Abstract
This article provides an overview of recent advances in chemotherapy that may be used for the treatment of patients with locally advanced or metastatic breast cancer (mbc). Key phase ii and iii trial data for eribulin mesylate, ixabepilone, and nab-paclitaxel, published since 2006, are discussed on the basis of recency, depth, and quality.
Eribulin mesylate is the first monotherapy to significantly increase overall survival in patients with pretreated mbc, but nab-paclitaxel offers a novel and safer mode of delivery in comparison with standard taxanes. By contrast, the use of ixabepilone will be limited for now, until the associated neurotoxicity can be better managed. Alongside a brief overview of the other major chemotherapies currently in use, we have aimed to provide a Canadian context for how these novel agents may be integrated into clinical practice.
Keywords: Pretreated, breast cancer, metastatic, mbc, chemotherapy, eribulin, ixabepilone, nab-paclitaxel
1. INTRODUCTION
Breast cancer is a leading cause of cancer mortality in women throughout many countries 1. Worldwide, more than 1 million new cases of breast cancer are diagnosed annually; in the developed world, one third of women with early-stage breast cancer will develop advanced disease 2,3. Because of breakthroughs in early detection and treatment, mortality rates from breast cancer have been declining steadily since the early 1990s 1. However, metastatic breast cancer (mbc) remains a major therapeutic challenge 4. Despite significant advances, this condition is still considered an incurable disease, with a median survival of 2–3 years 2.
Metastatic breast cancer may present itself in a variety of clinical scenarios, ranging from solitary metastatic lesions to diffuse and multiple organ involvement 5. Growing knowledge of tumour biology informs the understanding of prognostic and predictive factors, and continues to guide therapeutic decisions 2,5,6. Furthermore, new knowledge of the biologic heterogeneity of the disease permits a consideration of advanced breast cancer as a number of separate disease entities, to which therapy can be accordingly tailored 5,6.
Several molecular subtypes of mbc have been identified 7. Hormone-sensitive mbc is defined by the expression of the estrogen receptor or the progesterone receptor, or both 2. For women with this type of breast cancer, hormone therapy is often preferred because it offers a targeted approach with a favourable side-effect profile and general ease of administration. A second subtype is characterized by amplification or overexpression of the human epidermal growth factor receptor 2 (her2) 4,8. Metastatic breast cancer positive for her2 is a particularly aggressive clinical phenotype and represents up to 20% of invasive breast cancers 8. Patients with this subtype generally benefit from anti-her2 targeted therapy. In the mbc setting, cytotoxic chemotherapy is often used to treat triple-negative breast cancer (tnbc), defined as disease negative for her2 as well as for the estrogen and progesterone receptors 7. The tnbc subtype comprises a smaller fraction (10%–15%) of invasive breast carcinomas with an aggressive phenotype associated with poor prognosis and limited treatment options 1,7,9.
2. CHEMOTHERAPY FOR MBC: THE CURRENT STATE OF THE ART
The goals in mbc management are primarily disease control and palliation of symptoms, combined with a good quality of life for the patient 4,5. Long-term survival benefit also constitutes a significant objective, but the availability of multiple therapies and the use of crossover designs in various trials have made that outcome an elusive one 5,10. The choice of therapy for patients with mbc typically depends on the risks and benefits of each treatment option, the disease burden and subtype, prior therapeutic exposure, availability, and patient and physician preference 11,12. Hormonal and biologic therapies are widely used to treat patients with endocrine receptor– or her2-positive disease subtypes, but those modalities are beyond the scope of the present review. Our focus here is limited to the use of novel chemotherapeutic agents in the treatment of her2-negative mbc. In addition, we focus on novel single-agent therapies, because combinations are not routinely used and are often reserved for the small proportion of patients with symptomatic visceral involvement.
Since the 1960s, numerous chemotherapeutic agents and combination regimens have been investigated for the treatment of mbc 12–14. Early trials studied chemotherapy in patients who had not received prior adjuvant treatment 13. As adjuvant chemotherapy became increasingly common, anthracycline- and taxane-based regimens became embedded in practice as standard treatments in the adjuvant or neoadjuvant setting 12,13,15. Thus, the choice of therapy in the metastatic setting is now increasingly influenced by issues of cumulative toxicity and resistance. Debate continues over the value of combination chemotherapy compared with sequential monotherapy in mbc 13.
Inter-patient and inter-trial variability complicate efficacy and tolerability analyses of new agents 12. Treatment guidelines have not deemed any particular chemotherapeutic regimen superior for second- or further-line treatment, and no third-line or later standard of care has been established for treatment of mbc 1,12,14,16. However, many patients with mbc do benefit from later lines of therapy. Dufresne and colleagues 17 demonstrated that 40% of patients achieve disease control for more than 6 months with third-line chemotherapy 12. Currently, the only widely approved monotherapies for late-line treatment are ixabepilone (in the United States) and capecitabine 1,12,18. In addition, recent findings from studies of eribulin mesylate and nab-paclitaxel in patients who have received multiple lines of chemotherapy warrant attention. Various other agents—including liposomal doxorubicin, gemcitabine, and (with renewed interest) platinum analogs—have been studied for the treatment of mbc 19–26. However, although those agents may also be considered potential alternatives for the treatment of mbc, they have not been engaged as the standard of care, and limited prospective data have been generated to enable a systematic review of their efficacy in that setting.
Therefore, in our view, three main chemotherapeutic agents have emerged out of the most recent prospective clinical efforts to develop new alternatives for the management of mbc: eribulin mesylate, ixabepilone, and nab-paclitaxel. Considering the depth and quality of the data available on those agents, they are the focus of the present review. After a discussion of the clinical data, we consider how these novel agents may be incorporated into Canadian clinical practice.
3. ADVANCES IN CHEMOTHERAPY
3.1. Eribulin Mesylate: An Analog of a Natural Product
Recent studies have demonstrated that eribulin mesylate (E7389), a non-taxane inhibitor of microtubule dynamics, may be effective in patients with mbc resistant to other tubulin-targeting agents 12,27. Eribulin is a structurally modified synthetic analog of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai. It acts by driving the formation of nonfunctional tubulin aggregates, thereby depleting tubulin stores 12,27,28. In addition, in contrast to a number of other microtubule inhibitors, eribulin caps the end of microtubules, blocking polymerization without affecting depolymerization 12,27. As a result, it delivers two “hits” to induce apoptosis and eventual cell death. Moreover, eribulin has a rapid infusion time, is water-soluble, and requires no pre-medication for hypersensitivity.
3.1.1. Clinical Trials
After encouraging results from phase i and ii studies, Cortes and colleagues 27 conducted a global multicentre phase iii randomized open-label study of eribulin in mbc (Table i) 12. That study, known as the embrace (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) trial, aimed to compare overall survival (os) in pretreated women with mbc receiving eribulin or a treatment of the physician’s choice (tpc). The tpc group received a single agent chosen to emulate clinical practice in the management of patients with mbc 27. Eligible patients had received between two and five previous chemotherapy regimens, including an anthracycline and a taxane (two or more for advanced disease).
TABLE I.
Summary of phase ii and iii trials of eribulin mesylate in patients with metastatic breast cancer previously treated with two or more chemotherapy regimens
| Reference | Study design | Primary endpoint | Patient population | Treatment groups | Regimen | rr(%) | Median pfs or ttp (months) | Median os (months) | Grade 3/4 toxicities (%) |
|---|---|---|---|---|---|---|---|---|---|
| Vahdat et al., 2009 29 | Single arm Open-label Phase ii | orrd | Median of 4 prior chemotherapy treatments (range: 1–11)c | Eribulin (n=87) | Eribulin mesylate 1.4 mg/m2 over 2–5 minutes on days 1, 8, and 15 of a 28-day cyclee | 11.5 | 2.6 (pfs) | 9.0 | Neutropenia: 64 Leukopenia: 18 Fatigue: 5 Peripheral neuropathy: 5 Febrile neutropenia: 4 |
| Cortes et al., 2010 30 | Single-arm Open-label Phase ii | orrd | Median of 4 prior chemotherapy treatmentse | Eribulin (n=291) | Eribulin mesylate 1.4 mg/m2 over 2–5 minutes on days 1 and 8 of a 21-day cycle | 9.3 | 2.6 (pfs) | 10.4 | Neutropenia: 54 Febrile neutropenia: 5.5 Leukopenia: 14 Asthenia/fatigue: 10 Neuropathy (grade 3): 6.9 |
| Cortes et al., 2011 27 | Randomized Open-label Phase iii | os | 2–5 Prior chemotherapy treatments (≥2 for locally recurrent or metastatic breast cancer)a | Eribulin (n=508) tpc (n=254) |
Eribulin mesylate 1.4 mg/m2 over 2–5 minutes on days 1 and 8 of a 21-day cycle vs. tpc | 12 vs. 5b (p=0.002) | 3.6 vs. 2.2 (pfs) (p=0.002)a 3.7 vs. 2.2 (pfs) (p=0.137) |
13.1 vs. 10.6 (p=0.137) | Asthenia/fatigue: 54 vs. 40 Neutropenia: 52 vs. 30 Peripheral neuropathy (grade 3): 8 vs. 2c |
Prior chemotherapy treatments included anthracyclines, taxanes, capecitabine, vinorelbine, gemcitabine, cyclophosphamide, fluorouracil, carboplatin or cisplatin, methotrexate.
Objective response rate, including complete and partial responses.
Most common event leading to discontinuation—includes peripheral motor, sensory, sensorimotor, or demyelinating neuropathy; polyneuropathy; and paresthesia.
Assessed by independent review.
21-Day cycle after protocol amendment for improved tolerability.
rr = response rate; pfs = progression-free survival; ttp = time to progression; os = overall survival; tpc = treatment of physician’s choice; orr = overall response rate.
Using a 2:1 randomization scheme, 508 patients were assigned to the eribulin treatment group, who received a 2- to 5-minute intravenous bolus of 1.4 mg/ m2 eribulin mesylate on days 1 and 8 of a 21-day cycle for a median of 3.9 months (Table i) 12,27. The 254 patients randomized to the tpc group received single-agent chemotherapy (most commonly vinorelbine, gemcitabine, or capecitabine; n = 238, 93.7%), hormonal therapy (n = 9, 3.5%), or biologic therapy approved for the treatment of cancer and administered according to local practice. No patient received supportive care alone.
Of the 762 patients overall, 386 (51%) had metastatic disease involving three or more organs, with the most common metastatic sites being bone and liver. Patients with known brain metastases were not included in the study unless treated and stable.
The embrace trial demonstrated a significant increase in os for eribulin compared with tpc [hazard ratio (hr): 0.81; 95% confidence interval (ci): 0.66 to 0.99; p = 0.041] 27. Median os was 13.1 months (95% ci: 11.8 to 14.3 months) for patients receiving eribulin, and 10.6 months (95% ci: 9.3 to 12.5 months) for patients in the tpc group (Table i). Secondary endpoints were based on independent masked review of tumour assessments and were found to be generally consistent with the primary endpoints 12,27. Also based on that review, a nonsignificant increase in progression-free survival (pfs) was found in the eribulin group. By contrast, when pfs was evaluated according to an investigator assessment of the intention-to-treat population (conducted for the purpose of sensitivity analyses), pfs prolongation was found to be significant in the eribulin group compared with the tpc group (3.6 months vs. 2.2 months, Table i) 27. This apparent difference arose from the censoring of almost twice as many patients in the independent review as in the investigator review (241 vs. 127). Study scans stopped once the investigator declared disease progression, leading to many censored patients in the independent review, in which non-measurable disease could be assessed for progression only if nontarget lesions progressed or new lesions appeared. More progression events were therefore included in the investigator review than in the independent review (635 vs. 521).
Consistent with phase i and ii trials, eribulin exhibited a manageable toxicity profile 27. Adverse events (aes) occurred in 497 of 503 patients (99%) receiving eribulin and in 230 of 247 patients (93%) receiving tpc. The overall incidence of aes, treatment discontinuations, and treatment-related fatalities were comparable between the treatment groups 12,27. The most common aes in both arms were asthenia (or fatigue) and neutropenia, most of which were grade 1 or 2. Grade 3 or 4 events that were more frequent in the eribulin group than in the tpc group were leukopenia, neutropenia, and peripheral neuropathy (Table i). Notably, however, the incidences of grade 3 and 4 febrile neutropenia and peripheral neuropathy were low; both were generally manageable with dose modifications.
3.1.2. Conclusions
The results of the embrace study establish eribulin mesylate as a potential new alternative for women with pretreated (including an anthracycline and a taxane) mbc—a patient population for whom there was previously no single-agent chemotherapy treatment with demonstrated survival benefit 12,27. To date, eribulin is the only agent that has been shown, when administered as monotherapy, to prolong os in pretreated patients with mbc. In addition to a manageable toxicity profile, eribulin also offers a clinically significant 2.5-month increase in median survival for such patients 27. It should be noted that, in the embrace trial, patients received a median of four prior chemotherapy regimens, and more than 50% of patients had metastatic involvement of three or more organs. The overall objective response rate (orr) in patients treated with eribulin was 12% [compared with 5% in the tpc arm (p = 0.002)], including three complete responses (crs). No crs were seen with tpc. It might be argued that the choice of tpc as a comparator arm precluded detailed comparisons with eribulin and yielded limited quality-of-life interpretations, because tpc included a number of chemotherapies with unique benefits and toxic effects. But, despite the limitations, investigation of tpc as a comparator arm is clinically relevant because the results reflect real-life choices made by clinicians and their patients. Cortes and colleagues 27 argue that the comparison makes the benefits of eribulin more generalizable to clinical practice than if the control in the study had been artificially constrained to a single monotherapy.
Given the benefits that eribulin showed as a single agent in the pretreated mbc setting, further interest in the potential role of eribulin in early treatment is warranted27. Two ongoing phase ii trials are investigating the role of eribulin (monotherapy and combinations) as first-line therapy for mbc (search for NCT01268150 and NCT01269346 at http://clinicaltrials.gov/ct2/search). Additional phase i and ii trials are examining eribulin in combination with various chemotherapeutic agents in patients with advanced, metastatic, or unresectable solid tumours [NCT01372579 (carboplatin), NCT00415324 (cisplatin), NCT00410553 (gemcitabine), and NCT01323530 (capecitabine)] 12. Moreover, eribulin is being studied in phase ii trials with targeted agents such as trastuzumab (NCT01432886) and ramucirumab (NCT01427933) for the treatment of mbc.
3.2. Ixabepilone: A Novel Epothilone Analog
Epothilones are a new class of cytotoxic antineoplastic agents that affect microtubule dynamics to induce cell cycle arrest and apoptosis 31. Ixabepilone is a semisynthetic analog of the natural product epothilone B, derived from the myxobacterium Sorangium cellulosum 31–33. It is the first agent in its class specifically designed to provide enhanced antitumour activity 34. Ixabepilone acts by binding to a site on β-tubulin proteins and driving the formation of abnormal mitotic spindles, thereby enhancing microtubule stability 34–36. Although the mechanism of action of ixabepilone is similar to that of taxanes, preclinical studies have shown that ixabepilone is structurally different from taxanes and anthracyclines, and binds to tubulin in a distinct manner that makes it less susceptible to mechanisms of drug resistance 32,36–38. With low susceptibility to tumour resistance and potential for synergy with other cytotoxic agents (such as capecitabine), ixabepilone has been labelled one of the most active epothilone analogs for the treatment of mbc 31,32,35,37–39.
3.2.1. Efficacy Profile
Phase ii trials have reported activity for ixabepilone monotherapy, with orrs ranging from 12% (in patients refractory to anthracyclines, taxanes, and capecitabine) to 42% (in patients with metastatic disease after adjuvant anthracycline-based therapy) 31,37,40–42. Preclinical data also indicated a potential role for ixabepilone combinations in anthracycline- and taxane-pretreated mbc 31,39. A phase i/ii study showed a promising synergistic interaction between ixabepilone and capecitabine (a combination likely to enhance tumoricidal activity), prompting phase iii clinical trials (Table ii) 31,34,43.
TABLE II.
Summary of phase ii and iii trials of ixabepilone in patients with metastatic breast cancer (mbc) treated with prior chemotherapy regimens
| Reference | Study design | Primary endpoint | Patient population | Treatment groups | Regimen | rr(%) | Median pfs or ttp (months) | Median os (months) | Grade 3/4 toxicities (%) |
|---|---|---|---|---|---|---|---|---|---|
| Single-agent studies | |||||||||
| Perez et al., 200737 | Multicentre Single-arm Phase ii | orra | ≥2 Prior chemotherapy treatments (mbc setting) | Ixabepilone (n=113) | Ixabepilone 40 mg/m2 IV over 3 hours on day 1 of a 21-day cycle (median: 4 cycles) | 11.5 | 3.1 (pfs) | 8.6 | Peripheral sensory neuropathy: 14 Asthenia/fatigue: 13 Myalgia: 8 Stomatitis/mucositis: 6 |
| Combination studies | |||||||||
| Thomas et al., 200731 | Randomized Phase iii | pfsb | ≤3 Prior chemotherapy regimens (adjuvant or mbc setting) | Ixabepilone plus capecitabine (n=375) Capecitabine (n=612) |
Ixabepilone 40 mg/m2 IV on day 1 of a 21-day cycle, plus oral capecitabine 2 g/m2 on days 1–14 of a 21-day cycle vs. Capecitabine 2.5 g/m2 (same schedule) | 34 vs. 14 | 5.8 vs. 4.2 (pfs) (p<0.0001)c | nr (p=0.0003) | Sensory neuropathy: 21.0 vs. 0 Fatigue: 9 vs. 3 Neutropenia: 68 vs. 11 Rate of death as a result of toxicity: 3 vs. 1d,e |
| Sparano et al., 201044 | Randomized Phase iii | os | ≤2 Prior anthracycline and taxane treatments (including neoadjuvant/adjuvant) | Ixabepilone plus capecitabine (n=609) Capecitabine (n=612) |
Ixabepilone 40 mg/m2 IV on day 1, plus oral capecitabine 2 g/m2 on days 1–14 of a 21-day cycle vs. Capecitabine 2.5 g/m2 (same schedule) | 43 vs. 29 (p<0.0001) | 6.2 vs. 4.2 (pfs) (p=0.0005) | ns | Neuropathy: 24 |
Assessed by an independent radiology facility.
Assessed by blinded independent review.
Objective response rate based on assessment of radiologic evaluations and photographs of skin lesions by independent radiology review (blinded to treatment assignment and investigator), using the Response Evaluation Criteria in Solid Tumors.
Patients with liver dysfunction at greater risk.
Capecitabine-related toxicities were similar for both treatment groups.
rr = response rate; pfs = progression-free survival; ttp = time to progression; os = overall survival; orr = objective response rate; IV = intravenous; nr = not reported; ns = nonsignificant result.
A pivotal phase iii trial compared the efficacy and safety of the combination of ixabepilone and capecitabine with that of capecitabine alone in patients treated with up to three prior anthracycline- or taxane-based chemotherapies for locally advanced or metastatic disease (Table ii) 31. Eligible patients received either a 3-hour intravenous infusion of ixabepilone 40 mg/m2 on day 1 of a 21-day cycle, plus oral capecitabine 2.0 g/m2 administered in two doses on days 1–14 of a 21-day cycle; or just capecitabine 2.5 g/m2 on the same schedule. Treatment was continued until disease progression or unacceptable toxicity. Patients received a median of 5 cycles of combined ixabepilone–capecitabine or a median of 4 cycles of capecitabine alone. Of the 752 patients enrolled, 737 patients were treated, 369 being randomized to ixabepilone–capecitabine, and 368, to capecitabine monotherapy. The combination therapy proved to be superior, with a median pfs of 5.8 months (95% ci: 5.45 to 6.97 months) compared with 4.2 months for capecitabine alone (95% ci: 3.81 to 4.50 months)—almost a 40% increase in median pfs with combination treatment. Secondary endpoints—including tumour response rate, time to response, and duration of overall response—were consistent with the primary endpoint. Trial data was not mature for os analysis at the time of publication. A subsequent publication presented complete os data and reported a nonsignificant increase in os for the ixabepilone–capecitabine combination compared with capecitabine alone (12.9 months vs. 11.1 months; hr: 0.9; 95% ci: 0.77 to 1.05; p = 0.19) 45.
A similar phase iii trial studied 1221 patients with mbc that had been pretreated with anthracyclines and taxanes, but that were not necessarily chemotherapy-resistant (Table ii) 34,44. No significant difference in os (the primary outcome) was reported, but the pfs (6.2 months vs. 4.2 months; hr: 0.79; p = 0.0005) and response rate (43% vs. 29%, p < 0.0001) were significantly improved.
3.2.2. Toxicity Profile
Ixabepilone has a generally manageable toxicity profile as monotherapy and in combination, with minimal overlapping toxicities (Table ii) 46. However, a safety signal for peripheral neuropathy emerged that may affect the integration of ixabepilone into clinical practice.
The most common toxicity observed with ixabepilone was myelosuppression—namely, neutropenia (Table ii) 34. Grade 3 or 4 neutropenia occurred at a rate of 22%–58% with ixabepilone monotherapy and at 68% with ixabepilone–capecitabine 47, but febrile neutropenia was rare (5%–7%) 34. Neutropenia-related deaths have been reported in patients treated with combination therapy who had moderate to severe liver dysfunction at baseline; ixabepilone in combination with capecitabine is contraindicated for this patient subgroup.
As with some microtubule inhibitors, neuropathy is another relevant concern with ixabepilone 47,48. Grade 3 or 4 peripheral sensory neuropathy occurred in 0%–20% of patients treated with ixabepilone monotherapy, and in 21%–24% of patients treated with ixabepilone–capecitabine 44,47. This toxicity was reported to be cumulative in nature, but generally reversible to grade 1 or baseline within a median of 6 weeks with dose reductions 44,47,49. Development of neuropathy can be dose- or treatment-limiting in many patients and can lead to permanent neuronal dysfunction in a small proportion of patients; further research is required to identify clinical tools for the prediction and prevention of neurotoxicity 34. In the interim, the benefits associated with ixabepilone therapy should be carefully weighed against the risks 49.
3.2.3. Conclusions
Ixabepilone has shown activity across various advanced breast cancer settings. As monotherapy, it has activity rates that appear comparable with those of taxanes, capecitabine, vinorelbine, gemcitabine, and doxorubicin 32. Phase iii data support the use of ixabepilone in combination with capecitabine, but no crossover data are available for the sequential use of capecitabine followed by ixabepilone. The U.S. Food and Drug Administration has approved ixabepilone based on the available data. But challenges such as myelosuppression and peripheral neuropathy remain problematic with this agent. To date, the European Medicines Agency and Health Canada have not granted marketing authorization 34. Existing data suggest that ixabepilone may be a reasonable alternative in patients whose disease has progressed on a taxane, an anthracycline, and capecitabine, but, pending approval, this agent’s modest activity, problematic toxicities, and lack of phase iii data showing improvement in os will limit its clinical use as monotherapy or in combination with capecitabine.
The potential use of ixabepilone in early-stage breast cancer is currently under evaluation 46. A recent phase ii trial examined first-line trastuzumab plus weekly ixabepilone and carboplatin in patients with her2-positive mbc 50. Chemotherapy combinations with ixabepilone are being studied in phase i and ii trials, chiefly involving epirubicin 34,51. In addition, ongoing phase ii studies are evaluating ixabepilone in combination with various targeted agents, including trastuzumab (search for NCT00079326, NCT00490646, NCT00077376, and NCT00821886 at http://clinicaltrials.gov/ct2/search), lapatinib (NCT00634088), and bevacizumab (NCT00785291, NCT00370552) for the treatment of pretreated or untreated mbc 52.
3.3. Nab-Paclitaxel: A New Taxane Formulation
Taxanes such as paclitaxel and docetaxel have been described as some of the most active cytotoxic agents available for the treatment of breast cancer 53. These agents typically act by binding to tubulin and stabilizing polymerization, ultimately leading to cell death by apoptosis 54,55. Nanoparticle albumin-bound (nab)–paclitaxel is a novel, solvent-free formulation of paclitaxel recently developed to prevent the hypersensitivity reactions typically associated with this taxane, which are generally related to the solvent suspension of polyoxyethylated castor oil (for example, Cremophor: BASF, Ludwigshafen, Germany) 2,34,56. Serum albumin–bound nab-paclitaxel complexes safely deliver high intracellular concentrations of taxane to tumour cells by interacting with albumin receptors 2. This new formulation allows for the safe infusion of significantly higher doses of paclitaxel than can be administered with standard paclitaxel therapy. In addition, nab-paclitaxel can be administered over a shorter duration of infusion (30 minutes compared with 3 hours for standard paclitaxel) and without pre-medication for hypersensitivity 57.
3.3.1. Efficacy Profile
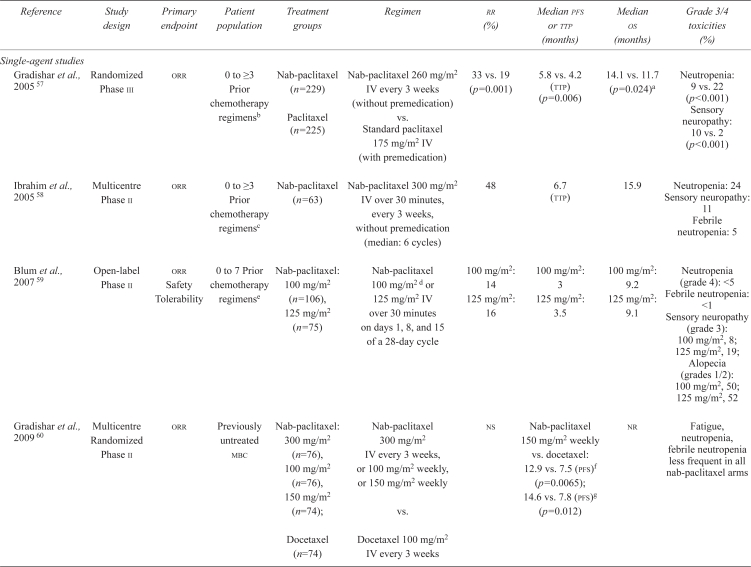
Numerous phase i and pharmacokinetic studies have been conducted to determine a maximum tolerated dose and optimal dose for nab-paclitaxel, with limited success 58. Phase ii studies have subsequently studied the safety and efficacy of this agent at various doses, reporting significant orr improvements (ranging from 14% to 48%) and a significant pfs prolongation for nab-paclitaxel compared with docetaxel (12.9 months vs. 7.5 months, p = 0.0065, Table iii). Notably, Blum and colleagues 59 reported modest orrs (14% and 16% with weekly nab-paclitaxel 100 mg/m2 and 125 mg/ m2 respectively) and a favourable safety profile in patients with mbc that had progressed with previous taxane therapy.
TABLE III.
Summary of phase ii and iii trials of nab-paclitaxel in patients with previously treated or untreated metastatic breast cancer (mbc)
| Reference | Study design | Primary endpoint | Patient population | Treatment groups | Regimen | rr(%) | Median pfs or ttp (months) | Median os (months) | Grade 3/4 toxicities (%) |
|---|---|---|---|---|---|---|---|---|---|
| Single-agent studies | |||||||||
| Gradishar et al., 200557 | Randomized Phase iii | orr | 0 to ≥3 Prior chemotherapy regimensb | Nab-paclitaxel (n=229) Paclitaxel (n=225) |
Nab-paclitaxel 260 mg/m2 IV every 3 weeks (without premedication) vs. Standard paclitaxel 175 mg/m2 IV (with premedication) | 33 vs. 19 (p=0.001) | 5.8 vs. 4.2 (ttp) (p=0.006) | 14.1 vs. 11.7 (p=0.024)a | Neutropenia: 9 vs. 22 (p<0.001) Sensory neuropathy: 10 vs. 2 (p<0.001) |
| Ibrahim et al., 200558 | Multicentre Phase ii | orr | 0 to ≥3 Prior chemotherapy regimensc | Nab-paclitaxel (n=63) | Nab-paclitaxel 300 mg/m2 IV over 30 minutes, every 3 weeks, without premedication (median: 6 cycles) | 48 | 6.7 (ttp) | 15.9 | Neutropenia: 24 Sensory neuropathy: 11 Febrile neutropenia: 5 |
| Blum et al., 200759 | Open-label Phase ii | orr Safety Tolerability | 0 to 7 Prior chemotherapy regimense | Nab-paclitaxel: 100 mg/m2 (n=106), 125 mg/m2 (n=75) | Nab-paclitaxel 100 mg/m2 d or 125 mg/m2 IV over 30 minutes on days 1, 8, and 15 of a 28-day cycle | 100 mg/m2: 14 125 mg/m2: 16 |
100 mg/m2: 3 125 mg/m2: 3.5 |
100 mg/m2: 9.2 125 mg/m2: 9.1 |
Neutropenia (grade 4): <5 Febrile neutropenia: <1 Sensory neuropathy (grade 3): 100 mg/m2, 8; 125 mg/m2, 19; Alopecia (grades 1/2): 100 mg/m2, 50; 125 mg/m2, 52 |
| Gradishar et al., 200960 | Multicentre Randomized Phase ii | orr | Previously untreated mbc | Nab-paclitaxel: 300 mg/m2 (n=76), 100 mg/m2 (n=76), 150 mg/m2 (n=74); |
Nab-paclitaxel 300 mg/m2 IV every 3 weeks, or 100 mg/m2 weekly, or 150 mg/m2 weekly vs. |
ns | Nab-paclitaxel 150 mg/m2 weekly vs. docetaxel: 12.9 vs. 7.5 (pfs)f (p= 0.0065); 14.6 vs. 7.8 (pfs)g (p=0.012) | nr | Fatigue, neutropenia, febrile neutropenia less frequent in all nab-paclitaxel arms |
| Docetaxel (n=74) | Docetaxel 100 mg/m2 IV every 3 weeks | ||||||||
| Combination studies | |||||||||
| Roy et al., 2009 61 | Multicentre Single-arm Phase ii | Proportion of confirmed responsesh | No previous chemotherapy for metastatic diseasei | Nab-paclitaxel (n=50) | Nab-paclitaxel 125 mg/m2 IV and gemcitabine 1 g/m2 on days 1 and 8 of a 21-day cycle until disease progression |
cr: 8 pr: 42 |
7.9 (pfs) | ns | Neutropenia: 42 Neurotoxicity: 4 |
Second-line nab-paclitaxel.
No prior paclitaxel or docetaxel; no relapse with metastatic disease within 1 year of adjuvant paclitaxel or docetaxel.
Prior chemotherapy was received by 76% of patients; 62% had received no prior mbc treatments of any kind.
Because minimal toxicity was observed with this dose and schedule, the protocol was amended to include an additional cohort of patients receiving 125 mg/m2 on the same schedule.
Patients must have developed progressive disease while receiving a taxane for their metastatic disease or have relapsed within 12 months of taxane-containing adjuvant therapy.
Assessed by independent radiologist review.
Assessed by investigator review.
Success was defined as a cr or pr according to the Response Evaluation Criteria in Solid Tumors on two consecutive evaluations at least 6 weeks apart.
Prior adjuvant chemotherapy was received 50% of patients; 2% of patients received trastuzumab as part of adjuvant chemotherapy.
rr = response rate; pfs = progression-free survival; ttp = time to progression; os = overall survival; orr = overall response rate; IV = intravenous; ns = nonsignificant result; nr = not reported; cr = complete response; pr = partial response.
An international open-label randomized phase iii study by Gradishar and colleagues 57 set out to demonstrate superior efficacy and reduced toxicity for nab-paclitaxel compared with standard paclitaxel in patients with three or fewer previous chemotherapy treatments (and other pre-specified criteria). Patients previously treated with paclitaxel or docetaxel in the metastatic setting were excluded from the study. Eligible patients were randomly assigned to receive either nab-paclitaxel 260 mg/m2 administered intravenously over 30 minutes without pre-medication or standard paclitaxel 175 mg/m2 administered intravenously over 3 hours every 3 weeks. A total of 229 patients were randomized to receive nab-paclitaxel, and 225, to receive standard paclitaxel. At least 6 cycles were administered to 129 patients (56%) in the nab-paclitaxel group and to 112 patients (50%) in the standard paclitaxel group. Based on the intention-to-treat population, the reported orr was significantly increased with nab-paclitaxel than with standard paclitaxel (33% vs. 19%, p = 0.001). The reported orr for patients who received first-line therapy with nab-paclitaxel was 42%, compared with 27% for patients in the standard paclitaxel group (p = 0.029). For patients who received 2 or more lines of therapy, the orrs were 27% and 13% respectively (p = 0.006). Significant differences in secondary endpoints were also reported. Median time to progression (ttp) was significantly longer with nab-paclitaxel than with standard paclitaxel for all patients (23.0 weeks vs. 16.9 weeks; hr: 0.75; p = 0.006). In addition, a statistically significant difference in os (median censoring time of 103 weeks for the nab-paclitaxel group and 101 weeks for the standard paclitaxel group) was observed for nab-paclitaxel compared with standard paclitaxel as second- or subsequent-line therapy (56.4 weeks vs. 46.7 weeks; hr: 0.73; p = 0.024).
Various phase ii studies have examined combinations of nab-paclitaxel with targeted agents—namely, trastuzumab and bevacizumab—for early treatment of mbc (orrs ranged from 30% to 60%) 62–64. Studied chemotherapy combinations have included nabpaclitaxel with gemcitabine and with capecitabine (orrs ranged from 50% to 61%) 61,64,65. Currently, nab-paclitaxel is being investigated in combination with targeted agents such as bevacizumab (search for NCT00618657 at http://clinicaltrials.gov/ct2/search).
3.3.2. Toxicity Profile
As expected, compared with paclitaxel, nab-paclitaxel was reported to be associated with less myelotoxicity (5%–9% grade 4 neutropenia), but a greater incidence of grade 3 sensory neuropathy (10% vs. 2%, p < 0.001, Table iii) 34,57,60. Sensory neuropathy was cumulative, dose-dependent, and partially reversible 52. Moreover, fewer hypersensitivity reactions were reported for patients treated with nab-paclitaxel than for patients treated with paclitaxel (<1% vs. 2%) despite the fact that only 8% of nab-paclitaxel–treated patients received pre-medication (99% of paclitaxel-treated patients received pre-medication) 33,57. Treatment discontinuations, dose reductions, and dose delays because of aes were infrequent in both treatment groups, and no quality-of-life differences were observed between them. Myelotoxicity, including the incidence of febrile neutropenia, was also reduced in comparisons of various doses of nab-paclitaxel with docetaxel 60.
3.3.3. Conclusions
With improved efficacy, lack of a need for pre-medication, a better toxicity profile, and a faster infusion time at high doses, nab-paclitaxel may overcome and surpass the limitations of the existing solvent-based taxanes in the treatment of mbc. A benefit in os for this agent in mbc has yet to be demonstrated 66. Encouraging results have prompted new clinical trials to further investigate and establish the role of nab-paclitaxel as a single agent or in combination with other cytotoxic or biologic agents in breast cancer.
3.4. Other Agents Currently in Use in Canada for MBC
3.4.1. Capecitabine
Capecitabine is a pro-drug unique in its ability to be converted to 5-fluorouracil by the enzyme thymidine phosphorylase, which is highly active in tumour tissue 2. Ultimately, 5-fluorouracil exerts its cytotoxic effect by inhibiting dna, rna, and protein synthesis. Capecitabine has been studied as a single agent in numerous phase ii trials, as well as in phase iii combination studies with other cytotoxic agents and novel targeted agents. In addition, capecitabine has been studied in first-, second-, and later-line mbc settings. A number of phase ii studies have demonstrated orrs ranging from 15% to 37%, and phase iii studies have reported more modest response rates ranging from 9% to 14% for capecitabine in patients who were previously treated with at least one chemotherapy regimen (adjuvant or mbc, or both) 2,67–73. Notably, those trials studied os only as a secondary endpoint. As a secondary endpoint, os was found to be significantly longer with first-line oral capecitabine than with classical cyclophosphamide–methotrexate– fluorouracil in the treatment of women with advanced breast cancer unsuited to more intensive regimens (median: 22 months vs. 18 months; hr: 0.72; 95% ci: 0.55 to 0.94; p = 0.02) 74. Overall survival was also significantly prolonged in a phase iii trial comparing the combination of capecitabine–docetaxel with single-agent capecitabine 75. However, the greater toxicity of the former combination and the prevailing practice of sequential monotherapies in mbc has kept capecitabine–docetaxel from gaining widespread acceptance. Capecitabine is generally well tolerated, with a low incidence of grade 3 or 4 events 2. It must be noted, however, that as clinical experience with capecitabine increased, many clinicians found that a large proportion of patients required dose reductions after initiation of treatment at the registered starting dose (2.5 g/m2 daily in two divided oral doses for 14 days, every 21-day cycle) 76. Based on a review of cumulative evidence of alternative capecitabine dosages and dosing schedules, Zielinski and colleagues76 recommend a starting dose of 1.0 g/m2 twice daily. Currently, the most common aes with this agent include fatigue, hand–foot syndrome, and gastrointestinal effects 2.
3.4.2. Vinorelbine
Vinorelbine is a vinca alkaloid, which—like eribulin, ixabepilone, and nab-paclitaxel—targets microtubules, leading to apoptosis 2. Currently, vinorelbine is commonly used as a second-line agent or beyond, often after treatment with anthracyclines and taxanes. Common aes with this agent include neutropenia, nausea, fatigue, constipation, peripheral neuropathy, and stomatitis 2,77. Neutropenia, while dose-limiting, is generally reversible and not cumulative over time. Because of cumulative neurotoxicity and a high incidence of neutropenia, continuous weekly dosing with vinorelbine 30 mg/m2 can be difficult, and dose reductions or schedule modifications are often required 2,78. Phase ii studies have examined orrs with this agent at a weekly dose of 20–30 mg/m2, with favourable results (orrs of 16%–36%) 2,78–80. A recent phase iii study of single-agent vinorelbine 30 mg/m2 (on days 1 and 8 of a 21-day cycle) compared with vinorelbine–gemcitabine, demonstrated a median pfs prolongation of 4.0 months compared with 6.0 months in favour of the combination. Overall tumour response rates were 26% and 36% (in favour of the combination, but nonsignificant: p = 0.093) 2,81. Febrile neutropenia occurred in 6% of patients receiving single-agent vinorelbine and in 11% of those receiving the combination (p = 0.15).
3.4.3. Gemcitabine
Gemcitabine is a dna nucleoside analog that inhibits dna synthesis, leading to tumour cell death 2. Most of the data on single-agent gemcitabine for the treatment of mbc is derived from phase ii studies. In patients pretreated with anthracyclines and taxanes, gemcitabine treatment (at doses ranging from 800 mg/m2 to 1.2 g/m2) resulted in orrs ranging from 12% to 30% 2,19,20. In a phase iii trial that studied anthracycline-pretreated patients with mbc, Albain and colleagues 22 demonstrated an improved os with gemcitabine–paclitaxel compared with single-agent paclitaxel 22. The most common toxicities with gemcitabine are hematologic, particularly neutropenia and thrombocytopenia 2.
4. NOVEL AGENTS: IMPLICATIONS FOR CLINICAL PRACTICE
Cumulative toxicity and resistance to commonly used antineoplastic agents such as anthracyclines and taxanes, whether in the adjuvant, neoadjuvant, or mbc setting, continue to be limiting factors in breast cancer treatment 81,82. Numerous cytotoxic agents are available for the treatment of locally advanced or metastatic disease, but no one agent is recommended or preferred in all situations, and the choice should be tailored to both tumour and patient characteristics 2,14. A thoughtful approach is therefore required to optimize therapy for mbc. In some cases, optimal therapy may involve a return to prior lines of therapy, including anthracyclines and taxanes, in an attempt to elicit a response and provide relief of symptoms.
The available agents are all active, well-tolerated single agents, with limitations posed by their respective toxicity profiles—namely, myelosuppression and cardiotoxicity 2. Use of platinum-based agents is common in patients with tnbc, but additional investigation is required to further understand the role of those agents in mbc. Capecitabine, with its convenient oral route of administration, is a wellstudied agent in mbc. Vinorelbine and gemcitabine have also shown activity in mbc, but data from wellconducted studies for those two agents are limited. Combinations offer improved response rates over single agents at the cost of increased toxicity, and for the most part, without an improvement in os or quality of life 19,22,75,83. Platinum-based agents may be particularly effective in the treatment of patients with tnbc, and trials are underway to assess their efficacy in this subtype of mbc.
For women with her2-negative metastatic disease, the lack of effective options for heavily pretreated patients (for example, those treated with anthracyclines, taxanes, and capecitabine) 8,27 indicates a need for novel treatments that would ideally be implemented early in the course of metastatic disease. The search for such chemotherapeutic interventions has proved challenging. Over recent decades, in-depth investigation of a number of novel compounds has been completed, and results point to potentially meaningful findings.
Ongoing shifts in clinical standards and significant differences in trial designs make specific trial comparisons with novel agents challenging. That situation is exemplified in the clinical trial that examined nab-paclitaxel against paclitaxel (dosed every 3 weeks), which used a control arm that was later found to be inferior in efficacy and safety to weekly paclitaxel 84. That control arm is therefore no longer reflective of the standard of care (weekly dosing) for patients treated with paclitaxel. Differences in patient populations assessed within each trial further complicate cross-trial comparisons. For example, results of the embrace trial 27, which investigated eribulin in patients previously treated with two or more chemotherapies for mbc, cannot be directly compared with those of the trial by Gradishar and colleagues 57, which investigated nab-paclitaxel in naïve to heavily pretreated patients with mbc.
Eribulin has shown very promising activity in the pretreated mbc setting 27. Although the choice of a tpc comparator arm limits specific comparisons of eribulin with other agents, eribulin was shown to provide a survival benefit over the variety of agents used in the tpc arm (including capecitabine, gemcitabine, and vinorelbine), in patients pretreated with a median of four previous chemotherapy regimens 12,27. To date, embrace is the first major single-agent study of a cytotoxic or biologic agent to show significantly increased os in patients pretreated for mbc 27. Nab-paclitaxel may have a role as an agent of choice in lieu of existing taxanes, which are typically used in the pretreated mbc setting 53,66. While the data for nab-paclitaxel compared with docetaxel are limited, nab-paclitaxel is preferred for its improved toxicity profile, and it is increasingly being considered in early lines of mbc treatment. In another recent finding, ixabepilone was found to be the first epothilone analog to demonstrate significant clinical activity in pretreated mbc 31,43. However, peripheral neuropathy remains a major concern with this particular agent, likely limiting its potential use in worldwide clinical practice for the treatment of mbc.
As novel cytotoxic agents are introduced into clinical practice, it is useful to outline key commonalities among the patients that may benefit from such interventions. An outline of this kind may help to facilitate treatment decisions and the incorporation of these agents into practice. Currently, the uniting criteria among the available data for novel agents in mbc are good organ function; prior treatment with an anthracycline, taxane, or capecitabine; and a need for further lines of chemotherapeutic treatment. Importantly, it is not yet possible to draw conclusions regarding the treatment of choice for women who have a heavy burden of disease and for whom treatment may help to relieve symptoms and improve quality of life 27.
Finally, with the imminent arrival of new and promising options for breast cancer, the appropriateness and value of survival endpoints used in clinical trial analyses are often debated. Overall survival is considered the “gold standard” in oncology trials 10,85, this endpoint being favoured because of its objectivity, clear indication of benefit, and ease and reliability of measurement 10. However, determination of os also requires a larger sample size and a prolonged follow-up period, and the endpoint itself may be influenced by therapies used after patient participation in a given trial has ended. In randomized mbc trials, pfs and ttp are often used as surrogate primary endpoints for survival. The merits of using pfs and ttp as measures of clinical benefit are that they are reached faster than os is, and they are not influenced by subsequent treatments. However, measurements of pfs and ttp are more involved and therefore more susceptible to error and bias. Moreover, although some degree of association has been detected between those endpoints and os, results remain inconsistent, and the nature of the relationship is uncertain.
5. SUMMARY
Eribulin, ixabepilone, and nab-paclitaxel are three novel agents developed to improve patient outcomes in the mbc setting. Eribulin mesylate is the first monotherapy to significantly increase os in patients with pretreated mbc, and nab-paclitaxel offers a novel, safer mode of delivery in comparison with standard taxanes 27,53,66. By contrast, the use of ixabepilone will be limited until the related neurotoxicity can be better managed 34. The clinical benefits of each of the foregoing agents may encourage their integration into Canadian clinical practice. To choose the right treatment, the clinician must consider regional availability and reimbursement status in addition to the efficacy and toxicity profiles of the available agents in the context of tumour biology and patient preference.
6. ACKNOWLEDGMENTS
Grant support was provided by Eisai Limited. Editorial assistance was provided by Meducom Health Inc.
Footnotes
7. CONFLICT OF INTEREST DISCLOSURES
JPMA has received research funding (Pfizer), honoraria (Eisai, BMS), and consultancy fees (Eisai, Novartis, Roche). ShV has received research funding (Eisai, Abraxis), honoraria (Eisai, Abraxis), and consultancy fees (Eisai). SuV has received research funding (Roche, Sanofi–Aventis), and honoraria (Celgene, Eisai, Roche).
All authors contributed equally to the development of this manuscript.
8. REFERENCES
- 1.Cardoso F, Senkus–Konefka E, Fallowfield L, Costa A, Castiglione M, on behalf of the esmo Guidelines Working Group Locally recurrent or metastatic breast cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v15–19. doi: 10.1093/annonc/mdq160. [DOI] [PubMed] [Google Scholar]
- 2.Morris PG, McArthur HL, Hudis CA. Therapeutic options for metastatic breast cancer. Expert Opin Pharmacother. 2009;10:967–81. doi: 10.1517/14656560902834961. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Geneva, Switzerland: WHO; 2003. Global cancer rates could increase by 50% to 15 million by 2020 [press release] [Available online at: http://www.who.int/mediacentre/news/releases/2003/pr27/en/; cited October 15, 2011] [Google Scholar]
- 4.El Saghir NS, Tfayli A, Hatoum HA, Nachef Z, Dinh P, Awada A. Treatment of metastatic breast cancer: state-of-the-art, subtypes and perspectives. Crit Rev Oncol Hematol. 2011;80:433–49. doi: 10.1016/j.critrevonc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sørlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40:2667–75. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Dawood S. Triple negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247–58. doi: 10.2165/11538150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Orlando L, Colleoni M, Fedele P, et al. Management of advanced breast cancer. Ann Oncol. 2007;18(suppl 6):vi74–6. doi: 10.1093/annonc/mdm230. [DOI] [PubMed] [Google Scholar]
- 9.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumour subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S, McLeod D, Batist G, Robidoux A, Martins IR, Mackey JR. In the end what matters most? A review of clinical endpoints in advanced breast cancer. Oncologist. 2011;16:25–35. doi: 10.1634/theoncologist.2010-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno–Aspitia A, Perez EA. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin Proc. 2009;84:533–45. doi: 10.4065/84.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradishar WJ. The place for eribulin in the treatment of metastatic breast cancer. Curr Oncol Rep. 2011;13:11–16. doi: 10.1007/s11912-010-0145-9. [DOI] [PubMed] [Google Scholar]
- 13.Mayer EL, Burstein HJ. Chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 2007;21:257–72. doi: 10.1016/j.hoc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 15.Dean–Colomb W, Esteva FJ. Emerging agents in the treatment of anthracycline- and taxane-refractory metastatic breast cancer. Semin Oncol. 2008;35(suppl 2):S31–8. doi: 10.1053/j.seminoncol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20:1771–85. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 17.Dufresne A, Pivot X, Tournigand C, et al. Impact of chemotherapy beyond the first line in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;107:275–9. doi: 10.1007/s10549-007-9550-7. [DOI] [PubMed] [Google Scholar]
- 18.Bristol–Myers Squibb . Ixempra (Ixabepilone) Prescribing Information. Princeton NJ: Bristol–Myers Squibb Company; 2010. [Google Scholar]
- 19.Martín M, Ruiz A, Muñoz M, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase iii Spanish Breast Cancer Research Group (geicam) trial. Lancet Oncol. 2007;8:219–25. doi: 10.1016/S1470-2045(07)70041-4. [DOI] [PubMed] [Google Scholar]
- 20.Possinger K, Kaufmann M, Coleman R, et al. Phase ii study of gemcitabine as first-line chemotherapy in patients with advanced or metastatic breast cancer. Anticancer Drugs. 1999;10:155–62. doi: 10.1097/00001813-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase ii trial. Oncology. 2002;62:2–8. doi: 10.1159/000048240. [DOI] [PubMed] [Google Scholar]
- 22.Albain KS, Nag SM, Calderillo–Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–7. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 23.Martín M, Sánchez–Rovira P, Muñoz M, et al. Pegylated liposomal doxorubicin in combination with cyclophosphamide and trastuzumab in her2-positive metastatic breast cancer patients: efficacy and cardiac safety from the geicam/2004–05 study. Ann Oncol. 2011;22:2591–6. doi: 10.1093/annonc/mdr024. [DOI] [PubMed] [Google Scholar]
- 24.Fiegl M, Mlineritsch B, Hubalek M, Bartsch R, Pluschnig U, Steger GG. Single-agent pegylated liposomal doxorubicin (pld) in the treatment of metastatic breast cancer: results of an Austrian observational trial. BMC Cancer. 2011;11:373. doi: 10.1186/1471-2407-11-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decatris MP, Sundar S, O’Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 26.Lorusso V, Manzione L, Silvestris N. Role of liposomal anthracyclines in breast cancer. Ann Oncol. 2007;18(suppl 6):vi70–3. doi: 10.1093/annonc/mdm229. [DOI] [PubMed] [Google Scholar]
- 27.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (embrace): a phase 3 open-label randomised study. Lancet. 2011;377:914–23. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 28.Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–95. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 29.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase ii study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–61. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 30.Cortes J, Vahdat L, Blum JL, et al. Phase ii study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–8. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 31.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–17. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 32.Denduluri N, Swain SM. Ixabepilone for the treatment of solid tumors: a review of clinical data. Expert Opin Investig Drugs. 2008;17:423–35. doi: 10.1517/13543784.17.3.423. [DOI] [PubMed] [Google Scholar]
- 33.Gerth K, Bedorf N, Höfle G, Irschik H, Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (myxobacteria). Production, psycho-chemical and biological properties. J Antibiot (Tokyo) 1996;49:560–3. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro JT, Macedo LT, Curigliano G, et al. Cytotoxic drugs for patients with breast cancer in the era of targeted treatment: back to the future? Ann Oncol. 2011. [Epub ahead of print]. [DOI] [PubMed]
- 35.Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with Taxol-like mechanism of action. Cancer Res. 1995;55:2325–33. [PubMed] [Google Scholar]
- 36.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on α,β-tubulin by electron crystallography. Science. 2004;305:866–9. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 37.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase ii study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–14. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 38.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–37. [PubMed] [Google Scholar]
- 39.Lee FY, Camuso A, Castenada C, et al. Preclinical efficacy evaluation of ixabepilone (BMS-247550) in combination with cetuximab or capecitabine in human colon and lung carcinoma xenografts [abstract 12017] J Clin Oncol. 2006;24 [Available online at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=34846; cited February 9, 2012] [Google Scholar]
- 40.Thomas E, Tabernero J, Fornier M, et al. Phase ii clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 41.Low JA, Wedam SB, Lee JJ, et al. Phase ii clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005;23:2726–34. doi: 10.1200/JCO.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Roché H, Yelle L, Cognetti F, et al. Phase ii clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25:3415–20. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 43.Bunnell C, Vahdat L, Schwartzberg L, et al. Phase i/ii study of ixabepilone plus capecitabine in anthracycline-pretreated/ resistant and taxane-resistant metastatic breast cancer. Clin Breast Cancer. 2008;8:234–41. doi: 10.3816/CBC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 44.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase iii trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–63. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hortobagyi GN, Gomez HL, Li RK, et al. Analysis of overall survival from a phase iii study of ixabepilone plus capecitabine versus capecitabine in patients with mbc resistant to anthracyclines and taxanes. Breast Cancer Res Treat. 2010;122:409–18. doi: 10.1007/s10549-010-0901-4. [DOI] [PubMed] [Google Scholar]
- 46.Rivera E, Gomez H. Chemotherapy resistance in metastatic breast cancer: the evolving role of ixabepilone. Breast Cancer Res. 2010;12(suppl 2):S2. doi: 10.1186/bcr2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodin S. Ixabepilone: a novel microtubule-stabilizing agent for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 2008;65:2017–26. doi: 10.2146/ajhp070628. [DOI] [PubMed] [Google Scholar]
- 48.Pivot X, Villanueva C, Chaigneau L, et al. Ixabepilone, a novel epothilone analog in the treatment of breast cancer. Expert Opin Investig Drugs. 2008;17:593–9. doi: 10.1517/13543784.17.4.593. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim NK. Ixabepilone development across the breast cancer continuum: a paradigm shift. Cancer Manag Res. 2010;2:169–79. doi: 10.2147/CMR.S10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moulder S, Li H, Wang M, et al. A phase ii trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with her2- positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat. 2010;119:663–71. doi: 10.1007/s10549-009-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gianni L, Dalenc F, De Benedictis E, et al. Clinical and pharmacokinetic study of ixabepilone (ixa) plus epirubicin (epi) as therapy for women with advanced breast cancer (bc) Cancer Res. 2009;69(suppl 3):2097. doi: 10.1158/0008-5472.SABCS-09-2097. [DOI] [Google Scholar]
- 52.Lee F, Jure–Kunkel MN, Salvati ME. Synergistic activity of ixabepilone plus other anticancer agents: preclinical and clinical evidence. Ther Adv Med Oncol. 2011;3:11–25. doi: 10.1177/1758834010386402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Kawano I, Iwase H. Nab-paclitaxel for the treatment of breast cancer: efficacy, safety and approval. Onco Targets Ther. 2011;4:123–36. doi: 10.2147/OTT.S13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ringel I, Horwitz SB. Studies with RP 56976 (Taxotere): a semisynthetic analogue of Taxol. J Natl Cancer Inst. 1991;83:288–91. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- 55.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 56.Nyman DW, Campbell KJ, Hersh E, et al. Phase i and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–93. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 57.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase iii trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 58.Ibrahim NK, Samuels B, Page R, et al. Multicenter phase ii trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–26. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Blum JL, Savin MA, Edelman G, et al. Phase ii study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7:850–6. doi: 10.3816/CBC.2007.n.049. [DOI] [PubMed] [Google Scholar]
- 60.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–19. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 61.Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM. On behalf of North Central Cancer Treatment Group. Phase ii trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531) Ann Oncol. 2009;20:449–53. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirtsching B, Cosgriff T, Harker G, Keaton M, Chidiac T, Min M. Single agent nab-paclitaxel given weekly (3/4) as first-line therapy for metastatic breast cancer (an International Oncology Network Study, #I-04-012) [abstract 1118] J Clin Oncol. 2008;26 [Available online at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=35599; cited February 9, 2012] [Google Scholar]
- 63.Danso MA, Blum JL, Robert NJ, et al. Phase ii trial of weekly nab-paclitaxel in combination with bevacizumab as first-line treatment in metastatic breast cancer [abstract 1075] J Clin Oncol. 2008;26 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=33192; cited February 9, 2012] [Google Scholar]
- 64.Chirgwin J, Chua SL. Management of breast cancer with nanoparticle albumin-bound (nab)–paclitaxel combination regimens: a clinical review. Breast. 2011;20:394–406. doi: 10.1016/j.breast.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Somer BG, Schwartzberg LS, Arena F, et al. Phase ii trial of nab-paclitaxel (nanoparticle albumin-bound paclitaxel: abx) + capecitabine (xel) in first line treatment of metastatic breast cancer (mbc) [abstract 1053] J Clin Oncol. 2007;25 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=47&abstractID=36215; cited February 9, 2012] [Google Scholar]
- 66.Vishnu P, Roy V. Safety and efficacy of nab-paclitaxel in the treatment of patients with breast cancer. Breast Cancer (Auckl) 2011;5:53–65. doi: 10.4137/BCBCR.S5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase ii study of capecitabine in paclitaxel-refractory metastatic breast carcinoma patients. Cancer. 2001;92:1759–68. doi: 10.1002/1097-0142(20011001)92:7<1759::AID-CNCR1691>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 68.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase ii study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–93. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 69.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase ii study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–42. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 70.O’Shaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase ii trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous cmf (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/ metastatic breast cancer. Ann Oncol. 2001;12:1247–54. doi: 10.1023/A:1012281104865. [DOI] [PubMed] [Google Scholar]
- 71.Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase ii trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86:1367–72. doi: 10.1038/sj.bjc.6600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bajetta E, Procopio G, Celio L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol. 2005;23:2155–61. doi: 10.1200/JCO.2005.02.167. [DOI] [PubMed] [Google Scholar]
- 73.Reichardt P, Von Minckwitz G, Thuss–Patience PC, et al. Multicenter phase ii study of oral capecitabine (Xeloda) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14:1227–33. doi: 10.1093/annonc/mdg346. [DOI] [PubMed] [Google Scholar]
- 74.Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498–504. doi: 10.1200/JCO.2010.33.9101. [DOI] [PubMed] [Google Scholar]
- 75.O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase iii trial results. J Clin Oncol. 2002;20:2812–23. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol. 2010;21:2145–52. doi: 10.1093/annonc/mdq069. [DOI] [PubMed] [Google Scholar]
- 77.Pierre Fabre Pharma Canada . Navelbine (Vinorelbine Tartrate) Injection. St-Bruno de Montarville, QC: Pierre Fabre Pharma Canada; 2005. [product monograph] [Google Scholar]
- 78.Zelek L, Barthier S, Riofrio M, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer. 2001;92:2267–72. doi: 10.1002/1097-0142(20011101)92:9<2267::AID-CNCR1572>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 79.Degardin M, Bonneterre J, Hecquet B, et al. Vinorelbine (Navelbine) as a salvage treatment for advanced breast cancer. Ann Oncol. 1994;5:423–6. doi: 10.1093/oxfordjournals.annonc.a058873. [DOI] [PubMed] [Google Scholar]
- 80.Gasparini G, Caffo O, Barni S, et al. Vinorelbine is an active antiproliferative agent in pretreated advanced breast cancer patients: a phase ii study. J Clin Oncol. 1994;12:2094–101. doi: 10.1200/JCO.1994.12.10.2094. [DOI] [PubMed] [Google Scholar]
- 81.Valero V, Hortobagyi GN. Are anthracycline-taxane regimens the new standard of care in the treatment of breast cancer? J Clin Oncol. 2003;21:959–62. doi: 10.1200/JCO.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 82.Bernard–Marty C, Cardoso F, Piccart MJ. Facts and controversies in the treatment of metastatic breast cancer. Oncologist. 2004;9:617–32. doi: 10.1634/theoncologist.9-6-617. [DOI] [PubMed] [Google Scholar]
- 83.Sledge GW, Neuberg D, Bernardo P, et al. Phase iii trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an Intergroup trial (E1193) J Clin Oncol. 2003;21:588–92. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase iii trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all her-2 overexpressors and random assignment to trastuzumab or not in her-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–9. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 85.Burzykowski T, Buyse M, Piccart–Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–92. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]