Abstract
Osteoarthritis (OA) is a chronic condition characterized by pain during joint movement. Additionally, patients with advanced disease experience pain at rest (i.e., ongoing pain)that is generally resistant to non-steroidal anti-inflammatory drugs (NSAIDs). Injection of monosodium iodoacetate (MIA) into the intra-articular space of the rodent knee is a well-established model of OA that elicits weight-bearing asymmetry and referred tactile and thermal hypersensitivity. Whether ongoing pain is present in this model is unknown. Additionally, the possible relationship of ongoing pain to MIA dose is not known. MIA produced weight asymmetry, joint osteolysis, and cartilage erosion across a range of doses (1, 3, and 4.8 mg). However, only rats treated with the highest dose of MIA showed conditioned place preference to a context paired with intra-articular lidocaine, indicating relief from ongoing pain. Diclofenac blocked the MIA-induced weight asymmetry but failed to block MIA-induced ongoing pain. Systemic AMG9810, a TRPV1 antagonist, effectively blocked thermal hypersensitivity, but failed to block high dose MIA-induced weight asymmetry or ongoing pain. Additionally, systemic or intra-articular HC030031, a TRPA1 antagonist, failed to block high dose MIA-induced weight asymmetry or ongoing pain. Our studies suggest that a high dose of intra-articular MIA induces ongoing pain originating from the site of injury that is dependent on afferent fiber activity but apparently independent of TRPV1 or TRPA1 activation. Identification of mechanisms driving ongoing pain may enable development of improved treatments for patients with severe OA pain and diminish the need for joint replacement surgery.
Introduction
Osteoarthritis (OA) is characterized by progressive loss of articular cartilage, new bone formation, and synovial proliferation that can result in pain, loss of joint function, and diminished quality of life[1]. Pain is the key complaint for OA patients, is the driving factor for visiting a primary care physician and is why many patients ultimately choose to undergo joint replacement surgery[21]. OA pain is often comprised of hyperalgesia, referred pain, and ongoing pain (pain at rest)[19]. Osteoarthritis patients report pain that can be broadly characterized into two categories: 1) dull aching, throbbing pain that becomes more constant over time and 2) shorter episodes of more intense or sharp pain, that become more frequent over time[21; 22]. Early OA pain is characterized by predictable sharp or other pain, usually brought on by a trigger whereas advanced OA is characterized by constant dull/aching pain that is punctuated by short episodes of often unpredictable intense pain [21]. OA pain is primarily treated with lifestyle changes, followed by pharmacological interventions including acetaminophen, NSAIDS, topical agents, intra-articular injections (e.g. steroids), and with non-pharmacological interventions, such as joint replacement, in cases where patients are not responsive to pharmacological therapy[11; 12; 36].
Injection of monosodium iodoacetate (MIA) into the intra-articular space of the knee is an established and well-characterized preclinical model of OA [8; 23; 33; 42]. Intra-articular MIA elicits transient inflammation followed by joint destruction consistent with clinical OA[23; 33]. Previous studies using this model have demonstrated that intra-articular MIA results in decreased weight bearing on the injured limb, movement-evoked pain, and hypersensitivity to acute application of noxious (hyperalgesia) and non-noxious (allodynia) stimulation to the hindpaw, suggesting referred pain[8; 9; 42]. We recently used a high dose of intra-articular MIA in an attempt to model advanced OA. Our studies showed that intrathecal clonidine produced place preference in rats treated 28 days earlier with 4.8 mg of intra-articular MIA reflecting negative reinforcement associated with the relief of persistent ongoing pain [28]. As pathological changes to the knee joint may result in ongoing activation of primary afferent nociceptors, we used this approach to explore whether peripheral nerve block would produce pain relief, i.e., to reveal MIA-induced ongoing pain, as well as determining whether lower doses of MIA would elicit ongoing pain. We further determined the potential effectiveness of diclofenac, a non-steroidal anti-inflammatory drug (NSAID) that is widely used clinically to treat pain and inflammation in arthritis [14] against MIA-induced shifts in weight bearing and ongoing pain.
Previous studies report that the transient receptor potential (TRP) cation channels, such as TRPV1 and TRPA1, are important transducers in evoked responses in models of inflammatory pain [7; 10; 13; 18; 24; 25; 30]. Blockade of TRPV1 has been shown to reverse MIA-induced shift in weight bearing and diminished grip force [8]. As TRPV1 and TRPA1 antagonists are in clinical development potentially for OA, we also determined whether blockade of the TRPV1 or the TRPA1 receptors might play a role in MIA-induced ongoing pain.
Materials and Methods
Animals
Male Sprague-Dawley (Harlan, Indianapolis, IN) rats weighing 275 to 300 g were maintained on a 12-hour light/dark cycle with food and water available ad libitum. All experiments were performed in accordance with the policies and recommendations of the International Association for the Study of Pain, National Institutes of Health, and the Institutional Animal Care and Use Committee (IACUC) of the University of Arizona. All behavioral experiments were performed by an experimenter blinded to the treatment conditions.
Intra-articular Injection and Drug Administration
Rats were anaesthetized with a 2% isofluorane O2 mixture and given a single intra-articular injection of monosodium iodoacetate (MIA, Sigma, USA) through the infra-patella ligament of the left knee at a dose of 1, 3 or 4.8 mg in 60 µl sterile saline. Control animals were given a single intra-articular injection of equivolume sterile saline. In order to produce an effective nerve block, lidocaine (Roxane Laboratories) was administered in an intra-articular injection (4% w/v, 200µl) in the ipsilateral knee. This volume of lidocaine is sufficient to fill the joint space and may additionally disperse to surrounding tissues. The TRPV1 receptor antagonist, AMG9810 (Tocris Bioscience) was mixed in PEG400 administered systemically (30 mg/kg, i.p.) at a dose previously demonstrated to effectively block CFA-induced thermal hyperalgesia [18]. Diclofenac was mixed in PEG400/dH20 (30:70 v/v) and administered systemically (30 mg/kg, p.o.), a dose previously demonstrated to effectively block MIA-induced reduction in grip force [8]. The TRPA1 antagonist, HC 030031 (Tocris Bioscience) was dissolved in 0.5% methylcellulose and administered systemically (100mg/kg, p.o.). This dose has been shown to effectively attenuate mustard oil induced paw lifting and CFA-induced mechanical hypersensitivity (19). Notably, higher doses of HC 030031 (300 mg/kg, p.o.) showed did not show greater efficacy [13]. HC 030031 was also administered in an intra-articular injection at a dose of 200µg dissolved in 100µl DMSO.
Behavioral Measures of Evoked Responses, Weight Bearing and Ongoing Pain
Evaluation of evoked pain behaviors, shift in weight bearing, and ongoing pain occurred 14 days following intra-articular injection of MIA or saline injection.
Assessment of Shift in Weight Bearing
Changes in hind paw weight distribution between the left (MIA) and right (contralateral) limbs were utilized as an index of joint discomfort in the MIA treated knee. An incapacitance tester (Stoelting) was employed for determination of hind paw weight distribution. Rats were placed in an angled plexiglass chamber positioned so that each hind paw rested on a separate force plate. The force exerted by each hind limb (measured in grams) is averaged over a 5sec period. Each data point is the mean of five readings. The data are normalized as % injured/noninjured weight bearing, such that sensitivity on the injured side is indicated by values < 100%, equal weight distribution is indicated by 100%.
Thermal hyperalgesia
The method of Hargreaves et al. [20] was used to assess paw-withdrawal latency to a thermal nociceptive stimulus. Rats were allowed to acclimate within a Plexiglas enclosure on a clear glass plate maintained at 30°C for 30 min. A radiant heat source (i.e., high intensity projector lamp) was directed onto the plantar surface of the left hindpaw. A motion detector halted both lamp and timer when the paw was withdrawn. The paw-withdrawal latency from the radiant heat source was determined both before and after drug or vehicle administration. Baseline latencies were established at 20 sec to allow a sufficient window for the detection of possible hyperalgesia. A maximal cutoff of 30 sec was used to prevent tissue damage.
Conditioned Place Preference (CPP) Testing
The conditioned place preference assay has been used extensively to study reward [4]. Treatments that are rewarding (e.g. cocaine) elicit positive reinforcement so that when they are paired with a specific context, animals are motivated to seek that context. As a result, animals will increase the time spent in a context paired with a treatment that is positively reinforcing compared to a neutral (e.g. vehicle) paired chamber. Aversive states, such as ongoing pain, also produce motivational drive. Thus, removal of an aversive state elicits negative reinforcement that can reveal the presence of ongoing pain [27]. Pain relief is a form of negative reinforcement that produces motivational drive that can be detected using conditioned place preference. Previous work has demonstrated that clinically effective analgesics that are not positively reinforcing agents will produce negative reinforcement selectively in the setting of tissue injury, revealing the presence of ongoing pain [26; 27; 34]. This approach was employed here to detect ongoing pain 2 weeks following injection of MIA. A three-chambered box was used to record behaviors. The box consisted of two end chambers (10 3/8” W × 8 1/8” D×13 1/8” H) with distinct visual, tactile, and odor cues as previously described [27]. The third, central chamber (6 1/4” W × 8 1/8” D × 13 1/8” H) was well lit and had gray walls. For the three (3) day pre-conditioning phase, rats were placed in the center chamber and allowed free access to all 3 chambers for 15 minutes. On day 3, time spent in each chamber was recorded to verify no pre-conditioning chamber bias. In the morning of Day 4 rats received vehicle and were restricted to one of the chambers for 30 minutes. Rats were returned to their home cages and 4 hours later they received drug treatment(s) and were restricted to the opposite chamber for 30 minutes. The chamber assignments for conditioning were counterbalanced. On day 5 the rats were allowed free access to all chambers for 15 minutes and the total time spent in each chamber was recorded. If ongoing pain is present, then treatments that produce pain relief (e.g., peripheral nerve block) are expected to result in more time in the chamber paired with the treatment and correspondingly less time in the alternate chamber. Importantly, such effects should occur selectively in animals with injury revealing the presence of an aversive state that likely reflects pain.
Intra-articular Lidocaine (Peripheral Nerve Block)
All rats underwent a 3-day habituation, in which rats were placed in the CPP boxes with access to all chambers for 30 min per day. Pre-conditioning (baseline) time spent in each of the boxes was recorded for 15 min on day 3. The following day, all rats received intra-articular saline and were placed immediately (within 2 min) into the appropriate chamber for 30 min. Four (4) hours later, all rats received intra-articular lidocaine and were placed immediately into the opposite chamber for 30 min. Testing occurred the following day (D5) wherein the rats were placed drug-free into the CPP boxes with access to all chambers for 15 min.
Systemic Diclofenac
All rats underwent the 3-day habituation and preconditioning (baseline) time spent in each of the boxes was recorded on day 3. The following day, all rats received systemic administration of vehicle (PEG400/dH20 (30:70 v/v), p.o.) followed 45 min later with intra-articular saline and immediate placement into the appropriate chamber. Four (4) hours later, all rats received systemic administration of diclofenac (30 mg/kg, p.o.) followed 45 min later with intra-articular lidocaine and immediate placement into the opposite chamber. Control rats received treatment with vehicle (PEG400/dH20 (30:70 v/v), p.o.) during the afternoon session followed by intra-articular lidocaine 45 minutes later. Testing occurred the following day (D5) wherein the rats were placed drug-free into the CPP boxes with access to all chambers for 15 min. Preference to intra-articular lidocaine in the presence of diclofenac indicates the presence of ongoing pain (i.e., the failure of the diclofenac to block such pain).
Systemic TRPV1 Antagonist
All rats underwent the 3-day habituation and preconditioning (baseline) time spent in each of the boxes was recorded on day 3. The following day, all rats received systemic administration of vehicle (PEG400, i.p.) followed 30 min later with intra-articular saline and immediate placement into the appropriate chamber. Four (4) hours later, all rats received systemic administration of the TRPV1 receptor antagonist, AMG9810 (30 mg/kg, i.p.) followed 30 min later with intra-articular lidocaine and immediate placement into the opposite chamber. Testing occurred the following day (D5) wherein the rats were placed drug-free into the CPP boxes with access to all chambers for 15 min. Preference to intra-articular lidocaine in the presence of TRPV1 blockade indicates the presence of ongoing pain (i.e., the failure of the TRPV1 antagonist to block such pain).
Systemic TRPA1 Antagonist
All rats underwent the 3-day habituation and preconditioning (baseline) time spent in each of the boxes was recorded on day 3. The following day, all rats received systemic administration of vehicle (0.5% methylcellulose, p.o.) followed 60 min later with intra-articular saline and immediate placement into the appropriate chamber. Four (4) hours later, all rats received systemic administration of the TRPA1 receptor antagonist, HC 030031 (100 mg/kg, p.o.) followed 60 min later with intra-articular lidocaine and immediate placement into the opposite chamber. Testing occurred the following day (D5) wherein the rats were placed drug-free into the CPP boxes with access to all chambers for 15 min. Preference to intra-articular lidocaine in the presence of TRPA1 blockade indicates the presence of ongoing pain (i.e., the failure of the TRPA1 antagonist to block such pain).
Intra-articular TRPA1 antagonist
All rats underwent a 3-day habituation, in which rats were placed in the CPP boxes with access to all chambers for 30 min per day. Pre-conditioning (baseline) time spent in each of the boxes was recorded for 15 min on day 3. The following day, all rats received intra-articular saline (100µl) and were placed immediately (within 2 min) into the appropriate chamber for 30 min. Four (4) hours later, all rats received intra-articular TRPA1 receptor antagonist, HC 030031 (200µg) and were placed immediately into the opposite chamber for 30 min. Testing occurred the following day (D5) wherein the rats were placed drug-free into the CPP boxes with access to all chambers for 15 min.
Radiographic imaging
One day following behavioral testing (D15 post-MIA), rats were lightly anesthetized with ketamine/xylazine (64 mg/kg, i.p.) and radiograph images of the knee joint were captured by a digital camera (Faxitron X-ray Corporation). Following radiograph imaging, rats were euthanized, and joints dissected for visual inspection of cartilage erosion.
Histology and analysis
Separate groups of animals were sacrificed at day 14 post-treatment with MIA or saline. Legs were fixed in 10% neutral buffered formalin for 24 hours and were decalcified (Decalcifier 1, Surgipath) for 48 hours. The joints were embedded in paraffin in the frontal plane and 8µm slices were taken at the center of the joint space. Toluidine blue (0.04%) staining was performed and the amount of cartilage lining the medial femoro-tibial joint was analyzed by calculating the area using imageJ software.
Data Analysis
Within each treatment group, post-administration means were compared with the baseline values by analysis of variance (ANOVA), followed by post hoc analysis of least significant difference for multiple comparisons. A probability level of 0.05 was used to establish significance. For conditioned place pairing, the effects of injury and conditioning chamber were analyzed by a two-way ANOVA. Bonferonni t-tests were used for post-hoc analysis of pre-conditioning (BL) vs post-conditioning values within each treatment group. Pairwise t-test was used to analyze the difference scores that were calculated as post-conditioning (test) – pre-conditioning (BL) time spent in the drug-paired chamber.
Results
MIA-induced loss of cartilage
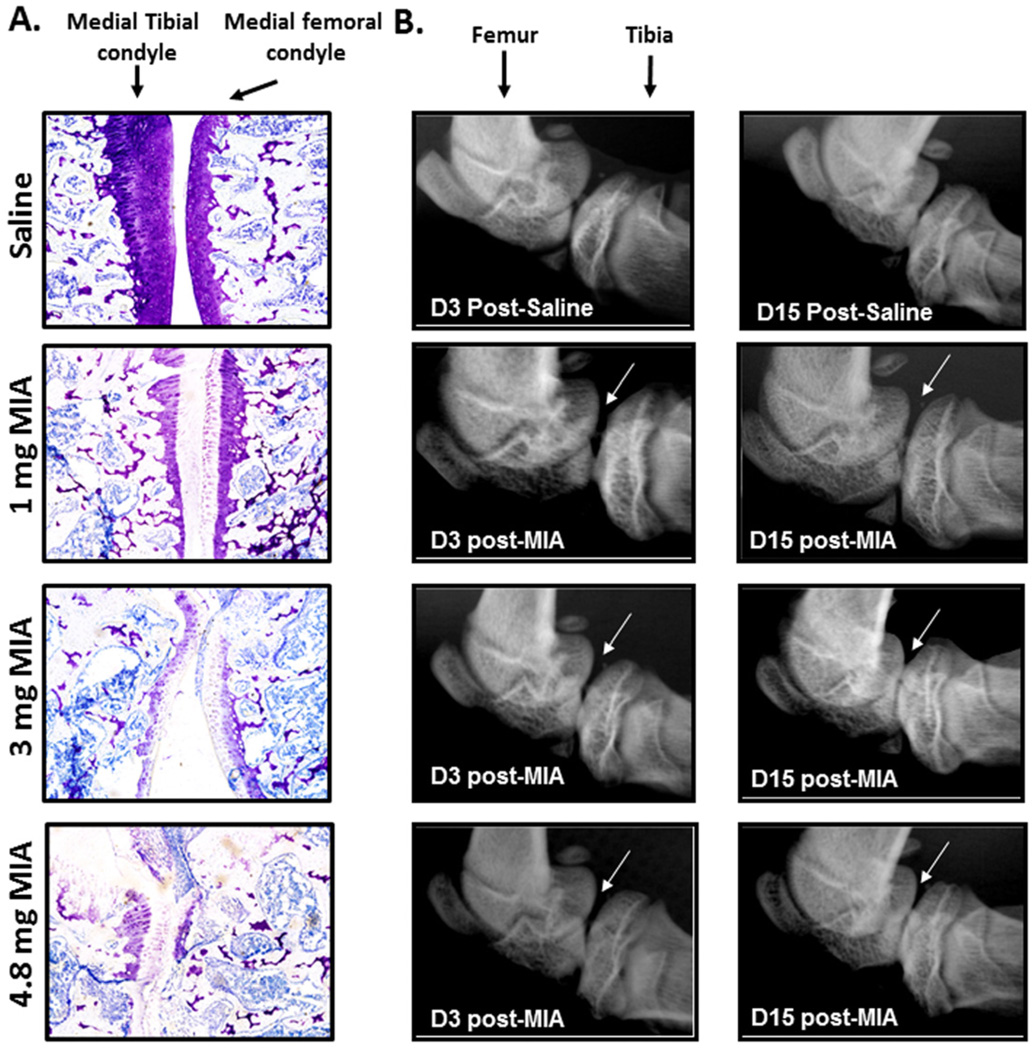
Intra-articular injection of 1, 3, or 4.8 mg of MIA produced an apparent dose-dependent increase in the amount of cartilage lost from the medial femoro-tibial joint compared to saline treated animals *p<.05, compared to saline. The 4.8 mg dose of MIA resulted in similar loss of cartilage as the 3 mg dose in 3 animals. However, in other animals (n=2) the 4.8 mg dose resulted in complete destruction of the articular cartilage (Fig 1A).
Figure 1.
A) Toluidine blue staining of the joint was performed 2 weeks post injection. MIA produced a decrease in the amount of cartilage lining the medial femoro-tibial joint compared to saline treated joints. B) Radiographic images of joints demonstrate that intra-articular injection of MIA results in an apparent dose-dependent joint degeneration. High-resolution radiographs of the knee joints demonstrate smooth femoral and tibial bones in naïve rats (control) whereas rough edges of the tibia and femur are observed in joints that had been treated with 1, 3, or 4.8 mg MIA. In addition, diminished space between the tibia and femur is apparent by 15 days post-MIA, particularly in the joints treated with 3 and 4.8 mg MIA.
MIA-induced joint degeneration
Intra-articular injection of 1, 3 or 4.8 mg of MIA induced time- and dose-dependent joint degeneration as indicated by diminished space between the femoral and tibial bones compared to control bones (Fig 1B). Arrows indicate joint degeneration characterized by osteolysis of the lateral and femoral condyles as compared to controls at all doses, with rough edges apparent on the femur and tibial bones indicative of bone lysis and swelling as previously demonstrated [33; 40].
MIA-induced shift in weight bearing and ongoing pain
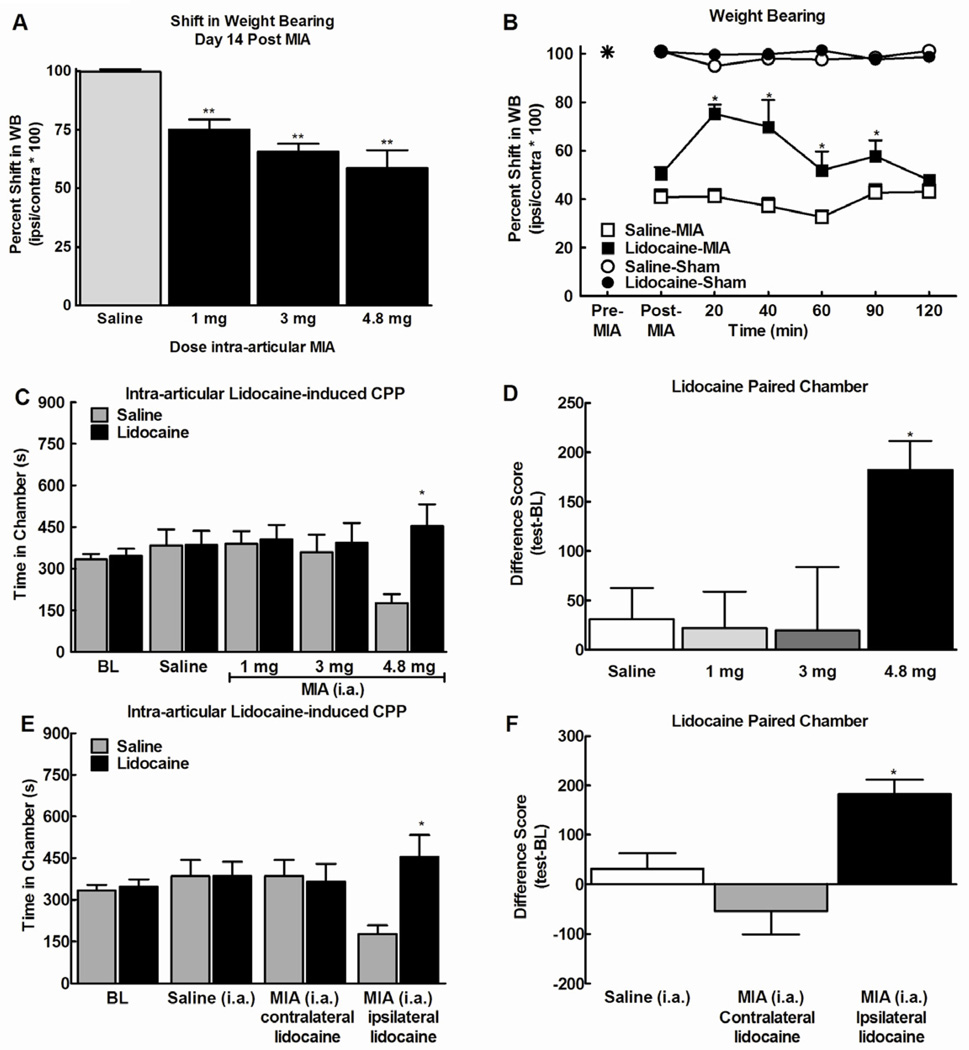
At day 14post-intra-articular injection of MIA, a significant decrease in weight bearing on the injured hindlimb was detected at all doses of MIA (Fig 2A, p<0.0001). Intra-articular administration of lidocaine (4% w/v, 200µl) reversed the MIA (4.8mg) induced shift in weight bearing, with a peak effect observed within 20 min (Fig 2B, *p<0.05 compared to post-MIA).
Figure 2.
A) Intra-articular (i.a.) injection of 1, 3 and 4.8 mg of MIA all induced a shift in weight bearing. B) Intra-articular (i.a.) administration of lidocaine (200 µl, 4% w/v) reversed the MIA-induced (4.8 mg) shift in weight bearing within 20 min at 14 days following MIA administration. This effect dissipated within 120 min. * indicates p<0.05 vs post-MIA, n=7. C) Rats treated with the 4.8 mg dose of MIA increased time spent in the lidocaine paired chamber at 14 days following MIA treatment. Rats treated with the lower doses of MIA or saline did not show preference for the lidocaine or the saline treated chambers. * indicates p<0.05 compared to pre-conditioning time spent in chamber (n=7 saline, n=12, 12, and 7 for MIA at 1, 3 and 4.8 mg, respectively). D) Difference scores (test time-preconditioning time) confirmed that rats treated with 4.8 mg of MIA increased time spent in the lidocaine paired chamber. * indicates p<0.05 compared to saline. E) Treatment with intra-articular lidocaine in the ipsilateral, but not contralateral joint produced conditioned place preference in rats treated with 4.8 mg of MIA. F) Difference scores confirmed that ipsilateral, but not contralateral intra-articular lidocaine leads to an increase in time spent in the lidocaine paired chamber.
We determined whether local nerve block with administration of lidocaine (4%w/v, 200µl) into the intra-articular space and surrounding tissues induced conditioned place preference 14 days following MIA injection. Pre-conditioning time spent in the saline versus lidocaine-paired chambers did not differ between groups (p>0.05), therefore these data were pooled for graphical representation (Fig 2C). Rats treated with the 4.8mg dose of MIA showed conditioned place preference for the intra-articular lidocaine paired chamber as indicated by a significant increase in time-spent in the paired chamber compared to pre-conditioning values (*p<0.05). In contrast, lidocaine failed to produce conditioned place preference in rats treated with the 1 or 3mg doses of MIA. Lidocaine also failed to produce conditioned place preference in the control rats that had received intra-articular saline injection instead of MIA. Difference scores confirm that intra-articular lidocaine produced CPP only in rats treated with the 4.8mg dose of MIA (Fig 2D, *p<0.05).
Intra-articular injection of the same dose of lidocaine into the contralateral knee joint failed to produce conditioned place preference in rats treated with 4.8 mg of MIA indicating that intra-articular lidocaine produced local and not systemic effects (Fig 2E). Comparison of difference scores (test-BL) for time spent in the lidocaine paired chamber confirmed that only MIA rats treated with ipsilateral lidocaine showed significantly increased time spent in the lidocaine paired chamber (Fig 2F, *p<0.05).
Systemic diclofenac blocks MIA-induced Shift in Weight Bearing, but not Ongoing Pain
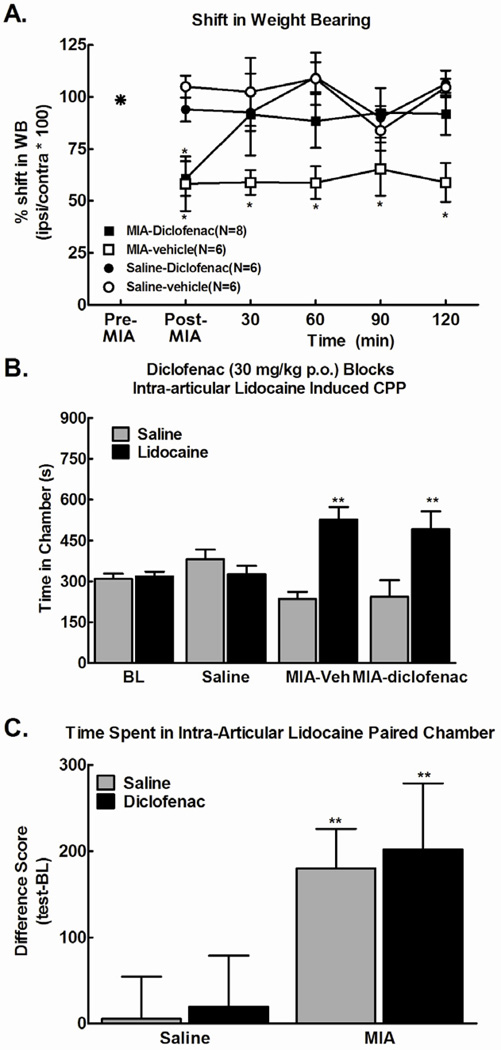
Intra-articular injection of 4.8mg of MIA induced a significant shift in weight bearing away from the MIA treated hindlimb when evaluated at day 14 post-MIA (Fig 3A). Systemic administration of diclofenac (30 mg/kg, p.o.) fully reversed the MIA-induced shift in weight bearing within 30 min, an effect that persisted throughout the 2 hour testing period.
Figure 3.
A) Systemic administration of diclofenac (30 mg/kg, p.o.) reversed the MIA-induced (4.8 mg) shift in weight bearing, *p<.05 vs post-MIA, n=8. B) Systemic administration of diclofenac (30 mg/kg, p.o.) failed to block conditioned place preference to a chamber paired with intra-articular lidocaine 45 minutes post-diclofenac,. No pre-conditioning differences were observed in any treatment group. Intra-articular lidocaine failed to produce CPP in the saline (i.a.) treated rats. * indicates p < 0.05 compared to pre-conditioning time spent in chamber (n=6–8). C) Difference from baseline scores (test time-preconditioning (BL) time) confirmed that intra-articular lidocaine produced equivalent preference in MIA treated rats irrespective of diclofenac treatment. * indicates p<0.05 compared to saline.
We determined whether intra-articular lidocaine produced place preference in rats treated with the same dose of diclofenac and over the same time period that reversed MIA-induced changes in weight bearing. Pre-conditioning time spent in the saline versus lidocaine-paired chambers did not differ between groups (p > 0.05), therefore these data were pooled for graphical representation (Fig 3B). Intra-articular injection of lidocaine produced conditioned place preference in both the vehicle- and the diclofenac-treated groups (Fig 3B). Importantly, difference from baseline scores confirmed that intra-articular lidocaine produced equivalent preference irrespective of diclofenac treatment (Fig 3C, **p<0.01 vs intra-articular saline (saline (i.a.)), indicating that the diclofenac failed to block MIA-induced ongoing pain.
Systemic TRPV1 Antagonist Blocks MIA-Induced Evoked, but not Ongoing Pain
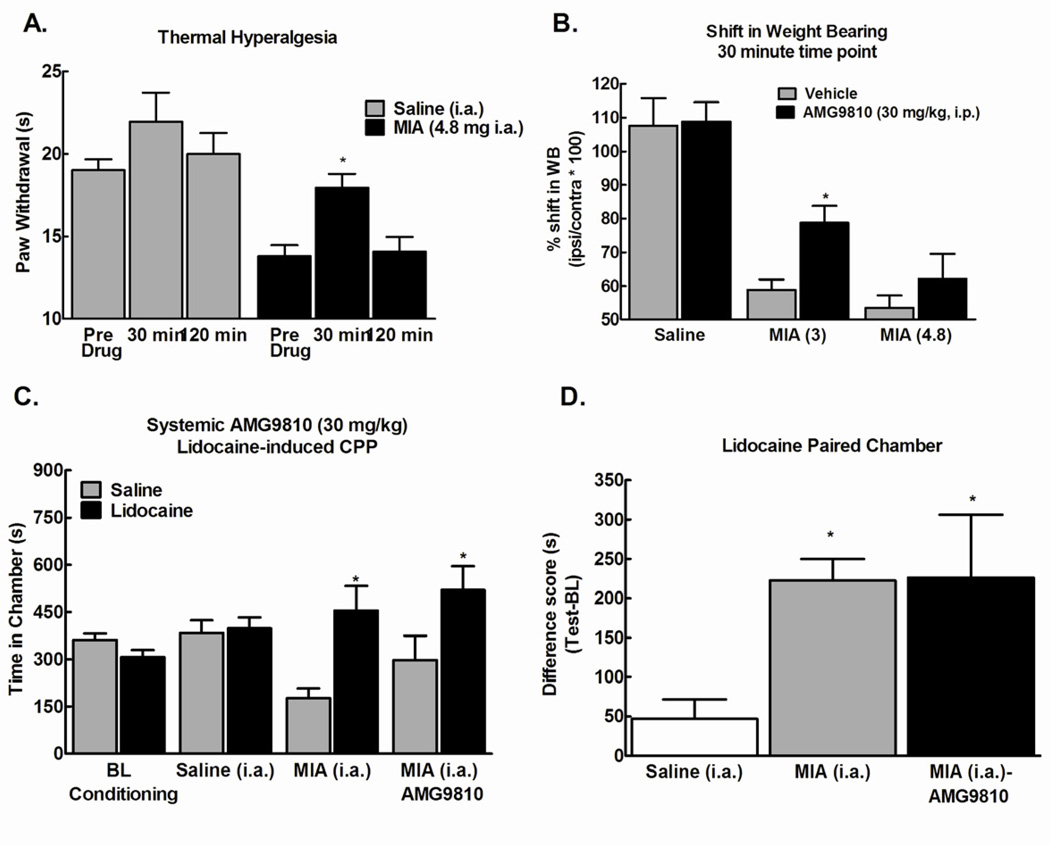
Intra-articular MIA injection induced thermal hyperalgesia evident at post-injection day 14. Consistent with previous reports, thermal hyperalgesia was fully reversed by the TRPV1 antagonist (AMG9810, 30 mg/kg, i.p.) within 15–30 min (Fig 4A, *p<0.05 vs. Pre-Drug). The anti-hyperalgesic effects of the TRPV1 antagonist dissipated within 120 min post-administration. Systemic administration of the AMG9810 (30 mg/kg, i.p.) partially blocked the MIA-induced shift in weight bearing at the 3mg dose, but not at the 4.8mg dose of MIA(Fig 4B).
Figure 4.
A) Systemic administration of AMG9810 (30 mg/kg, i.p.) reversed MIA-induced thermal hyperalgesia observed at 14 days post-MIA injection (i.a.), *p<0.05 vs Pre-drug, n=8–10. B) Systemic administration of AMG9810 (30 mg/kg, i.p.) partially reversed the MIA-induced shift in weight bearing at the 3 mg, but not the 4.8 mg dose of MIA (n=8). C) Systemic administration of AMG9810 (30 mg/kg, i.p.) failed to block conditioned place preference to a chamber paired with intra-articular lidocaine 30 min post-AMG9810, **p<0.01 vs pre-conditioning, n=6. No pre-conditioning differences were observed in any treatment group. Intra-articular lidocaine failed to produce CPP in the saline (i.a.) treated rats. D) Difference from baseline scores (test time-preconditioning (BL) time) confirmed that intra-articular lidocaine produced equivalent preference in MIA treated rats irrespective of AMG9810 treatment, **p<0.01.
We determined whether pretreatment with the TRPV1 receptor antagonist at the same dose that effectively blocked thermal hyperalgesia in the MIA rats was sufficient to block the preference elicited by intra-articular lidocaine. On conditioning day, rats received systemic administration of the AMG9810 (30 mg/kg, i.p.) 30 min prior to administration of the intra-articular lidocaine and placement into the pairing chamber. Rats pre-treated with the TRPV1 receptor antagonist demonstrated equivalent conditioned place preference to the intra-articular lidocaine paired chamber as the animals that had not received the antagonist (Fig 4C, **p<0.01 vs BL). Importantly, difference from baseline scores demonstrated that intra-articular lidocaine produced equivalent preference irrespective of TRPV1 antagonist treatment (Fig 4D, **p<0.01 vs saline (i.a.)), indicating that the TRPV1 antagonist did not eliminate MIA-induced ongoing pain.
Systemic and Site Specific TRPA1 Antagonist Attenuates Mustard-oil Induced Paw Lifting, but Fails to Block the MIA-induced Shift in Weight Bearing or Ongoing Pain
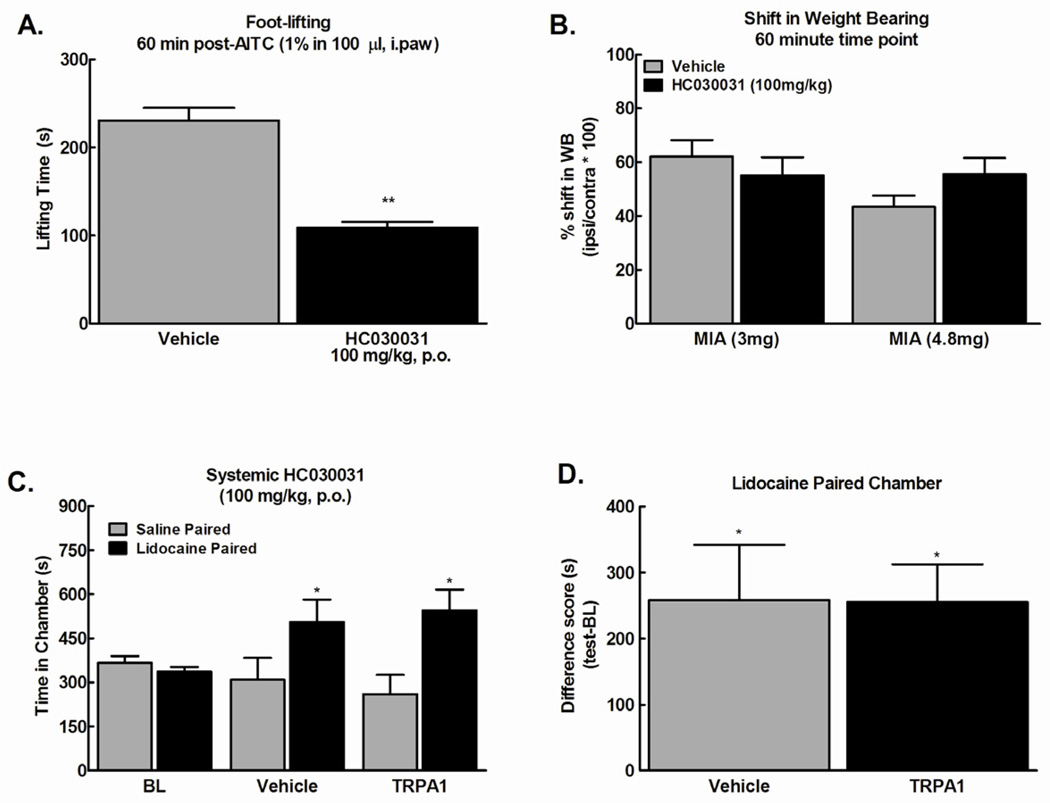
Systemic administration of the TRPA1 antagonist (HC030031, 100mg/kg p.o.) significantly attenuated mustard oil (AITC, 1%)-induced paw lifting behavior 1 hour after administration (Fig 5A, **p<0.01), consistent with previous reports [13]. We determined whether this dose of the TRPA1 antagonist reverses MIA-induced shift in weight bearing or blocks ongoing pain. Systemic administration of TRPA1 antagonist (100mg/kg, p.o) failed to reverse the MIA-induced shift in weight bearing throughout the 2 hour observation period (Fig 5B, p>0.05). Systemic administration of the TRPA1 antagonist (100mg/kg, p.o.) failed to block place preference elicited by intra-articular lidocaine (Fig 5C, *p<0.05 vs. BL). Difference scores confirm that lidocaine produced equivalent preference in the group that received the antagonist and the vehicle treated group (Fig 5D, *p<0.05). This indicates that systemic TRPA1 antagonist does not block MIA-induced ongoing pain.
Figure 5.
A) Mustard oil (1%, 100 µl) injected into the plantar surface of the hind paw induced paw lifting behavior that was attenuated by systemic administration of HC030031 (100 mg/kg, p.o.) 60 min prior to mustard oil injection, **p<.01 vs vehicle, n=6. B) Systemic administration of HC030031(100 mg/kg, p.o.) failed to reverse the MIA-induced shift in weight bearing (n=7 and 8 for 3 and 4.8 mg MIA, respectively). C) Systemic administration of HC 030031 (100 mg/kg, p.o.) failed to block conditioned place preference to a chamber paired with intra-articular lidocaine 60 min post-HC030031. * indicates p<0.05 vs pre-conditioining (n=9–12). D) Difference from baseline scores (test time-preconditioning (BL) time) confirmed that intra-articular lidocaine produced equivalent preference in MIA treated rats irrespective of HC030031 treatment. * indicates p<0.05 vs saline.
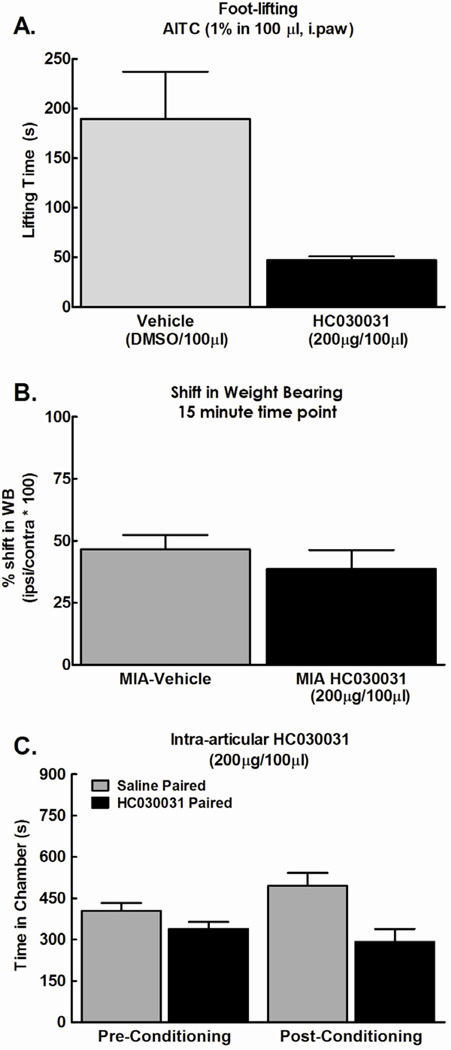
We also investigated whether intra-articular administration of the TRPA1 antagonist blocks MIA-induced shift in weight bearing or ongoing pain. Direct injection of TRPA1 antagonist (200µg/100µl) into the hindpaw 5 minutes prior to injection of mustard oil attenuated paw lifting behavior (Fig 6A, *p<0.05). An intra-articular injection of the same dose of TRPA1 antagonist (200µg/100µl) failed to block the MIA-induced shift in weight bearing (Fig 6B). An intra-articular injection of TRPA1 antagonist (200µg/100µl) also failed to elicit conditioned place preference in MIA-treated rats (Fig 6C); no effects were observed in rats treated with saline. These results indicate that local blockade of the TRPA1 receptor at the site of injury does not provide relief from MIA-induced ongoing pain.
Figure 6.
A) Mustard oil (1%, 100 µl) injected into the plantar surface of the hind paw induced paw lifting behavior that was attenuated by a intraplantar injection of HC030031 (200 µg/100 µl) given 5 min prior to mustard oil injection. * indicates p<0.05 vs vehicle, n=6. B) Intra-articular HC030031 (200 µg/100 µl) failed to reverse the MIA-induced shift in weight bearing (n=8). C) Intra-articular HC030031 (200 µg/100 µl) failed to produce conditioned place preference (n=12).
Discussion
The disease processes that lead to the structural changes and pain associated with OA are complex and poorly understood. Intra-articular MIA injection in rats induces synovitis lasting through 3 days post injection that is followed by thinning of articular cartilage and subsequent lesion of subchondral bone at days 8–14 and onwards [23]. Numerous studies have demonstrated that MIA in rodents produces behaviors that might be relevant to pain experienced by OA patients including a shift in weight bearing, hyperalgesia, and referred pain measured as decreased paw withdrawal thresholds to evoked stimuli [8; 23; 33; 42]. However, mechanistic evaluation of ongoing pain, such as that experienced at rest by patients with advanced OA [21] has not previously been possible. Here, we show that: 1) MIA at the highest dose tested (4.8 mg), but not at lower doses, induces ongoing pain that is driven by afferent input from the injured joint; 2) diclofenac, an NSAID effective against mild OA pain, but not advanced OA pain in patients, blocks MIA-induced shifts in weight bearing, but does not block ongoing pain, and 3) blockade of TRPV1 or TRPA1 channels under the conditions used in this study do not block weight bearing associated with high dose MIA or ongoing pain.
Ipsilateral intra-articular lidocaine induced CPP selectively in high dose MIA, but not saline, pretreated rats suggesting relief of ongoing pain. Importantly, intra-articular lidocaine given in the contralateral knee did not elicit CPP in rats with high dose MIA suggesting that effects observed with ipsilateral lidocaine were the result of local blockade of afferent drive. Intra-articular lidocaine, however, did not induce CPP when lower MIA doses were used. This result suggests that these doses of MIA either do not elicit ongoing pain, or do not establish a sufficiently aversive state if some level of ongoing pain is present that can be detected with place preference from pain relief. MIA induced long-term, ongoing damage to the knee joint that appeared to be dose-related. Loss of cartilage was observed at all MIA doses with almost complete destruction of the articular surface seen at the highest dose.
Sensitization of afferents has been demonstrated following MIA [37–39] and could lead to enhanced mechanosensation reflected as allodynia, hyperalgesia, and shift in weight bearing[8; 9; 33; 37; 38; 42]. The present study used higher doses of MIA than normally evaluated to model advanced OA and resulted in ongoing pain that might reflect nerve damage. Peripheral nerve block produces relief of pain in OA patients [32]. Additionally, many patients show improvement in their pain following joint replacement, supporting the likelihood that pain in OA can be driven from the joint [6; 31]. Consistent with pain arising from the site of injury, our data show that peripheral nerve block produced a reversal of weight bearing as well as CPP[2; 3; 5; 17]. Whether such ongoing pain results from nerve damage is not known as it is also possible that ongoing pain might reflect enhanced activation of sensitized afferent fibers resulting from movement. Previous studies, however, have not demonstrated changes in locomotor activity in MIA treated animals and the shift in weight bearing suggests a protective behavior that would diminish the influence of movement-evoked pain.
Systemic administration of diclofenac did not prevent intra-articular lidocaine induced CPP suggesting a failure of this NSAID to block MIA-induced ongoing pain. At the same dose, diclofenac fully blocked the MIA-induced shift in weight bearing, indicating that this dose was effective in ameliorating some aspects of MIA-induced pain. The ineffectiveness of diclofenac on MIA-induced ongoing pain is consistent with the ineffectiveness of NSAIDs, such as diclofenac, in patients with advanced OA-induced pain who often have intractable pain and must undergo joint replacement[11; 12; 14; 36]. The primary mechanism of action of diclofenac’s anti-inflammatory, antipyretic, and analgesic action is an inhibition of prostaglandin synthesis through inhibition of the COX1 and COX2 enzymes [35]. Our data suggest that the anti-inflammatory effects of diclofenac are not sufficient to block MIA-induced ongoing pain at a dose sufficient to block MIA-induced shift in weight bearing. Structural pathology following MIA appears time dependent with early inflammation (e.g. 3 days post-administration) that diminishes with time while ATF-3, a nerve-injury marker is observed at later time-points (days 8–14 post-MIA) [23]. Consistent with this sequence, anti-inflammatory drugs show improved blockade of MIA-induced mechanical and tactile hypersensitivity as well as weight bearing at early time-points following MIA injection (day 3), compared to later time-points (day 14 or later) [16; 23; 33]. Notably, agents such as gabapentin and amitriptyline effectively blocked MIA-induced weight-bearing asymmetry at later time-points (day 14, 21, 28) suggesting the possibility of a potential neuropathic pain component [23; 42].
The role of specific transducers inOA pain is not well understood. The TRPV1 channel has been implicated as a potential target for treatment of OA pain and up-regulation of TRPV1 has been observed in neurons innervating the knee following MIA injection [15]. Further, selective blockade of the TRPV1 receptor with ABT-102, a selective TRPV1 antagonist, was demonstrated to block shift in weight bearing and diminished grip force following injection of 3 mg of MIA [8]. Wetherefore investigated possible effects of TRPV1 blockade in MIA-induced ongoing pain. In these experiments, we used AMG9810 to block the TRPV1 receptor so that MIA-induced thermal hypersensitivity was fully reversed, suggesting that the dose used was sufficient to engage this target. This dose of AMG9810 also reversed the shift in weight bearing induced by 3mg of MIA. Unlike diclofenac, however, this dose of AMG 9810 was insufficient to reverse the shift in weight bearing observed in animals treated with the 4.8mg dose. The effect of blocking TRPV1 on MIA-induced ongoing pain was examined by administration of the same dose of AMG9810 followed by intra-articular lidocaine at a time-point at which full reversal of the MIA-induced thermal hyperalgesia is observed. If MIA-induced ongoing pain was blocked by the TRPV1 antagonist, then no CPP should result from intra-articular lidocaine. However, AMG 9810 had no effect on CPP induced by intra-articular lidocaine. These findings suggest that while the TRPV1 receptor may contribute to heat and mechanical hypersensitivity following MIA, blockade of this channel is insufficient to modulate theaversive state that associated with MIA-induced ongoing pain. Notably, a recent preliminary report indicated no difference between a TRPV1 antagonist and placebo in NSAID resistant OA pain patients [41]. Collectively, the data suggest that TRPV1 blockade might be effective against mild to moderate, but not in advanced OA. This possibility will require further experimentation with TRPV1 antagonists that may have different characteristics as well as with different administration schedules that could involve repeated dosing.
Previous studies suggest that TRPA1 mediates nerve-injury and inflammation induced mechanical allodynia [13; 25; 30], suggesting this channel as a potentially attractive target for OA pain. Consistent with previous studies, systemic administration of the TRPA1 antagonist HC 030031(100mg/kg, p.o.) attenuated mustard oil-induced paw lifting measured 1 hr post administration [13]. In our studies, this dose of HC 030031 failed to reverse the MIA-induced shift in weight bearing or to block intra-articular lidocaine induced CPP (i.e., did not block ongoing pain). In addition, local administration of the HC 030031 into the intra-articular space failed to block MIA-induced shift in weight bearing and additionally, did not elicit CPP indicating a failure to block ongoing pain following local administration. Notably, this dose given into the hind paw effectively blocked mustard-oil induced foot-lifting, suggesting that this dose is capable of engaging the target. Collectively, these data suggest that blocking TRPA1 does not block ongoing pain induced by MIA. Recently it was shown that blockade of TRPA1 reduces the evoked responses of WDR neurons to high intensity stimulation, but did not reduce the spontaneous firing of WDR neurons in the MIA model(3mg).[29]. This observation is consistent with our findings and suggests that blocking TRPA1 may not provide sufficient relief from ongoing pain in this preclinical model of advanced OA pain.
In summary, our data suggest that MIA can produce ongoing pain and that such pain is likely to be driven from the injury site. MIA-induced ongoing pain was resistant to diclofenac, suggesting relevance to patients with advanced OA disease. Selective blockade of TRPV1 or TRPA1 receptors under the conditions of this study was also insufficient to block advanced MIA-induced ongoing pain indicating that MIA-induced pathological changes might drive ongoing pain in a TRPV1- and TRPA1-independent manner. The effective blockade of ongoing pain by lidocaine administration at the site of injury indicates that determining the pathological changes and the molecular mechanisms driving afferent input within the injured joint may allow the identification of novel peripheral targets that may be appropriate for drug development. Development of peripherally restricted drugs that block ongoing afferent drive might provide pain management with better efficacy, diminished side effects, and better safety profiles across long-term administration required for most patients with OA than currently available medications and help to avoid eventual joint replacement.
Acknowledgements
This work was supported by NS 066958.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
MIA-induced ongoing pain and weight bearing depend on input from the joint; blockade of TRPV1 and TRPA1 channels fails to block MIA-induced ongoing pain.
Contributor Information
Alec Okun, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Ping Liu, Institute of Clinical Pharmacology, Qilu Hospital of Shandong University, Jinan 250012, China.
Peg Davis, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Jiyang Ren, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Bethany Remeniuk, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Triza Brion, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Michael H. Ossipov, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA
Jennifer Xie, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Gregory O. Dussor, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA
Tamara King, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Frank Porreca, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
References
- 1.Abramson S, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Research & Therapy. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argoff CE. Conclusions: chronic pain studies of lidocaine patch 5% using the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20 Suppl 2:S29–S31. doi: 10.1185/030079904X12979. [DOI] [PubMed] [Google Scholar]
- 3.Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 5.Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open-label effectiveness trial. Osteoarthritis Cartilage. 2004;12(3):253–255. doi: 10.1016/j.joca.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement. A meta-analysis. JAMA. 1994;271(17):1349–1357. [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol. 2009;613(1–3):39–45. doi: 10.1016/j.ejphar.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370(2–3):236–240. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 11.Dieppe P, Brandt KD. What is important in treating osteoarthritis? Whom should we treat and how should we treat them? Rheumatic Disease Clinics of North America. 2003;29(4):687–716. doi: 10.1016/s0889-857x(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 12.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. The Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 13.Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emery P, Koncz T, Pan S, Lowry S. Analgesic effectiveness of celecoxib and diclofenac in patients with osteoarthritis of the hip requiring joint replacement surgery: a 12-week, multicenter, randomized, double-blind, parallel-group, double-dummy, noninferiority study. Clin Ther. 2008;30(1):70–83. doi: 10.1016/j.clinthera.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neuroscience Letters. 2005;388(2):75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112(1–2):83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Galer BS, Sheldon E, Patel N, Codding C, Burch F, Gammaitoni AR. Topical lidocaine patch 5% may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opin. 2004;20(9):1455–1458. doi: 10.1185/030079904X2754. [DOI] [PubMed] [Google Scholar]
- 18.Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]. dioxin-6-yl)acrylamide, a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313(1):474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- 19.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Care & Research. 2009;61(9):1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 20.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 21.Hawker GA. Experiencing painful osteoarthritis: what have we learned from listening? Current Opinion in Rheumatology. 2009;21(5):507–512. doi: 10.1097/BOR.0b013e32832e99d7. [DOI] [PubMed] [Google Scholar]
- 22.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, Suarez-Almazor M, Gooberman-Hill R. Understanding the pain experience in hip and knee osteoarthritis - an OARSI/OMERACT initiative. Osteoarthritis and Cartilage. 2008;16(4):415–422. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128(3):272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 25.Kerstein PC, del Camino D, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152(9):1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neuroscience Letters. 2011;493(3):72–75. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsdotter AK, Toksvig-Larsen S, Roos EM. A 5 year prospective study of patient-relevant outcomes after total knee replacement. Osteoarthritis Cartilage. 2009;17(5):601–606. doi: 10.1016/j.joca.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of Local Anesthetics on Articular Cartilage. Am J Sports Med. 2011 doi: 10.1177/0363546511402780. [DOI] [PubMed] [Google Scholar]
- 33.Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, Walker K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114(3):339–346. doi: 10.1016/j.pain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152(7):1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 36.Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, Atzeni F, Canesi B. Osteoarthritis: An Overview of the Disease and Its Treatment Strategies. Seminars in Arthritis and Rheumatism. 2005;35(1, Supplement 1):1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54(5):1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 38.Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60(6):2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- 39.Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP6-28 reduces nociception in an animal model of osteoarthritis. Osteoarthritis Cartilage. 2006;14(11):1155–1162. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian Li X, Kennedy JD, Glasson SS, Whiteside GT. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis Cartilage. 2010;18(10):1319–1328. doi: 10.1016/j.joca.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Svensson O, Thorne C, Miller F, Bjornsson M, Reimfelt A, Karlsten R. A phase II randomized controlled trial evaluating the efficacy and safety of the TRPV1-antagonist AZD1386 in osteoarthritis of the knee. 13th World Congress on Pain. 2010 [Google Scholar]
- 42.Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]