated to suppress aberrant inflammatory responses in the intestinal mucosa. Over the last few decades, CD4+ regulatory T (Treg) cells have been demonstrated to prevent T-cell-mediated chronic inflammatory diseases including IBDs (4, 5). Several possible mechanisms for this suppressive effect of Treg cells have been proposed. Regulatory dendritic cells have also been implicated in the immune tolerance by inducing Treg cells (6, 7). In tumor models, myeloid-derived suppressor cells (MDSCs) have been reported to suppress T-cell-mediated responses through several mechanisms (8, 9).
Several subsets of intestinal innate phagocytic cells have recently been identified that modulate intestinal homeostasis (10–12). In particular, CD103+ CX3CR1− CD11b− dendritic cells (DCs) and CX3CR1+ CD11b+ DCs have been well characterized (13–15). CD103+ CX3CR1− CD11b− DCs have been shown to generate and activate gut-tropic CD8+ T cells (16, 17). These DCs have further been shown to induce development of Foxp3+ Treg cells (18–20). CX3CR1+ CD11b+ DCs have been shown to mediate inflammatory responses through the induction of Th1 and Th17 cell development (15, 21–24). In addition to these cell populations, CD103+ CX3CR1− CD11b+ cells and CD11b+CD11c− macrophages have been identified in the intestinal lamina propria (13, 14, 22, 25). Other intestinal myeloid cell populations inducing Treg cells have also been characterized (26–28). However, it remains unclear whether cell populations other than Treg cells directly contribute to the suppression of inflammatory responses.
In this study, we characterized intestinal CX3C chemokine receptor 1high (CX3CR1high) CD11b+ CD11c+ cells, which show a cell contact-dependent suppression of T-cell proliferation, leading to prevention of intestinal inflammation.
Results
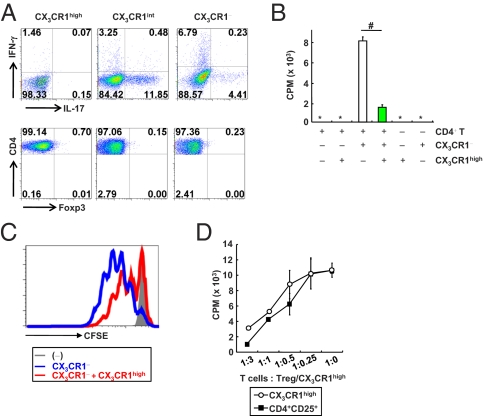
Intestinal CX3CR1high CD11b+ CD11c+ Cells Suppress T-Cell Growth.
Several unique subsets of innate immune cells in the intestinal lamina propria have been identified (12–14, 16–26, 28–30). Among these subsets, CD103+ CX3CR1− CD11b− CD11c+ cells and CX3CR1+ CD11b+ CD11c+ cells have been reported to be major subsets in the intestine (13, 14). The functions of CX3CR1+ CD11b+ CD11c+ cells have been characterized in several aspects (13, 14, 17). However, the CD11b+ CD11c+ cell population could be divided into three subsets based on the expression level of CX3CR1: CX3CR1high, CX3CR1intermediate (int), and CX3CR1negative (−) cells (Fig. S1A) (13, 17). Although previous studies have indicated the presence of CX3CR1high and CX3CR1int cells, differential functions of these cell subsets in T-cell differentiation have not been characterized (13, 17). Therefore, we examined the effects of three subsets on induction of Th1, Th17, and Treg cells (Fig. 1A). CX3CR1high, CX3CR1int, and CX3CR1− cells were isolated from the colonic lamina propria and cultured with splenic naïve CD4+ T cells for 4 d. CD4+ T cells cocultured with CX3CR1int or CX3CR1− cells predominantly produced IL-17 or IFN-γ, respectively. In contrast, expression of IFN-γ, IL-17, or Foxp3 was not induced in CD4+ T cells cocultured with the CX3CR1high cells. Next, we examined the effects on T-cell proliferation. CD4+ T cells were cocultured with the CX3CR1high, CX3CR1int, or CX3CR1− cells for 72 h, and their proliferation was analyzed by assessing incorporation of [3H]thymidine (Fig. S1B). CD4+ T cells cocultured with CX3CR1− and CX3CR1int cells showed robust proliferative responses, indicating that the CX3CR1− and CX3CR1int cells had similar properties as DCs in enhancing T-cell responses. In contrast, CD4+ T cells cocultured with CX3CR1high cells did not show any enhanced proliferation. These findings indicate that CX3CR1high cells are not typical DCs. Indeed, CX3CR1high cells express several macrophage-related molecules (CD14, CD68, and F4/80), as well as DC-related molecules (CD11c and DEC205) (Fig. S1C). In addition, CX3CR1high cells contain cytoplasmic vacuolar structures characteristic of macrophages (Fig. S1D) (14). We further found that the addition of CX3CR1high cells into a coculture of CD4+ T cells and CX3CR1− DCs profoundly reduced T-cell proliferation (Fig. 1B). The suppression of T-cell proliferation by the CX3CR1high cells was further confirmed by reduced dilution of the fluorescence intensity of CD4+ T cells labeled with carboxyfluorescein succinimidyl ester (CFSE) (Fig. 1C). We then compared the suppressive ability of CX3CR1high cells on T-cell proliferation with that of Treg cells. CD4+ T cells were cultured with DCs and anti-CD3 mAb in the presence of various numbers of Treg cells or CX3CR1high cells (Fig. 1D). The CX3CR1high cells showed a dose-dependent suppression of T-cell proliferation in a very similar manner to that induced by Treg cells. CX3CR1high cells isolated from CX3CR1+/GFP mice, in which CX3CR1 Ab staining was well correlated with GFP expression (Fig. S1E), also inhibited T-cell proliferation (Fig. S1F). CX3CR1high cells were not present in the CD11b+ CD11c+ population in the spleen, mesenteric lymph nodes (MLNs), or thymus (Fig. S1G). Collectively, our in vitro analyses suggested that a CX3CR1high CD11b+ CD11c+ subset of intestinal myeloid cells inhibited T-cell proliferation independently of Treg induction.
Fig. 1.
CX3CR1high CD11b+ CD11c+ cells in the intestinal lamina propria suppress T-cell proliferation. (A) Flow cytometric plots of IL-17-, IFN-γ-, or Foxp3-expressing CD4+ T cells cocultured with the indicated cells for 72 h. (B) [3H]thymidine uptake of CD4+ T cells cultured with the indicated cells. #P < 0.022. (C) The fluorescence intensity of CFSE-labeled CD4+ T cells cultured with the indicated cells at a ratio 1:1:1 for 72 h. (D) [3H]thymidine uptake by CD4+ T cells cocultured with CX3CR1− DCs in the presence of increasing ratios of splenic CD4+ CD25+ Treg cells (closed rectangle) or colonic CX3CR1high cells (open circle). All data are representative of two independent experiments (means ± SD of duplicate well measurements).
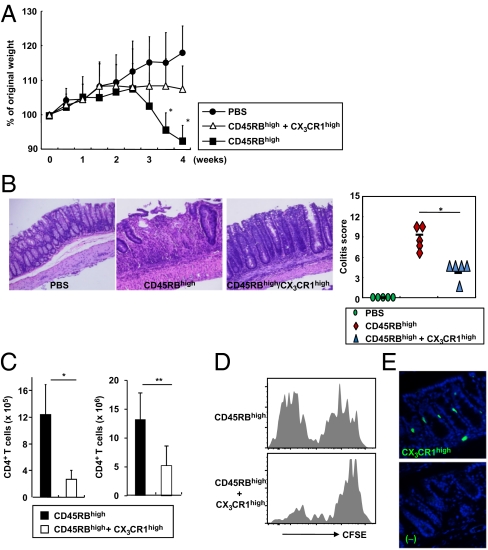
CX3CR1high Myeloid Cells Prevent Intestinal Inflammation.
We next assessed the in vivo function of the CX3CR1high subset of intestinal myeloid cells using a T-cell-dependent colitis model. Severe combined immunodeficiency (SCID) mice given CD45RBhigh CD4+ T cells showed severe weight loss with severe intestinal pathology (Fig. 2 A and B). Cotransfer of CX3CR1high cells dramatically reduced their weight loss and the severity of intestinal inflammation. The cotransfer of CX3CR1high cells did not induce any change in the frequency of IL-17-, IFN-γ-, IL-4-, or IL-10-producing CD4+ T cells or in the frequency of Foxp3-expressing CD4+ T cells in the colonic lamina propria (Fig. S2). However, the total number of CD4+ T cells in the lamina propria was markedly reduced by CX3CR1high cell coadministration (Fig. 2C). Assessment of CFSE dilution in transferred CD45RBhigh CD4+ T cells demonstrated robust T-cell proliferation in the colonic lamina propria of Rag2−/− mice. However, cotransfer of CX3CR1high cells substantially inhibited CFSE dilution in transferred T cells (Fig. 2D). Transferred CX3CR1high cells were observed just beneath the epithelial cell layers of the intestine but not in MLNs or spleen (Fig. 2E and Fig. S3 A and B). Transferred CX3CR1high cells were in close proximity to T cells in the lamina propria (Fig. S3C). In addition, the number of CD4+ T cells was reduced where CX3CR1high cells were present (Fig. S3 C and D). Total number of CX3CR1high cells increased in the colonic lamina propria of the transferred mice (Fig. S4). Thus, the CX3CR1high subset of intestinal myeloid cells suppresses T-cell proliferation in the intestinal lamina propria, thereby preventing intestinal inflammation; hereafter, we call this subset CX3CR1high regulatory myeloid (Mreg) cells.
Fig. 2.
CX3CR1high myeloid cells alleviate T-cell-dependent intestinal inflammation. (A) SCID mice were injected i.p. with 3 × 105 CD45RBhigh CD4+ T cells or PBS (closed circles). After 2 h, 3 × 105 CX3CR1high cells were transferred (open triangles) or not (closed rectangles). Body weight change was monitored and is presented relative to initial body weight. *P < 0.005 (n = 8 per group). (B) Hematoxylin and eosin staining of colon sections at 4 wk after the transfer described in A (Left) and the colitis score (Right). *P < 0.0012. (Original magnification, 200×.) (C) Numbers of large intestinal lamina propria CD4+ T cells at 2 wk (n = 4 per group) (Left) and 4 wk (n = 5 per group) (Right) after transfer. *P < 0.02; **P < 0.015. (D) CD45RBhigh T cells (3 × 105) were labeled with CFSE and transferred into Rag2−/− mice with or without 3 × 105 CX3CR1high cells. After 12 d, CFSE dilution in colonic CD4+ T cells was analyzed. (E) Cryosection of the colon from a SCID mouse at 3 d after i.v. injection of CFSE-labeled CX3CR1high cells. (Original magnification, 200×.) Data are representative of three independent experiments (D and E).
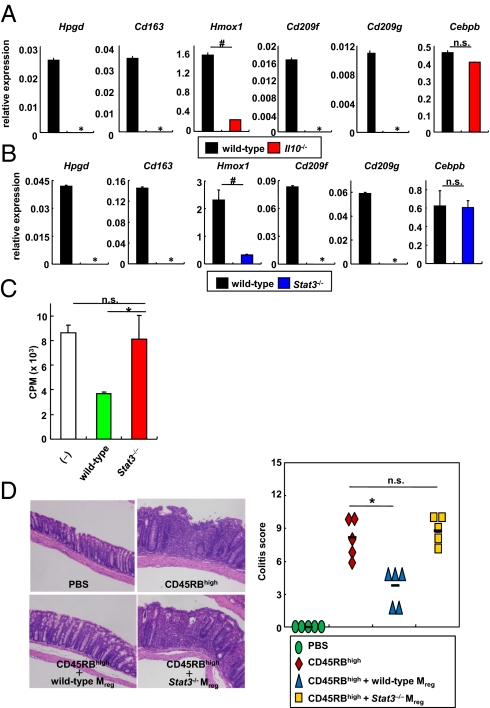
IL-10/Stat3-Dependent Suppressive Ability of CX3CR1high Mreg Cells.
To determine how CX3CR1high Mreg cells exert their immunosuppressive function, we performed a comprehensive analysis of gene expression profiles in CX3CR1high Mreg cells. CX3CR1high Mreg cells expressed several IL-10-inducible genes such as Hpgd, Cd163, Hmox1, Cd209f, and Cd209g (Fig. S5A). Hence, we analyzed CX3CR1high Mreg cells in Il10−/− mice. Because normal numbers of CX3CR1high Mreg cells were observed in the colon of Il10−/− mice (Fig. S5B), we isolated these cells and analyzed the expression of the genes that were selectively expressed in wild-type CX3CR1high Mreg cells. Expression of Hpgd, Cd163, Hmox1, Cd209f, and Cd209g was severely decreased in Il10−/− cells despite normal expression of the myeloid cell-related gene Cebpb (Fig. 3A). CX3CR1high Mreg cells from LysM-cre; Stat3fl/fl mice (Stat3−/− CX3CR1high Mreg cells) also showed profoundly decreased levels of expression of these genes (Fig. 3B). To evaluate whether Stat3−/− CX3CR1high Mreg cells suppress the T-cell proliferative response, wild-type or Stat3−/− CX3CR1high Mreg cells were added to coculture of CD4+ T cells with wild-type DCs (Fig. 3C). Stat3−/− CX3CR1high Mreg cells were not able to suppress T-cell proliferation. Il10−/− CX3CR1high Mreg cells were also defective in their suppression of T-cell proliferation (Fig. 4E). Furthermore, Stat3−/− CX3CR1high Mreg cells showed impaired prevention of intestinal inflammation in Rag2−/− mice given CD45RBhigh CD4+ T cells (Fig. 3D). Thus, the suppressive function of CX3CR1high Mreg cells in vitro and in vivo was impaired in the absence of IL-10/Stat3 signaling.
Fig. 3.
Defective activity of Stat3−/− Mreg cells. (A and B) Expression of Hpgd, Cd163, Hmox1, CD209f, CD209g, and Cebpb mRNA in CX3CR1high Mreg cells from wild-type, Il10−/−, and LysM-cre; Stat3fl/fl mice. Data are presentative of two independent experiments (means ± SD of at least triplicate PCRs on the identical sample). *, not detected; #P < 0.025. (C) [3H]thymidine uptake by CD4+ T cells cultured with CX3CR1− DCs in the presence of wild-type or Stat3−/− CX3CR1high Mreg cells. Data are representative of four independent experiments (means ± SD of triplicate well measurements). *P < 0.047. (D) Hematoxylin and eosin staining of colon sections of Rag2−/− mice given 3 × 105 CD45RBhigh CD4+ T cells with 3 × 105 Mreg cells from wild-type or LysM-cre; Stat3fl/fl mice (Left) and colitis score (Right). (Original magnification, 200×.) *P < 0.025 (n = 5 per group).
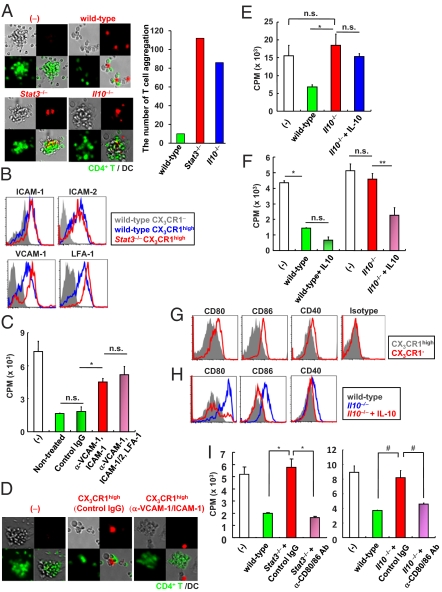
Fig. 4.
Two-step mechanism for suppression of T-cell growth by Mreg cells. (A) Green dye-labeled CD4+ T cells were cultured with nonlabeled CX3CR1− DCs and red dye-labeled Mreg cells from wild-type, LysM-cre; Stat3fl/fl, or Il10−/− mice (Left). The number of T-cell aggregation in twenty fields (Right). (Original magnification, 100×.) (B) Expression of adhesion molecules on the indicated cells from wild-type and LysM-cre; Stat3fl/fl mice. (C) CD4+ T cells and wild-type CX3CR1− DCs were cocultured with Mreg cells pretreated with the indicated blocking Abs or control Ig for evaluation of T-cell proliferation. *P < 0.012. (D) Red dye-labeled CX3CR1high Mreg cells were treated with blocking Abs to ICAM-1 and VCAM-1 then added to the mixture of green dye-labeled CD4+ T cells and nonstained CX3CR1− DCs. (Original magnification, 100×.) (E) IL-10 (100 ng/mL) was added to the coculture of CD4+ T cells, wild-type DCs, and Il10−/− Mreg cells. Then, T-cell proliferation was measured. *P < 0.02. (F) Wild-type and Il10−/− Mreg cells were preincubated with or without 100 ng/mL IL-10 for 72 h. Then, the cells were analyzed for the suppressive activity of T-cell proliferation. *P < 0.016; **P < 0.034. (G) Surface expression of CD80, CD86, CD40, and MHC class II on CX3CR1high Mreg cells and CX3CR1− DCs. (H) Expression of CD80, CD86, and CD40 on Mreg cells from wild-type and Il10−/− mice cultured for 48 h with or without 100 ng/mL IL-10. (I) Stat3−/− and Il10−/− Mreg cells were pretreated with the indicated blocking Abs. Then, Mreg cells were cultured with CD4+ T cells and wild-type CX3CR1− DCs, and T-cell proliferation was measured. *P < 0.025; #P < 0.045. All data are representative of at least two independent experiments (mean values ± SD of triplicate well measurements).
CX3CR1high Mreg Cells Inhibit T-Cell Growth by Two Steps.
We then assessed the mechanism underlying the suppression of T-cell proliferation by CX3CR1high Mreg cells. Because MDSCs have been reported to suppress T-cell response through arginase-1, inducible NOS, and reactive oxygen species (ROS) (8, 9), we analyzed the effects of inhibitors of these mediators. However, the inhibitors did not cancel the suppressive activity of CX3CR1high Mreg cells (Fig. S6 A–E). Expression of indoleamine 2, 3-dioxygenase (IDO) in several regulatory DCs inhibits T-cell responses (6, 20). However, T-cell proliferation was not increased by addition of an IDO inhibitor (Fig. S6F). Moreover, CX3CR1high Mreg cells did not express Treg-related genes such as Foxp3, Ctla4, and Folr4 (Fig. S6G). Thus, CX3CR1high Mreg cells possess distinct mechanisms from those used by those cells previously shown to inhibit T-cell proliferation. Coculture of CX3CR1high Mreg cells with CD4+ T cells in transwell plates did not suppress T-cell proliferation, indicating that cell–cell contact is required for suppression (Fig. S6H). Addition of CX3CR1high Mreg cells to cocultures of CD4+ T cells and DCs substantially decreased T-cell aggregation around DCs; instead, T cells preferentially associated with CX3CR1high Mreg cells (Fig. 4A). Inhibition of T-cell aggregation was not observed in Stat3−/− and Il10−/− CX3CR1high Mreg cells. Thus, CX3CR1high Mreg cells had a higher affinity to interact with T cells than DCs and, thereby, suppressed T-cell responses. These findings prompted us to investigate the expression of adhesion molecules that are involved in DC–T-cell interactions. Surface expression of intercellular adhesion molecule (ICAM)-1, ICAM-2, lymphocyte function-associated antigen (LFA)-1, and vascular cell adhesion molecule (VCAM)-1 was considerably higher in CX3CR1high Mreg cells than in CX3CR1− DCs (Fig. 4B). Therefore, we analyzed whether these adhesion molecules are involved in the CX3CR1high Mreg suppressive activity. Treatment of CX3CR1high Mreg cells with blocking mAbs to ICAM-1, ICAM-2, LFA-1, and VCAM-1 canceled the CX3CR1high Mreg suppressive activity on T-cell proliferation (Fig. 4C). Treatment of CX3CR1high Mreg cells with each mAb did not abrogate the suppressive activity (Fig. S7A), but the combination of ICAM-1 and VCAM-1 mAbs substantially induced T-cell proliferation. In addition, treatment of CX3CR1high Mreg cells with mAbs to ICAM-1 and VCAM-1 resulted in increased aggregation of CD4+ T cells around DCs (Fig. 4D). These results indicate that an ICAM-1/VCAM-1-mediated interaction is required for suppression. Expression of ICAM-1 and VCAM-1 was high in Stat3−/− and Il10−/− CX3CR1high Mreg cells, which showed impaired suppressive activity (Fig. 4B and Fig. S7B). In addition, T cells aggregated around Stat3−/− and Il10−/− CX3CR1high Mreg cells (Fig. 4A). Therefore, we analyzed how CX3CR1high Mreg cells with high affinity for T cells show IL-10-dependent suppression of T-cell proliferation. CX3CR1high Mreg cells produced IL-10 constitutively (Fig. S8 A and B). However, supplementation of exogenous IL-10 into cocultures of CD4+ T cells and Il10−/− CX3CR1high Mreg cells did not induce the reduction of T-cell proliferative responses (Fig. 4E). In addition, the suppressive activity of wild-type CX3CR1high Mreg cells was not blocked in the presence of neutralizing Abs to IL-10 and the IL-10 receptor (Fig. S8C). Thus, IL-10 is not directly involved in the suppression of T-cell proliferation. However, IL-10 pretreatment of Il10−/−, but not Stat3−/−, CX3CR1high Mreg cells before coculture led to a substantial reduction of T-cell proliferative responses, indicating that IL-10 provides a key signal for CX3CR1high Mreg cells to acquire suppressive activity (Fig. 4F and Fig. S8D). Expression of molecules that transduce coinhibitory signals toward T cells, including B7-H4, herpesvirus entry mediator (HVEM), programmed death ligand (PD-L)1, and PD-L2 was increased in CX3CR1high Mreg cells compared with CX3CR1− DCs (Fig. S8E). However, expression of these coinhibitory molecules remained high in Stat3−/− CX3CR1high Mreg cells. Furthermore, the possible involvement of these inhibitory molecules in the suppressive activity of CX3CR1high Mreg cells was ruled out in experiments using neutralizing Abs and knockout mice (Fig. S8 F–H). In contrast, expression of CD80 and CD86 was severely decreased in CX3CR1high Mreg cells compared with that in CX3CR1− DCs, although MHC class II was equally and highly expressed in both populations (Fig. 4G and Fig. S1C). Notably, expression of CD80 and CD86 was considerably higher in Il10−/− and Stat3−/− CX3CR1high Mreg cells than in wild-type CX3CR1high Mreg cells (Fig. 4H and Fig. S9A). Furthermore, IL-10 treatment of Il10−/− CX3CR1high Mreg cells decreased the expression of CD80 and CD86 (Fig. 4H). Therefore, we suspected that CX3CR1high Mreg cells with decreased expression of costimulatory molecules compete with DCs to suppress T-cell proliferation. To test this, Stat3−/− and Il10−/− CX3CR1high Mreg cells were pretreated with blocking mAbs to CD80 and CD86, and then cocultured with CD4+ T cells (Fig. 4I). Stat3−/− and Il10−/− CX3CR1high Mreg cells pretreated with mAbs to CD80 and CD86 suppressed T-cell proliferation. These findings clearly indicate that CX3CR1high Mreg cells suppress T-cell responses via a two-step mechanism: CX3CR1high Mreg cells interact with T cells with high affinity through high expression of adhesion molecules and then show IL-10-dependent suppression of CD80/CD86-mediated costimulatory signals, leading to inhibition of T-cell proliferation.
Defective CX3CR1high Mreg Function Results in Development of Colitis.
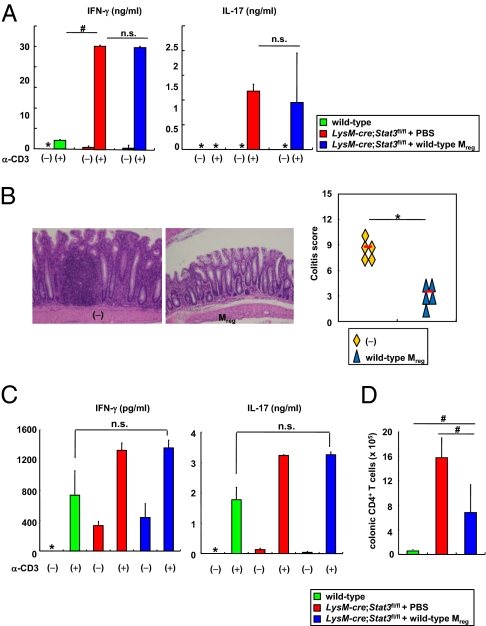
The aberrant Th1/Th17-mediated responses attributable to enhanced DC activity have been considered to induce intestinal inflammation in Il10−/− mice and innate immune cell-specific Stat3 mutant mice (31–34). Because the function of CX3CR1high Mreg cells was impaired in the absence of IL-10/Stat3, we assessed the involvement of CX3CR1high Mreg cells in the pathogenesis of intestinal inflammation in LysM-cre; Stat3fl/fl mice. LysM-cre; Stat3fl/fl mice were transferred with wild-type CX3CR1high Mreg cells and were analyzed for Th1 and Th17 activities. In LysM-cre; Stat3fl/fl mice, Th1 and Th17 activities, as determined by IFN-γ and IL-17A production from splenic CD4+ T cells, respectively, were increased (Fig. 5A). Even in LysM-cre; Stat3fl/fl mice transferred with wild-type CX3CR1high Mreg cells, Th1 and Th17 activity remained considerably enhanced. However, the severity of intestinal inflammation was greatly improved in LysM-cre; Stat3fl/fl mice transferred with wild-type CX3CR1high Mreg cells (Fig. 5B). CD4+ T cells in the colonic lamina propria produced increased amounts of IFN-γ and IL-17A even in LysM-cre; Stat3fl/fl mice transferred with wild-type CX3CR1high Mreg cells; however, the total number of CD4+ T cells was markedly decreased in the lamina propria (Fig. 5 C and D). These findings demonstrate that the defective activity of CX3CR1high Mreg cells is critically involved in the development of spontaneous colitis when T cells are overactivated and that transfer of normal CX3CR1high Mreg cells is able to ameliorate the colitis.
Fig. 5.
Defective Mreg cell function leads to development of colitis. (A, C, and D) LysM-cre; Stat3fl/fl mice were transferred i.p. with 7 × 104 wild-type CX3CR1high Mreg cells at 4 and 6 wk of age. At 2 wk after the last transfer, splenic (A) or colonic lamina propria (C) CD4+ T cells were analyzed for production of IFN-γ and IL-17A. *, not detected; #P < 0.008. Total number of CD4+ T cells in the colonic lamina propria was analyzed (D). #P < 0.0079. Data are from two independent experiments with four mice per group. (B) Hematoxylin and eosin staining of colon sections at 2 wk after the last transfer (Left) and the colitis score (Right). (Original magnification, 200×.) *P < 0.0002.
Discussion
In the present study, we characterized the intestinal CX3CR1high CD11b+ CD11c+ Mreg cell subset, which directly inhibits T-cell proliferation and, thereby, prevents T-cell-dependent intestinal inflammation. The IL-10/Stat3 pathway is critically involved in the suppressive activity of CX3CR1high Mreg cells.
To date, CD103+ CX3CR1− DCs and CX3CR1+ CD11b+ CD11c+ cells have been identified as major DC subsets in the intestine. Previous studies indicated that intestinal CX3CR1+ CD11b+ CD11c+ cells are further divided into two subsets based on the expression level of CX3CR1, but these studies did not analyze differential functions of CX3CR1high and CX3CR1int cells (13, 17). This study clearly demonstrates that CX3CR1high CD11b+ CD11c+ cells are a regulatory myeloid cell subset possessing unique functions that directly suppress T-cell proliferation. Previous studies indicated that CX3CR1+ CD11b+ CD11c+ cells mediate inflammatory responses such as Th17 cell induction (21–23). In this regard, when splenic naïve CD4+ T cells were cocultured with unsorted CX3CR1+ CD11b+ CD11c+ cells, including CX3CR1high and CX3CR1int cells, T cells did not vigorously proliferate, although they produced IL-17. In contrast, splenic naïve CD4+ T cells cocultured with CX3CR1int subset robustly proliferated and produced higher amounts of IL-17 compared with T cells cocultured with unsorted CX3CR1+ CD11b+ CD11c+ cells (Fig. S9B). These observations would be attributable to suppression of T-cell proliferation by CX3CR1high Mreg cells present within the CX3CR1+ CD11b+ CD11c+ cell population. Thus, CX3CR1high and CX3CR1int cells are shown to possess distinct functions suppressing and activating T cells, respectively. At present, these functionally distinct populations can only be separated by expression level of CX3CR1, indicating that both populations are related. It is possible that CX3CR1int cells are precursors of CX3CR1high cells, and both cell populations show plasticity. Several previous reports have shown that CX3CR1+ cells have macrophage-like properties (14, 15, 35, 36). Indeed, CX3CR1high Mreg cells show macrophage-like morphology and express macrophage-related surface markers. On the other hand, we found that Stat3−/− and Il10−/− CX3CR1high Mreg cells induced T-cell proliferation, indicating that CX3CR1high Mreg cells show DC-like properties in the absence of the IL-10/Stat3 signaling in vitro (Fig. S9C). Therefore, it is possible that CX3CR1int cells are precursors of CX3CR1high cells and terminally differentiate into CX3CR1high Mreg cells sharing some macrophage-like properties in response to IL-10.
Several regulatory subsets of myeloid cells, which might be related to Mreg cells, have been reported. Colonic IL-10-producing F4/80+ CD11b+ myeloid cells have been reported to mediate the maintenance of Foxp3 expression in Treg cells (26). This subset is observed in MLNs, where Mreg cells are not present. Intestinal macrophages suppress T-cell responses via IL-10-dependent induction of Treg cells (22). Unlike Mreg cells, these macrophages do not express CD11c, CD14, or DEC205. Most recently, the activity of intestinal macrophages has been shown to be regulated by CX3CR1 (37). Indeed, mice deficient in CX3CR1 were highly sensitive to intestinal inflammation induced by dextran sodium sulfate. These intestinal cells might include CX3CR1high Mreg cells. It is interesting to analyze the suppressive activity of CX3CR1high Mreg cells in CX3CR1-deficient mice. MDSCs are also the cell population similar to CX3CR1high Mreg cells. However, MDSCs do not express CD11c or MHC class II and are hardly detected in healthy mice (9, 38), whereas CX3CR1high Mreg cells express CD11c and MHC class II and are abundant in the intestinal lamina propria of healthy mice. Expression of CX3CR1high Mreg cell-related genes (Hpgd, Cd163, Hmox1, Cd209f, and Cd209g) was severely reduced in MDSCs, whereas MDSC-related genes (Arg1, Nos2, Cybb, S100a8, and S100a9) were not expressed in CX3CR1high Mreg cells (Fig. S9 D and E).
CD103+ CX3CR1− DCs have been shown to promote intestinal immune tolerance through the generation of Foxp3+ Treg cells (15, 16, 18, 19). Intestinal macrophages are also reported to induce Foxp3+ Treg cells (22). Intestinal epithelial cells have been implicated in promoting differentiation of CD103+ DCs possessing a property to induce Treg cells (39, 40). CX3CR1high Mreg cells localize very close to intestinal epithelial cells. Therefore, intestinal epithelial cells might be involved in the final maturation (or differentiation) of CX3CR1high Mreg cells in the intestinal lamina propria through modulation of IL-10 production.
In the present study, we characterize intestinal CX3CR1high CD11b+ CD11c+ cells (CX3CR1high Mreg cells) that suppress intestinal inflammation through direct inhibition of T-cell proliferation in the intestinal lamina propria. Treg cells with a normal suppressive activity are abundantly present in LysM-cre/Stat3f/f mice (33), indicating that defective activity of CX3CR1high Mreg cells can cause intestinal inflammation even in the presence of Treg cells. Therefore, CX3CR1high Mreg cells maintain the intestinal homeostasis together with Treg cells, as well as several innate cell subsets that have regulatory functions. Identification of an CX3CR1high Mreg population in the human intestines and characterization of human CX3CR1high Mreg function in patients with IBD will be a critical future issue in establishing their role in the pathogenesis of intestinal inflammation in humans.
Materials and Methods
Mice.
C57BL/6J mice and BALB/c mice at 6–8 wk of age were purchased from CLEA Japan or Japan SLC. Male 6-wk-old CB17-SCID mice were purchased from CLEA Japan. LysM-cre; Stat3fl/fl mice and CX3CR1-EGFP knock-in (heterozygous) mice were generated as described (32, 41, 42). Il10−/− mice were purchased from The Jackson Laboratory. Each mutant mouse strain was backcrossed onto a C57BL/6J background for at least five generations. All animal experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Osaka University.
The details of reagents, isolation of lamina propria cells, histopathological score, and proliferation assay are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Sakaguchi and T. Hirano for fruitful discussions; C. Hidaka for secretarial assistance; E. Morii for histological analysis; J. Kikuta and E. Ohata for microscopy analysis; and K. Atarashi, D. Dodd, and Y. Magota for technical assistance. This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology; the Ministry of Health, Labour and Welfare; The Kato Memorial Trust for Nambyo Research; the Osaka Foundation for the Promotion of Clinical Immunology; and the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114931109/-/DCSupplemental.
References
- 1.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: How do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 6.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 7.Wakkach A, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laffont S, Powrie F. Immunology: Dendritic-cell genealogy. Nature. 2009;462:732–733. doi: 10.1038/462732a. [DOI] [PubMed] [Google Scholar]
- 11.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 16.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matteoli G, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 21.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 22.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 23.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 24.Manocha M, et al. Blocking CD27-CD70 costimulatory pathway suppresses experimental colitis. J Immunol. 2009;183:270–276. doi: 10.4049/jimmunol.0802424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda Y, et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22:953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 26.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada Y, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 30.Jung S. Dendritic cells: A question of upbringing. Immunity. 2010;32:502–504. doi: 10.1016/j.immuni.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melillo JA, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol. 2010;184:2638–2645. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niess JH. What are CX3CR1+ mononuclear cells in the intestinal mucosa? Gut Microbes. 2010;1:396–400. doi: 10.4161/gmic.1.6.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: Master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Medina-Contreras O, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 40.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 41.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.