Abstract
OBJECTIVE
Impaired insulin sensitivity is linked to cognitive deficits and reduced brain size. However, it is not yet known whether insulin sensitivity involves regional changes in gray matter volume. Against this background, we examined the association between insulin sensitivity, cognitive performance, and regional gray matter volume in 285 cognitively healthy elderly men and women aged 75 years from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study.
RESEARCH DESIGN AND METHODS
Insulin sensitivity was calculated from fasting serum insulin and plasma glucose determinations using the homeostasis model assessment of insulin resistance (HOMA-IR) method. Cognitive performance was examined by a categorical verbal fluency. Participants also underwent a magnetic resonance imaging (MRI) brain scan. Multivariate analysis using linear regression was conducted, controlling for potential confounders (sex, education, serum LDL cholesterol, mean arterial blood pressure, and abdominal visceral fat volume).
RESULTS
The HOMA-IR was negatively correlated with verbal fluency performance, brain size, and temporal lobe gray matter volume in regions known to be involved in speech production (Brodmann areas 21 and 22, respectively). No such effects were observed when examining diabetic (n = 55) and cognitively impaired (n = 27) elderly subjects as separate analyses.
CONCLUSIONS
These cross-sectional findings suggest that both pharmacologic and lifestyle interventions improving insulin signaling may promote brain health in late life but must be confirmed in patient studies.
A high density of insulin receptors occurs in the temporal lobe, a brain region associated with memory functions (1). In addition to its essential role for declarative memory (i.e., the ability to recollect facts and events) (2), the temporal lobe has also been linked to verbal fluency (VF) (3). Highlighting an important role of insulin for temporal lobe–dependent functions, previous studies show that intranasal insulin, providing a direct route to the brain (4), improves declarative and verbal memory in both healthy and cognitively impaired humans (5,6). Contrasting these direct effects of insulin on central nervous system (CNS) function, reduced peripheral insulin sensitivity has been linked to subtle cognitive deficits and reduced spontaneous cortical activity in otherwise cognitively healthy humans (7,8), suggesting that the ability of insulin to benefit memory functions is attenuated when peripheral insulin signaling is disrupted.
Against this background, in the present community-based study of elderly men and women, we aimed to examine, by using linear regression models, whether cognitive functions are linked to fluctuations in fasting insulin sensitivity, as indexed by the homeostasis model assessment (HOMA) (9). To this aim, we administered the 7-minute screen (7MS) test, a brief cognitive instrument used for clinical assessment of cognitive ability (10). The 7MS consists of four components involving temporal lobes: Benton temporal orientation (BTO) (11), enhanced cued recall (ECR) (12), clock drawing (CD) (13), and a categorical VF test. Previous findings show that insulin has neuroprotective properties in that it reduces the attachment of amyloid-β–derived diffusible ligands at neural synapses (14). This process is widely accepted to play a prominent role in triggering cognitive deficits and neurodegeneration (15). Thus, in addition to the cognitive measures, we examined magnetic resonance imaging (MRI) brain scans for links between alterations in insulin sensitivity and changes in total and regional brain volume. Our main question concerned how fluctuations in peripheral insulin sensitivity link to changes in cognition and brain structure in a cognitively healthy elderly population. To this aim, cognitively impaired subjects were not considered eligible for the main analysis (i.e., those who have a mini–mental examination score <27) (16) and, thus, were tested separately from the main cohort. Furthermore, since insulin sensitivity in diabetic subjects treated with insulin, or in those taking insulin secretagogues, might simply reflect the mechanism of action of the drug (17), those who were diabetic patients were also tested separately.
On the basis of the memory-improving and neuroprotective properties of insulin (5,6,14), it was hypothesized that HOMA scores would negatively correlate with brain structure and cognitive function.
RESEARCH DESIGN AND METHODS
Setting and participants
Data was derived from the project Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) (18), a population-based prospective cohort study that initially included 1,016 (50% females) individuals aged 70 years living in the community of Uppsala, Sweden. After their inclusion between 2001 and 2003, subjects participated in a follow-up investigation 5 years later (i.e., at age 75).
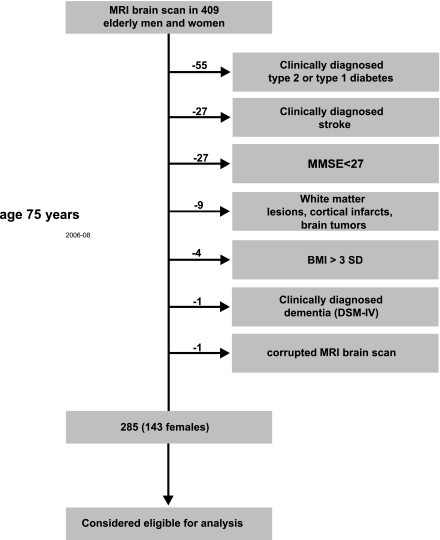
To minimize potential confounding of variables generally related to our main measures (e.g., cognition, insulin sensitivity, and brain volume), the following factors were used to exclude subjects from analysis of the main cohort: type 1 and type 2 diabetes (n = 55); stroke (n = 27); mini–mental state examination score <27, indicating cognitive impairment (n = 2); white matter lesions, cortical infarcts, and tumors (n = 9); excessive body weight (>3 SD from the population mean, n = 3); and dementia (n = 1). On the basis of these exclusion criteria, 285 elderly participants (134 females) were considered eligible for analysis (corresponding 70% of 409 subjects who agreed to undergo an MRI scan at age 75 years) (Fig. 1). Note that the participants in this subsample, as a result of time and cost constraints, were randomly assigned to an MRI session. Independent t tests and χ2 tests yielded no significant physical or cognitive characteristic differences between the MRI subsample and the whole cohort with similar exclusion criteria, indicating a representative subsample (Table 1). The study was approved by the ethics committee of the Faculty of Medicine, Uppsala University. All participants gave their written informed consent. The study was conducted according to the Declaration of Helsinki.
Figure 1.
Subject inclusionary criteria and sample sizes. Initially, 1,016 individuals (509 females) were examined at age 70 years. From these individuals, 409 underwent an MRI whole-brain scan at age 75. At the same age, the participant’s ability to generate spontaneously words of a certain category was tested by means of a VF task. To ensure a cognitively healthy population, those participants with a history of dementia or stroke, a diagnosis of type 1 or type 2 diabetes, a BMI >3 SD, pathological lesions on MRI (including arachnoidal cyst, bilateral hygroma, meningioma, and schwannoma), and who scored <27 on the mini–mental state examination (MMSE) to control for mild cognitive impairment were excluded. Furthermore, we examined each individual’s MRI scan and excluded one that was affected by movement artifacts. Thus, 285 elderly men and women were considered eligible to analyze the association between the HOMA-IR, cognitive functions, and gray matter volume at age 75 years.
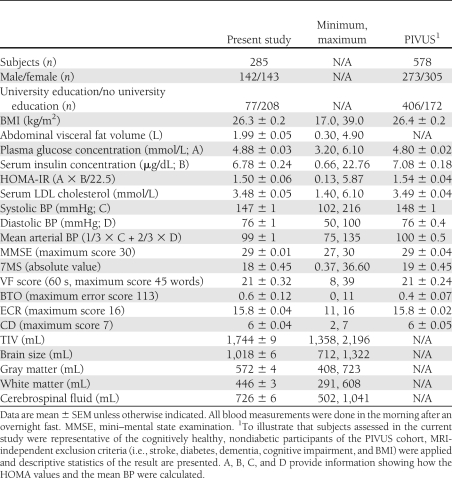
Table 1.
Descriptive characteristics of study participants aged 75 years
Clinical and biochemical investigation
At age 75 years, blood samples were collected in the morning after an overnight fast. No medication or smoking was allowed after midnight. The instrument used for biochemical analysis was an Architect (Abbott, Abbott Park, IL), unless otherwise stated. After recording height and weight, allowing the calculation of BMI (kg/m2), the subjects were supine in a quiet room maintained at a constant temperature, and blood pressure (BP) was measured by a calibrated mercury sphygmomanometer in the noncannulated arm to the nearest mmHg. The systolic and diastolic BP of this recording were used to calculate the mean arterial BP in the current study. LDL cholesterol was calculated using Friedewald formula. The abdominal visceral fat volume was measured by abdominal MRI and was quantified by an automated postprocessing approach, as previously described (19). The educational level (i.e., university level vs. no university level education) for each subject was assessed by means of a standardized questionnaire.
7MS test
At age 75 years, a Swedish translation of the 7MS test was administered to the participants by trained study nurses. This test is clinically used to screen for dementia and cognitive decline and has been described previously (10). The 7MS consists of four brief cognitive tests:
BTO (11): In this test, the orientation in time is measured and quantified in degree of error. The maximum score is 113 (10 error points for 1 year, 5 points for 1 month, 1 point for the date and the day of a week, and 1 point for each 30-min deviation in time).
ECR (12): This test requires the subject to recall 16 figures. The score is the total number of items recalled in both free and cued recall (range 0–16).
CD (13): In this test, the subject has to draw the face of a clock and place the hands of the clock at a given time. The maximum score is 7 points.
Categorical VF: This test requires the subject to name as many different animals as possible in 1 min. There is a maximum score of 45.
The raw scores of the four subtests of the 7MS were summed with the logistic regression formula described previously (11):
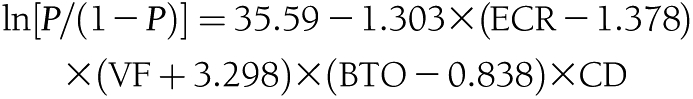 |
where P is the probability of having dementia. Solomon et al. (10) estimated the formula by using the scores of the four tests from the screening battery as predictor variables. The ln of P/(1 − P) is equal to the total 7MS score of the above logistic regression formula. In the current study, total scores obtained by the formula are presented as absolute numbers (i.e., the lower the obtained value, the higher the probability of having dementia). According to Solomon et al. (10), an absolute total score of >4.6 equals a probability of having dementia <1%.
MRI acquisition and processing
At age 75 years, regional measures of brain volume were acquired with MRI. A high-resolution three-dimensional T1-weighted volumetric turbo field echo scan was acquired using a Philips 1.5 Tesla scanner (Gyroscan NT; Philips Medical Systems, Best, the Netherlands). The three-dimensional gradient echo sequence was used with scan parameters TR 8.6 ms, TE 4.0 ms, and flip angle 8°. Sagittal slices with a field of view of 240 × 240 mm, a slice thickness of 1.2 mm, and an in-slice resolution of 0.94 mm2 were reconstructed.
Images were processed using voxel-based morphometry (VBM), an MRI-based technique that uses statistical parametric mapping to determine local concentrations of gray matter volume on a voxel-by-voxel basis (20). Morphological changes in gray matter were calculated by segmenting gray matter from white matter and cerebrospinal fluid using the unified segmentation approach (21). After this segmentation procedure, probability maps of gray matter were “modulated” to account for the effect of spatial normalization by multiplying the probability value of each voxel by its relative volume in native space before and after warping. Gray matter images based on probability maps at each voxel were normalized into Montreal Neurological Institute (MNI) standard space with a voxel size of 2 mm × 2 mm × 2 mm. Modulated images were smoothed with an 8-mm full-width half-maximum Gaussian kernel, in line with other recent VBM studies (22). This smoothing kernel was applied prior to statistical analysis to reduce signal noise and to correct for image variability. VBM analyses were carried out using SPM8 (University College London, London, U.K.) (20).
Statistical analysis
Data were analyzed using multivariate linear regression models. Normal distribution of all variables was confirmed by Kolmogorov-Smirnov testing. Because of a skewed distribution, the HOMA of insulin resistance (HOMA-IR) variable was included in the multivariate linear regression models as ln-transformed index: ln [100 × (x − min)/(max − min)] (Kolmogorov-Smirnov P > 0.49). Unless otherwise specified, HOMA shall refer to the transformed index score for HOMA-IR. The primary analysis (model A) was a multivariate linear regression adjusting for sex to examine the association between HOMA and cognitive functions and gray matter volume, respectively. To assess the robustness of the association between these variables, in secondary analyses, we additionally adjusted for factors that have been previously described to be associated with cognition and brain structure in the general population (model B): education (23), serum concentration of LDL cholesterol (24), mean arterial BP (25), and abdominal visceral fat volume (26). To control for individual differences in head size, the VBM regression models described above were both additionally controlled for the total intracranial volume (TIV; defined as the sum of gray matter, white matter, and cerebrospinal fluid). All clusters and peak voxels of gray matter t statistic brain maps reported were thresholded at P < 0.05 by using family-wise error (FWE). To avoid possible edge effects between different tissue types, we excluded from our analyses all voxels with gray matter probabilities of <0.1 (absolute threshold masking). For cognitive measurements, the level of significance was set to P < 0.05.
RESULTS
Descriptive characteristics of participants, subject inclusionary criteria, and sample sizes are shown in Table 1 and Fig. 1, respectively.
7MS score and VF performance
Regression analyses in the main cohort revealed a negative association between the HOMA and the score that was obtained on the 7MS (model A: β = −0.154, SE = 0.69, P = 0.01; model B: β = −0.188, SE = 0.77, P = 0.005). Using model A (i.e., covarying for sex), further regression analysis revealed that the negative association between the HOMA and the 7MS test score was associated with a variance in the VF subtest score (β = −0.165, SE = 0.75, P = 0.005). This effect also remained in cumulative regression model B (β = −0.187, SE = 0.012, P = 0.005).
Brain volumes
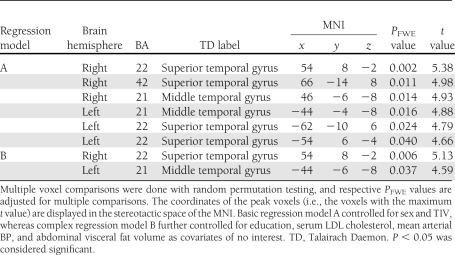
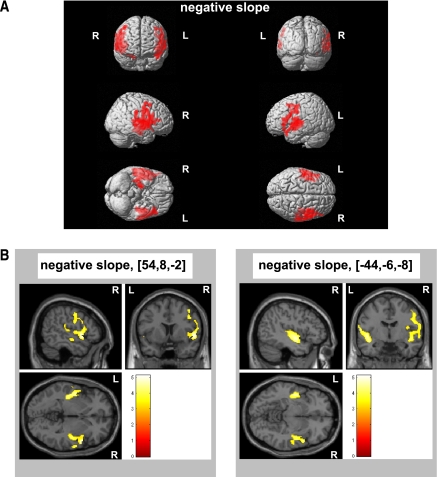
Significant peak voxel statistics and MNI coordinates are presented in Table 2. The VBM regression analysis showed that the HOMA was negatively correlated with two major clusters in model A (i.e., covarying for sex and TIV) comprising Brodmann areas (BAs) 22, 42, and 21 of the right temporal lobe (cluster size: 3,715 voxels, PFWE = 0.0001) and BAs 21 and 22 of the left temporal lobe (cluster size: 2,321 voxels, PFWE = 0.0001) (Fig. 2A). Using cumulative model B (i.e., covarying for sex, education, serum concentration of LDL, mean arterial BP, abdominal visceral fat volume, and TIV), both clusters survived FWE correction (cluster size: right temporal lobe, 2,231 voxels, PFWE = 0.0001; left temporal lobe, 948 voxels, PFWE = 0.0001) (Fig. 2B). The VBM regression analysis did not produce positive associations between the HOMA and gray matter volume.
Table 2.
Peak voxel statistics for the negative association between insulin resistance and regional gray matter
Figure 2.
Insulin resistance is linked to deficits in temporal lobe gray matter volume in elderly men and women. VBM in 285 elderly men and women aged 75 years illustrates a significant inverse association between insulin resistance as indexed by the HOMA-IR and two major clusters situated in the left and right temporal lobe of the brain, as indicated by colored areas. A: Basic regression model rendering controlled for sex and TIV. B: Complex regression model further controlled for education, serum LDL concentration, mean arterial BP, and abdominal visceral fat volume as covariates of no interest. Neurologic sections were focused at the significant peak voxels (MNI coordinates shown in brackets), and the colored bar indicates the t values. All clusters illustrated were thresholded at P < 0.05 by using FWE. R, right brain hemisphere; L, left brain hemisphere. (A high-quality digital representation of this figure is available in the online issue.)
In line with recent observations (27), both model A (i.e., covarying for sex and TIV) and model B (i.e., covarying additionally for education, serum concentration of LDL, mean arterial BP, and abdominal visceral fat volume) revealed that the HOMA was inversely linked to the total brain volume (i.e., gray matter volume + white matter volume) (β = −0.096, SE = 5.928, P = 0.008 and β = −0.102, SE = 6.797, P = 0.013, respectively).
For all analyses presented, the interaction term of the HOMA variable by sex did not reach significance. Regarding the cognitively impaired (n = 27) and diabetic subgroups (n = 55), separate regression analyses (using model A and B) revealed no significant association between the HOMA score on one hand and cognitive functions and brain structure on the other hand.
CONCLUSIONS
Insulin sensitivity as indexed by the HOMA value in our community-based data shows strong links to deficits in speech production. We are the first to demonstrate this in a negative association between the HOMA value and VF scores in cognitively healthy, nondiabetic elderly men and women. Furthermore, we found that HOMA is negatively coupled with gray matter volume in the temporal lobe (specifically BAs 21 and 22) brain regions that support language ability (3,28,29). Corroborating previous findings (27), we show that the HOMA value is negatively correlated with total brain size as well. In contrast, separate analyses in the cognitively impaired and diabetic subgroups did not produce significant associations. These results provide evidence that fluctuations in insulin sensitivity in cognitively healthy, nondiabetic elderly men and women (independent of sex effects) are linked to changes in cognitive performance and brain volume.
Both impaired glucose tolerance and increased HOMA values have been previously associated with subtle impairments in cognitive functions, including VF (30,31). We found a similar pattern in that the HOMA value was inversely associated with the VF test score. The HOMA value was also negatively linked to a reduction in gray matter volume, specifically to BA 22 and BA 21 of the right and left temporal lobes, respectively. These brain areas play an important role in language processing and speech production (3,28,29). This is intriguing inasmuch as disturbed speech production is a common trait of Alzheimer disease (32), a disease that has been previously linked to CNS insulin resistance (33). However, a larger set of cognitive tests that assess brain functions involving language should be examined in the future to determine if they also show similar associations with insulin resistance and temporal lobe gray matter reduction.
The cross-sectional nature of our study precludes any assumption about the cause and effect relationship between variables. However, there is convincing evidence that CNS insulin signaling enhances synaptic long-term potentiation (34), modulates the action of neurotransmitters involved in memory processing (e.g., norepinephrine and acetylcholine) (35), and attenuates cortisol secretion (36). Moreover, insulin has been shown to mitigate pathological binding of amyloid-β–derived diffusible ligands (potent CNS neurotoxins) to synapses of neurons (14). Thus, a condition of peripheral insulin resistance may represent a metabolic state in that the ability of insulin to exert its neuroprotective and neuromodulatory effects in the CNS is disrupted. This may therefore accelerate the process of neurodegeneration in brain regions that are sensitive to changes in CNS insulin signaling, such as the temporal lobes, which express high densities of insulin receptors (1). Supporting this assumption, peripheral insulin resistance has been associated with reduced insulin transport across the blood-brain barrier (37); reduced cerebral glucose metabolic rate in frontal, parietotemporal, and cingulate brain regions (7); and reduced cortical excitability in response to intravenous insulin infusion (8). Furthermore, a chronically elevated blood glucose concentration, the hallmark metabolic abnormality associated with somatic insulin resistance, has been shown to accelerate formations of advanced glycation end products, which are hypothesized to promote the development of Alzheimer disease (38). However, alternative mechanisms may also play a role in the observed associations in elderly people. For instance, since vascular damage is linked to deficits in cognitive function and brain volume (39), the negative associations between the HOMA score and both cognitive function and brain structure might be secondary to the detrimental effect of impaired insulin sensitivity on vascular health (40).
In summary, our results provide a potential rationale for hypothesizing that interventions improving insulin sensitivity, such as exercise, are attractive strategies to deter cognitive ageing in cognitively healthy, nondiabetic elderly men and women. However, we did not find any cross-sectional association between the HOMA score, cognitive function, and brain structure in the cognitively impaired and diabetic subgroups. These negative findings are most probably due to the relatively low sample size available in these subpopulations, but they also might be caused by the biasing influence of antidiabetes and psychopharmacologic treatments. Thus, when interpreting our main results (i.e., that fluctuations in insulin sensitivity are associated with speech production and temporal lobe gray matter volume in elderly men and women), caution is warranted. The reader must be careful in making assumptions about the influence of insulin sensitivity on cognitive performance and brain structure in cognitively impaired and diabetic patients.
Acknowledgments
This study was funded by the Swedish Research Council, the Brain Research Foundation (Sweden), the Olle Engkvist Byggmästare Foundation (Sweden), the Tore Nilsons Foundation (Sweden), the Gunvor och Josef Anérs Foundation (Sweden), the Åke Wibergs Foundation (Sweden), the Åhlens Foundation (Sweden), and Uppsala University (Sweden).
Funding for the study also was provided by Novo Nordisk (Denmark) and AstraZeneca (Sweden). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript. No other potential conflicts of interest relevant to this article were reported.
C.B. and S.J.B. contributed to study concept and design, analyzed and interpreted data, contributed to statistical analysis, and wrote the manuscript. J.K. contributed to study concept and design; acquired, analyzed, and interpreted data; contributed to statistical analysis; and critically revised the manuscript for important intellectual content. J.B. contributed to study concept and design, analyzed and interpreted data, contributed to statistical analysis, and wrote and critically revised the manuscript for important intellectual content. M.J.K. and R.No. analyzed and interpreted data, contributed to statistical analysis, and critically revised the manuscript for important intellectual content. R.Ny. contributed to study concept and design, acquired data, and critically revised the manuscript for important intellectual content. L.K. acquired data and critically revised the manuscript for important intellectual content. S.C. analyzed and interpreted data and critically revised the manuscript for important intellectual content. E.-M.L., L.J., and H.A. contributed to study concept and design; acquired data; obtained funding; provided administrative, technical, or material support; critically revised the manuscript for important intellectual content; and supervised the study. L.L. contributed to study concept and design, acquired data, analyzed and interpreted data, obtained funding, critically revised the manuscript for important intellectual content, and supervised the study. H.B.S. contributed to study concept and design, analyzed and interpreted data, obtained funding, critically revised the manuscript for important intellectual content, and supervised the study.
All authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis. C.B. is the guarantor of this work.
References
- 1.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978;272:827–829 [DOI] [PubMed] [Google Scholar]
- 2.Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus 2006;16:795–808 [DOI] [PubMed] [Google Scholar]
- 3.Pihlajamäki M, Tanila H, Hänninen T, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 2000;47:470–476 [PubMed] [Google Scholar]
- 4.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 5.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004;29:1326–1334 [DOI] [PubMed] [Google Scholar]
- 6.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008;70:440–448 [DOI] [PubMed] [Google Scholar]
- 7.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tschritter O, Preissl H, Hennige AM, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci U S A 2006;103:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med 2002;19:527–534 [DOI] [PubMed] [Google Scholar]
- 10.Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol 1998;55:349–355 [DOI] [PubMed] [Google Scholar]
- 11.Benton AL. Contributions to Neuropsychological Assessment. New York, : Oxford University Press, 1983 [Google Scholar]
- 12.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;38:900–903 [DOI] [PubMed] [Google Scholar]
- 13.Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis D. Clock Drawing: A Neuropsychological Analysis. New York, : Oxford University Press,; 1994. [Google Scholar]
- 14.De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 2009;106:1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krafft GA, Klein WL. ADDLs and the signaling web that leads to Alzheimer’s disease. Neuropharmacology 2010;59:230–242 [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 18.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol 2005;25:2368–2375 [DOI] [PubMed] [Google Scholar]
- 19.Kullberg J, Ahlström H, Johansson L, Frimmel H. Automated and reproducible segmentation of visceral and subcutaneous adipose tissue from abdominal MRI. Int J Obes (Lond) 2007;31:1806–1817 [DOI] [PubMed] [Google Scholar]
- 20.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851 [DOI] [PubMed] [Google Scholar]
- 22.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010;31:1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foubert-Samier A, Catheline G, Amieva H, et al. Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging 2012;33:423 [DOI] [PubMed]
- 24.Ward MA, Bendlin BB, McLaren DG, et al. Low HDL cholesterol is associated with lower gray matter volume in cognitively healthy adults. Front Aging Neurosci 2010;2: 29 [DOI] [PMC free article] [PubMed]
- 25.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI, SMART Study Group Blood pressure, cerebral blood flow, and brain volumes. The SMART-MR study. J Hypertens 2010;28:1498–1505 [DOI] [PubMed] [Google Scholar]
- 26.Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol 2010;68:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 2011;34:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 2010;1191:62–88 [DOI] [PubMed] [Google Scholar]
- 29.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 2004;92:67–99 [DOI] [PubMed] [Google Scholar]
- 30.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging 2011;32:1942–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A 2003;100:2019–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folstein MF, Whitehouse PJ. Cognitive impairment of Alzheimer disease. Neurobehav Toxicol Teratol 1983;5:631–634 [PubMed] [Google Scholar]
- 33.Hallschmid M, Schultes B. Central nervous insulin resistance: a promising target in the treatment of metabolic and cognitive disorders? Diabetologia 2009;52:2264–2269 [DOI] [PubMed] [Google Scholar]
- 34.Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging 2009;30:377–387 [DOI] [PubMed] [Google Scholar]
- 35.Gerozissis K. Brain insulin: regulation, mechanisms of action and functions [corrected to Kyriaki G. in: Cell Mol Neurobiol 2003;23:873–874]. Cell Mol Neurobiol 2003;23:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282 [DOI] [PubMed] [Google Scholar]
- 37.Kern W, Benedict C, Schultes B, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 2006;49:2790–2792 [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi M, Yamagishi S. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des 2008;14:973–978 [DOI] [PubMed] [Google Scholar]
- 39.Miralbell J, Soriano JJ, Spulber G, et al. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol Aging. 18 October 2011 [Epub ahead of print] [DOI] [PubMed]
- 40.Petrie JR, Ueda S, Webb DJ, Elliott HL, Connell JM. Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation 1996;93:1331–1333 [DOI] [PubMed] [Google Scholar]