Abstract
Several AU-rich RNA binding element (ARE) proteins were investigated for their possible effects on transcription of hepatic 3-hydroxy-3-methyglutaryl coenzyme A reductase (HMGR) in normal rats. Using in vivo electroporation, four different siRNAs to each ARE protein were introduced together with HMGR promoter (−325 to +20) luciferase construct and compared to saline controls. All four siRNAs to tristetraprolin (TTP) completely eliminated transcription from the HMGR promoter construct. Since insulin acts to rapidly increase hepatic HMGR transcription, the effect of TTP siRNA on induction by insulin was tested. The 3-fold stimulation by insulin was eliminated by this treatment. In comparison, siRNA to AU RNA binding protein/enoyl coenzyme A hydratase (AUH) had no effect. These findings indicate a role for TTP in the insulin-mediated activation of hepatic HMGR transcription.
Keywords: AU-rich RNA binding proteins, HMG-CoA reductase transcription, siRNA, insulin, in vivo electroporation
Introduction
3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), the enzyme that catalyzes the rate-limiting step in cholesterol biosynthesis, responds rapidly to various stimuli. In rats subjected to controlled light and dark periods, hepatic HMGR activity and immunoreactive protein levels increase rapidly upon entering the dark period when the animals start eating [1]. Administration of insulin to diabetic rats increases hepatic HMGR activity, immunoreactive protein, mRNA levels and the rate of transcription within 1 hour [2–4]. Also HMGR activity is increased 3-fold within 1 hour after sub-culturing L1210 tumor cells1, reaching a maximum of nearly 20-fold after 10 hours. The half-life of hepatic HMGR protein is 2.5 hr [5] while the half-life of hepatic mRNA is about 3 hours in normal rats [6]. Thus, HMGR can be regarded as a rapid response gene.
Rapid response genes typically have AU-rich sequences in the 3’-untranslated regions (3’UTR) of their mRNAs. These mofits consist of AUUUA pentamers and UUAUUUAUU nomers [7]. The 3’UTR of HMGR has several AUUUA sequences and a UUAUUUAUU sequence located at 3122 to 3130 of the rat sequence [8]. These AU-rich sequences are generally thought to function as targets for the binding of AU-rich binding proteins (ARE-BPs) that promote degradation of these short-lived mRNAs by removal of the poly A tail [9]. However, some ARE-BPs such as HUR act to stabilize mRNAs [10]. T-cell inhibitor of apoptosis-1 (TIA-1) and TIA-1 related protein (TIAR) act as translational repressors [11]. Although tristetraprolin (TTP) has been widely shown to be involved in mRNA degradation [7], recent reports have demonstrated that this CCCH zinc finger-containing protein acts to inhibit NF-kappaB-dependent transcription [12,13].
Since hepatic HMGR is a rapid response gene, we sought to determine whether any of these ARE-BPs play a role in modulating its expression. We carried out in vivo electroporation studies with siRNAs to several ARE-BPs to determine whether any of these proteins affect transcriptional activation of hepatic HMGR. Surprisingly, all four siRNAs to TTP abolished transcription of hepatic HMGR.
Materials and methods
Experimental animals
Male Sprague-Dawley rats weighing 125 to 150 g were purchased from Harlan (Madison, WI). The rats were housed in a reversed lighting cycle room (12 hr dark/12 hr light) and fed Harland Teklad 22/5 rodent chow. The animals were killed at the third hr of the dark period when hepatic HMGR expression is at its maximum. Diabetes was induced in rats by a single subcutaneous injection of streptozotocin (Sigma), 65 mg/kg in 0.1 M sodium citrate, pH 5.5. Diabetes was confirmed by the presence of urinary glucose detected with Clinistix (Bayer). Some of the diabetic rats were injected subcutaneously with 3.0 units/100g of recombinant human insulin, Novolin 70/30 (Novo Nordisk) two hrs before killing the animals.. All procedures were carried out according to protocol 3571 approved by the University of South Florida Institutional Animal Care and Use Committee.
Materials
Four different (A-D) siRNAs each to TTP (cat# LQ-098940-01-0010), HuR (cat# LQ-080370-00-0010), AUF1 (cat# LQ-099225-01-0010), AUH (cat# LQ-090133-01-0010), TIA1 (cat# LQ-095224-01-0010), KSRP (cat# LQ-093278-01-0010) were purchased from Dharmacon. Polyclonal antibodies to TTP (#ab33058), and AUF1 (#ab61193) were purchased from Abcam. A polyclonal antibody to AUH (#sc-82518) was purchased from Santa Cruz Biotechnology. A mouse monoclonal antibody to HuR (sc-5261) was purchased from Santa Cruz Biotechnology. SuperSignal West Pico Chemiluniscent Substrate was purchased from Pierce.
In vivo electroporation
Ten μg of −325/+20 HMGR promoter in pGL3-Basic [4] with and without 5 μg of siRNAs to several ARE-BPs was introduced in duplicate in the left, right and medial liver lobes of the rats. pHRL-CMV Renilla vector was co-electroporated at a 1 to 2,000 dilution to control for electroporation efficiency. The electroporation procedure and parameters used were as previously described [14,15].
Luciferase assays
Twenty-four hr after in vivo electroporation, the electroporated sites in the liver lobes were removed using a 5 mm cork-borer. The liver piece, approximately 0.1 g, was homogenized in passive lysis buffer (Promega) and processed for luciferase activity using the dual luciferase assay kit from Promega [15]. Luciferase activity was calculated as the ratio of firefly (reporter) to Renilla (transfection control).
Western blotting analysis
Liver nuclear extracts were prepared as previously described [16]. Protein concentrations were determined using the Bradford assay. Twenty μg of protein in 20 μl of 0.25 M sucrose were mixed with 20 μl of Laemmli sample buffer containing 2% SDS and 5% 2-mercaptoethanol and denatured at 94°C. The denatured samples were subjected to electrophoresis on pre-cast 4–20% SDS PAGE gels. Dual color precision plus protein standard markers were applied to one lane. After electrophoresis for 1 hr the proteins were transferred to nitrocellulose membranes. Detection of TTP and AUF1 was done with 1 to 1,000 dilutions of the primary rabbit antibodies and 1 to 10,000 dilutions of goat secondary antibodies. Detection of HuR was preformed using a 1 to 100 dilution of the primary mouse monoclonal antibody and a 1 to 10,000 dilution of the goat secondary antibody. Immunoreactive proteins were visualized using SuperSignal West Pico Chemiluniscent substrate and exposure times ranging from 2 seconds to 5 minutes. Quantitation was performed with a Bio-Rad Quantity One 4.6.6 densiometer equipped with a ChemiDoc XRS scanner.
Results
Effects of ARE-BP siRNAS on hepatic HMGR promoter activity
Since it has been established that insulin acts to rapidly increase expression of hepatic HMGR by increasing the rate of transcription, we embarked on a series of experiments using siRNAs to several ARE-BPs to see if they would act to decrease HMGR promoter activity. To test this in a physiological setting, HMGR promoter constructs with and without the siRNAs were introduced into liver lobes of normal rats by in vivo electroporation.
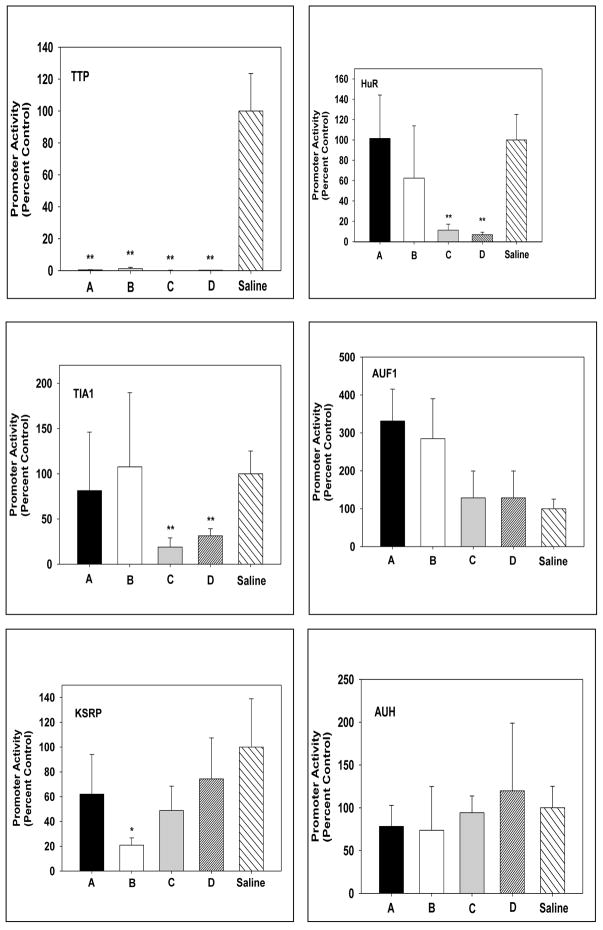
All four of the siRNAs to TTP completely abolished HMGR promoter activity (Fig. 1). Two of the four siRNAS to HuR and TIA1 significantly reduced HMGR promoter activity; but the other two had no effect. siRNA B to KH-type splicing regulatory protein (KSRP) significantly reduced promoter activity but the other three siRNAs did not. siRNAs to AUH had no effect while two siRNAs to AUF1 appeared to stimulate promoter activity.
Fig. 1.
Effects of siRNAs to several AU-rich RNA binding proteins on transcription of HMGR. Normal rats were transfected with HMGR promoter luciferase with siRNAs to ARE-BPs or saline. Four siRNAs were used for each ARE-BP. Typically, two siRNAs in duplicate HMGR promoter were introduced by in vivo electroporation into the left and right liver lobes while HMGR promoter with saline was introduced into the medial lobe. The data are expressed as means ± SD for at least four duplicates of siRNAs, A-D for each ARE-BP. Significant differences are p< 0.01 ** and p< 0.05 *.
Effects of diabetes and insulin treatment on hepatic ARE-BP protein levels
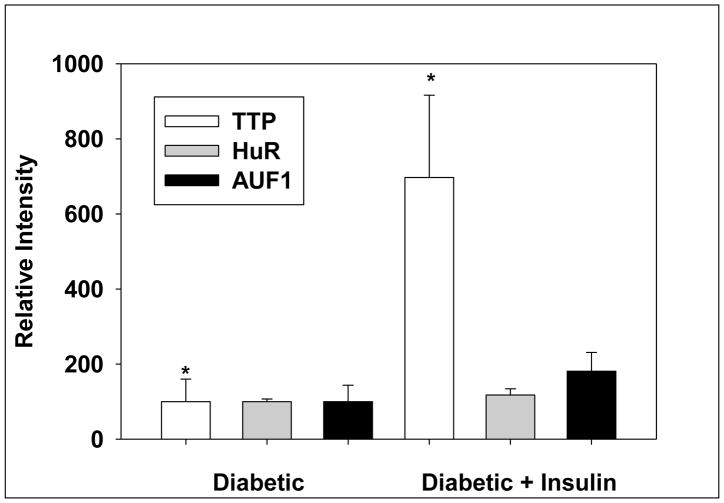
Since hepatic HMGR is rapidly induced by insulin [4] and ARE-BP proteins are known to participate in modulating the expression of “immediate-early response” genes [17, 18], we sought to determine whether the levels of any of these proteins are quickly changed in response to insulin. Western blotting analysis of liver nuclear extracts was performed. Administration of insulin markedly increased TTP levels (Fig. 2). This is in agreement with a previous report showing that insulin treatment dramatically increased TTP mRNA levels within 10 minutes (19). AUF1 protein levels were increased slightly but not significantly by insulin while HUR levels were not affected (Fig.2). AUH could not be detected.
Fig. 2.
Effect of insulin treatment on protein levels of AU-rich RNA binding proteins in liver nuclear extracts. Nuclear extracts were subjected to Western blotting analysis followed by densitometric analysis. The data are expressed as means ± SD for 5 animals in each group. Significant difference is denoted as p< 0.05.
Effect of TTP siRNA on the rapid activation of hepatic HMGR promoter activity
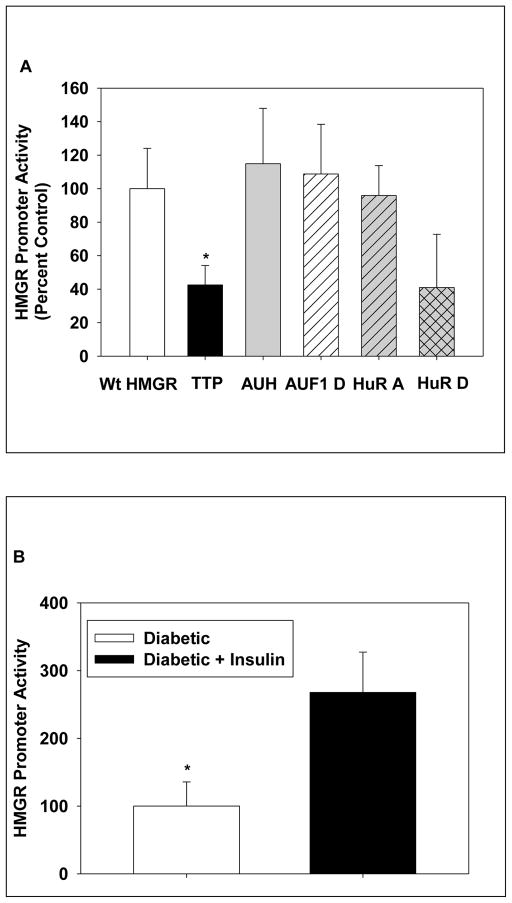
Since siRNAs to TTP abolished HMGR promoter activity in livers of normal rats, we wished to determine their effect on the rapid, near 3-fold increase in HMGR transcription caused by insulin treatment of diabetic rats [4]. The HMGR promoter luciferase construct was introduced by in vivo electroporation in duplicate into the left, right and medial lobes of diabetic rats. TTP siRNA was co-electroporated into the left lobe while AUH siRNA was co-electroporated into the right lobe. The medial lobe had only the HMGR construct. Twenty-two hrs later, the rats were given 3 units per 100 g of insulin. Two hrs later, the rats were killed and the electroporated areas were punched out and analysized for luciferase activity. Introduction of TTP siRNA decreased HMGR promoter activity to about 38% (Fig. 3A), which agrees with the magnitude of the increase in transcription caused by insulin in these animals (Fig. 3B). In contrast, siRNA to AUH had no effect (Fig. 3A). In parallel experiments, siRNA to AUF1 also had no effect while HUR D siRNA caused a 50% reduction, which was not statistically significant, and HUR A siRNA had no effect (Fig. 3A). However, insulin did not alter levels of HUR in liver nuclear extracts (Fig. 2).
Fig. 3.
Effects of siRNAs to AU-rich RNA binding proteins on the insulin-promoted increase in hepatic HMGR transcription. In A, experiments were performed as described in Fig.1 except that the diabetic rats were injected with insulin at the time of surgery. The animals were killed two hrs later. The promoter activity is expressed relative to the luciferase activity observed in punches of HMGR promoter with saline (Wt HMGR). A pool of siRNAs A-D were used for TTP and AUH. HuR A and HuR D siRNAs were also evaluated. The data are expressed as means ± SD for at least four duplicates in each group. In B, the effect of insulin treatment of HMGR promoter (luciferase activity) activity is presented for at least five duplicate punches as means ± SD. Significant differences are denoted as p< 0.05.
Discussion
The in vivo experiments presented in this report show that siRNAs to TTP dramatically reduce hepatic HMGR transcription as well as the insulin promoted increase in HMGR transcription. In the original report describing TTP, it was noted that other proteins with proline-rich regions are involved in transcriptional activation [19]. The finding that insulin induces TTP mRNA within 10 min led to speculation that TTP might be involved in stimulating transcription of genes by insulin.
Subsequent to this report [19], numerous studies have appeared showing that TTP is an RNA-binding protein that recognizes AU-rich elements found in mRNAs with short half-lives. [20–25]. The binding of TTP to these mRNAs causes rapid degradation. TTP−/− mice develop an inflammatory syndrome [20]. The macrophages from these mice overexpress TNFα due to prolonged mRNA half-live. TTP was shown to specifically bind to the ARE of TNFα and cause its rapid degradation [21]. A recent genome-wide analysis found 250 mRNAs to be stabilized in TT−/− mouse embryonic fibroblasts [23].
Nevertheless the data reported in this communication indicate a role for TTP in transcription activation of hepatic HMGR by insulin. There are several possible ways by which TTP could be involved. It could directly bind the HMGR promoter. CTF/NF-1, a CCAAT box binding protein has a C-terminal region that is a transcriptional activation domain containing approximately 25% proline residues [26]. TTP has three repeats of Pro-Pro-Pro-Pro. TTP could also bind to a DNA binding protein to stimulate transcription of HMGR; perhaps in response to phosphorylation of TTP via insulin signaling. Another possibility might relate to the mRNA degrading activity of TTP. TTP could target an HMGR transcriptional repressor; leading to derepression.
Highlights.
siRNAs to tristetraprolin blocks transcription of HMGR in vivo in rat liver.
siRNAs to tristetraprolin inhibits insulin activation of HMGR transcription.
Insulin acts to rapidly increase tristetraprolin in liver nuclear extracts.
Acknowledgments
This work was supported by a grant from the National Institutes of Diabetes and Digestive and kidney Diseases (R01DK075414) and does not necessarily represent the official views of the NIDDK or the National Institutes of Health.
Abbreviations
- ARE
AU-rich RNA binding element
- ARE-BP
AU-rich RNA binding element binding protein
- TTP
tristetraprolin
- HMGR
3-hydroxy-3-methylglutaryl coenzyme A reductase
- AUH
AU RNA binding protein/enoyl coenzyme A hydratase
- HUR
human antigen R
- TIA-1
T-cell inhibitor of apoptosis-1
- TIAR
tein
- AUF1
A+U-rich element-binding factor 1
- KSRP
KH-type splicing regulatory protein
- TNF-α
tumor necrosis factor α
Footnotes
G.C. Ness and M. Watkins, unpublished observations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ness GC, Chambers CM. The diurnal variation of hepatic HMG-CoA reductase activity is due to changes in the level of immunoreactive protein. Arch Biochem Biophys. 1996;327:41–44. doi: 10.1006/abbi.1996.0090. [DOI] [PubMed] [Google Scholar]
- 2.Ness GC, Wiggins L, Zhao Z. Insulin increases hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA and immunoreactive protein levels in diabetic rats. Arch Biochem Biophys. 1994;309:193–194. doi: 10.1006/abbi.1994.1102. [DOI] [PubMed] [Google Scholar]
- 3.Ness GC, Zhao Z, Wiggins L. Insulin and glucagon modulate hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity by affecting immunoreactive protein levels. J Biol Chem. 1994;269:29168–29172. [PubMed] [Google Scholar]
- 4.Lagor WR, de Groh ED, Ness GC. Diabetes alters the occupancy of the hepatic 3-hydroxy-3-methylglutaryl-CoA reductase promoter. J Biol Chem. 2005;280:36601–36608. doi: 10.1074/jbc.M504346200. [DOI] [PubMed] [Google Scholar]
- 5.Keller RK, Zhao Z, Chambers CM, Ness GC. Farnesol is not the nonsterol regulator mediating degradation of HMG-CoA reductase in rat liver. Arch Biochem Biophys. 1996;328:324–330. doi: 10.1006/abbi.1996.0180. [DOI] [PubMed] [Google Scholar]
- 6.Simonet WS, Ness GC. Transcriptional and posttranscriptional regulation of rat hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase by thyroid hormones. J Biol Chem. 1988;263:12448–12453. [PubMed] [Google Scholar]
- 7.Stoecklin G, Tenenbaum SA, Mayo T, Chittur SV, George AD, Baroni TE, Blackshear PJ, Anderson P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem. 2008;283:11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds GA, Basu SK, Osborne TF, Chin DJ, Gil G, Brown MS, Goldstein JL. HMG-CoA reductase: a negatively regulated gene with unusal promoter and 5’ untranslated regions. Cell. 1984;38:275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- 9.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 10.Chen CYA, Xu N, Shyu AB. Highly selective actions of HuR in antagonizing AU-rich element-mediated mRNA destabilization. Mol Cell Biol. 2002;22:7268–7278. doi: 10.1128/MCB.22.20.7268-7278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda K, Marasa B, Martindale JL, Halushka MK, Gorospe M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging. 2009;1:681–698. doi: 10.18632/aging.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, Lei T, Song Y, Yanes N, Qi Y, Fu M. RNA-destabilizing factor tristetraprolin negatively regulates NF-kB signaling. J Biol Chem. 2009;284:29383–29390. doi: 10.1074/jbc.M109.024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schichl YM, Resch U, Hofer-Warbinek R, de Martin R. Tristetraprolin impairs NF-kB/p65 nuclear translocation. J Biol Chem. 2009;284:29571–29581. doi: 10.1074/jbc.M109.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagor WR, Heller R, de Groh ED, Ness GC. Functional analysis of the hepatic HMG-CoA reductase promoter by in vivo electroporation. Exp Biol Med. 2007;232:353–361. [PubMed] [Google Scholar]
- 15.Boone LR, Niesen MI, Jaroszeski M, Ness GC. In vivo identification of promoter elements and transcription factors mediating activation of hepatic HMG-CoA reductase by T3. Biochem Biophys Res Commun. 2009;385:466–471. doi: 10.1016/j.bbrc.2009.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771:1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Sanduja S, Blanco FF, Dixon DA. The roles of TTP and BRF proteins in regulated mRNA decay. Wiley Interdiscip Rev RNA. 2010;2:42–57. doi: 10.1002/wrna.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Shyu AB. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994;14:8471–8482. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai WS, Stumpo DJ, Blackshear PJ. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J Biol Chem. 1990;265:16556–16563. [PubMed] [Google Scholar]
- 20.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Hayes BF, Blackshear PJ. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 21.Carballo E, Lai WS, Blackshear PJ. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 22.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai WS, Parker JS, Grisson SF, Stumpo DJ, Blackshear PJ. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficients fibroblasts. Mol Cell Biol. 2006;26:9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoecklin G, Tenenbaum SA, Mayo T, Chittur SV, George AD, Baroni TE, Blackshear PJ, Anderson P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem. 2008;283:11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvie RL, Sternjohn JR, Rattenbacher B, Vlasova IA, Williams DA, Hau HH, Blackshear PJ, Bohjanen PR. Tristetraprolin mediates interferon-gamma mRNA decay. J Biol Chem. 2009;284:11216–11223. doi: 10.1074/jbc.M901229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mermod N, O’Neill EA, Kelly TJ, Tjian R. The proline-rich transcriptional activator of CTF/NF-1 is distinct from the replication and DNA binding domain. Cell. 1989;58:741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]