Summary
Here, we report that temporally-patterned, coherent spiking activity in posterior parietal cortex (PPC) coordinates the timing of looking and reaching. Using a spike-field approach, we identify a population of parietal area LIP neurons that fire spikes coherently with 15 Hz beta frequency LFP activity. The firing rate of coherently-active neurons predicts the reaction times (RTs) of coordinated reach-saccade movements but not of saccades when made alone. Area LIP neurons that do not fire coherently do not predict RT of either movement type. Similar beta-band LFP activity is present in the parietal reach region but not nearby visual area V3d. This suggests that coherent spiking activity in PPC can control reaches and saccades together. We propose that the neural mechanism of coordination involves a shared representation that acts to slow or speed movements together.
Introduction
Vision is essential for guiding accurate arm movements. The tight link between vision and reaching means that arm movements are coordinated with eye movements (Song and McPeek, 2009; Crawford et al., 2004). Coordinated reach and saccade movements are a central aspect of our natural behavior and lead to faster and more accurate movements (Neggers and Bekkering, 2002). An intriguing feature of coordinated reach and saccade movements is that the reaction time (RT) of the reach is correlated with the RT of the saccade (Lünenburger et al., 2000). Although RTs are influenced by non-specific factors like motivation and arousal (Broadbent, 1971; Barry et al., 2005), non-specific influences alone cannot explain saccade and reach RTs. Therefore, RT correlations may result from movement coordination (Dean et al., 2011).
Movement coordination depends on the posterior parietal cortex (PPC), which constructs representations of space for different movements (Andersen and Buneo, 2002; Bisley and Goldberg, 2010). Damage to the PPC gives rise to a range of deficits of visual-motor coordination which suggests that the ability to coordinate gaze with arm and hand movements fundamentally depends on parietal mechanisms (Gaveau et al., 2008). Neural firing within the lateral intraparietal area (area LIP) and the parietal reach region (PRR), two subdivisions of the PPC, encodes spatial representations that guide saccadic eye movements and arm movements, respectively (Snyder et al., 1997). Coordinated saccade and reach movements may result from spatial representations in posterior parietal circuits that are shared between effectors.
Local field potentials (LFPs) in area LIP and PRR also encode spatial representations for saccades and reaches (Pesaran et al., 2002; Scherberger et al., 2005). LFP activity is generated by temporally coherent patterns of activity in neural circuits (Mitzdorf, 1985; Pesaran, 2009). Since spatial representations are observed in posterior parietal LFP activity, coherent patterns of neural activity in posterior parietal circuits may coordinate movements through the formation of shared movement representations.
To identify shared representations supporting coordinated movement, we recorded spiking and LFP activity in area LIP of two monkeys making either coordinated reach and saccade movements or isolated saccades after a short, 1-1.5 s, memory delay. We also made recordings in PRR and the dorsal part of visual area 3 (V3d), for comparison. By taking a spike-field approach (Pesaran, 2010; Pesaran et al., 2008), we found that RT was predicted by the activity of area LIP neurons that fired coherently in a 15 Hz beta frequency band. Area LIP neurons that did not participate in the coherent activity did not predict RT. Strikingly, area LIP activity only predicted RT before coordinated movements and not when saccades were made alone. The same pattern of results was present in beta band LFP power in area LIP. Beta band LFP power also predicted RT in PRR, but not in V3d. We propose that coherent beta band activity in area LIP and PRR coordinates the timing of eye and arm movements through a shared representation that can be used to slow or speed both movements together.
Results
Linking neural activity and coordination
Figure 1 presents two potential mechanisms for how neural activity could control reaches and saccades. Reach and saccade movements could rely on separate representations for each movement (Fig 1a, left): a saccade representation which guides eye movements and a reach representation which guides arm movements. If so, increases in saccade preparation will shorten saccade RTs without affecting reach RTs (Fig 1a, upper right), and increases in reach preparation will shorten reach RTs without affecting saccade RTs (Fig 1a, lower right). As a result, effector-specific representations cannot coordinate movements because they do not give rise to correlated RTs without other influences.
Figure 1. Schematic illustrating link between preparation and combined movement RTs.
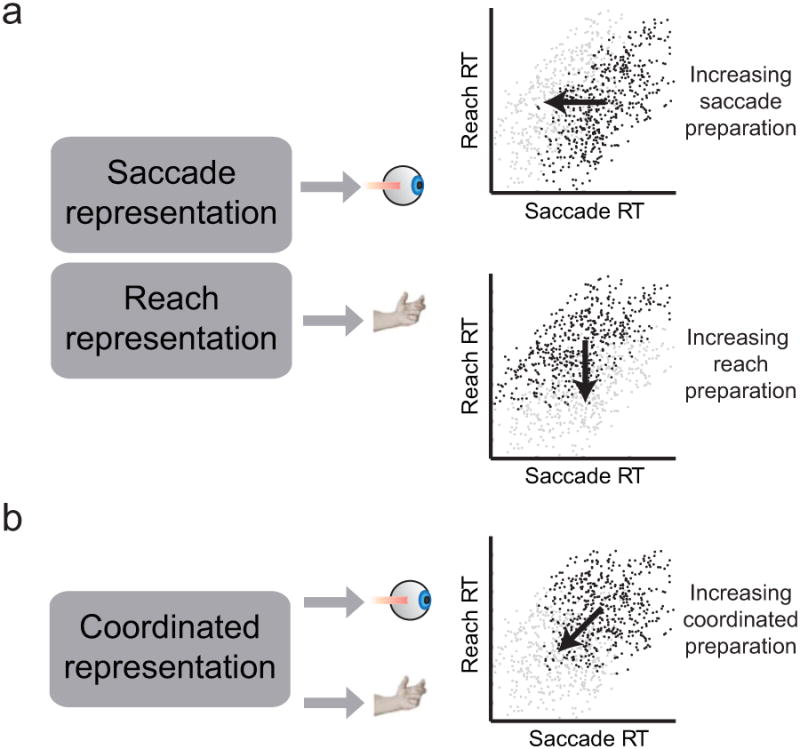
(a) Correlations between saccade and reach RTs when movements are prepared separately. Upper right: Dark points show RTs during groups of trials when saccade preparation is low. Gray points show RTs during groups of trials when saccade preparation is high. Lower right: Dark points show RTs during groups of trials when reach preparation is low. Gray points show RTs during groups of trials when reach preparation is high (b) Correlations between saccade and reach RTs when movement preparation is coordinated. Dark points show RTs during groups of trials when coordinated preparation is low. Gray points show RTs during groups of trials when coordinated preparation is high. In this figure, RTs are illustrative and do not reflect experimental data.
A neural mechanism of coordinated reach and saccade movements could, instead, depend on a shared representation that controls both movements so that they are made together (Fig 1b, left). If there is a shared representation, increases in coordinated movement preparation will shorten both saccade and reach RTs (Fig 1b, right), and neural activity related to coordination will predict both saccade and reach RTs. Covariations in coordinated preparation in this model could give rise to saccade and reach RT correlations. Analyzing the link between RT and neural activity might reveal shared representations that control both movements together.
Area LIP displays selective spike firing and gamma- and beta-band LFP activity
We trained two monkeys to make either coordinated reaches and saccades (Fig 2a) or saccades alone (Fig 2b) to a visually cued target. Before coordinated movements, saccade RTs (SRTs) were correlated with reach RTs (RRTs; example in Fig 2c, R = 0.69, mean SRT =190 ms, mean RRT = 280 ms). Across 105 experimental sessions, SRT-RRT correlations were 0.50 ± 0.24 (mean ± std). Mean SRT across the population was also significantly faster when the saccade was made with a reach (243 ± 0.6 ms, mean ± sem) than when it was made alone (252 ± 0.6 ms; p < 0.001). These results demonstrate that correlations exist between RTs for saccades and reaches such that saccades can be initiated more quickly when made with a reach.
Figure 2. Behavioral task and RT correlations.
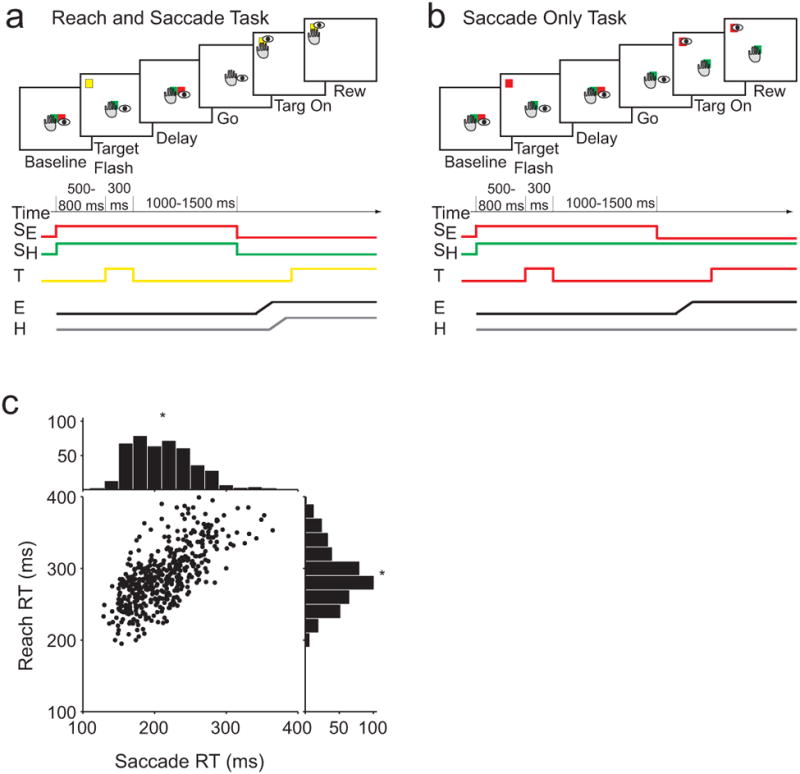
(a) Reach and saccade task. (b) Saccade only task. (c) Scatter plot of reach RT and saccade RT for each trial during the Reach and saccade task in an example session. Histograms show the distributions of each RT. Asterisk denotes mean RT for each effector.
We recorded spiking and LFP activity from 105 sites in area LIP (74 in Monkey H; 31 in Monkey J), 135 sites in PRR (53 in Monkey H; 82 in Monkey J) and 36 visually responsive sites in V3d (36 in Monkey J; Fig 3a, Fig S1). We first present example activity from a single session recorded in area LIP during the reach and saccade task. Spiking and LFP activity in area LIP showed robust selectivity for the preferred (Fig 3bi) compared with the null (Fig 3ci) direction. Spatial tuning was present in LFP activity with different dynamics at different frequencies. One pattern of power changes was present before movements to the preferred direction (Fig 3bii), and another pattern was present before movements to the null direction (Fig 3cii). LFP power was generally greatest around 15-17 Hz, in the beta-frequency band, and decreased relative to baseline for preferred direction trials (Fig 3d). In contrast, LFP power increased at frequencies above ∼30 Hz, in the gamma-frequency band, and the opposite pattern was present for trials in the null direction (Fig 3e). Thus, reach and saccade movements influence the rate of spiking as well as LFP power in both gamma- and beta-frequency bands.
Figure 3. Recording sites and example area LIP data.
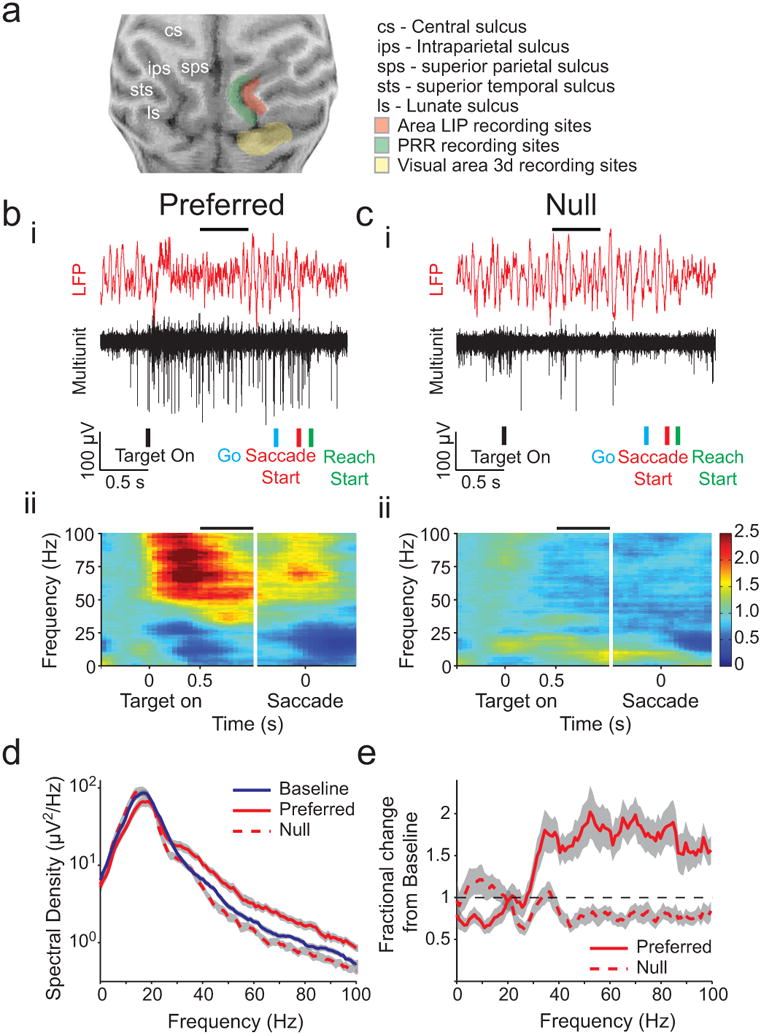
(a) Structural magnetic resonance images showing recording sites for fields in Monkey J. (b) i. Trace from example area LIP recording filtered to show LFP (red) and spiking activity (black). ii. Color map presents average percent change in spectrum for example field during the Reach and saccade task aligned on target onset and on saccade start for preferred direction. Horizontal black line above data in i. and ii. indicates the time of the analysis shown in d. (c) Example data as in b for the null direction, (d) Line plot of spectral density at each frequency for preferred and non-preferred directions 1.5 s after target onset in the Reach and saccade task for the example field shown in b and c (red) as well as baseline activity in the 0.5 s before target onset (blue). (e) Line plot of the change from baseline for preferred (solid line) versus null (dashed line) directions. Gray indicates 95% confidence interval in d and e. See also Figure S1.
We then related LFP power and spike firing rate to saccade and reach RTs in order to build a link between neural activity and coordination. We started by considering LFP power.
Beta-band but not gamma-band LFP power predicts RTs
We examined whether LFP activity predicts movement RTs by grouping LFP power during trials with the slowest or fastest RTs. We selected LFP activity from 72 sites in area LIP with at least 60 trials in each direction and for each task (Monkey H: 57 sites. Monkey J: 15 sites). Before reach and saccade movements in the preferred direction, beta-band LFP power (15 Hz) was significantly greater during the 33% of trials with the slowest SRTs than for the 33% of trials with the fastest SRTs (Fig 4a; p<0.05, rank-sum test). The effect was even stronger when the data was grouped according to RRTs (Fig 4b; p<0.001, rank-sum test). In the following, we will present gamma band activity by analyzing signals at 45 Hz because this activity displayed the strongest spatially-selective persistent memory activity across the population of recordings (presented in detail below, Movement selectivity of LFP power in parietal but not occipital areas). At 45 Hz, we found that LFP power was not significantly selective for either RT (SRT: p=0.32, RRT: p=0.67, rank-sum test). We obtained similar results at other frequencies above 30 Hz (For example, at 65 Hz, SRT: p=0.11, RRT: p= 0.23, rank-sum test). Since greater beta-band LFP power is associated with slower RTs, decreasing beta-band LFP power may speed movement initiation.
Figure 4. LFP reaction time analysis in area LIP.
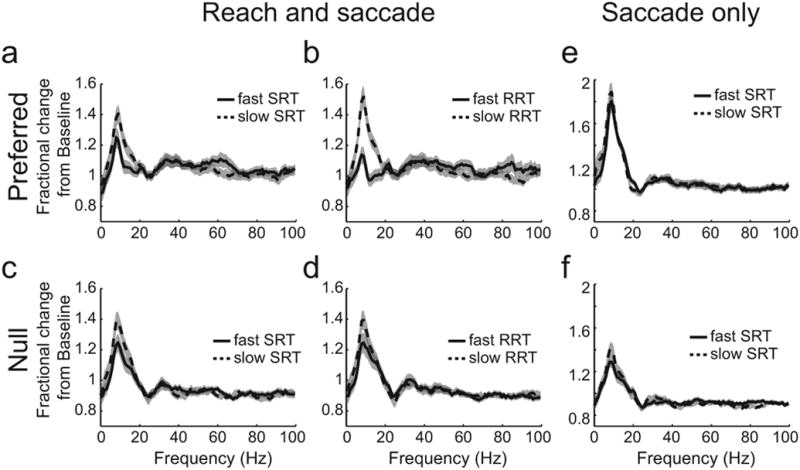
(a) Reach and saccade task. Mean fractional change over baseline during delay for 33% of trials with fastest SRT (solid) and slowest SRT (dashed) before movements to the preferred direction; (b) 33% of trials with fastest RRT (solid) and 33% of trials with slowest RRT (dashed) before movements to the preferred direction; (c) same as a for null direction; (d) Same as b for null direction, (e) Saccade only task Mean fractional change over baseline during delay for 33% of trials with fastest SRT (solid) and 33% of trials with slowest SRT (dashed) for preferred direction; (f) same as e for null direction. In each panel, gray shading indicates the standard error of the mean.
The RT selectivity of beta-band LFP power before a reach and saccade is spatially specific and present before movements to the preferred direction. Before movements to the null direction, the activity was not significantly greater during slow trials regardless of whether activity was sorted by saccade RT or reach RT (Figs 4c,d; RRT: p=0.43. SRT: p=0.27, rank-sum test).
Beta band LFP power does not predict RT before saccades made alone
To further establish the specificity of beta-band activity for specific interactions between reach and saccade processes, we asked whether RT selectivity is also present when saccades are made alone. Strikingly, there was no significant difference between activity across the population for the fast versus slow RTs when saccades were made alone in the preferred direction (Fig 4e; at 15 Hz, p=0.18, rank-sum test) or the null direction (Fig 4f; at 15 Hz, p=0.63, rank-sum test). Lack of RT selectivity before saccades is also not associated with a lack of spatial selectivity. LFP activity was significantly greater for saccades in the preferred direction than in the null direction (Fig 4e,f; Supplementary Results). Therefore, beta-band LFP power in area LIP correlates with SRT only when a saccade is made with a coordinated reach in the preferred direction.
The level of beta-band power before movements to the preferred direction, however, is greater before saccades made alone than before coordinated movements. Since SRTs are faster before coordinated reach and saccade movements than before saccades made alone, this is consistent with increasing beta-band LFP power slowing down movement initiation. The overall picture is that beta-band activity exerts a ‘braking’ mechanism to control the timing of saccades with reaches.
Coherent area LIP spike firing rate predicts RT before coordinated movement
Next, we determined whether beta-band selectivity for RT was also present in the spiking activity of area LIP neurons. We recorded isolated action potentials from 59 neurons that showed spatially tuned activity before a coordinated reach and saccade (p<0.05; permutation test, 48 neurons in Monkey H; 11 neurons in Monkey J). To determine whether spiking activity that is coherent with beta-band LFP activity also predicts RT, we first divided neurons into two groups: coherent cells and not coherent cells. We defined coherent cells as those cells with activity that is significantly correlated with nearby beta-band LFP activity in area LIP. We defined not coherent cells as those cells whose activity is not significantly correlated with nearby beta-band LFP activity. 34 cells (34/59, 58%) were significantly correlated with LFP at 15 Hz in the late delay epoch, 500 – 1000 ms after target onset (coherent cells; p<0.05). The remaining 25 cells (25/59, 42%) were not significantly correlated with LFP activity (not coherent cells; p>0.05).
Strikingly, the firing rate of coherent cells showed stronger spatially tuning than the activity of not coherent cells (Fig 5). The difference in firing rate before movements in the preferred and null directions was greater for coherent cells than not coherent cells for both tasks (Fig 5a,b; Coherent cell average firing rate = 14.9 sp/s. Not coherent cell average firing rate = 7.3 sp/s.). In general, firing rate was higher for coherent versus not coherent cells throughout the trial, including during the baseline epoch. Note that although firing rate is elevated during the delay as opposed to the baseline epoch, LFP directional selectivity and power (see Fig 3bii) drop off at frequencies > 60 Hz during the delay. This suggests that the band-limited effects that we see at frequencies < 60 Hz are not due to increased spiking activity associated with upcoming movements in the preferred direction.
Figure 5. Encoding of coordinated movement RTs in spiking activity coherent with LFP in area LIP.
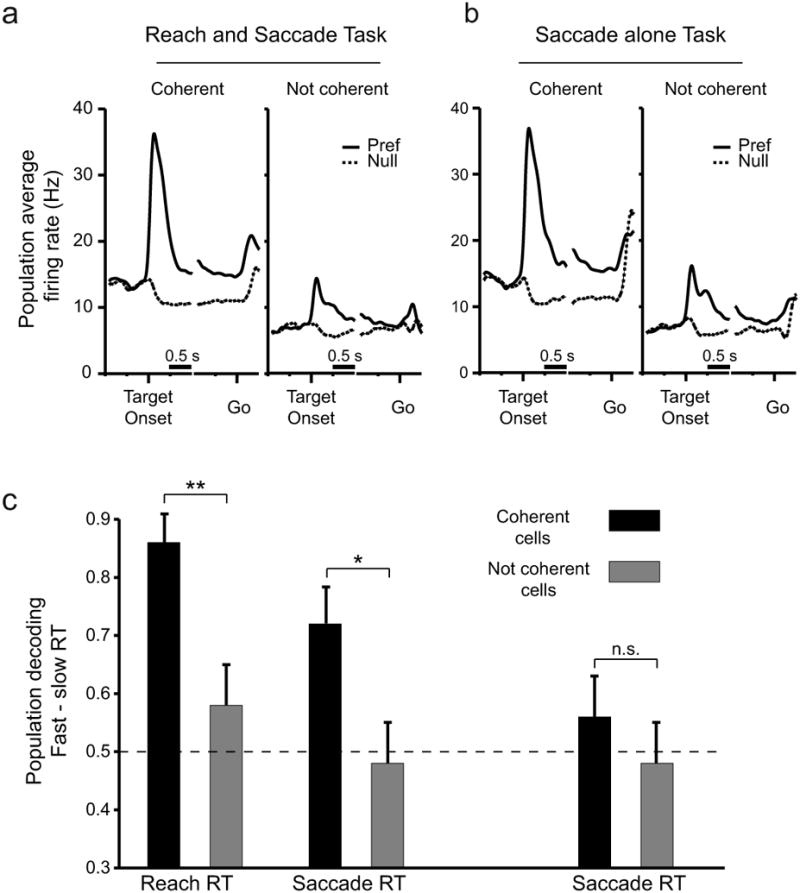
(a) Population average peri-stimulus time histograms (PSTHs) of firing rate during reach and saccade trials for cells with spiking activity that is coherent (left; Coherent cells) or not coherent (right; Not coherent cells) with LFP activity at 15 Hz. Spike-field coherence was calculated during the late delay period (horizontal bar). Preferred direction trials (solid lines) and null direction trials (dashed) are plotted separately, (b) Population average PSTHs for the same cells in a during the Saccade alone task. Conventions as in a. (c) Probability of correct classification of trials in the preferred direction as fast or slow RT trials based on firing rate in the 8 cells with the highest RT selectivity based on an ANOVA. Dashed line indicates chance performance. Error bars indicate standard deviation. * indicates p < 0.05, ** p < 0.01. See also Figure S2.
To determine whether the definition of a cell as coherent or not coherent was consistent across the trial, we also analyzed spike-field coherence during the target epoch, 0 – 500 ms after target onset, and during the baseline epoch, 500 ms immediately before target onset. Almost the same proportion of cells was defined as coherent during the target epoch (Coherent: 35/59, 59%. Not coherent: 24/59 41%) as during the late delay epoch. The definition of a cell as coherent was consistent between target and late delay epochs for 44 out of 59 cells (44/59, 75%). We observed consistent results based on the baseline epoch. A similar proportion of cells was defined as coherent during the baseline epoch (Coherent: 31/59, 53%. Not coherent: 28/59, 47%). The definition of a cell as coherent was again consistent between baseline and late delay epochs, with 42 cells (42/59, 71%) having the same definition for both epochs. Therefore, the definition of a cell as coherent or not coherent did not vary substantially across the trial. Since we observed beta-band selectivity for RT in the LFP during the delay, we chose to focus our analysis of spiking using the definition of coherence during the delay.
The difference in spike-field coherence was not simply due to an increase in firing rate. First, coherence is normalized by the firing rate. Second, if coherence were an artifact of higher firing rates we would expect that the largest differences in firing rate between coherent and not coherent cells would be present during the late delay epoch, when coherence was estimated. However, the largest differences in firing rate were not present during the late delay epoch. The largest differences in firing rate were present immediately following the target onset. Third, the same proportions of neurons were coherently active immediately following target onset and during the late delay epoch despite the difference in firing rates between these epochs. Fourth, although coherent activity can be easier to detect when the firing rate is higher (Zeitler et al., 2006), if coherent activity is not present, the number of false positives that result from the statistical testing procedure we use does not vary with firing rate (see Supplementary Methods, see also (Maris et al., 2007)). Finally, we recalculated SFC after decimating the firing rate of the significantly coherent units by 50% to match the firing rate of those units not coherent with the local fields. We found that after decimation, 29/34 (85%) remained significantly coherent with LFP. Consequently, although there was a difference between the firing rate of coherent and not coherent cells, the difference in firing rate we report was not due to a confounding influence of firing rate on coherence.
To determine whether coherent and not coherent spiking predicted RT, we performed an ANOVA to determine whether individual neurons showed significant differences in firing rate between the fast and slow RT trials. We found that before a reach and saccade, 21% of coherent cells have significant (p<0.05) differences in firing rate between fast and slow RRT groups and 9% have significant differences between fast and slow SRT groups. Of these recordings, 70% showed a decrease in firing rate with faster RTs and the remaining 30% showed an increase in firing rate. We also found that only 3% of coherently-active cells are significantly selective for SRT during the saccade alone task, which is within the expected proportion of false positives (5%). Finally, and most importantly, when cells are not coherently-active, fewer than 5% of cells show significantly selective differences in firing rate for the fast and slow reaction times for all combinations of task and RT type (Reach and saccade, RRT: 4%. Reach and saccade, SRT 0%, Saccade alone, SRT 4%).
To quantify the extent to which populations of cells with coherent and not coherent spiking predicted RT, we used a decoding algorithm to predict the RT from each cell population (Fig 5c; see Methods). Unlike the LFP analysis, which was done using fixed proportions of fast and slow trials, the population decoding algorithm required that we use a fixed number of trials in each group. We analyzed the fastest or slowest 25 trials (SRT or RRT) in the preferred direction. Ideally, more trials would be available to perform a multiple neuron decoding analysis, but this was the largest number of trials available in the database of neuronal recordings for which there was no overlap between the RTs for the fast and slow groups. Quantifying the extent to which RT could be decoded from neural populations allowed us to summarize and compare the strength of the results across tasks and movements. We computed the probability of correct classification of trials in the preferred direction as fast or slow RT trials based on firing rate in the eight cells with significant RT selectivity based on an ANOVA. Before coordinated movements to the preferred direction, coherent cells significantly predicted whether a trial had a fast or slow RRT (decode probability correct: 0.86; p<0.001 Binomial test) and a fast or slow SRT (decode probability correct: 0.72; p<0.001). In contrast, not coherent cells did not significantly predict RRT (decode probability correct: 0.58, p=0.16) or SRT (decode probability correct: 0.48; p=0.56). Coherently-active cells predicted RRT significantly better than cells that were not coherently active (p<0.005, Two-sample Binomial test). Coherently-active cells also predicted SRT significantly better than not coherent cells (p<0.05).
Importantly, coherent cells encoded the speed of RTs only when movements were coordinated. When saccades were made alone, despite the fact that mean firing rate did not differ for reach and saccade versus saccade alone trials (Fig 5a,b), the decoder performed at chance (decode probability correct: 0.56, p=0.16). The not coherent cells also did not predict SRT (decode probability correct: 0.48, p=0.56).
The performance advantage of the coherent cell population in decoding RT was not due to the fact that there were more cells in the coherent population than the not coherent population. We repeated the analysis for increasing sizes of coherent and not coherent populations up to the number of available cells. The coherent population out-performed the not coherent population for all cell subsets greater than two (Fig S2). Though we report here the results for eight cells, note that for all numbers of coherent cells greater than three, the decoder performs best and above chance for RRT during coordinated movements. The decoder also performs well and usually above chance for SRT during coordinated movements at all numbers of cells but not for SRT during saccades made alone or for not coherent cells.
We also examined whether the better decoding performance of the coherent cells could be due to their higher overall firing rate. When we subtracted the mean firing rate from each cell before decoding the firing rate, we found that the results maintained the same pattern of significance. Coherently-active cells predicted coordinated movement RT significantly better than cells that were not coherently active (RRT: p<0.05. SRT: p<0.01). Neither group of cells predicted SRT before saccades made alone (Coherent decode probability correct = 0.48. Not coherent decode probability correct = 0.46). Additionally, we decimated the firing rate of the significantly coherent units by 50% to match the firing rate of the not coherent units (see Methods). When we performed the RT decoding analysis on the coherent units after decimating the firing rates, we found no significant change in performance when decoding RRT (decode probability correct = 0.70, p<0.05) or SRT (decode probability correct = 0.62, p<0.05) before a coordinated movement.
Therefore, beta-band LFP activity reflects a population of neurons whose firing rate reliably predicts the RT of coordinated eye-hand movements but not saccades made alone. Neurons which do not participate in the coherent beta-band LFP activity do not predict RT of either movement type.
Beta-band activity could act to control saccades and reaches together
Beta-band activity may reflect the coordinated control of reach and saccade RTs together. We have shown that beta-band spiking and LFP activity varies with both SRT and RRT across a population of sites, but this is not necessarily sufficient to demonstrate that the control of saccade and reach RTs occurs together. Activity at some sites may be involved in controlling one effector, while activity at different sites may control the other effector. To link beta-band activity to the coordinated control of movement timing, we examined whether selectivity for both saccade and reach RTs is present in activity at the same sites.
We determined RT selectivity by grouping LFP power during trials with the slowest 33% of RTs and LFP power during trials with the fastest 33% of SRTs and computing a z-score using random permutations (see Methods) and found that RT selectivity does exist for both movements at the same sites (Fig 6a). At 15 Hz, LFP activity was significantly selective for both SRT and RRT at 10/72 sites (14%; p<0.01, Binomial test). In comparison, LFP activity at 45 Hz was selective for both RTs at only 2/72 sites (3%; p=0.88. Binomial test. Fig 6b). The strength of the effect at single sites is limited by the number of trials available for analysis. When we restrict our analysis to recording sites with at least 135 trials per direction and task, 30% of recording sites were significantly selective for both SRT and RRT in the beta-band.
Figure 6. Selectivity for both SRT and RRT in area LIP.
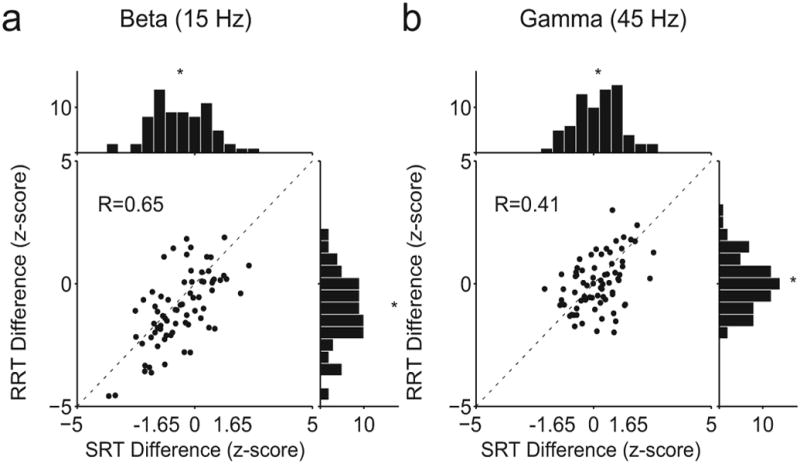
(a) Scatter plot of selectivity of activity at 15 Hz for SRT against selectivity of activity at 15 Hz for RRT. Selectivity is measured as a z-score for difference in power against the null hypothesis that there is no change in power. (b) Same as a for activity at 45 Hz. Histograms show the marginal distributions. Each dot represents data from one LFP session.
We found a high degree of correlation between SRT selectivity and RRT selectivity in both the beta-band (R = 0.65 at 15 Hz) and the gamma-band (R = 0.41 at 45 Hz). Thus, LFP activity at each recording site predicts the RT of both the saccade and the reach in a similar manner, with the strongest effects present in the beta band.
These data suggest that if changes in beta-band power change the RT for both movements, beta-band activity could coordinate movement timing. If beta-band power reflects the joint control of movement RTs, variations in the level of beta-band power could give rise to correlations in the behavioral RTs, and lack of power variation could lead to a reduction or even elimination in the RT correlations. To test this prediction, we calculated the relationship between saccade and reach RTs across groups of trials when beta-band power is relatively constant (see Methods). This approach is similar to a partial correlation analysis, which is defined by the relationship between two variables while controlling for a third variable, but it does not require us to correlate LFP power and RT trial-by-trial. We found that average RT correlations calculated during groups of trials when beta-band power was relatively constant (R = 0.32) were significantly lower than correlations calculated in the same way when beta-band power varied (R = 0.37). The difference in RT correlation was significant (p<0.05, rank-sum test). An average of 18% of the correlation between saccade and reach RTs could be explained by variations in beta-band power in area LIP. At some sites, beta-band power could explain over 60% of the RT correlations. Since SRT and RRT are less correlated when beta-band power does not vary, variation in the level of beta-band activity can contribute to RT correlations.
Beta-band activity in parietal but not occipital areas predicts RT
Beta-band power is selective for RT in other areas of posterior parietal cortex and is not selective for RT in nearby occipital cortex. We analyzed a complementary data set of 122 LFP recordings in PRR and 36 visually responsive recordings in V3d, located along the lunate sulcus, with at least 60 trials in each condition, and we plotted RT selectivity from all three areas as the trial progressed (Fig 7). Beta-band LFP activity in area LIP was increasingly selective for RT as the memory period progressed (Fig 7a,b). The RT effect was also robust in PRR where 28/122 sessions (23%) were significantly selective when trials were sorted by RRT, and 18/122 sessions (15%) were significantly selective when trials were sorted as a function of SRT (Fig 7c,d). In comparison, at 45 Hz, only 12/122 sessions (10%, not shown) were significantly selective for RRT, and 7/122 sessions (6%, not shown) were selective for SRT, which is not statistically significant (Binomial test). Beta-band power in the visual areas we studied, in contrast, is not selective throughout the trial (Fig 7e,f). PRR LFP recordings also showed RT selectivity for both movements at the same site (not shown). As in area LIP, LFP activity at 15 Hz in PRR was significantly selective for both SRT and RRT at 22/122 sites (18%; p<0.01), while at 45 Hz, LFP was selective for both RTs at only 4/122 sites (3%) which, as in area LIP, is not statistically significant. Therefore, LFP beta-band RT selectivity is a feature of areas within the intraparietal sulcus of the posterior parietal cortex and is not present in nearby visual cortex.
Figure 7. Time course of RT selectivity in area LIP, PRR and V3d.
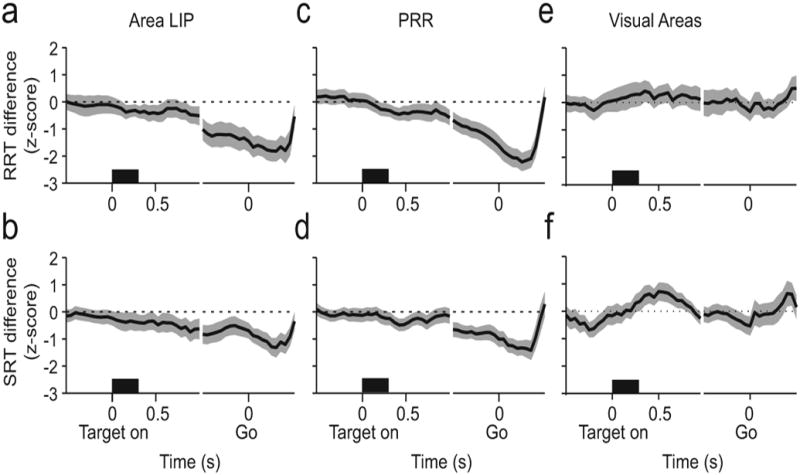
(a) Line plot of correlation of activity (z-score) in area LIP at 15 Hz with RRT (top row) and with SRT (bottom row) aligned on target onset and go cue before reach-and-saccade movements to the preferred direction. (b) Same as a for PRR. (c) Same as a for V3d. In each panel, gray indicates 95% confidence interval.
Movement selectivity of LFP power in parietal but not occipital areas
To be involved in guiding movements, neural activity should be selective for the properties of the movement, such as the direction of the movement and the type of movement (coordinated or isolated). Therefore, we examined the directional and movement type selectivity of LFP power in all 105 recordings in area LIP and compared this with LFP power in the 135 recordings from PRR and 36 visually responsive recordings from nearby V3d (Fig 8).
Figure 8. Directional selectivity in area LIP, PRR and V3d.
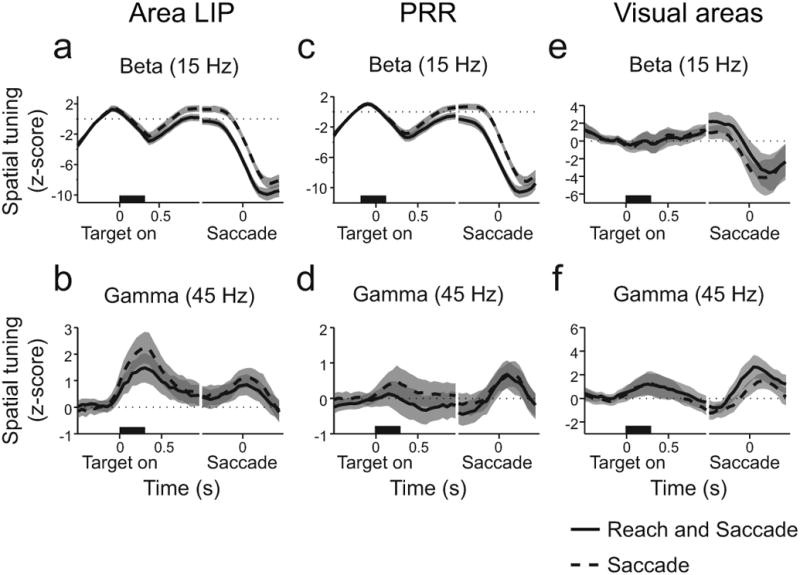
(a) Time course of average z-score aligned on target onset and on saccade start for 15 Hz, beta-band LFP power in area LIP. (b) Same as a for 45 Hz gamma frequency band. (c) Time course of average z-score aligned on target onset and on saccade start for 15 Hz, beta-band LFP power in PRR. (d) Same as c for 45 Hz gamma frequency band. (e) Time course of average z-score aligned on target onset and on saccade start for 15 Hz, beta-band LFP power in V3d. (f) Same as e for 45 Hz, gamma frequency band. Reach and saccade (solid). Saccade only (dashed). Gray indicates 95% confidence interval. See also Figures S3-5.
To analyze the directional selectivity of LFP power at each time and frequency, we subtracted LFP power before movements in the null direction from LFP power before movements in the preferred direction and computed a z-score using random permutations (see Methods and also Supplementary Results. Figs S3, S4, S5). At individual recording sites in area LIP, LFP activity at both 45 Hz and 15 Hz exhibited strong spatial tuning (Fig S3). Across the population of recording sites in area LIP, average LFP power at 15 Hz developed after target onset and differed according to whether or not a reach was made with a saccade (Fig 8a, memory period, p ≪ 0.001, rank-sum test). Gamma-band, 45 Hz, LFP power was directionally selective but did not depend on whether a coordinated saccade was made with the reach (Fig 8b, memory period, p = 0.74, rank-sum test). Consequently, selectivity of area LIP gamma-band LFP power for saccades does not change with a reach movement. In contrast, beta-band LFP power in area LIP is selective for both movement direction and type, consistent with a role in the control of coordinated movements.
Beta-band but not gamma-band LFP power in PRR showed similar signatures of coordination (Fig 8c,d, see Fig S4). In contrast, in V3d, not only was there no movement specificity in beta-band signals, the initial significant decrease in beta-band selectivity immediately following target onset was not present (Fig 8e,f, Fig S5). Therefore, movement specificity of beta-band LFP power is a feature of activity within PPC circuits and is not a global feature of brain activity.
Discussion
Here, we use a spike-field approach to identify a neural mechanism of coordination and find that only area LIP neurons that coherently fire with beta-band LFP activity predict movement RT before coordinated movement. Decreasing beta-band activity speeds movement initiation. On average, RTs are faster on trials when there is less beta-band activity and slower on trials when there is more beta-band activity. Beta-band activity encodes the properties of coordinated movement: it is selective for the direction of the movement as well as whether a coordinated reach is being made with a saccade. These properties of beta-band activity are a feature of area LIP and PRR and are not present in visual cortical areas. Therefore, we propose that posterior parietal beta-band activity coordinates the timing of reaches with saccades through the formation of a shared movement representation.
To uncover the shared movement representation responsible for coordinated timing, we correlate the activity of individual neurons to nearby LFP activity. Our results demonstrate how correlating spiking with LFP activity can help us to define distinct neuronal populations in terms of the circuits in which they are active. By dividing neurons into two populations, those which coherently fire action potentials with LFP activity and those which do not, we show that only coherent spiking predicts movement RTs. We quantify the amount of information about movement timing present in each of these two populations using a decoding analysis in which we decode the RT of either reach or saccade movements on each trial from the firing rates. The decoding analysis is limited by the number of trials available, but the conclusions are consistent with the results of an ANOVA which demonstrate that only coherent spiking predicts coordinated movement RTs.
We also find that the role beta-band activity in area LIP plays in movement preparation depends on whether movements are coordinated. Beta-band activity and the spiking coherent with it in area LIP predict coordinated RTs but not saccade RTs when saccades are made alone. The lack of association between area LIP activity and RT when saccades are made alone suggests that performing a coordinated movement alters the role that beta-band activity in area LIP plays in the generation of movement. Beta-band activity in area LIP could be a measure of a linking of areas involved in the preparation of each movement. Coordinating two movements requires information about the timing of one to be shared with the other. This involves constructing a shared representation of movement preparation that recruits beta-band activity in area LIP. Note that this need not contradict data showing that area LIP has more saccade-related activity than reach-related activity. Beta-band activity may simply modulate already-existing activity in area LIP in order to coordinate saccades with reaches.
Area LIP is one of several posterior parietal regions situated between visual and motor areas. These areas contain spatial representations for visual spatial attention (Bisley and Goldberg, 2010), decision-making (Sugrue et al., 2004; Gold and Shadlen, 2007; Kable and Glimcher, 2009) and movement intention (Andersen and Cui, 2009). Spatial representations in PPC are effector-specific (Colby, 1998; Andersen et al., 1998). Area LIP activity encodes space for the guidance of saccades in eye-centered coordinates, and PRR encodes space for reaching in eye-centered coordinates (Batista et al., 1999; Pesaran et al., 2006). These properties of area LIP and PRR position them to share effector-specific representations to control coordinated movements. While previous work studying PPC has emphasized spatial representations, extensive behavioral work shows that eye-hand coordination reliably influences movement RTs, and evidence for spatial coupling is relatively less clear (Carey et al., 2002; Sailer et al., 2000).
The eye leads the hand in many tasks, allowing vision to guide the hand to the target (Prablanc et al., 2003; Johansson et al., 2001). When a reach and a saccade are made simultaneously, reach and saccade RTs are correlated (Dean et al., 2011; Lünenburger et al., 2000). These correlations mean that the eye tends to arrive at a target at a predictable time before the hand. Hence, RT correlations could support improved visual-motor performance (Neggers and Bekkering, 2002). Reaction times and their correlations are not necessarily due to processes that support visual-motor performance and could be due to several other factors, including processing bottlenecks (Pashler, 1984), common sensory inputs (Lee et al., 2010) and non-specific influences such as motivation or arousal (Boucher et al., 2007). Recent behavioral and computational modeling work, however, indicates that as saccade and reach movements are dissociated in time, correlations in RTs decay rapidly. RT correlations cannot be fit by a family of models featuring non-specific interactions and are best fit by models invoking specific interactions between movement representations (Dean et al., 2011). Consequently, saccade and reach RT correlations may be due to interactions that form an effectively shared movement representation.
We provide convergent evidence that beta band signals reflect movement preparation shared between saccades and reaches, which may be sufficient for generating RT correlations and could ultimately influence movement initiation. The relationship between coordination and RT correlations is likely to involve areas in addition to PPC. PPC works in concert with other areas that prepare and initiate movements, including areas in the frontal cortex and basal ganglia (Hanes and Schall, 1996; Requin and Riehle, 1995). PPC also contains direct connections to the cerebellum (Prevosto et al., 2010), a structure that has been implicated in the timing of coordinated movements (Miall and Reckess, 2002). If the RT selectivity of beta-band activity we observe is also present in other areas, this aspect of beta-band activity may reflect processing across a network of areas which work together to control the timing of movements and coordinate saccades with reaches.
Several other lines of convergent evidence support the hypothesis that beta-band activity reflects distributed processing. Correlated beta-band LFP activity is present across long-range circuits (Rougeul et al., 1979) and could underlie long-range communication between brain regions (Roelfsema et al., 1997; Brovelli et al., 2004; Bressler et al., 1993; Donner and Siegel, 2011). Beta-band activity may be involved in bottom-up/top-down influences (Buschman and Miller, 2007) and maintaining a motor state, the “status quo” (Engel and Fries, 2010), leading to slower responding. Beta-band activity is widely modulated during movement tasks (Sanes and Donoghue, 1993) and could be related to attended motor behavior (Bouyer et al., 1987) and sensory-motor integration (Murthy and Fetz, 1992). Beta-band LFP activity in human and monkey motor cortex may work to influence processing of visual cues and targets (Reimer and Hatsopoulos, 2010; Rubino et al., 2006; Saleh et al., 2010). In monkeys, after a ‘Go’ cue has been delivered, increased beta-band LFP power in supplementary motor areas is correlated with slower reach RTs, and increased beta-band activity after a stop cue is also correlated with canceled movement plans in a countermanding task (Chen et al., 2010). In humans, decreased beta-band power in sub-thalamic nuclei is correlated with faster RT indicating that beta-band activity can also reflect the motor command to initiate movement (Kuhn et al., 2004). Beta-band activity is also observed in EEG and boosting beta-band EEG activity using TMS in humans slows movements themselves (Pogosyan et al., 2009), which is broadly consistent with our results.
Since beta-band activity is a widespread property of skeletal-motor circuits, a concern that naturally arises is that beta-band signals in area LIP are not locally-generated and are due to activity that arises in PRR, for example, and passively spreads, through volume conduction, to area LIP. While we cannot rule out the influence of volume conduction on our results, the evidence suggests that area LIP beta-band activity is a property of local neural processing within area LIP and is not simply due to volume conduction from PRR. First, beta-band activity in V3d that is within 10 mm of PRR and as close as area LIP does not show similar selectivity. Second, we also show that beta-band activity is coherent with spike timing within area LIP, demonstrating a role in local processing. Recent studies show that LFP activity recorded in V1 is predominantly local and does not significantly spread beyond 250 μm (Katzner et al., 2009; Xing et al., 2009), but this remains controversial (Kajikawa and Schroeder, 2011).
Another concern is that the correlation between beta-band power and SRT (beta-SRT correlation) results from behavioral correlations between the RTs. However, we believe that beta-SRT correlations do not simply result from RT correlations for two main reasons. First, we show that beta-band power before the ‘Go’ cue is correlated with RT following the ‘Go’ cue. Hence, beta-band power does not result from RT. Second, SRT and RRT are not sufficiently correlated that beta-SRT correlations imply beta-RRT correlations: we observe that beta-band power can be correlated with SRT but not RRT, and vice versa. We also show that SRT-RRT correlation is smaller during trials when beta-band power in area LIP does not vary. Thus, the data suggest that RT correlations can result from variations in beta-band power and that beta-band power cannot result from RT correlations.
To reveal a neural mechanism of coordination, we have used saccade and reach RTs to link neural activity to behavioral coordination. Our results indicate that coherent spike-LFP beta-band activity in PPC reflects spatial representations that guide coordinated movement and supports the hypothesis that eye-hand coordination involves coordinated movement preparation that is shared between effectors.
Experimental Procedures
Experimental preparation
Two male rhesus monkeys (Macaca Mulatta) participated in the experiments. Each animal was first implanted with an MRI-compatible head cap under general anesthesia. A structural MRI was obtained and used to guide the placement of a recording chamber over the posterior parietal cortex of the right (Monkey J) or left (Monkey H) hemisphere in a second surgical procedure. Chamber placement and electrode recording sites were registered to the structural MRI to within 1 mm (BrainSight, Rogue Research, Canada; Fig 3a and Fig S1). The structural MRIs were also used to estimate distances between the area LIP, PRR, and V3d recordings reported here. All surgical and animal care procedures were done in accordance with National Institute of Health guidelines and were approved by the New York University Animal Care and Use Committee.
Behavioral Tasks
Eye position was monitored with a video-based eye tracker (l-Scan, MA). Visual stimuli were presented on an LCD display (Dell Computers, TX) placed behind the touchscreen. Eye position and touch position on the screen were sampled at 1 kHz. Each signal was time-stamped and streamed to disk along with data about each trial from the LabVIEW behavioral control program. The time of cue presentation was recorded as the time at which a photosensor detected a simultaneous stimulus change on the monitor. For all tasks, reaches were made to a touch-sensitive screen (ELO Touch Systems, CA) with the arm contralateral to the recording chamber. Red squares instructed the animal where to fixate the eye. Green squares instructed the animal where to touch. Yellow squares instructed the animal where to make combined look-reach movements. All trials began with the illumination of a red and green square which the animal needed to fixate with his eye and touch with his hand, respectively, and hold for a baseline period (500-800 ms).
We studied a reach and saccade task and a saccade only task (Fig 2a,b). In the Reach and saccade task, a yellow square was then illuminated for 300 ms at a peripheral location indicating the target of the reach. A memory delay period (1-1.5 s) followed during which the animal had to withhold his response. After the delay, the initial green and red squares the animal had to touch and fixate were extinguished, providing the go signal for the animal to look and reach to the yellow target. 100-150 ms after the look-reach movement was completed, the target reappeared, and the animal had to touch and fixate the yellow square for an additional 300 ms. In the Saccade only task, a second red square was illuminated for 300 ms after the baseline period, indicating the target of the saccade. After a delay period (1-1.5 s), the red square the animal was fixating was extinguished, providing the go signal for the animal to saccade to the red target while maintaining touch on the initial green square. 100-150 ms after the saccade, the target reappeared and the animal had to fixate the red square while maintaining a touch on the green square for 300 ms. Reach and saccade and Saccade only tasks were interleaved trial-by-trial in equal proportions.
On each trial, targets were presented at one location on a grid spaced 10° around the central red square. We first mapped response fields of neurons in LIP in both tasks by placing the target at any one of the eight peripheral targets. Once we determined the response field, we presented the target at the location in the response field of the LIP neuron under study (preferred direction) or the opposite location (null direction). Preferred and null target locations were interleaved trial-by-trial in equal proportions.
Neuronal Recordings and Analysis
Neural recordings were made using multiple-electrode microdrives (Double MT, Alpha Omega, Israel). Spiking and LFP activity were recorded with glass-coated tungsten electrodes (Alpha Omega, Israel) with impedance 0.7-1.4 MOhm measured at 1 kHz (Bak Electronics, MD). Neural signals were amplified (×10,000; TDT Electronics, Alachua, FL), digitized at 20 kHz (National Instruments), and continuously streamed to disk during the experiment (custom C and Matlab code). Broad-band neural activity was pre-processed to obtain single unit spike times and LFP activity. Recordings in area LIP and V3d were acquired with respect to a reference placed at the cortical surface on the lateral bank of the intraparietal sulcus. Recordings in PRR were acquired with respect to a reference placed at the cortical surface on the medial bank of the intraparietal sulcus. See also Supplementary Experimental Procedures.
To analyze the relationship between RTs and LFP power at each time and frequency, we subtracted LFP power before movements in trials with the slowest 33% of RTs from LFP power before movements in trials with the fastest 33% of RTs and computed a z-score using 1000 random permutations (Maris et al., 2007). By fixing the proportions of trials across sessions, we were able to effectively control the degree that RT differed between fast and slow trial groups. The z-score was approximated to be normally distributed with mean 0 and variance 1, and values with an absolute value greater than 1.96 were taken to be significant with probability p < 0.05. Similarly, to examine the spatial selectivity of LFP power at each time and frequency, we subtracted LFP power before movements in the null direction from LFP power before movements in the preferred direction and computed a z-score using 1000 random permutations. We confirmed that the null distribution of permuted power differences satisfied the normal approximation (Kolmogorov-Smirnow test, p>0.05).
Correlations between SRT and RRT were calculated using Pearson's correlation coefficient. Similar results were obtained using Spearman's Rank correlation coefficient (not shown). To examine the relationship between SRT and RRT while controlling for beta-band power, we estimated RT correlations across groups of trials when beta band power was held constant and compared the results with RT correlations across groups of trials when beta band power varied. This approach is similar to a partial correlation analysis which is defined by the relationship between two variables while controlling for a third variable but does not require us to correlate LFP power and RT trial-by-trial. We calculated the RT correlations in the following way. We sorted the trials according to the LFP power at 15 Hz during the 500 ms memory period interval immediately before the Go cue was delivered. The window over which the LFP power was computed was centered 250 ms before the Go cue so that the result contained no activity due to the cue itself. We then grouped the trials into quantiles [0,20%), [20%,40%)… [80,100%], calculated the correlation coefficient for each quantile and then averaged the correlation coefficients across quantiles. To compare the results with the correlation coefficient calculated without constraining LFP power so that beta power varied, we randomly ordered the trials before assigning them to quantiles and calculating the correlation coefficient by averaging across quantiles.
Spike-field coherence was calculated on a 500 ms analysis window with ±10 Hz frequency smoothing. Significant spike-field coherence was calculated against the null hypothesis that there was no spike-field coherence. A permutation test was used to estimate significance by comparing the estimated coherence against 10,000 random permutations generated by changing the order of the trials in the LFP activity before computing the coherence. In order to avoid any contamination of the LFP due to spike activity from the isolated unit (Zanos et al., 2010), we estimated the relationship between single unit and LFP activity from recordings on pairs of electrodes separated by at least 550 μm.
In order to determine how firing rate influences spike-field coherence, we decimated the firing rate of significantly coherent units by removing each spike with 50% probability. We then recomputed spike-field coherence and checked for significance as described above.
In order to analyze spike rates for cells coherent or not coherent with LFP activity, we defined a database for cells of each type. Out of the 120 spike-field sessions, we took the 48 sessions with significant coherence and extracted the 34 unique spike sessions (coherent cells) with at least 50 trials for the preferred direction and the 25 unique spike sessions with at least 50 trials for the preferred direction that did not show significant spike-field coherence (not coherent cells). For each trial, we calculated the spike rate during the delay epoch. Because our analyses of spike rate required a set number of trials in each group for each cell, we could not use a fixed proportion for this analysis as in the analysis of LFP.
We first performed an ANOVA to determine whether individual neurons were selective for fast and slow RTs. We next used linear discriminant analysis to decode whether single trials were from the fastest or slowest RTs in reach and saccade trials in the preferred direction. We decoded the identity (fast or slow RT) of each trial in a cross-validated manner to control for over-fitting as follows: For each trial, we defined a test set which contained the trial and a training set which contained all the trials except for the trial in the test set. We ranked the cells according to their RT selectivity computed using an ANOVA based on the training set and fit a linear discriminant to the training set. We then decoded the test set on an increasing subset of cells from two to the maximum number available ranked according to the results of the ANOVA on the training set. We repeated this analysis for each trial and computed the probability of correct classification. We report the results of decoding across all conditions based on the same number of cells, 8 cells, in Figure 5 and present the data across increasing subsets of cells in Figure S2. This procedure ensured that the decoding results were not influenced by over-fitting. Significant differences between the performance of the decoding for each group were determined using a binomial test.
The mean firing rate of cells in the coherent and not coherent groups was different. To test whether the mean firing rate affected the decoding probability, we subtracted the mean firing rate across all trials from each cell and re-ran the decoding algorithm. Additionally, we performed the same decoding analysis for the significantly coherent units using firing rates that were decimated by removing each individual spike with 50% probability in order to match the mean firing rate of the units that were not coherent with the LFP.
Supplementary Material
Highlights.
Correlating area LIP spiking activity to nearby LFP activity reveals a mechanism of coordination.
Coherently-active spiking in area LIP predicts movement timing.
Spiking that does not fire coherently does not predict movement timing.
Coordination involves coherently-active neurons that can coordinate movement timing.
Acknowledgments
We thank Eva Tsui for assistance with animal training, Gerardo Moreno for surgical assistance, Roch Comeau, Stephen Frey and Brian Hynes for customizations to the Brainsight system and Bob Shapley for comments on the manuscript. This work was supported, in part, by CRCNS Program award R01 MH-087882, NSF CAREER Award BCS-0955701, a Fellowship in Brain Circuitry from the Patterson Trust (HLD), NIH Training grant T32 MH-19524 (HLD), NIH Training grant T32 EY-007136 (MAH), a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (BP), a Watson Investigator Program Award from NYSTAR (BP), a McKnight Scholar Award (BP), and a Sloan Research Fellowship (BP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Snyder LH, Batista AP, Buneo CA, Cohen YE. Posterior parietal areas specialized for eye movements (LIP) and reach (PRR) using a common coordinate frame. Novartis Found Symp. 1998;218:109–122. doi: 10.1002/9780470515563.ch7. discussion 122–128, 171–175. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Rushby JA. Arousal and Activation in a Continuous Performance Task. J Psychophysiol. 2005;19:91–99. [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher L, Stuphorn V, Logan GD, Schall JD, Palmeri TJ. Stopping eye and hand movements: are the processes independent? Percept Psychophys. 2007;69:785–801. doi: 10.3758/bf03193779. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Montaron MF, Vahnée JM, Albert MP, Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987;22:863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366:153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- Broadbent D. Decision and Stress. London: Academic Press; 1971. [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Carey DP, Della Sala S, Ietswaart M. Neuropsychological perspectives on eye-hand coordination in visually-guided reaching. Prog Brain Res. 2002;140:311–327. doi: 10.1016/S0079-6123(02)40059-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary Motor Area Exerts Proactive and Reactive Control of Arm Movements. J Neurosci. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL. Action-oriented spatial reference frames in cortex. Neuron. 1998;20:15–24. doi: 10.1016/s0896-6273(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Medendorp WP, Marotta JJ. Spatial transformations for eye-hand coordination. J Neurophysiol. 2004;92:10–19. doi: 10.1152/jn.00117.2004. [DOI] [PubMed] [Google Scholar]
- Dean HL, Marti D, Tsui E, Rinzel J, Pesaran B. Reaction Time Correlations during Eye-Hand Coordination: Behavior and Modeling. J Neurosci. 2011;31:2399–2412. doi: 10.1523/JNEUROSCI.4591-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends Cogn Sci. 2011;15:191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Gaveau V, Pélisson D, Blangero A, Urquizar C, Prablanc C, Vighetto A, Pisella L. Saccade control and eye-hand coordination in optic ataxia. Neuropsychologia. 2008;46:475–486. doi: 10.1016/j.neuropsychologia.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci. 2001;21:6917–6932. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. How Local Is the Local Field Potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim HGR, Lee C. Trial-to-trial variability of spike response of V1 and saccadic response time. J Neurophysiol. 2010;104:2556–2572. doi: 10.1152/jn.01040.2009. [DOI] [PubMed] [Google Scholar]
- Lünenburger L, Kutz D, Hoffmann K. Influence of arm movements on saccades in humans. Eur J Neurosci. 2000;12:4107–4116. doi: 10.1046/j.1460-9568.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences. J Neurosci Methods. 2007;163:161–175. doi: 10.1016/j.jneumeth.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ. The cerebellum and the timing of coordinated eye and hand tracking. Brain Cogn. 2002;48:212–226. doi: 10.1006/brcg.2001.1314. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of Dynamic Brain Imaging Data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SFW, Bekkering H. Coordinated control of eye and hand movements in dynamic reaching. Hum Mov Sci. 2002;21:349–376. doi: 10.1016/s0167-9457(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Pashler H. Processing stages in overlapping tasks: evidence for a central bottleneck. J Exp Psychol Hum Percept Perform. 1984;10:358–377. doi: 10.1037//0096-1523.10.3.358. [DOI] [PubMed] [Google Scholar]
- Pesaran B. Neural correlations, decisions, and actions. Curr Opin Neurobiol. 2010;20:166–171. doi: 10.1016/j.conb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B. Uncovering the mysterious origins of local field potentials. Neuron. 2009;61:1–2. doi: 10.1016/j.neuron.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prablanc C, Desmurget M, Gréa H. Neural Control of Space Coding and Action Production. Prog Brain Res. 2003;142:155–170. doi: 10.1016/S0079-6123(03)42012-8. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–228. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Hatsopoulos NG. Periodicity and evoked responses in motor cortex. J Neurosci. 2010;30:11506–11515. doi: 10.1523/JNEUROSCI.5947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin J, Riehle A. Neural correlates of partial transmission of sensorimotor information in the cerebral cortex. Acta psychologica. 1995;90:81–95. doi: 10.1016/0001-6918(95)00039-w. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, König P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Rougeul A, Bouyer JJ, Dedet L, Debray O. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogr Clin Neurophysiol. 1979;46:310–319. doi: 10.1016/0013-4694(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Rubino D, Robbins KA, Hatsopoulos NG. Propagating waves mediate information transfer in the motor cortex. Nat Neurosci. 2006;9:1549–1557. doi: 10.1038/nn1802. [DOI] [PubMed] [Google Scholar]
- Sailer U, Eggert T, Ditterich J, Straube A. Spatial and temporal aspects of eye-hand coordination across different tasks. Exp Brain Res. 2000;134:163–173. doi: 10.1007/s002210000457. [DOI] [PubMed] [Google Scholar]
- Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG. Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron. 2010;65:461–471. doi: 10.1016/j.neuron.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci U S A. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberger H, Jarvis MR, Andersen RA. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron. 2005;46:347–354. doi: 10.1016/j.neuron.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Song JH, McPeek R. Eye-hand coordination during target selection in a pop-out visual search. J Neurophysiol. 2009;102:2681–2692. doi: 10.1152/jn.91352.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci. 2009;29:11540–11549. doi: 10.1523/JNEUROSCI.2573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Pack CC. Removal of spurious correlations between spikes and local field potentials. J Neurophysiol. 2010 doi: 10.1152/jn.00642.2010. [DOI] [PubMed] [Google Scholar]
- Zeitler M, Fries P, Gielen S. Assessing neuronal coherence with single-unit, multi-unit, and local field potentials. Neural Comput. 2006;18:2256–2281. doi: 10.1162/neco.2006.18.9.2256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


