Background: Brd4 plays a central role in cellular growth control and cancer development.
Results: Brd4 depletion leads to chromatin decondensation, while dissociation of Brd4 from chromatin triggers severely fragmented chromatin morphology.
Conclusion: Brd4 is crucial for maintaining normal chromatin structure.
Significance: The mechanistic insight into Brd4 function will contribute to understanding how perturbing this bromodomain protein function can lead to oncogenic progression.
Keywords: Chromatin Histone Modification, Chromatin Remodeling, Tumor Cell Biology, Tumor Suppressor Gene, Tumor Therapy, Brd4, Bromodomain, Chromatin Fragmentation, Chromatin Structure, Nucleosome Assembly
Abstract
Chromatin structure organization is crucial for regulating many fundamental cellular processes. However, the molecular mechanism that regulates the assembly of higher-order chromatin structure remains poorly understood. In this study, we demonstrate that Brd4 (bromodomain-containing protein 4) protein participates in the maintenance of the higher-order chromatin structure. Brd4, a member of the BET family of proteins, has been shown to play important roles in cellular growth control, cell cycle progression, and cancer development. We apply in situ single cell chromatin imaging and micrococcal nuclease (MNase) assay to show that Brd4 depletion leads to a large scale chromatin unfolding. A dominant-negative inhibitor encoding the double bromodomains (BDI/II) of Brd4 can competitively dissociate endogenous Brd4 from chromatin to trigger severely fragmented chromatin morphology. Mechanistic studies using Brd4 truncation mutants reveal that the Brd4 C-terminal domain is crucial for maintaining normal chromatin structure. Using bimolecular fluorescence complementation technology, we demonstrate that Brd4 molecules interact intermolecularly on chromatin and that replacing Brd4 molecules by BDI/II causes abnormal nucleosome aggregation and chromatin fragmentation. These studies establish a novel structural role of Brd4 in supporting the higher chromatin architecture.
Introduction
Chromatin structure plays key roles in chromosomal functional events such as transcription, DNA replication, repair, and recombination. Mutations in genes encoding enzymes that modulate chromatin structure can result in perturbations of these important cellular processes and have been linked to many human genetic diseases (1). Aberrant chromatin structure is also manifested in cancer (2). Consequently, different processes involved in chromatin assembly and remodeling have become the potential drug targets for diseases such as cancer, neurodegenerative diseases, AIDS etc. A more complete understanding of the molecular mechanisms underlying the regulation of the chromatin structure is therefore vital for developing more rational drug therapies for these diseases.
Nucleosomes are the basic unit of chromatin, consisting of ∼147 base pairs of DNA wrapped around a histone octamer containing two copies each of the core histones H2A, H2B, H3, and H4 (3). Neighboring nucleosomes are connected through a 10–50-base pair linker DNA to form a 10-nm “beads on a string” array. Nucleosome-nucleosome interaction drives the folding of a nucleosomal array into the 30-nm chromatin fiber and eventually into large scale structure (4). Besides compaction into chromosomes during mitosis, the chromatin fiber is organized, either in euchromatin that contains actively transcribed genes, or in heterochromatin that contains inactive genes (5). The folding of chromatin into a 30-nm fiber in vitro has been revealed by x-ray crystallography and electron microscopy studies (6–8). However, it is still unknown how the nucleosome fibers compact into condensed chromatin within a cell. Although a number of contributors to chromatin dynamics are known, their functional relationships have not been mechanistically defined. The mechanisms involved in higher-order chromatin architecture assembly and maintenance have remained elusive.
Reversible post-translational modifications of the N-terminal tails of histones are crucial for regulating the higher-order states of chromatin compaction (9). The bromodomain is an evolutionarily conserved motif present in many chromatin-associated proteins and histone acetyltransferases (10). It is the only known protein module capable of specifically binding to acetylated lysines at the histone tails (11). Structural studies have established the bromodomain/acetyl-lysine recognition as a pivotal mechanism for regulating protein-protein interactions in histone-directed chromatin remodeling (11). The crystal structure of the nucleosome core particle reveals that the basic N-terminal tail of histone H4 plays an important role in chromatin compaction (6), suggesting that bromodomain proteins binding to the acetylated H4 tail may be directly involved in this process.
Brd4 is a member of the BET family of proteins that harbor two bromodomains and an extra terminal domain (12). It is normally expressed as a long isoform (Brd4L, aa 1–1362)4 and a short isoform (Brd4S, aa 1–722). Brd4 binds to acetylated histones through its double bromodomains and becomes associated with both interphase chromatin and mitotic chromosomes (13). Brd4 knock-out in mice is embryonic lethal (14). This is consistent with its role in host cellular growth control and cell cycle progression (12, 15–18). Previous works from our group and others have identified Brd4 as an important target for a number of oncogenic viruses (19–23). The human Brd4 gene is also the target of translocation t(15;19) that defines a highly lethal carcinoma (24). Brd4 activation in human breast carcinomas induces a gene expression signature that efficiently predicts survival (25). It has also been identified as a novel target required for maintenance of acute myeloid leukemia (26). Most of the Brd4 functions have been linked to its role in transcription regulation (27–29). However, in our study, we observed that Brd4 depletion leads to enlarged nuclei indicating chromatin decondensation. Because the closely related Brd2 protein has been shown to possess a histone chaperone activity (30) and another bromodomain protein BrdT can bind hyperacetylated histone tail to induce a large scale chromatin reorganization (31), we decided to further examine how Brd4 binding to acetylated histones affects the chromatin structure.
In this study, we use micrococcal nuclease (MNase) assay and in situ chromatin imaging to show that Brd4 knockdown in human cells leads to highly decondensed chromatin structure. A mutant encoding the double bromodomains of Brd4 functions in a dominant-negative manner to dissociate Brd4L from chromatin and to cause a highly fragmented chromatin structure. Mechanistic studies demonstrate that the Brd4 CTD is crucial for maintaining normal chromatin structure. Bimolecular fluorescence complementation (BiFC) reveals that Brd4 molecules interact intermolecularly on chromatin through the N terminus and that replacing Brd4 molecules with the double-bromodomain mutant lacking the CTD causes abnormal chromatin fragmentation. Our study therefore provides mechanistic evidence to support a novel structural role of Brd4 in higher-order chromatin structure organization.
EXPERIMENTAL PROCEDURES
Recombinant Plasmid Construction
Plasmids encoding the Xpress-tagged Brd4L (pcDNA4C-Brd4L), BDI/II (pcDNA4C-NLS-BDI/II), and CTD (pcDNA4C-NLS-CTD) have been described (19, 32). pCMV2-mBrd4 was kindly provided by Dr. Keiko Ozato. SV2-YFP-LacI was a generous gift from Dr. Susan M. Janicki. To generate Xpress-tagged Brd4 aa 1–594, 1–730, and 1–1046 mutants, the coding sequences were PCR amplified, and subcloned into pcDNA4C vector using BamHI and NotI sites. For generating the constructs encoding the Xpress-tagged BDI/II-LacI, BDI/II-(731–1046), BDI/II-(1047–1362), and BDI/II-C50, the coding sequences for LacI, Brd4 aa 731–1046, 1047–1362, and 1313–1362, were amplified by PCR and cloned individually into pcDNA4C-NLS-BDI/II using NotI and XhoI sites. The pOZN-Brd4L, pOZN-NLS-BDI/II, and pOZN-NLS-CTD were also constructed by cloning the PCR-amplified cDNA fragments into pOZN vector using XhoI and NotI sites. pOZN-NLS-BDI/II-C50 was constructed by cloning the PCR amplified coding sequence for C50 (Brd4 aa 1313–1362) into pOZN-NLS-BDI/II using NotI site. Brd4L-pOZC and BDI/II-pOZC containing the C-terminal FLAG-HA tags were cloned by ligating the PCR amplified coding sequences (without stop codon) into pOZC vector using XhoI and NotI sites. For pOZN-VN (encoding Venus N for Venus aa 1–155) and pOZN-VC (encoding Venus C for Venus aa 156–238), the coding sequences were amplified from pCS2-Venus provided by Dr. Atsushi Miyawaki. A small linker sequence (GGSGG) was introduced in the N-terminal end of each fragment by PCR and the amplified DNA fragments were cloned into pOZN vector at NotI site. BDI/II or Brd4L DNA fragments excised from BDI/II-pOZC or Brd4L-pOZC using XhoI and NotI digestion were ligated into pOZN-VN and pOZN-VC to generate in-frame fusion of the Brd4 molecules with Venus N or Venus C. All plasmid constructs were verified by DNA sequencing.
Cell Culture, Transfection, Knockdown, and Retrovirus Infection
C33A and 293T cells were maintained as monolayers in high glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Hyclone). U2OS cells were cultured using McCoy's 5A medium (Invitrogen) with 10% FBS (Hyclone). U2OS 2-6-5 cells provided by Dr. Susan M. Janicki were maintained in DMEM GlutaMAXTM-I high glucose medium with sodium pyruvate (Invitrogen) containing 10% tet system approved FBS (Clontech).
FuGENE 6 transfection reagent (Roche Applied Science) was used for transient transfection in U2OS cells. For 293T cells, the calcium phosphate transfection method was applied as previously described (32). For C33A cells, both FuGENE 6 (Roche Applied Science) and calcium phosphate transfection methods were used. For Brd4 knockdown in C33A cells, four Brd4 siRNAs (si1–4) targeting different regions of the gene and the control siRNA have been described previously (33). The siRNA were transfected into cells using DharmaFECT 2 (Thermo). For the experiment described in Fig. 1, C and D, U2OS 2-6-5 cells were first transfected with either Brd4 si1 or control siRNA using DharmaFECT 4 (Thermo). Forty-eight hours post-transfection, the YFP-LacI construct was transfected into the cells using FuGENE 6 (Roche Applied Science). The cells were split onto coverslips 24 h later and harvested at 96 h after the first transfection.
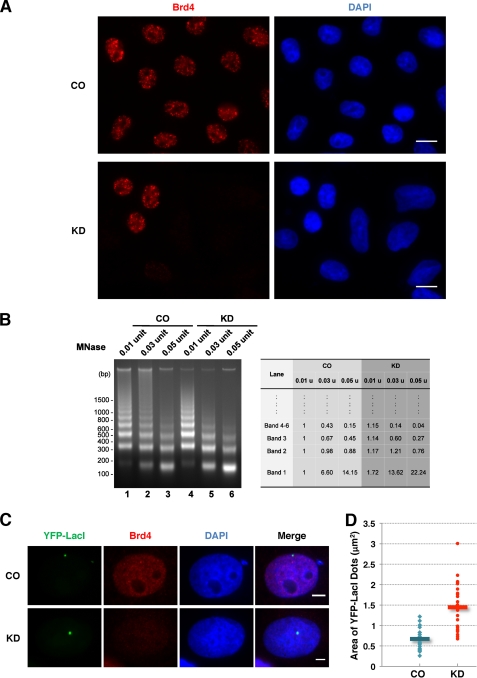
FIGURE 1.
Brd4 knockdown leads to decondensed chromatin structure. A, C33A cells were transfected with either Brd4 siRNA1 (KD) or control siRNA (CO). Seventy-two h later, the cells were fixed and stained with Brd4C antibody (red) and DAPI. Bar, 10 μm. B, C33A cells were transfected with either control siRNA (CO) or Brd4 siRNA1. At 72 h post-transfection, nuclei from 3 × 106 cells were isolated and analyzed in a MNase assay using three different doses of MNase. The band intensities quantified using ImageJ are shown on the right. C, U2OS 2-6-5 cells were transfected with either control siRNA (CO) or Brd4 siRNA1 (KD) and the YFP-LacI construct. The cells were fixed and stained with Brd4N antibody (red) and DAPI. Bar, 5 μm. D, scatter plot of the quantification of the YFP-LacI dot area (unit: μm2) in control (CO) or Brd4 knockdown (KD) cells. Data were collected from 26 control cells and 27 Brd4 knockdown cells using ImageJ. This experiment was repeated twice with similar results. Bars indicate the mean of all cells examined.
Recombinant retroviruses were prepared using standard methods and infections of the monolayer cells were performed as described previously (19). GFP-H2B retrovirus was kindly provided by Dr. Randall King (Harvard Medical School).
Immunofluorescent Staining
Cells cultured on coverslips were fixed with 3% paraformaldehyde in PBS for 20 min. Immunofluorescent staining was performed as previously described (32, 34). The following primary antibodies were used: anti-Brd4N (recognizing Brd4 aa 156–284, 1:40,000), anti-Brd4C (recognizing Brd4 aa 1313–1362, 1:40,000), and anti-Brd2 (provided by Gerald Denis, 1:500), anti-Xpress (Invitrogen, 1:200), and anti-FLAG (Sigma, 1:20000). Secondary antibodies used were: Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen, 1:1,000), Alexa Fluor 594 goat anti-mouse IgG (Invitrogen, 1:1,000), and Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, 1:500).
Microscopy and Image Analysis
All immunofluorescent images were collected using an inverted fluorescence microscope (Olympus, IX81) equipped with UPlanSApo ×40/0.95 NA lens (Olympus), UPlanSApo ×100/1.4 oil immersion lens (Olympus), and a high-resolution charge-coupled device camera (QImaging, FAST1394). Image data were analyzed and presented using SlideBook 5.0 software (Intelligent Imaging Innovations, Inc.). The scale bars were added using ImageJ software. Three-dimensional imaging of DAPI stained cells was performed using the “three-dimensional capture” mode of SlideBook 5.0 software with the step size set at 0.3 μm. The photos were assembled for presentation using the “make montage” function of ImageJ software. The immunofluorescent images of U2OS 2-6-5 cells were also analyzed using ImageJ software. The “adjust threshold” function of the software was used to select the YFP-LacI dots in the photos and the area data were acquired using “measure” function of the software.
Micrococcal Nuclease Assay
5 × 106 C33A cells were resuspended in 300 μl of HNB (15 mm Tris-HCl, pH 7.6, 0.5 m sucrose, 60 mm KCl, 0.25 mm EDTA, 0.125 mm EGTA, 0.5% Triton X-100, 1 mm DTT, 0.5 mm PMSF). After 20 min incubation on ice, nuclei were isolated by centrifugation at 240 × g for 5 min. Nuclei were gently resuspended in 50 μl of nuclear buffer (20 mm Tris-HCl, pH 7.6, 70 mm NaCl, 20 mm KCl, 5 mm MgCl2, and 3 mm CaCl2). Nuclei suspension was incubated with 0.06 unit of micrococcal nuclease (Sigma) at 37 °C for 10 min. The digestion was terminated by addition of EDTA and EGTA to a 5 mm final concentration each. The nuclear pellets were collected by centrifugation at 6000 × g for 5 min, and resuspended in 200 μl of lysis buffer (50 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm EDTA, 0.5% SDS) supplemented with RNase (Roche Applied Science, 100 μg/ml). After incubation at 37 °C for 20 min, Proteinase K (Roche Applied Science, 200 μg/ml) was added into the samples and incubated at 37 °C for 4 h. After phenol/chloroform extraction and ethanol precipitation, DNA was resuspended in H2O. Equal amounts of DNA samples were separated on a 1.2% agarose gel and detected with ethidium bromide. All MNase assay experiments were repeated more than three times.
Immunoprecipitation and Western Blot Analysis
At 48 h post-transfection, 293T cells were lysed in H buffer (10 mm HEPES, pH 7.9, 150 mm NaCl, 3 mm MgCl2, 1 mm DTT, 1 mm PMSF, 3 mm sodium butyrate, supplemented with protease inhibitor mixture and phosphatase inhibitor mixture, from Roche Applied Science) by passing through a 26-gauge needle 10 times. After a 20-min incubation on ice, the supernatant (S1) and the nuclear pellets were separated by centrifugation at 2,700 × g for 5 min. The pellets were resuspended in digestion buffer (H buffer with 20 mm MgCl2) supplemented with 18 units of DNase I (Roche Applied Science) for every 107 cells. The mixture was incubated at 37 °C for 30 min, and centrifuged at 2,700 × g for 5 min. The supernatant (S2) was combined with S1 to make the whole cell lysates. Cell lysates for all of IPs except the one presented in Fig. 5C were prepared using this method. Nuclear extracts used in Fig. 5C were prepared as described previously (32, 34).
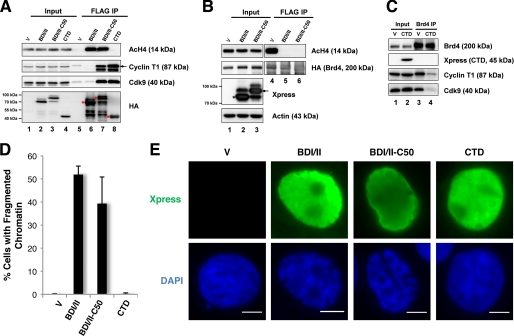
FIGURE 5.
The BDI/II-induced chromatin structure defect is independent of the role of Brd4 in P-TEFb-mediated transcriptional regulation. A, 293T cells were transfected with pOZN vector or pOZN encoding FLAG-HA-tagged BDI/II, BDI/II fused to the Brd4 C-terminal 50 amino acids (C50), or Brd4 CTD. Cell lysates were IPed with FLAG antibody. The precipitates were immunoblotted with the antibodies as indicated. Asterisks indicate the FLAG-HA-tagged proteins pulled down by FLAG antibody. B, 293T cells were co-transfected with a FLAG-HA-tagged Brd4L construct and a pcDNA4C vector, BDI/II plasmid, or BDI/II-C50 plasmid. Cell lysates were IPed with FLAG antibody. The precipitates were immunoblotted with the antibodies as indicated. The asterisk and arrow mark the expressed Xpress-tagged proteins. C, nuclear extracts of 293T cells transfected with a pcDNA4C vector or CTD plasmid were IPed with Brd4N antibody. The precipitates were immunoblotted with the specific antibodies listed. D, 293T cells transfected with a pcDNA4C vector or pcDNA4C expressing BDI/II, BDI/II-C50, or CTD were fixed 24 h post-transfection and stained with Xpress antibody and DAPI. The percentage of cells showing the fragmented chromatin defect was quantified from ∼200 positively transfected cells. Mean ± S.D. shown in the diagram were calculated from three independent experiments. E, representative images of the cells transfected and stained as described in D. Bar, 5 μm.
After preblocking in 1% BSA/PBS at 4 °C for 1 h, anti-FLAG M2-agarose beads (Sigma) were incubated with cell lysates at 4 °C for 4 h. For Brd4 IP, the lysates were immunoprecipitated using protein A beads (pre-blocked with 1% BSA in PBS at 4 °C for 1 h) mixed with 2 μg of either normal rabbit IgG or Brd4 antibody at 4 °C overnight. The beads were washed and eluted as described previously (32). Input and IP samples were resolved on a SDS-PAGE gel, transferred onto PVDF membrane, and immunoblotted with specific antibodies as indicated in the figure legends (ECL detection). Antibodies employed in the Western blot analysis include: Brd4C (1:80,000), Brd4N (1:80,000), AcH4 (Upstate, 1:2,000), Xpress (Invitrogen, 1:5,000), Actin (Chemicon, 1:150,000), HA-HRP (Roche, 1:1000), FLAG (Sigma, 1:1,000), Cyclin T1 (Santa Cruz, 1:200), and Cdk9 (Santa Cruz, 1:200). All of the IP experiments were repeated at least three times.
RESULTS
Brd4 Knockdown Leads to Decondensed Chromatin Structure
In our previous study, aberrant chromosome segregation and failures in cytokinesis were consistently observed when Brd4 was knocked down by RNA interference (33). We also noticed that, prior to the onset of the chromosome segregation defects, Brd4 knockdown cells first demonstrated enlarged nuclear shape that resembles chromatin decondensation. In this study, we further examined the chromatin structure induced by Brd4 knockdown in C33A cells. Efficient Brd4 knockdown was achieved using each of the four siRNAs (si1–4) targeting different regions of Brd4 gene (supplemental Fig. S1A). Brd4 knockdown frequently leads to increased cell size and enlarged/lobulated nuclei that are absent in the control siRNA-treated cells (Fig. 1A). Similar chromatin defects were observed in several other cell lines including 293T, U2OS, and primary human foreskin keratinocytes (data not shown).
Previous studies have demonstrated that Brd4 knockdown leads to G1 cell cycle arrest and does not increase DNA contents in cells (17, 18), the enlarged nuclei in Brd4 siRNA-treated cells therefore indicate chromatin decondensation (Fig. 1A). We then performed limited MNase digestion to further assess the chromatin structural defect induced by Brd4 knockdown. MNase digestion provides a useful tool for analyzing chromatin structure because it cleaves the linker DNA between adjacent nucleosomes, and depending on the extent of digestion, generates chromatin fragments with different sizes corresponding to nucleosome number, which could be detected as a specific ladder of DNA. The patterns of permeability to nuclease digestion reveal the chromatin organization of cells under different experimental conditions. As shown in Fig. 1B, compared with the control siRNA-treated cells, MNase digestion of chromatin from cells treated with Brd4 siRNA resulted in increased lower molecular weight DNA fragments especially the 1st, 2nd, and 3rd smallest bands and significantly reduced large DNA fragments migrating in the upper half of the gel. The changes in the DNA band pattern were confirmed by quantification using ImageJ (Fig. 1B, right panel). This increased chromatin accessibility to MNase reveals a decondensed chromatin structure associated with Brd4 knockdown, suggesting that the physiological level of Brd4 is essential for maintaining properly condensed chromatin architecture.
In a rescue experiment, co-expression of the full-length mouse Brd4 gene resistant to the human Brd4 siRNA could reverse the increased MNase cleavage in Brd4 knockdown cells, resulting in a digestion pattern similar to those observed in the control sample (supplemental Fig. S1B). This study demonstrates that mouse Brd4 protein is able to partially rescue the decondensed chromatin structure in Brd4 knockdown cells, providing further evidence to support the role of Brd4 in chromatin structure organization.
Because Brd4 knockdown leads to decondensed chromatin, we speculated that it might also induce chromatin unfolding. We then used a lac repressor-lac operator (lacR-lacO) array system in the U2OS 2-6-5 cell line to directly visualize the impact of Brd4 on the large scale chromatin structure in living cells. The U2OS 2-6-5 cell line contains a condensed heterochromatic region consisting of an array of 256 copies of the lacO repressor sequence (35). These cells were first transfected with either control or Brd4 siRNAs. Forty-eight hours later, the cells were re-transfected with a construct encoding a yellow fluorescent protein and lacR fusion protein (YFP-LacI) to directly visualize the lacO chromatin array by fluorescence microscopy. As shown in Fig. 1C, the lacO array was detected as a compact green nuclear dot in the control cells. However, in most of Brd4 knockdown cells, the sizes of these nuclear dots increased significantly (Fig. 1C). The areas of YFP-LacI dots were calculated using ImageJ to be 0.67 ± 0.27 μm2 in the control cells and 1.45 ± 0.58 μm2 in the Brd4 knockdown cells (Fig. 1D). The average area of the lacO region was 2.15 times larger in the Brd4 knockdown cells than in the control cells with a p value of 2.48E-07 (Student's t test). This result reveals the unfolding of the subnuclear chromatin structure induced by Brd4 knockdown. Together, these studies demonstrate that the absence of Brd4 on chromatin leads to chromatin decondensation, supporting the Brd4 function in linking the nucleosome together and maintaining a properly condensed chromatin structure.
The Brd4 Double Bromodomain Mutant Functions as a Dominant-negative Inhibitor to Dissociate Brd4L from Chromatin and Cause Abnormally Fragmented Chromatin Structure
Brd4 associates with chromatin and mitotic chromosomes by binding to acetylated histone 3 (AcH3) and histone 4 (AcH4) through its double bromodomains (13). To further determine how this interaction contributes to chromatin structure organization, we developed a dominant-negative inhibitor encoding the double bromodomains of Brd4 (aa 1–470, a.k.a. BDI/II) to prevent Brd4L from localizing to cellular chromatin. In C33A cells, endogenous Brd4L normally localizes to punctate dots on chromatin as seen in the non-transfected cells (Fig. 2A). However, in cells expressing BDI/II, endogenous Brd4L was prevented from localizing to punctuate chromatin dots (Fig. 2A). Co-immunoprecipitation (co-IP) experiments showed that the amount of Brd4 binding to AcH4 is dramatically reduced in cells expressing BDI/II (supplemental Fig. S2A). Notably, inhibition of Brd4 and AcH4 co-IP by BDI/II was not rescued by trichostatin A, a histone deacetylase inhibitor (supplemental Fig. S2A), suggesting that the decreased Brd4-AcH4 interaction is not due to any change in H4 acetylation. Western blot analysis also confirmed that the BDI/II expression does not affect H4 acetylation in cells (supplemental Fig. S2B). These studies demonstrate that the BDI/II molecule functions as a dominant-negative inhibitor to competitively bind to AcH4 and dissociate endogenous Brd4L from acetylated histones on chromatin. This effect is specific for Brd4, as BDI/II does not affect the nuclear localization pattern of another bromodomain protein, Brd2 (Fig. 2A).
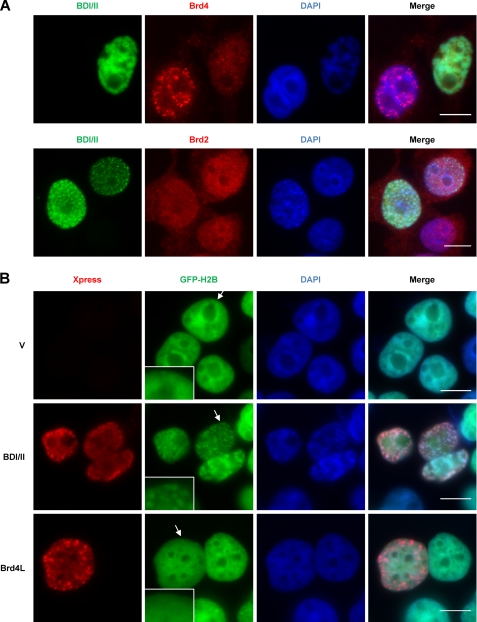
FIGURE 2.
Dissociation of Brd4L from chromatin by a dominant-negative inhibitor, BDI/II, leads to chromatin fragmentation. A, C33A cells were transfected with pcDNA4C Xpress-BDI/II (BDI/II). Forty-eight hours later, the cells were double-stained with Xpress antibody (green) and either Brd4C antibody (red) or Brd2 antibody (red). The cells were also counterstained with DAPI. Bar, 10 μm. B, C33A/GFP-H2B stable cells were transfected with pcDNA4C (V), pcDNA4C-NLS-BDI/II (BDI/II), or pcDNA4C-Brd4L (Brd4L). Forty-eight h post-transfection, the cells were stained with Xpress antibody (red) and counterstained with DAPI. Arrows indicate the regions magnified in the insets. Bar, 10 μm.
Immunofluorescence microscopy demonstrated that most of the BDI/II expressing cells not only show dispersed Brd4 staining but also exhibit severely fragmented chromatin as indicated by 4,6-diamidino-2-phenylindole (DAPI) DNA staining (Fig. 2A). The BDI/II-positive cells are negatively stained for Annexin V, suggesting that the fragmented chromatin is not caused by cell death (data not shown). To confirm that the abnormal DAPI staining induced by BDI/II reflects a change in chromatin structure, we expressed this protein in C33A cells stably expressing GFP-H2B. GFP-H2B binds to chromatin efficiently and paints the nuclear chromatin with GFP fluorescent signal, allowing a clearer visualization of chromatin structure. The BDI/II expression in C33A/GFP-H2B stable cells also causes a highly fragmented chromatin structure as shown by the GFP-H2B fluorescent signal as well as DAPI staining (Fig. 2B, middle panel). We therefore use the term “chromatin fragmentation” to describe this phenomenon of chromatin re-organization that appears to break chromatin into multiple fragments inside the nucleus (Fig. 2B, middle panel). In contrast to the BDI/II-positive cells, neighboring cells that do not express BDI/II display normal chromatin structure (Fig. 2B). To rule out an overexpression artifact, we also expressed Brd4L at a similar level in cells. Notably, exogenous expression of Brd4L does not induce visible changes in chromatin structure (Fig. 2B). This result indicates that association of Brd4 with chromatin is vital for maintaining the normal chromatin structure. The nuclear reorganization induced by BDI/II is consistent with a previous study showing that the shorter isoform of BrdT triggers a dramatic compaction of acetylated chromatin (31). Together, these studies demonstrate a structural role for double-bromodomain proteins in remodeling acetylated chromatin.
We then performed MNase analysis to examine the BDI/II-induced abnormal chromatin structural change at the molecular level. Compared with the control cells, MNase digestion of chromatin isolated from cells expressing BDI/II generates significantly more intense lower molecular weight DNA fragments (especially the 2nd, 3rd, and 4th smallest bands from the bottom of the gel) but less intense higher molecular weight DNA fragments (Fig. 3A). These increased lower molecular weight bands represent DNA fragments isolated from nucleosome dimers, trimers, and tetramers (Fig. 3A), indicating increased MNase cleavage between these nucleosome oligomers in the BDI/II expressing cells. Notably, the intensity for the smallest band from the bottom of the gel representing DNA fragments isolated from nucleosome monomers was not increased but slightly decreased in the BDI/II sample. This result suggests that BDI/II does not simply induce a global chromatin decondensation but rather an abnormally fragmented chromatin structure, which represents an uneven compaction of chromatin caused by aggregation of nucleosomes into different oligomers that could not be easily cut by MNase into monomers. Consistent with a normal nuclear morphology in cells expressing Brd4L (Fig. 2B), expression of full-length Brd4 does not cause a dramatic change in the MNase cleavage pattern compared with the control cells (Fig. 3A).
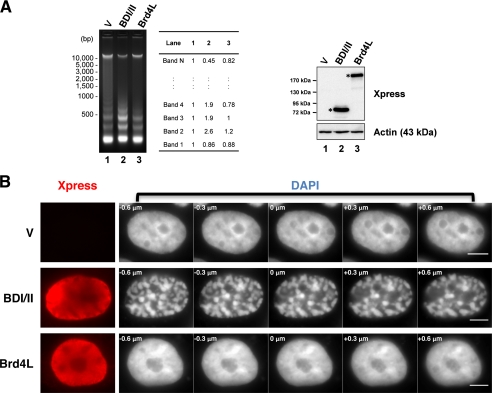
FIGURE 3.
The BDI/II-induced chromatin fragmentation was visualized by MNase assay and high-resolution imaging. A, C33A cells were transfected with pcDNA4C (V), pcDNA4C-NLS-BDI/II (BDI/II), or pcDNA4C-Brd4L (Brd4L). Cells were harvested 48 h post-transfection. Nuclei from 5 × 106 cells were analyzed using MNase assay. The DNA band intensities were quantified using ImageJ. Protein samples were immunoblotted using the antibodies indicated (right panel). Asterisks indicate the expressed BDI/II and Brd4L protein. B, 293T cells transfected with pcDNA4C (V), pcDNA4C-NLS-BDI/II (BDI/II), or pcDNA4C-Brd4L (Brd4L) were fixed 24 h post-transfection and stained with Xpress antibody (red) and DAPI. Three-dimensional images of the DAPI signal focusing within a −0.6 to +0.6 μm range were captured on an Olympus inverted microscope by setting the most focused plane as 0 μm. The step size in μm is shown in the top left corner of each image. Bar, 5 μm.
To further investigate how changing the Brd4 status modulates chromatin architecture at a higher resolution, we performed three-dimensional imaging of the chromatin structure in 293T cells expressing either BDI/II or Brd4L. As shown in Fig. 3B, Brd4L expression does not cause an obvious chromatin structural change when compared with the control cells. In contrast, in most of the BDI/II expressing cells, chromatin appears to aggregate into highly compact regions (Fig. 3B). This abnormal chromatin pattern is consistent with the result obtained in C33A cells and also supports the abnormal nucleosome oligomerization observed in Fig. 3A. In conclusion, our data show that dissociation of Brd4L from chromatin by the BDI/II dominant-negative inhibitor leads to abnormal chromatin reorganization. These observations support an important role of Brd4L in maintaining the properly folded chromatin structure.
The C-terminal Domain of Brd4 Is Important for Maintaining Normal Chromatin Structure
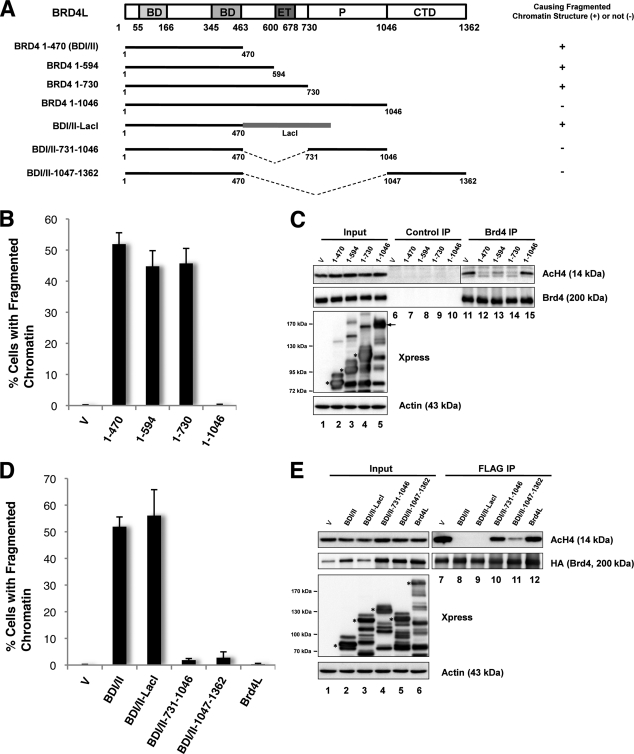
Because expression of the N-terminal double bromodomains of Brd4 can lead to abnormal chromatin reorganization that is absent in the Brd4L expressing cells, we decided to identify the molecular component(s) of Brd4L that allows it to maintain normal chromatin structure. We constructed a series of Xpress-tagged Brd4 C-terminal truncation mutants encoding aa 1–470 (BDI/II), 1–594, 1–730, and 1–1046 (Fig. 4A). All of these mutants can interact efficiently with AcH4 (supplemental Fig. S3), demonstrating that these proteins are folded correctly. We then examined chromatin morphology in cells expressing these truncation mutants. Quantification of the percentage of transfected cells with the fragmented chromatin structure indicates that shorter fragments such as 1–730, 1–594, or 1–470 all cause chromatin fragmentation in 40–50% of the transfected cells, whereas expression of the 1–1046 molecule does not generate any obvious chromatin structure defect (Fig. 4B).
FIGURE 4.
The C-terminal domain of Brd4 is important for maintaining normal chromatin structure. A, schematic diagrams of Brd4 molecules. Their abilities to cause fragmented chromatin structure are summarized on the right. Brd4 domains: BD, bromodomain; ET, extra-terminal domain; P, proline-rich region. B, 293T cells transfected with pcDNA4C vector or pcDNA4C encoding Xpress-tagged Brd4 truncation mutants were fixed 24 h post-transfection and stained with Xpress antibody and DAPI. The percentage of cells showing fragmented chromatin defects was quantified from ∼200 positively transfected cells. Mean ± S.D. shown in the diagram were calculated from three independent experiments. C, cell lysates from 293T cells transfected with pcDNA4C vector or a pcDNA4C Brd4 truncation mutant were IPed with either normal rabbit IgG (Control IP) or Brd4C antibody (Brd4 IP). Co-IPed proteins were immunoblotted with specific antibodies as indicated. Arrow and asterisks mark the Xpress-tagged Brd4 mutants expressed in cells. D, 293T cells transfected with pcDNA4C vector, or pcDNA4C plasmid expressing Brd4 molecules were fixed 24 h post-transfection and stained with Xpress antibody and DAPI. The percentage of cells showing the fragmented chromatin defect was quantified from ∼200 positively transfected cells. Mean ± S.D. shown in the diagram were calculated from three independent experiments. E, 293T cells were co-transfected with a FLAG-HA-Brd4L plasmid and either pcDNA4C vector or a pcDNA4C encoding BDI/II, BDI/II fusions, or Brd4L. Cell lysates were IPed with FLAG antibody. The precipitates were immunoblotted with specific antibodies as indicated. Asterisks mark the Xpress-tagged proteins expressed in the cells.
To test whether these Brd4 mutants could compete for AcH4 binding and dissociate endogenous Brd4L from chromatin, co-IP of Brd4 and AcH4 using an antibody that specifically recognizes the Brd4 C terminus was performed in cells transfected with either a vector control or a Brd4 mutant construct. Normal rabbit IgG was used as a negative control to show that there was not any nonspecific pulldown of AcH4 (Fig. 4C). In contrast, Brd4 co-IP pulled down significant amounts of AcH4 in the vector control sample (Fig. 4C). A similar amount of AcH4 was pulled down by the Brd4 antibody in the 1–1046-transfected cells (Fig. 4C), indicating that endogenous Brd4L molecules can still interact with AcH4 in the presence of 1–1046. However, the amount of AcH4 co-IP with Brd4 was dramatically reduced in cells expressing 1–470, 1–594, and 1–730 (Fig. 4C). Because the Brd4 antibody and the AcH4 antibody were both generated from rabbit, the co-IPed samples show some rabbit IgG reactive nonspecific bands in the AcH4 Western blot. However, it is clear that expression of the shorter Brd4 truncation mutants (1–470, 1–594, and 1–730) can efficiently inhibit the binding of Brd4 to AcH4, whereas the longest mutant (1–1046) cannot. The ability of these Brd4 mutants to cause competitive dissociation of Brd4L from chromatin clearly correlates with their efficiency in triggering the fragmented chromatin structure; further supporting that proper binding of Brd4L on chromatin is essential for maintaining normal chromatin structure (Fig. 4, B and C). This study also suggests that the shorter Brd4 mutants may associate with chromatin more tightly than Brd4L, providing a mechanistic basis for the dominant-negative effect of BDI/II in Brd4L chromatin association.
Expression of Brd4-(1–1046) and Brd4L generates barely any detectable chromatin structural defect (Figs. 2–4), whereas 1–470, 1–594, and 1–730 mutants can dissociate Brd4L from chromatin to induce dramatic chromatin fragmentation. The data indicate that the aa 731–1362 region of Brd4 may harbor important molecular component(s) for maintaining normal chromatin structure. To test this further, we generated constructs encoding the BDI/II domain fused directly to either aa 731–1046 or 1047–1362 (the CTD domain of Brd4) to determine whether these regions could rescue the BDI/II-induced abnormal chromatin structure (Fig. 4A). The LacI molecule was also fused to BDI/II to generate a fusion control with a similar molecular weight (Fig. 4A). Similar to the BDI/II molecule, BDI/II-(731–1046) and BDI/II-(1047–1362) fusions could efficiently interact with AcH4 (supplemental Fig. S4). Furthermore, just as for the Brd4 CTD, the BDI/II-(1047–1362) fusion protein could pulldown the Cdk9 and Cyclin T1 subunits of P-TEFb (positive transcription elongation factor b), which is known to bind to the last 50 amino acids of Brd4 (32) (supplemental Fig. S4). These results confirm that the mutant proteins are folded correctly. They were then expressed in 293T cells for chromatin structure analysis. Similar to the BDI/II expressing cells, the BDI/II-LacI-positive cells displayed a fragmented chromatin pattern (supplemental Fig. S5). However, the BDI/II-(731–1046) and BDI/II-(1047–1362) positive cells showed normal chromatin texture (supplemental Fig. S5). As shown in Fig. 4D, the percentage of BDI/II-LacI-positive cells that displayed fragmented chromatin (56.1%) was similar to the BDI/II-positive cells (51.9%), whereas this percentage was much lower in cells expressing BDI/II-(731–1046) (1.8%), BDI/II-(1047–1362) (2.8%), or Brd4L (0.3%).
To test whether the BDI/II-(731–1046) and BDI/II-(1047–1362) fusions could compete for AcH4 binding and dissociate Brd4L from chromatin, lysates from cells co-transfected with a FLAG-HA-Brd4L construct and either one of the Xpress-tagged Brd4 fusion constructs were subjected to FLAG IP. As shown in Fig. 4E, FLAG antibody could efficiently IP FLAG-HA-Brd4L in all samples. In cells co-transfected with a vector control, a significant amount of AcH4 was co-IP with FLAG-HA-Brd4L. Consistent with their abilities to cause chromatin fragmentation, expression of BDI/II-LacI and BDI/II abolished FLAG-HA-Brd4L and AcH4 co-IP (Fig. 4E). This result shows that BDI/II-LacI and BDI/II may cause chromatin fragmentation by dissociating Brd4L from AcH4 on chromatin. In contrast, expression of either Brd4L or the BDI/II-(731–1046) fusion did not significantly affect the FLAG-HA-Brd4L and AcH4 interaction (Fig. 4E), suggesting that these two molecules could not efficiently dissociate Brd4L from acetylated chromatin, therefore allowing endogenous Brd4L sitting on chromatin to maintain normal chromatin morphology. Remarkably, the BDI/II-(1047–1362) expression led to a significant inhibition of the FLAG-HA-Brd4L and AcH4 co-IP (Fig. 4E), indicating that this molecule could dissociate most Brd4L proteins from chromatin. Because the BDI/II-(1047–1362) expressing cells showed normal chromatin structure (supplemental Fig. S5 and Fig. 4D), this result demonstrates that the 1047–1362 aa region of Brd4 is able to abrogate the BDI/II-induced chromatin fragmentation defect, providing direct evidence to support that the CTD domain of Brd4 is important for maintaining normal chromatin structure.
The Brd4 Function in Chromatin Structure Maintenance Is Independent of Its Role in P-TEFb-mediated Transcriptional Regulation
The major protein complex that binds the Brd4 CTD is P-TEFb, composed of the Cdk9-Cyclin T1 dimer (27–29). Brd4 recruits the P-TEFb complex to reconstitute the active form of the complex, which phosphorylates the C-terminal domain of RNA polymerase II and stimulates transcriptional elongation (27, 29). Because our data suggest that the Brd4 CTD domain is important for maintaining normal chromatin structure, we wondered if recruitment of P-TEFb for transcription regulation was involved in this function. To test this possibility, we fused the minimum P-TEFb binding domain, the last 50 amino acids of Brd4 (C50) described previously (32), to BDI/II to determine whether it could rescue the BDI/II-induced chromatin structural defect. Similar to BDI/II, FLAG-BDI/II-C50 could efficiently pulldown AcH4 (Fig. 5A). It could also bind to P-TEFb subunits to a similar extent as the Brd4 CTD (Fig. 5A). Co-IP also showed that expression of BDI/II-C50 could completely dissociate Brd4L molecules from AcH4 on chromatin (Fig. 5B). Interestingly, the BDI/II-C50 expressing cells showed a clear fragmentation of chromatin structure just as in the BDI/II-positive cells (Fig. 5E). Quantification showed that BDI/II-C50 could cause chromatin fragmentation nearly as efficiently as BDI/II (Fig. 5D). This study shows that although the BDI/II-C50 can recruit P-TEFb to chromatin, it is unable to rescue the BDI/II-induced chromatin defect, demonstrating that P-TEFb binding is not sufficient to maintain the normal chromatin structure. We also tested how dissociation of P-TEFb from Brd4 on the chromatin template could affect chromatin structure. As shown in Fig. 5C, expression of the Brd4 CTD leads to efficient dissociation of P-TEFb from Brd4. However, the CTD expression does not lead to any changes in chromatin structure (Fig. 5, D and E), indicating that dissociation of P-TEFb and Brd4L from chromatin is not the cause of the chromatin structural defects in BDI/II cells. In conclusion, this study suggests a more direct structural role of the Brd4 C-terminal domain in maintaining normal chromatin architecture and demonstrates that this function is not likely to involve the P-TEFb-associated transcription regulation.
Brd4 Molecules Interact with Each Other through BDI/II Region
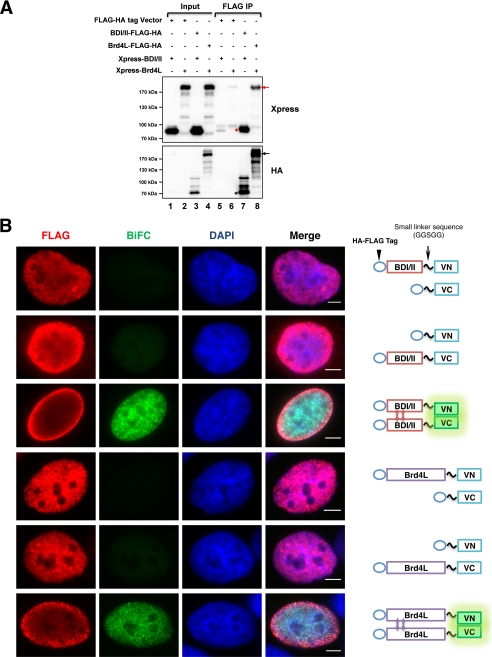
The crystal structure of the first bromodomain of Brd2 revealed that it could form an intact homodimer (36). Because Brd4 shares high sequence homology with Brd2, we investigated if Brd4 could interact intermolecularly to contribute to chromatin structure maintenance. Preliminary studies using glutathione S-transferase fusion pulldown identified a number of potential interactions between Brd4 domains (data not shown), which may contribute to either an intra- or intermolecular interaction of Brd4 in vivo. To test this further, Brd4L-FLAG-HA and Xpress-Brd4L were co-expressed in cells. FLAG co-IP showed that Brd4L-FLAG-HA could efficiently pulldown Xpress-Brd4L, whereas no major band was detected in the co-IP using cells transfected with a FLAG-HA vector (Fig. 6A). Using a similar approach, BDI/II-FLAG-HA was shown to efficiently pulldown Xpress-BDI/II (Fig. 6A). Because the cell lysates used in the co-IP were pretreated with DNase I to digest the DNA linkers between nucleosomes (“Experimental Procedures”), it rules out the possibility that the protein-protein interaction detected by this co-IP was mediated by the chromatin. This biochemical analysis demonstrates that Brd4 can interact intermolecularly and this interaction is mostly mediated by the N terminus 1–470 aa domain of Brd4.
FIGURE 6.
Brd4 intermolecular interaction is mediated by the aa 1–470 region. A, 293T cells were co-transfected with either pOZC vector or pOZC encoding FLAG-HA-tagged Brd4 molecules and pcDNA4C encoding Xpress-tagged Brd4 molecules. Cell lysates were IPed with FLAG antibody. The precipitates were immunoblotted with Xpress and HA antibodies. B, BiFC analysis of BDI/II-BDI/II interaction and Brd4L-Brd4L interaction. U2OS cells were co-transfected with pairs of Venus N (VN, BDI/II-VN, and Brd4L-VN) and Venus C (VC, BDI/II-VC, and Brd4L-VC) constructs as indicated on the right panel. Forty-eight h post-transfection, the cells were fixed and stained with FLAG antibody (red) and DAPI. Bar, 5 μm.
To ensure that the Brd4-Brd4 interaction detected by co-IP reflects true interaction in cells, we further examined the Brd4-Brd4 interaction using BiFC technology. For BiFC, two interacting proteins are fused individually to complementary fragments of a fluorescent reporter and expressed in live cells. Interaction of these proteins will bring the fluorescent fragments within proximity, allowing the reporter protein to reform its native three-dimensional structure and emit fluorescent signal. Therefore, protein interaction can be identified and located within the cell through visualization of the intensity and distribution of fluorescence in these cells (37). To test the BDI/II-BDI/II and Brd4L-Brd4L intermolecular interaction, these molecules were fused individually to either the N terminus (VN) or C terminus (VC) of Venus (38). Venus signal was detected only when both BDI/II-VN and BDI/II-VC constructs were co-expressed in cells (Fig. 6B). On the other hand, expression of either construct with the VN or VC control generated only very dim background signals (Fig. 6B). These observations support that BDI/II molecules can interact intermolecularly in cells. Similarly, co-expression of Brd4L-VN and Brd4L-VC fusion proteins in cells generated a strong BiFC positive signal, which is absent in cells transfected with either Brd4L-VN with the VC control or Brd4L-VC with the VN control (Fig. 6B). This result provides direct evidence to demonstrate that the Brd4 molecules can interact efficiently in cells through the BDI/II domain. FLAG staining was performed to detect the FLAG-tagged BiFC molecules. We noticed that most of the BiFC-positive cells show a dimmer FLAG signal in the center of each cell possibly because the BiFC interaction interferes with FLAG staining (Fig. 6B). Signals for both BDI/II BiFC and Brd4L BiFC are shown in a pattern of evenly spaced minute punctate dots on chromatin that closely resembles the staining pattern of endogenous Brd4 (Fig. 6B), suggesting that the interactions occur when either Brd4L or BDI/II binds to the native Brd4 binding sites on acetylated chromatin. These observations are significant as we speculate that this Brd4 interaction may contribute to its function in nucleosome binding and chromatin compaction.
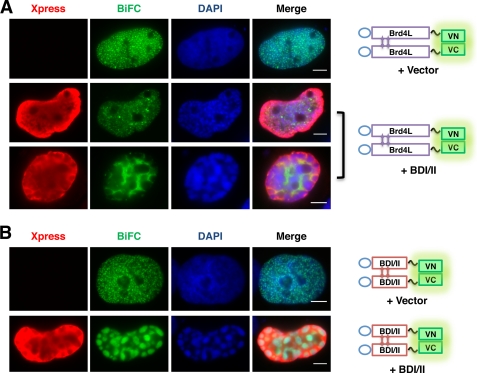
Replacement of Brd4L by BDI/II on Chromatin Causes Abnormal Chromatin Fragmentation
Because the BiFC assay showed a clear pattern of Brd4 intermolecular interaction on chromatin, we tested BDI/II in this system to visualize how it affects the Brd4L-Brd4L interaction on chromatin to cause chromatin fragmentation. U2OS cells were transfected with Brd4L-VN, Brd4L-VC, and either a control vector or the Xpress-BDI/II construct. The vector control cells showed a normal Brd4L BiFC signal indicating a nicely spaced Brd4 interaction on chromatin (Fig. 7A). However, in most of the Xpress-BDI/II-positive cells, the BiFC signal was significantly reduced to a dimmer diffused pattern, indicating dissociation of Brd4L from chromatin (Fig. 7A). In addition, the Xpress-BDI/II expression led to significant chromatin fragmentation as observed in previous experiments (Fig. 7A, DAPI, middle and bottom panels). In many cases, the green BiFC signal is mainly detected in the nuclear space without DAPI staining, suggesting that the Brd4L BiFC molecules were excluded from the fragmented chromatin (Fig. 7A, bottom panel). The resulting chromatin fragmentation morphology suggests that although Brd4 molecules retain the ability to interact with each other when they are dissociated from chromatin by BDI/II, they can no longer support the normal chromatin structure. This result provides direct visualization of how BDI/II causes the fragmented chromatin phenomenon by disrupting the Brd4L-Brd4L interaction on chromatin, offering further evidence to support that normal localization of Brd4L on chromatin is important for maintaining the properly organized chromatin structure.
FIGURE 7.
Dissociation of Brd4L by BDI/II leads to chromatin fragmentation at the BDI/II binding sites on chromatin. A, U2OS cells were transfected with Brd4L-VN and Brd4L-VC BiFC constructs and either pcDNA4C vector or Xpress-BDI/II plasmid. Forty-eight h post-transfection, the cells were fixed and stained with Xpress (red) and DAPI. Bar, 5 μm. B, U2OS cells were transfected with BDI/II-VN and BDI/II-VC BiFC constructs and either pcDNA4 vector or Xpress-BDI/II plasmid. Forty-eight hours post-transfection, the cells were fixed and stained as in A. Bar, 5 μm.
To further visualize how BDI/II causes chromatin fragmentation, U2OS cells were transfected with BDI/II-VN, BDI/II-VC, and either a control vector or the Xpress-BDI/II construct (Fig. 7B). In contrast to the normal BDI/II BiFC signal detected in the control cells, co-expression of Xpress-BDI/II causes a more severe chromatin fragmentation and redistribution of the BDI/II BiFC signal into large green fluorescent islands (Fig. 7B). Interestingly, this BDI/II BiFC signal precisely matches the DAPI staining pattern, suggesting that BDI/II BiFC occurs on fragmented chromatin in the presence of Xpress-BDI/II. This is significantly different from the dissociation of Brd4L BiFC from chromatin induced by Xpress-BDI/II (Fig. 7A, bottom panel). The phenomenon is consistent with the notion that Xpress-BDI/II could bind to chromatin with similar affinity as the BDI/II BiFC molecules and therefore could not compete away the BDI/II BiFC from chromatin. Because the BDI/II molecules could interact with each other, expression of Xpress-BDI/II could potentially further promote the BDI/II BiFC on chromatin. The BDI/II BiFC present on the fragmented chromatin provides direct evidence to demonstrate that binding of BDI/II to chromatin can replace/disrupt the Brd4L-Brd4L interaction on chromatin to introduce structural defects that lead to chromatin fragmentation.
DISCUSSION
Chromatin structure dynamics are crucial for regulating many fundamental cellular events. Aberrant chromatin structure that perturbs these important biological processes has been linked to many human genetic diseases and cancer. Elaborate mechanisms have been demonstrated for the assembly of primary and secondary chromatin structure. However, due to the technical difficulties of traditional approaches that prevented a clear visualization of large scale chromatin structure, the molecular mechanism(s) that regulate the assembly of the higher-order chromatin structure have yet to be fully elaborated.
In our study, we combined a set of complementary new techniques developed in recent years to examine the role of Brd4 in chromatin structure maintenance. We observed that Brd4 knockdown leads to enlarged nuclei. Using MNase assay and the lac-based chromatin dynamic in situ single cell imaging, we confirmed that Brd4 knockdown causes chromatin decondensation. BDI/II encoding the double bromodomains of Brd4 functions as a dominant-negative inhibitor to competitively bind to AcH4 and dissociate endogenous Brd4L from chromatin. Disruption of the Brd4-AcH4 interaction by BDI/II abolishes chromatin structure maintenance and results in highly disorganized chromatin fragmentation. Molecular analysis of Brd4 C-terminal truncation mutants demonstrated that shorter Brd4 fragments bind to AcH4/chromatin with higher affinity to competitively inhibit association of the long Brd4 isoform with chromatin, leading to abnormal chromatin reorganization. By fusing different C-terminal domains of Brd4 to BDI/II, we determined that the aa 1047–1362 region, the CTD domain, of Brd4 is crucial for maintaining normal chromatin structure. Co-IP and BiFC analyses further demonstrated that Brd4L proteins interact through the N-terminal aa 1–470 region and that displacement of Brd4L by BDI/II on chromatin leads to chromatin fragmentation. Our data supports a molecular model for Brd4-mediated chromatin structure maintenance. Although binding to acetylated nucleosomes on chromatin through its double bromodomains, Brd4 interacts intermolecularly through the N terminus to reconstitute a chromatin-associated molecular scaffold, in which the Brd4 C-terminal region provides additional structural support to maintain properly organized chromatin architecture. This function of Brd4 could be accomplished by promoting either nucleosome-nucleosome or chromatin fiber-fiber interactions.
The collapse of chromatin structure upon Brd4 knockdown supports that Brd4 binding to acetylated histones may provide molecular forces to maintain local nucleosome-nucleosome interactions. Each Brd4 molecule carries two bromodomains, which may bind to adjacent acetyl-lysines either within a single histone or on different nucleosomes to promote the nucleosome array interaction, contributing to chromatin fiber-fiber oligomerization. The intermolecular interaction may allow Brd4 to act in multimeric complexes to bridge acetylated nucleosomes together and to support higher-order chromatin folding.
Knockdown of both Brd4L and Brd4S isoforms leads to a highly decondensed chromatin structure, whereas dissociation of Brd4L from chromatin by BDI/II triggers a severely fragmented chromatin. These observations again suggest that binding of the Brd4L isoform to chromatin is essential for maintaining a properly organized chromatin conformation. BDI/II binding causes a more dramatic change in chromatin structure than Brd4 knockdown. One possibility is that it could eliminate native Brd4 from chromatin more effectively or could displace other BET proteins besides Brd4. In addition, unlike Brd4 knockdown that removes Brd4 molecules from chromatin, binding of BDI/II on chromatin may maintain the regional nucleosome organization and chromatin architecture through intermolecular interaction. However, the higher intrinsic affinity of BDI/II for chromatin described above may cause these molecules to bind nucleosomes more tightly than Brd4L, leading to nucleosome aggregation and chromatin fragmentation. This augmented chromatin structural defect suggests that the Brd4 C-terminal domain, which is absent in BDI/II, harbors important properties for maintaining the properly folded chromatin structure.
The Brd4 C terminus has been implicated in recruiting P-TEFb complex for stimulating transcription elongation (27–29). Our studies show that the Brd4 C terminus function in chromatin structure maintenance is independent of the P-TEFb-associated transcription regulation, providing a novel structural role of Brd4 in chromatin architecture maintenance. The Brd4 C-terminal domain may provide steric support for an optimal spacing between nucleosomes to prevent abnormal chromatin condensation. It could also harbor additional post-translational modifications and/or important protein-protein interactions (either between Brd4 molecules or for Brd4 and other cellular proteins) to promote the chromatin scaffold function. In addition, the Brd4 C-terminal domain could negatively regulate Brd4L interaction with chromatin to ensure an optimal chromatin compaction. This notion is supported by a previous study (39), and is also consistent with our data showing that the shorter Brd4 molecules bind more tightly to AcH4 to compete the Brd4L and AcH4 interaction (Fig. 4). Because native Brd4 short isoform exists in cells, our observation also points to a possibility that the long and short forms of Brd4 may bind to chromatin in a tightly regulated manner to provide a balanced distribution for proper chromatin condensation. The molecular basis for this differential chromatin affinity of Brd4 isoforms and how it pertains to the Brd4 physiological function in chromatin structure organization remain to be investigated.
In our study, we noticed that the severity of the chromatin defect caused by BDI/II paralleled its expression level. This is probably because BDI/II could interact with Brd4L and therefore higher concentrations of the dominant-negative inhibitor can better overcome this interaction to dissociate endogenous Brd4L from chromatin. However, expression of Brd4L, Brd-(41–1046), BDI/II-(731–1046), or BDI/II-(1047–1362) at the similar level as BDI/II does not cause chromatin fragmentation, suggesting that the BDI/II phenotype is not likely to be an overexpression artifact.
The BDI/II-induced chromatin reorganization is in line with previous studies reporting a histone chaperone activity of Brd2 (30). Furthermore, expression of the short form of BrdT has been shown to induce large scale chromatin condensation in a trichostatin A-dependent manner (31). In addition to studying the chromatin morphology, we further determined the protein-protein interaction between Brd4 molecules and Brd4 with acetylated histones. This study thus provides the first mechanistic insight into Brd4 function as a chromatin adaptor to support molecular interactions on nucleosome array for chromatin structure maintenance. Our studies also identified the Brd4 C terminus as a critical domain for maintaining a properly condensed higher-order chromatin structure. Interestingly, among all the known human BET family proteins, Brd4L is the only member that carries the unique C terminus, the rest of the Brd4 domains are highly conserved in all BET proteins. The biological significance of this unique Brd4 domain remains to be further investigated.
Brd4 is targeted by a number of oncogenic viruses, highlighting the significance of its biological function in ensuring normal cellular growth and cellular defense against viral infection (19–23). In addition, translocation t(15;19) deletes the C-terminal half of the Brd4 long isoform, resulting in a novel fusion oncogene Brd4-Nut in a highly aggressive carcinoma (24). It is tempting to speculate that viral protein interactions and the Brd4-Nut oncogenic mutation may trigger structural and functional changes in Brd4, contributing to the oncogenic mechanism for the virus- and translocation-associated carcinomas. Dysregulation of Brd4-associated pathways has been shown to contribute to breast cancer progression (25). Brd4 has also been identified as an important therapeutic target for acute myeloid leukemia (26). The widespread presence of bromodomains in leukemogenic and cancer genes highlights the significance of studying bromodomain proteins in malignant diseases. Comprehensive understanding of the Brd4 function will provide a paradigm for investigating the molecular mechanisms of other bromodomain proteins. Important Brd4 activities uncovered in the context of higher-order chromatin organization and normal cellular growth may offer promising leads for developing efficient therapeutic strategies to cure Brd4-associated human cancers.
Supplementary Material
Acknowledgments
We thank Dr. Susan M. Janicki (The Wistar Institute) for the U2OS 2-6-5 cell line, Dr. Atsushi Miyawaki (RIKEN) for the Venus-pCS2 plasmid, and the members of our laboratory for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA148768 and R01CA142723 from the NCI (to J. Y.) and a fellowship from the China Scholarship Council (2007-3079) (to Q. L.).

This article contains supplemental Figs. S1–S5.
- aa
- amino acid(s)
- Brd4
- bromodomain 4
- MNase
- micrococcal nuclease
- CTD
- C-terminal domain
- IP
- immunoprecipitation
- BiFC
- bimolecular fluorescence complementation
- P-TEFb
- positive transcription elongation factor b
- AcH
- acetylated histone
- VN
- N terminus of Venus
- VC
- C terminus of Venus.
REFERENCES
- 1. Bickmore W. A., van der Maarel S. M. (2003) Perturbations of chromatin structure in human genetic disease. Recent advances. Hum. Mol. Genet 12, R207–213 [DOI] [PubMed] [Google Scholar]
- 2. Davis P. K., Brackmann R. K. (2003) Chromatin remodeling and cancer. Cancer Biol. Ther. 2, 22–29 [DOI] [PubMed] [Google Scholar]
- 3. Mohd-Sarip A., Verrijzer C. P. (2004) Molecular biology. A higher order of silence. Science 306, 1484–1485 [DOI] [PubMed] [Google Scholar]
- 4. Tremethick D. J. (2007) Higher-order structures of chromatin. The elusive 30 nm fiber. Cell 128, 651–654 [DOI] [PubMed] [Google Scholar]
- 5. Morales V., Giamarchi C., Chailleux C., Moro F., Marsaud V., Le Ricousse S., Richard-Foy H. (2001) Chromatin structure and dynamics. Functional implications. Biochimie 83, 1029–1039 [DOI] [PubMed] [Google Scholar]
- 6. Dorigo B., Schalch T., Kulangara A., Duda S., Schroeder R. R., Richmond T. J. (2004) Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306, 1571–1573 [DOI] [PubMed] [Google Scholar]
- 7. Schalch T., Duda S., Sargent D. F., Richmond T. J. (2005) X-ray structure of a tetranucleosome and its implications for the chromatin fiber. Nature 436, 138–141 [DOI] [PubMed] [Google Scholar]
- 8. Robinson P. J., Fairall L., Huynh V. A., Rhodes D. (2006) EM measurements define the dimensions of the “30-nm” chromatin fiber. Evidence for a compact, interdigitated structure. Proc. Natl. Acad. Sci. U.S.A. 103, 6506–6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelato K. A., Fischle W. (2008) Role of histone modifications in defining chromatin structure and function. Biol. Chem. 389, 353–363 [DOI] [PubMed] [Google Scholar]
- 10. Filetici P., Ornaghi P., Ballario P. (2001) The bromodomain. A chromatin browser? Front. Biosci. 6, D866–876 [DOI] [PubMed] [Google Scholar]
- 11. Mujtaba S., Zeng L., Zhou M. M. (2007) Structure and acetyl-lysine recognition of the bromodomain. Oncogene 26, 5521–5527 [DOI] [PubMed] [Google Scholar]
- 12. Dey A., Ellenberg J., Farina A., Coleman A. E., Maruyama T., Sciortino S., Lippincott-Schwartz J., Ozato K. (2000) A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2 to M transition. Mol. Cell. Biol. 20, 6537–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dey A., Chitsaz F., Abbasi A., Misteli T., Ozato K. (2003) The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. U.S.A. 100, 8758–8763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houzelstein D., Bullock S. L., Lynch D. E., Grigorieva E. F., Wilson V. A., Beddington R. S. (2002) Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22, 3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maruyama T., Farina A., Dey A., Cheong J., Bermudez V. P., Tamura T., Sciortino S., Shuman J., Hurwitz J., Ozato K. (2002) A mammalian bromodomain protein, Brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell Biol. 22, 6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishiyama A., Dey A., Miyazaki J., Ozato K. (2006) Brd4 is required for recovery from antimicrotubule drug-induced mitotic arrest. Preservation of acetylated chromatin. Mol. Biol. Cell 17, 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mochizuki K., Nishiyama A., Jang M. K., Dey A., Ghosh A., Tamura T., Natsume H., Yao H., Ozato K. (2008) The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 283, 9040–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z., He N., Zhou Q. (2008) Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell. Biol. 28, 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. You J., Croyle J. L., Nishimura A., Ozato K., Howley P. M. (2004) Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117, 349–360 [DOI] [PubMed] [Google Scholar]
- 20. You J., Srinivasan V., Denis G. V., Harrington W. J., Jr., Ballestas M. E., Kaye K. M., Howley P. M. (2006) Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80, 8909–8919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ottinger M., Christalla T., Nathan K., Brinkmann M. M., Viejo-Borbolla A., Schulz T. F. (2006) Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80, 10772–10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin A., Wang S., Nguyen T., Shire K., Frappier L. (2008) The EBNA1 protein of Epstein-Barr virus functionally interacts with Brd4. J. Virol. 82, 12009–12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu S. Y., Lee A. Y., Hou S. Y., Kemper J. K., Erdjument-Bromage H., Tempst P., Chiang C. M. (2006) Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 20, 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. French C. A., Miyoshi I., Aster J. C., Kubonishi I., Kroll T. G., Dal Cin P., Vargas S. O., Perez-Atayde A. R., Fletcher J. A. (2001) BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19). Am. J. Pathol. 159, 1987–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crawford N. P., Alsarraj J., Lukes L., Walker R. C., Officewala J. S., Yang H. H., Lee M. P., Ozato K., Hunter K. W. (2008) Bromodomain 4 activation predicts breast cancer survival. Proc. Natl. Acad. Sci. U.S.A. 105, 6380–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuber J., Shi J., Wang E., Rappaport A. R., Herrmann H., Sison E. A., Magoon D., Qi J., Blatt K., Wunderlich M., Taylor M. J., Johns C., Chicas A., Mulloy J. C., Kogan S. C., Brown P., Valent P., Bradner J. E., Lowe S. W., Vakoc C. R. (2011) RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukemia. Nature 478, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Z., Yik J. H., Chen R., He N., Jang M. K., Ozato K., Zhou Q. (2005) Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545 [DOI] [PubMed] [Google Scholar]
- 28. Bisgrove D. A., Mahmoudi T., Henklein P., Verdin E. (2007) Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc. Natl. Acad. Sci. U.S.A. 104, 13690–13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jang M. K., Mochizuki K., Zhou M., Jeong H. S., Brady J. N., Ozato K. (2005) The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 [DOI] [PubMed] [Google Scholar]
- 30. LeRoy G., Rickards B., Flint S. J. (2008) The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pivot-Pajot C., Caron C., Govin J., Vion A., Rousseaux S., Khochbin S. (2003) Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol. Cell. Biol. 23, 5354–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan J., Li Q., Lievens S., Tavernier J., You J. (2010) Abrogation of the Brd4-positive transcription elongation factor B complex by papillomavirus E2 protein contributes to viral oncogene repression. J. Virol. 84, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. You J., Li Q., Wu C., Kim J., Ottinger M., Howley P. M. (2009) Regulation of aurora B expression by the bromodomain protein Brd4. Mol. Cell Biol. 29, 5094–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan J., Diaz J., Jiao J., Wang R., You J. (2011) Perturbation of BRD4 protein function by BRD4-NUT protein abrogates cellular differentiation in NUT midline carcinoma. J. Biol. Chem. 286, 27663–27675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janicki S. M., Tsukamoto T., Salghetti S. E., Tansey W. P., Sachidanandam R., Prasanth K. V., Ried T., Shav-Tal Y., Bertrand E., Singer R. H., Spector D. L. (2004) From silencing to gene expression. Real-time analysis in single cells. Cell 116, 683–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura Y., Umehara T., Nakano K., Jang M. K., Shirouzu M., Morita S., Uda-Tochio H., Hamana H., Terada T., Adachi N., Matsumoto T., Tanaka A., Horikoshi M., Ozato K., Padmanabhan B., Yokoyama S. (2007) Crystal structure of the human BRD2 bromodomain. Insights into dimerization and recognition of acetylated histone H4. J. Biol. Chem. 282, 4193–4201 [DOI] [PubMed] [Google Scholar]
- 37. Kerppola T. K. (2006) Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 1, 1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 39. Farina A., Hattori M., Qin J., Nakatani Y., Minato N., Ozato K. (2004) Bromodomain protein Brd4 binds to GTPase-activating SPA-1, modulating its activity and subcellular localization. Mol. Cell Biol. 24, 9059–9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.