Abstract
The thermo-transient receptor potentials (TRPs), a recently discovered family of ion channels activated by temperature, are expressed in primary sensory nerve terminals where they provide information about thermal changes in the environment. Six thermo-TRPs have been characterised to date: TRP vanilloid (TRPV) 1 and 2 are activated by painful levels of heat, TRPV3 and 4 respond to non-painful warmth, TRP melastatin 8 is activated by non-painful cool temperatures, while TRP ankyrin (TRPA) 1 is activated by painful cold. The thermal thresholds of many thermo-TRPs are known to be modulated by extracellular mediators, released by tissue damage or inflammation, such as bradykinin, PG and growth factors. There have been intensive efforts recently to develop antagonists of thermo-TRP channels, particularly of the noxious thermal sensors TRPV1 and TRPA1. Blockers of these channels are likely to have therapeutic uses as novel analgesics, but may also cause unacceptable side effects. Controlling the modulation of thermo-TRPs by inflammatory mediators may be a useful alternative strategy in developing novel analgesics.
Keywords: pain, nociceptor, hyperalgesia, sensitization, TRP ion channels, thermo-TRP, TRPV1, neurotrophic factor, NGF, GDNF, artemin
Introduction
Nociception refers to the initial detection of noxious stimuli at the terminals of primary sensory neurons, first named nociceptors by Sherrington a century ago (Sherrington, 1906). Nociceptive nerve axons are either small-diameter, unmyelinated C fibres or medium-diameter myelinated Aδ fibres (Wood and Perl, 1999). The cell bodies lie within the sensory ganglia, the trigeminal ganglia (TG) innervating the face and head, and the dorsal root ganglia (DRG) for the rest of the body. Nociceptors respond to noxious mechanical, chemical or thermal stimuli, generating action potentials that are propagated via the dorsal horn of the spinal cord to higher brain centres to result in a sensation of pain. In the last decade, roles for several ion channels in the initiation of action potentials in nociceptors have been identified. Cloning and characterization of a thermally sensitive subclass of the transient receptor potential (TRP) channels (the thermo-TRPs) have opened up our understanding of the molecular basis of thermal sensation. More than 30 members of mammalian TRP channel family have been characterized and can be subdivided into seven main classes according to their sequence homology: TRP ankyrin (TRPA), TRP canonical (TRPC), TRP melastatin (TRPM), TRP melastatin-like (TRPML), TRP NOMPC (TRPN), TRP polycystic (TRPP) and TRP vanilloid (TRPV) (Montell, 2005). Six are recognized as thermo-TRPs, in the sense that they are expressed in primary somatosensory neurons and are activated at specific temperatures in the range from noxious heat to painful cold. TRPV1–4 transduce elevated temperatures ranging from warm (TRPV4 and TRPV3) to noxious heat (TRPV1 and TRPV2). TRPM8 and TRPA1, on the other hand, are activated respectively by moderate and more extreme cooling. All thermo-TRPs are also activated by a wide range of natural compounds present in foodstuffs and in the environment. Three other TRPs (TRPM2, TRPM4 and TRPM5) also show temperature sensitivity but are not usually included in the thermo-TRP family because they are not expressed in primary somatosensory neurons. Note though that TRPM5 is expressed in primary taste receptors where it plays a role in temperature sensitivity of taste (Talavera et al., 2005), and there is recent evidence that these TRPMs may, in fact, be expressed in somatosensory neurons (Lechner et al., 2009; Staaf et al., 2010).
In this review we first briefly present the properties of the thermo-TRP channels. We then present our current knowledge on their modulation by inflammatory mediators or by growth factors, which act via intracellular signalling pathways to alter the thermal thresholds of agonist sensitivity of the thermo-TRPs. Finally we present some recent findings from the rapidly developing field of thermo-TRP agonists and antagonists. We point out that directly blocking these channels may not be the only useful pharmaceutical strategy in controlling pain, and that compounds attacking the modulation of these channels by intracellular signalling pathways may also have value as analgesics.
Properties of thermo-TRP channels
The basic architecture of TRP channels is similar to that of the voltage-gated K+ channel family – four identical or similar subunits, each with six transmembrane (TM) domains (TM1–TM6) and cytosolic N- and C-terminal tails. TM5, TM6 and the connecting loop form the central cation-conducting pore whereas TM1–TM4 and the cytoplasmic N- and C-terminal parts are thought to contain the regulatory domains that control channel gating. Much of what we assume about the structure of the thermo-TRPs is extrapolated from the known structure of other ion channels, but some structural information is now becoming available (Gaudet, 2009; Moiseenkova-Bell and Wensel, 2009). Figure 1 and Table 1 summarize the activation of the thermo-TRPs by temperature and by non-thermal agonists, and outline probable functions of these ion channels.
Figure 1.
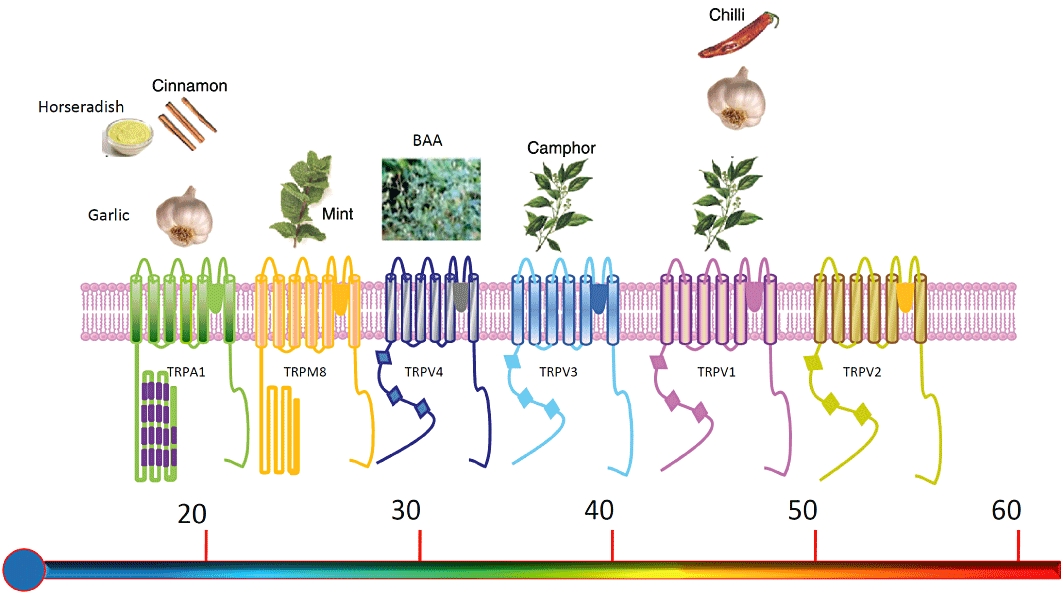
Schematic depiction of the six mammalian thermo-TRP channels. Each subunit consists of six transmembrane domains (S1–S6), a hydrophobic pore loop linking transmembrane segments five (S5) and six (S6), and large cytoplasmic N- and C- terminals (NB: not drawn to scale). All thermo-TRPs have a variable number of ankyrin repeat domains in the N-terminus (except TRPM8, which has none). Thermo-TRPs display distinct thermal thresholds from very hot (TRPV2) to cold (TRPA1). Each thermo-TRP is also activated by specific natural compounds and by synthetic substances, which are also known to induce the relevant thermal and pain sensations in humans. This figure provides a schematic overview of some of the main agonists – see Table 1 for a more complete list. BAA, bisandrographolide A.
Table 1.
TRP channels and their temperature sensitivity, with examples of agonists acting on each receptor and suggested functions of the receptor in the intact animal
| Channel | Temperature sensitivity | Non-thermal agonists | Function |
|---|---|---|---|
| TRPV1 | ≥42°C | Capsaicin, low pH, ethanol, anadamide, NADA, 12-HPETE, camphor, resiniferatoxin, allicin, 2-APB, lidocaine, gingerol, shogaol, piperine monoacylglycerols, ω-3 fatty acids, membrane stretch | Noxious heat sensor; also involved in inlammatory pain, thermal hyperalgesia, hippocampal long-term depression, diabetes, obesity, bladder function, hypertension, gastrointestinitis, hypothermia, renal excretory function. |
| TRPV2 | ≥52°C | 2-APB, cannabidiol, membrane stretch | Possible extreme heat sensor; innate immune system |
| TRPV3 | 32°C∼39°C | 2-APB, camphor, carvacrol (from oregano), incensole acetate, thymol, eugenol | Warmth; possible involvement in noxious heat detection |
| TRPV4 | 27°C∼34°C | Membrane stretch, phorbol ester, 5,6-EET, anandamide, arachidonic acid, BAA | Warm temperature sensation and volume regulation; possible involvement in noxious mechanical pain and thermal hyperalgesia |
| TRPM8 | 25°C∼34°C | Menthol, icilin, eucalyptol | Innocuous cold perception, behavioural thermoregulation, cold-mediated analgesia; cold nociception in some neurons |
| TRPA1 | ≤17°C | Cinnamaldehyde, acrolein, chlorine, ROS, formalin, fatty acids, mustard oil, allicin, icilin, gingerol, prostanoids, NSAIDs, isoflurane, propofol, etomidate, dihydropyridines, clotrimazole, nicotine, menthol | Cold, mechanical and chemically induced nociception, cold hyperalgesia |
NADA, N-Arachidonyl dopamine AA-DA; 12-HPETE, 12- hydroperoxyeicosatetraenoic acid; 2-APB, 2-aminoethoxydiphenyl borate; 5,6-EET, 5,6 epoxyeicosatrienoic acid; BAA, bisandrographolide A; NSAIDs, non-steroidal anti-inflammatory drugs; ROS, reactive oxygen species.
TRPV1
The activation of heat-sensitive ion channels in sensory neurons was first characterized by work from our group (Cesare and McNaughton, 1996). A heat-sensitive ion channel that seems to be responsible for much of the heat sensitivity of primary sensory neurons (discussed further below) was subsequently cloned using an innovative expression-cloning strategy and named vanilloid receptor 1 (VR1), although this was later changed to TRPV1 to emphasize its membership of the TRP ion channel family (Caterina et al., 1997). TRPV1 is mainly detected in small- to medium-diameter neurons of primary sensory ganglia (DRG, TG and sympathetic ganglia such as the nodose ganglia). TRPV1 is expressed by all the major classes of nociceptive neurons, namely the nociceptive subset of Aδ myelinated-fibre neurons, and the peptidergic and non-peptidergic classes of small unmyelinated C-fibre primary afferents. TRPV1 is the only TRP family member that is activated by vanilloids such as capsaicin, a pungent chemical found in hot chilli peppers. Capsaicin has been known for many years to be a selective activator of unmyelinated and small myelinated nociceptive afferents (Oh et al., 1996). Studies using membrane-permeable and impermeable analogues of capsaicin and the competitive antagonist capsazepine demonstrated that capsaicin binds to an intracellular site of TRPV1 to activate the channel (Jung et al., 1999). TRPV1 is a promiscuous channel that is activated by a wide range of agonists (see Table 1), by low pH and by physical factors such as heat and membrane depolarization (Tominaga et al., 1998; Voets et al., 2004; Zhu, 2007). Noxious heat directly gates the channel with a threshold for activation at >43°C. (Note that the concept of ‘threshold’ is not strictly applicable to a channel such as TRPV1 where increases in temperature activate the channel by increasing its probability of activation, and where the channel, therefore, has some degree of activity at any temperature. We use here the term ‘threshold’ as an operational definition to indicate the temperature at which the inward current through a thermo-TRP channel becomes large enough to fire action potentials in an afferent nerve fibre). However, the temperature threshold can be lowered by the action of proinflammatory mediators that are released during tissue injury or inflammation. These mediators can be classified into two broad classes, as discussed further below: those that activate serine–threonine kinases, such as PKA and PKC, via a GPCR; and those which activate tyrosine kinase (TK) via growth factor receptors. The former include bradykinin (Cesare and McNaughton, 1996; Vellani et al., 2001; Bhave et al., 2003), PGs (Bhave et al., 2002; Moriyama et al., 2005), ATP (Moriyama et al., 2003), endothelins (Plant et al., 2007) and prokineticins (Vellani et al., 2006). The second and more recently discovered class of sensitizing agents include nerve growth factor (NGF) (Lewin and Mendell, 1993; Bonnington and McNaughton, 2003; Zhang et al., 2005), insulin and insulin-like growth factor (IGF) (Van Buren et al., 2005; Camprubi-Robles et al., 2009) and stem cell factor (SCF) (Milenkovic et al., 2007).
TRPV1 is directly gated by acidic conditions, pH < 6. Lowering the extracellular pH from 7.6 to 6.4 also sensitized TRPV1 to both capsaicin and heat (Tominaga et al., 1998). TRPV1 can be directly activated by ethanol (Trevisani et al., 2002), and by a variety of endogenous lipids such as anandamide (Ross, 2003) and lipoxygenase products (Hwang et al., 2000). Thus, TRPV1 is viewed as a signalling integrator for many noxious stimuli.
Genetic studies showed that TRPV1 is the only receptor for capsaicin, because vanilloids evoked no pain behaviours in TRPV1−/− mice (Caterina et al., 2000). TRPV1−/− mice largely failed to develop thermal hyperalgesia in response to mustard oil, complete Freund's adjuvant (CFA) or carrageenan, showing that TRPV1 is a key element in the generation of thermal hyperalgesia (Caterina et al., 2000; Davis et al., 2000; Moriyama et al., 2005). Mechanical hyperalgesia was little affected by deletion of TRPV1 (Caterina et al., 2000), but pharmacological studies using capsazepine and other more selective TRPV1 antagonists demonstrated reduced mechanical hyperalgesia in different pain models, supporting the hypothesis that TRPV1 contributes at least to some extent to the development of mechanical hyperalgesia (Davis and Perkins, 1996; Hua et al., 1997; Kwak et al., 1998; Caterina, 2003).
Although there is general agreement that TRPV1 mediates thermal hyperalgesia following inflammation, the precise role of TRPV1 in detecting heat stimuli still remains surprisingly elusive. Mice lacking the TRPV1 receptor are relatively normal in their response to noxious heat stimuli (Caterina et al., 2000; Davis et al., 2000). Consistent with this, in culture many isolated sensory neurons (23%) generate an inward current in response to heat but not to the TRPV1 agonist capsaicin (Nagy and Rang, 1999a,b). A heat-detecting mechanism other than TRPV1 must therefore be present. TRPV2 is the obvious candidate (Caterina et al., 2000), but TRPV2 null mice show no apparent deficit in thermal sensation (Park et al., 2011). There is also evidence at the level of single neurons for heat-detecting mechanisms not mediated by either TRPV1 or TRPV2, because only a minority of heat-sensitive neurons in intact preparations express either of the heat-sensitive ion channels TRPV1 or TRPV2 (Woodbury et al., 2004; Lawson et al., 2008; Koerber et al., 2010). Of particular interest is a recent paper showing that thermal hyperalgesia is communicated via nerve fibres which do not express TRPV1 (Koerber et al., 2010). These studies show that ion channels other than TRPV1 or TRPV2 must play important roles in sensing painful heat, and possibly also in thermal hyperalgesia.
TRPV2
In contrast to TRPV1, TRPV2 is insensitive to capsaicin and responds to higher temperature stimuli, with an activation threshold of 52°C. TRPV2 is highly expressed in a subpopulation of medium to large diameter sensory neurons that predominantly give rise to Aδ fibres (Caterina and Julius, 1999; Bender et al., 2005). TRPV2 is activated in vitro by physical stimuli such as heat, osmotic stress, mechanical stretch (Caterina et al., 1999; Muraki et al., 2003), and non-selective chemical activators such as 2-aminoethoxydiphenyborate (2-APB) (Hu et al., 2004) and cannabidiol (Qin et al., 2008). It has been suggested that TRPV2 is up-regulated in DRG neurons after nerve injury and inflammation (Shimosato et al., 2005; Frederick et al., 2007), which may indicate a potential role for TRPV2 in pain sensation. Compared to TRPV1, TRPV2 has a wider distribution pattern, including lung, spleen, bladder and immune cells, suggesting a broader range of physiological functions than TRPV1. However, the specific physiological and pathological roles of TRPV2 in thermal nociception remain unclear, as TRPV2 null mice showed no evident deficits in thermal sensation (Park et al., 2011; Link et al., 2010).
TRPV3 and TRPV4
These thermo-TRPs are activated by moderate temperatures, with thresholds of 34°C–38°C and 27°C–34°C respectively (Smith et al., 2002; Xu et al., 2002; Peier et al., 2002b; Watanabe et al., 2002b). Mice lacking TRPV3 or TRPV4 have been reported to exhibit deficits in both innocuous and noxious heat sensation indicating an involvement of both channels in thermosensation (Todaka et al., 2004; Lee et al., 2005; Moqrich et al., 2005). In addition to temperature, both are activated by chemical stimuli which in humans give a sensation of pleasant warmth. For example, thymol from thyme, carvacrol from oregano and eugenol, found in cloves, nutmegs, cinnamon, basil and bay leaves, activate TRPV3 (Xu et al., 2006). The synthetic compound 2-aminoethyl diphenylborate (2-APB) is a promiscuous activator of TRPV1, TRPV2 and TRPV3 (Hu et al., 2004; but note that effects of 2-APB on sensory neurons are mediated via TRPV1, see Zimmermann et al., 2005). Hypotonic stress and phorbol esters activate many TRPV channels including TRPV4 (Watanabe et al., 2002a; Fan et al., 2009). TRPV4 is activated by the compound bisandrographolide from the Chinese herbal plant Andrographis paniculata (Smith et al., 2006). Both TRPV3 and TRPV4 are found to be predominantly expressed in skin keratinocytes, suggesting that warm stimuli might be detected initially in keratinocytes and then excitation transferred to primary afferent terminals by an extracellular mediator, which has been proposed to be ATP (Peier et al., 2002b; Mandadi et al., 2009) or PGE2 (Huang et al., 2008). TRPV4 is also expressed in mouse and rat urothelium and vascular endothelium, and studies with knockout animals demonstrated a role for TRPV4 in voiding behaviour, raising the possibility that TRPV4 plays a critical role in urothelium-mediated transduction of intravesicular mechanical pressure (Gevaert et al., 2007). Knockout studies suggest that TRPV4 plays a role in thermal hyperalgesia (Todaka et al., 2004), and studies in which TRPV3 was overexpressed in keratinocytes suggest a possible similar role for TRPV3 (Huang et al., 2008). TRPV4 has been suggested to play a potential role in mechanosensation (Alessandri-Haber et al., 2005) and in normal hearing (Nilius and Voets, 2005).
TRPM8 and TRPA1
Soon after the discovery of TRPV1, two cold-sensitive TRP channels, TRPM8 and TRPA1, were cloned and characterized (McKemy et al., 2002; Peier et al., 2002a; Story et al., 2003). Like TRPV1, both are Ca2+-permeable cation channels. TRPM8 is activated by menthol, which produces a sensation of pleasant cooling in human subjects, and responds to non-noxious cool temperatures, being first activated on cooling at 25°C in expression systems, though the activation threshold of TRPM8 in native sensory neurons is ∼6°C higher than in HEK cells (De la Pena et al., 2005). TRPM8 is activated by chemicals that mimic cooling, such as menthol, icilin and eucalyptol (McKemy et al., 2002; Peier et al., 2002a; Chuang et al., 2004; Voets et al., 2004). Low levels of menthol elevate the threshold for TRPM8 activation to warmer temperatures, and repeated menthol stimulation rapidly desensitises TRPM8 in a Ca2+-dependent manner (McKemy et al., 2002; Reid et al., 2002). The expression of TRPM8 in small-diameter sensory neurones lacking nociceptor markers, such as TRPV1 and calcitonin gene-related peptide (CGRP), supports the idea that TRPM8 acts as a non-noxious cool thermosensor in vivo (McKemy et al., 2002; Peier et al., 2002a), although TRPM8 is also co-expressed with TRPV1 in probable nociceptive neurons, for reasons that are currently unclear (Babes et al., 2004; Okazawa et al., 2004). In vivo studies of TRPM8−/− mice showed deficits in sensation of non-noxious cool temperatures, demonstrating that this channel is a major sensor of peripheral innocuous coolness (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). However, these mice are not deficient in responses to noxious cold, and therefore, other receptors must exist to detect more extreme cold.
TRPA1 is activated by cooling to the noxious cold range of temperatures (∼17°C), suggesting that this channel may be responsible for detecting painful levels of cold (Story et al., 2003). TRPA1 is directly activated by intracellular Ca2+ binding to an EF-hand domain in its N-terminal region, suggesting that the apparent cold sensitivity may result not from direct temperature-sensitive gating but instead from a cold-induced increase in intracellular Ca2+ (Doerner et al., 2007; Zurborg et al., 2007). Against this idea, more recent data show that the response to cold is still seen in the absence of extracellular Ca (Karashima et al., 2009).
There is some difference of opinion as to whether TRPA1 does actually fulfil a physiological function as a cold detector (Bautista et al., 2006; Kwan et al., 2006; 2009), but evidence in favour of this idea has accumulated in recent years (Fajardo et al., 2008; Belmonte et al., 2009; Karashima et al., 2009). In experiments on isolated sensory neurons, several groups have found that a substantial fraction of cold-sensitive DRG neurons does not express either TRPM8 or TRPA1, implying the existence of cold-sensing mechanisms not dependent on either TRPM8 or TRPA1 (Babes et al., 2006; Munns et al., 2007; Karashima et al., 2009). TRPA1-deficient mice were initially found to exhibit normal cold sensitivity (Bautista et al., 2006), and double TRPM8/TRPA1 knockout mice are not very different in their responses to cold from TRPM8−/− mice (Knowlton et al., 2010); significantly, both still show aversive responses to cold stimuli below 15°C. Other groups have since found that TRPA1-deficient mice do in fact show some deficits in painful cold sensation (Kwan et al., 2006; Karashima et al., 2009), and TRPA1 does appear to play a role in cold hyperalgesia or allodynia after injuries, because TRPA1 mRNA is up-regulated after tissue inflammation and peripheral nerve injury, and TRPA1 knock-down mice have reduced cold hyperalgesia (Obata et al., 2005; Katsura et al., 2006). Taken overall, these experiments agree in showing a role for TRPM8 in mild cold sensation and in some aspects of more extreme cold sensation, and a role for TRPA1 in some aspects of painful cold sensation, but all experiments agree that extreme cold sensation is not completely accounted for by either TRPM8 or TRPA1. The idea that TRPA1 can act as a physiological temperature sensor is given credence by a recent report showing that snake TRPA1 channels are highly temperature-sensitive (Gracheva et al., 2010).
There is little doubt, however, that TRPA1 acts as a detector of environmental irritants such as acrolein, a pollutant found in smoke (Bautista et al., 2005). TRPA1 is also activated by pungent compounds of the isothiocyanate family, such as mustard oil and allicin from garlic (Bandell et al., 2004; Bautista et al., 2005). Many TRPA1 agonists irreversibly activate the channel by covalently modifying N-terminal cysteines (Hinman et al., 2006; Macpherson et al., 2007) but it has recently been shown that non-electrophilic non-steroidal anti-inflammatory drugs can also selectively activate TRPA1 by conventional non-covalent reversible ligand–receptor interactions (Hu et al., 2010). Menthol can also activate TRPA1 by a non-covalent action (Karashima et al., 2007; Xiao et al., 2008). Unlike TRPM8, TRPA1 is found to be expressed exclusively in nociceptive TRPV1 and CGRP-positive neurons, consistent with an exclusive role for this channel as a sensor of noxious stimuli (Dhaka et al., 2006). This also raises the possibility that the expression of TRPA1 in some but not all TRPV1-expressing neurons may delineate subsets of polymodal nociceptors (Bautista et al., 2006; Kwan et al., 2006). TRPA1 is also strongly expressed in hair cells of the inner ear (Corey et al., 2004), but TRPA1−/− mice have normal hearing, making a role for TRPA1 in auditory transduction unlikely (Bautista et al., 2006; Kwan et al., 2006).
Modulation of thermo-TRP ion channels by GPCRs
Many inflammatory mediators released during tissue injury or inflammation, such as PGs, bradykinin, ATP and proteinases, bind to GPCRs and cause downstream activation of PKs such as PKA and PKC. These serine/threonine kinases phosphorylate thermo-TRP ion channels, modulating their thermal thresholds and also influencing their gating by a wide range of agonists.
TRPV1
TRPV1 contains multiple phosphorylation sites for serine/threonine PKs such as PKC, PKA, calcium/calmodulin-dependent kinase (CaMK)II and sarcoma (Src) kinase, all of which regulate TRPV1 activity by a dynamic interplay between receptor phosphorylation and dephosphorylation (Huang et al., 2006). Several sites on TRPV1 are known to be phosphorylated by PKC, but only Ser502 and Ser800 play important functional roles in modulation of TRPV1 (Numazaki et al., 2002; Bhave et al., 2003; Mandadi et al., 2006). PKC-dependent phosphorylation of TRPV1 reduces the temperature threshold for TRPV1 activation and potentiates the capsaicin-induced channel open probability (Studer and McNaughton, 2010). PKA is also important in regulating the sensitivity of TRPV1 to many activators, including heat (Lopshire and Nicol, 1998; Bhave et al., 2002; Rathee et al., 2002). The critical residues have been reported to be Ser116 and Thr370 (Bhave et al., 2002), although more recent evidence shows that Ser502 is also important (Zhang et al., 2008). Recent studies have demonstrated that the scaffolding protein A-kinase anchoring protein (AKAP)79 (AKAP150 in rodents) is critical in phosphorylation of TRPV1 by both PKA and PKC. AKAP79 binds PKA and PKC and recognizes a C-terminal domain on TRPV1, thus bringing the kinases into proximity to TRPV1 to form a signalling complex, without which phosphorylation, and therefore sensitization, is completely abolished (Zhang et al., 2008). In vivo studies have confirmed that PKC-induced sensitization to a thermal stimulus is abrogated in AKAP150 knockout mice (Jeske et al., 2009).
The normal activation threshold of TRPV1 is around 43°C, but following sensitization by phosphorylation, the threshold can fall below body temperature, suggesting that part or all of the pain associated with inflammation may be due to the activation of TRPV1 by the normal body temperature (Cesare and McNaughton, 1996; Premkumar and Ahern, 2000; Vellani et al., 2001; Bhave et al., 2003). There is also a substantial increase in the sensitivity of TRPV1 to protons (Tominaga et al., 1998), which may also contribute to ongoing pain in the presence of elevated H+ levels in inflamed tissues. The possible involvement of TRPV1 in inflammatory pain has led to a huge effort to develop blockers of TRPV1 as analgesic therapies (discussed later).
TRPV2/3/4
TRPV4 is regulated by Ser/Thr phosphorylation in a basically similar manner to TRPV1. Both PKC and PKA enhance activation of the TRPV4 ion channel by phosphorylation at specific residues (PKC phosphorylates TRPV4 at Ser162, Thr175, and Ser189 and PKA phosphorylates TRPV4 at Ser824), and the phosphorylation depends on the assembly of PKC and PKA by AKAP79 into a signalling complex with TRPV4 (Fan et al., 2009). Xu et al. (2003a,b) suggested that hypotonic stress increases the Tyr phosphorylation level of TRPV4 via Tyr253, while Wegierski et al. (2009) identified Tyr110 to be the critical phosphorylation site of TRPV4 by Src in inflammatory hyperalgesia.
Information on the regulation of TRPV2 and TRPV3 by PKs is very limited. Some evidence suggests a role for PKA in modulation of TRPV2 function by phosphorylation (Stokes et al., 2004) and other studies showed PI3-kinase (PI3K) may regulate TRPV2 gating (Penna et al., 2006). In addition, it has been suggested that TRPV3 activity is enhanced by PLC-dependent GPCR activation, but no in vivo studies have shown a role for TRPV3 in the sensitization of nociceptors (Xu et al., 2006).
TRPM8
In comparison with TRPV1, whose activation is enhanced by phosphorylation, modulation of TRPM8 by PKs appears to function as a negative regulator. Activity of TRPM8 was found to be down-regulated by PKC activation, which has been proposed to initiate dephosphorylation of TRPM8 by activation of a phosphatase (Premkumar et al., 2005). Recent evidence also shows that TRPM8 is desensitized by phosphorylation by PKA (Linte et al., 2007; Bavencoffe et al., 2010).
TRPA1
It has been suggested that mustard oil activation of TRPA1 is sensitized by bradykinin in a mechanism involving PKA and PLC both in vitro (Wang et al., 2008) and in vivo (Schmidt et al., 2009). PLC-induced degradation of phosphatidylinositol bisphosphate (PIP2) may reduce desensitization of TRPA1, suggesting that PIP2 inhibits TRPA1 activity (Dai et al., 2007; Karashima et al., 2008; Kim et al., 2008). Besides bradykinin, protease-activated receptor 2 was also shown to sensitize TRPA1 through PLC/PIP2 signalling in sensory neurons (Dai et al., 2007). A recent paper has shown that inflammatory signals or acute activation of TRPA1 by mustard oil induces translocation of TRPA1 to the membrane in sensory neurons, and that this incorporation of new channels is PKA- and PLC-dependent (Schmidt et al., 2009). However, the molecular mechanisms mediated by PKA, PLC and PIP2, and leading to TRPA1 sensitization and translocation, still remain to be elucidated.
Modulation of thermo-TRP ion channels by growth factors
Growth factors such as neurotrophins of the NGF and glial cell line-derived nerve growth factor (GDNF) families typically bind to and activate receptors coupled to downstream TK. Sensory neurons express receptors for many growth factors, and it has become clear that these receptors perform different functions in the developing embryo, where they determine the survival of neurons, and in the adult, where neurons are no longer dependent on growth factors for survival but where their properties are subject to modulation by growth factors released during infection and inflammation, and acting much more like inflammatory mediators than growth factors in the conventional sense (Lewin and Barde, 1996; Farinas, 1999).
NGF
NGF is released from a range of cell types following inflammation or injury (Weskamp and Otten, 1987). Injection of NGF in animals produces a rapid hyperalgesic effect, and also causes a sustained hyperalgesia lasting for days or weeks (Lewin and Mendell, 1993; Lewin et al., 1994; Pezet and McMahon, 2006). Two receptors [Tyr receptor kinase type 1 (TrkA) and p75NTR] interact with NGF, but the effect of NGF in thermal hyperalgesia is predominantly mediated via TrkA receptors (McMahon et al., 1994). In rat DRG neurons, TRPV1 is expressed in ∼50% of TrkA-positive cells, and ∼50% of TRPV1-positive cells express TrkA. TRPM8 is found in ∼40% of TrkA-positive cells, and virtually all TRPM8-positive cells express TrkA (Kobayashi et al., 2005).
NGF has a long-term effect in up-regulating TRPV1 expression via the Ras-MAPK pathway. In particular, retrograde transport of NGF from a peripheral site of inflammation acts via p38MAPK activation to increase translation of TRPV1 in the cell body, resulting in an increased transport of TRPV1 to peripheral terminals (Ji et al., 2002). A short-term effect of NGF is also important, and acts via two distinct signalling pathways present in the nerve terminal (Bonnington and McNaughton, 2003; Zhang et al., 2005). A major pathway is initiated by autophosphorylation of the Tyr760 site of TrkA and, subsequently, the activation of PI3K. The non-receptor TK Src is activated downstream and directly phosphorylates TRPV1 at Tyr200, triggering the translocation of TRPV1 from a subcellular vesicular store and its insertion into the cell surface membrane (Zhang et al., 2005; Stein et al., 2006). The specific Src inhibitor PP2 eliminated both NGF-induced phosphorylation of TRPV1 and translocation to the surface membrane (Zhang et al., 2005). Tyr200 is well conserved among TRPV1s of various species and also other TRPV family members, TRPV2–4, suggesting that Tyr200 may play an important role in regulating the surface membrane expression of other thermo-TRPs. In addition to the PI3K–Src pathway, the PKCε pathway, as outlined above, plays a more minor role in NGF-induced sensitization of TRPV1 (Zhang et al., 2005). In addition, NGF has also been reported to alter the activation threshold of TRPV1 via the p42/44 MAPK pathway (Zhu and Oxford, 2007). NGF was initially proposed to enhance TRPV1 function via activation of PLCγ and consequent depletion of PIP2, which some experiments suggested acts as an inhibitor of TRPV1 activation (Chuang et al., 2001), but there is now strong evidence against this idea, as follows: (i) all of the regulation of TRPV1 by NGF can be explained by the PIP2-independent pathways outlined above (Zhang et al., 2005; Zhang and McNaughton, 2006); (ii) the supposed C-terminal TRPV1 modular PIP2-binding domain thought to underlie the effect (Prescott and Julius, 2003) in fact promotes TRPV1 activation by enhancing by Src-dependent phosphorylation rather than by binding PIP2 (Zhang et al., 2005); and (iii) PIP2 actually promotes TRPV1 activation, rather than inhibiting it as required by the original PIP2 hypothesis (Stein et al., 2006; Sowa et al., 2010).
GDNF family
GDNF was originally purified from the supernatant of a rat glial cell line lysate, and was later found to be synthesized and secreted by a variety of tissues. The four GDNF family members (GDNF, neurturin, artemin and persephin) share the common receptor TK ‘rearranged during transfection’ (RET), and specificity is conferred by the RET-binding partners GFRα1–4 as follows: GDNF–GFRα1, neurturin–GFRα2, artemin–GFRα3 and persephin–GFRα4 (Airaksinen et al., 1999). Although initial studies of sensory neurotrophic factors focused on their importance for neuronal survival and the regulation of differentiation during development, we now know that, as in the case of NGF, they also regulate functional properties of adult sensory neurons (Stucky et al., 2002; Albers et al., 2006). Like NGF, artemin potentiates TRPV1 activity in over 60% of capsaicin responsive sensory neurons in vitro and application of both NGF and artemin potentiate of the TRPV1 response fourfold greater than that seen with either growth factor alone (Malin et al., 2006). When injected into the hind paw of adult mice, artemin produced acute thermal hyperalgesia that lasted for up to 4 h. Using reverse transcriptase-PCR, the mRNA expression of artemin was found to be significantly up-regulated in response to inflammation induced by hindpaw injection of complete Freund's adjuvant (CFA) (Malin et al., 2006). These experiments show that GDNF family members regulate the sensitivity of thermal nociceptors and moreover that they play a role in inflammatory hyperalgesia.
The mechanism of sensitization of TRPV1 by artemin was investigated by observing the effects of inhibitors of artemin-activated second messenger signalling pathways (unpublished data from our lab). Pharmacological blockade of PKC, PI3K and Src kinase all prevented enhancement of TRPV1 by artemin, whereas inhibition of PKA, MAPK and Akt had no effect. These data support the hypothesis that sensitization of TRPV1 by artemin is mediated by two pathways, much as was the case for NGF. A signalling cascade involving PKC, PI3K and Src kinase and resulting in translocation of TRPV1 to the surface membrane is the major mediator of artemin-induced TRPV1 sensitization, whereas the PLCδ/PKCε signalling pathway plays a more minor role.
Insulin and IGFs
Insulin and IGFs play a pivotal role in regulating nutrient metabolism and maintain vital neuronal functions. Insulin and IGF-I potentiate TRPV1-mediated membrane currents both in DRG neurons and in heterologous expression systems. Enhancement of membrane current is dependent on the activation of PI3K and, as in the case of NGF, results in enhanced translocation of TRPV1 to the plasma membrane (Van Buren et al., 2005). TRPV2 activity is also regulated by IGF, which promotes, via the PI3K pathway, a dynamic and transient translocation of the TRPV2 from the cytosol to the plasma membrane (Kanzaki et al., 1999; Boels et al., 2001). However, some recent data show that PI3K directly enhances TRPV2 activity instead of regulating surface expression of TRPV2 (Penna et al., 2006). Other studies also showed TRPV2 is regulated by insulin in an autocrine manner in pancreatic beta cells (Hisanaga et al., 2009).
SCF
SCF has recently been shown to bind to and activate a receptor TK, c-Kit, in DRG neurons, leading to a reduced thermal threshold and a potentiation of heat-activated currents mediated by TRPV1 in a similar way to the factors discussed in the preceding paragraphs (Milenkovic et al., 2007).
Recycling of TRPV receptors from the cell membrane
Pathways mediating recovery of TRPV channels from the membrane have been little studied. An interesting recent paper (Shukla et al., 2010) showed that internalization of TRPV4 is promoted by β-arrestin, which, following binding of angiotensin to its receptor, is recruited to a complex of TRPV4 and the angiotensin receptor where it binds to and activates a ubiquitin ligase, which ubiquitinates TRPV4 and so mediates its down-regulation.
Thermo-TRP ion channels: therapeutic implications
Members of the thermo-TRP family are attractive targets for novel analgesics effective in a wide range of pathophysiological conditions (reviewed in Nilius et al., 2007), but in view of the recent discovery of the channels, the development of clinically effective agonists and antagonists of these ion channels as analgesic compounds is in its infancy. Many potent agonists are present in natural compounds used for herbal and folk remedies and in culinary flavourings, and are often found in over-the-counter analgesic preparations. Examples include the TRPV1 agonist capsaicin, the active ingredient of chilli peppers, which is used in topical creams to stimulate blood flow and thus to relieve mild muscle pain (Hautkappe et al., 1998; Chrubasik et al., 2010); the TRPM8 agonist menthol, used as a topical analgesic (Proudfoot et al., 2006); and agonists of TRPV3, such as camphor, cajuput oil and extracts of oregano, which are used in proprietary topical analgesic preparations.
TRPV1 agonists
Potent TRPV1 agonists, such as capsaicin or resiniferatoxin, produce pain but also profoundly desensitize the receptor (Novakova-Tousova et al., 2007). This inactivation reduces the sensitivity to heat and other ligands and can be used to reduce pain, albeit with the problem of the initial pain produced before desensitization (which can be alleviated by lidocaine pretreatment). Pain reduction was seen in patients with human immunodeficiency virus-associated distal sensory polyneuropathy, in patients with postherpetic neuralgia and in patients with urinary tract inflammation (Simpson et al., 2008; Backonja et al., 2009).
TRPV1 antagonists
Knockout studies provided clear evidence for an involvement of TRPV1 in inflammatory heat hyperalgesia, and pharmacological studies showed some effect of antagonists in reducing the clinically more important inflammatory mechanical hyperalgesia (see earlier discussion). Most efforts to date have therefore focussed on the development of antagonists to this member of the thermo-TRP family. Several TRPV1-selective antagonists have recently been found to be particularly potent and efficacious in acute and chronic inflammatory pain models associated with thermal hyperalgesia (recently reviewed by Szallasi et al., 2007; Gomtsyan and Faltynek, 2010). The behavioural effects of TRPV1 antagonists are in broad agreement with the phenotype observed in TRPV1 knockout mice when challenged with agonists such as capsaicin or inflammatory agents such as carrageenan and CFA. However, in the initial TRPV1 genetic deletion experiments, no change in susceptibility of null mice to neuropathic pain was seen (Caterina et al., 2000), but some TRPV1 antagonists have, in fact, been found to reduce neuropathic pain, for reasons not currently understood. A possible action in counteracting neuropathic pain, a common condition poorly treated by existing pharmaceuticals, has attracted considerable attention. Several TRPV1 antagonists have entered clinical trials, including ABT-102 (Abbott; IC50 = 3–4 nM for block of activation by capsaicin and N-arachidonoyl dopamine, 0.7 nM for block of activation by pH 5.5; Gomtsyan et al., 2008), AMG-517 (Amgen; IC50 1 nM for block of activation by capsaicin, acid or heat; Doherty et al., 2007), SB-705498 (GSK), GRC-6211 (Lilly/Glenmark) and MK 2295 (Merck; Crutchlow, 2009).
There are two major drawbacks to the systemic use of TRPV1 antagonists. First, it has become apparent that at least some TRPV1-expressing afferent nerve fibres are partially activated at normal bodily temperature. Blocking TRPV1 removes this ongoing afferent input and, thus, causes a mild hyperthermia (Gavva et al., 2008), but one that could be dangerous in a patient already suffering from hyperthermia for other reasons. Antagonist-induced hyperthermia was not observed in TRPV1 knockout mice, showing that it is an on-target effect, and it is seen with both brain penetrant (AMG8163) and peripherally restricted (AMG1692 and AMG3731) TRPV1 antagonists in rats (Gavva et al., 2007) and with AMG 517 in humans (Gavva et al., 2008), showing that it is a peripheral rather than a central effect. The main site of action in inducing hyperthermia appears to be the viscera, because desensitizing TRPV1 in the viscera by prior application of the potent TRPV1 agonist resiniferatoxin blocks the hyperthermic effect of subsequent antagonist administration (Steiner et al., 2007). Hyperthermia may not be a fatal weakness for the use of TRPV1 antagonists, provided they are administered under careful supervision, as the hyperthermia lessens after a few days (Honore et al., 2009). There have been suggestions that hyperthermia is less obvious with some TRPV1 antagonists than with others, raising the possibility that hyperthermia may arise from a differential block of only one of the three main modes of activation of TRPV1 (Gavva et al., 2007; Lehto et al., 2008; Kym et al., 2009). For instance, if hyperthermia is caused by a block of the action of endogenous agonists on TRPV1, while the relevant modality for analgesia is thermal activation of TRPV1, then an antagonist that blocks thermal but not agonist activation of TRPV1 may get round the problem of hyperthermia. With these thoughts in mind, a number of companies are currently seeking to develop modality-selective antagonists (Wong and Gavva, 2009).
Secondly, the threshold for detection of noxious heat is somewhat elevated following genetic deletion of TRPV1 in mice (Caterina et al., 2000; Davis et al., 2000). In humans treated with TRPV1 antagonists, the thermal threshold is also elevated, although the elevation seems to be more pronounced for some antagonists than for others (Chizh et al., 2007; Crutchlow, 2009). The elevation in thermal threshold raises the possible complication that block of TRPV1 may make patients susceptible to accidental burns.
Modulation of TRPV1 threshold
The problems arising from a direct block of TRPV1 suggest that an indirect action on the modulation of TRPV1 may be a more fruitful avenue to explore. Inflammatory mediators cause heat hyperalgesia by lowering the thermal threshold of TRPV1 (Cesare and McNaughton, 1996; Tominaga et al., 1998; Vellani et al., 2001), raising the interesting possibility that hyperalgesia could be counteracted without affecting the normal thermal threshold of TRPV1 by blocking processes leading to its sensitization. The scaffolding protein AKAP79 has recently been shown to be crucial for the actions of PKA and PKC by binding to TRPV1 and promoting its sensitization by anchoring these kinases adjacent to critical phosphorylation sites on TRPV1 (Jeske et al., 2008; 2009; Schnizler et al., 2008; Zhang et al., 2008). The interaction between AKAP79/150 and TRPV1 occurs at a small region in the TRPV1 C-terminal cytoplasmic tail, and the introduction of a peptide with the same sequence is able to displace AKAP79/150 from TRPV1 and, thus, completely switch off sensitization by inflammatory mediators acting through both PKA and PKC (Zhang et al., 2008). This finding suggests that small-molecule antagonists could be developed that would antagonize binding between AKAP79/150 in a similar manner, and thus abolish heat hyperalgesia without affecting the underlying heat sensitivity of TRPV1.
Analgesia using TRPV1 as a Trojan horse
TRPV1 shows a time and agonist-dependent increase in permeability to large cations owing to changes in the TRPV1 selectivity filter that reflect increases in pore diameter (Chung et al., 2008). This property allows cationic molecules with molecular masses up to 500 Da to enter the cell through TRPV1. In an exciting recent development, it has been shown that QX-314, a charged quaternary amine derivative of the local anaesthetic lidocaine (ineffective in blocking sodium channels when administered extracellularly because it cannot gain access to the inner face of the channels) can produce a long-lasting analgesia specific to TRPV1-expressing neurones when applied in conjunction with a TRPV1 agonist (Binshtok et al., 2007). To use the technology successfully in patients, a non-pungent activator of TRPV1 would be ideal to minimize patient discomfort until sodium channel blockade is achieved on entry of the charged membrane-impermeant sodium channel blocker, and this requirement appears to be met by the use of the local anaesthetic lidocaine (Binshtok et al., 2009; Roberson et al., 2011). This simple but elegant idea is currently being tested as a possible basis for the potential development of next generation local anaesthetics.
TRPM8
TRPM8 is often thought of as an ion channel giving rise only to non-painful sensations but it is co-expressed with TRPV1 in some sensory neurones and there is evidence that TRPM8 channels may play a role in noxious cold sensation and cold allodynia (Proudfoot et al., 2006; Fleetwood-Walker et al., 2007; Belmonte et al., 2009). The possibility that antagonists of TRPM8 may have analgesic effects in particular situations, for example in the cornea where TRPM8 is the dominant thermo-TRP expressed in sensory nerve endings, deserves further investigation. Recently it has been shown that several TRPM8 antagonist compounds act as negative allosteric modulators of channel gating, shifting rather than abolishing activation by voltage and cold (Malkia et al., 2007).
TRPA1
TRPA1 is specifically expressed in a subclass of TRPV1-expressing nociceptors and is activated by both noxious cold (Story et al., 2003) and by a wide range of environmental irritants (Bautista et al., 2006). NGF evokes the functional up-regulation of TRPA1 (Obata et al., 2005; Diogenes et al., 2007), and various knock-down or knockout studies in rodents have demonstrated reduced nociceptive behaviour in a variety of pain models (Bautista et al., 2006; Kwan et al., 2006; McNamara et al., 2007) and attenuated airway inflammation in models of asthma (Caceres et al., 2009). Cold and pollutants, such as those present in smog, can cause cough and reflex bronchconstriction in asthmatics (Colsoul et al., 2009), and a number of TRPA1 antagonists are currently under investigation as antitussives and to counteract airway constriction (reviewed by Viana and Ferrer-Montiel, 2009). Pharmacological inhibition of TRPA1 significantly attenuated CFA-induced mechanical hypersensitivity in wild-type but not TRPA1 knockout mice (Petrus et al., 2007). A selective TRPA1 antagonist, HC-030031was effective in reducing leucocyte infiltration and airway hyperreactivity in a model of lung inflammation (Caceres et al., 2009) and pain behaviours in inflammatory and neuropathic models (Eid et al., 2008).
Conclusion
Several TRPV1 antagonists are in advanced clinical trials and it seems likely that at least some of these will enter the clinic. The two main problems currently identified are hyperthermia, which could lead to dangerously elevated core temperatures particularly if combined with a pre-existing pyrexia, and reduced sensitivity to painful or damaging heat stimuli, which could lead to damaging accidental burns. The bright spot is that these features do not seem to be invariable accompaniments of TRPV1 antagonism – some antagonists cause less hyperthermia than others, and, in a similar way, the elevation of heat threshold seems to be less pronounced for some antagonists than for others. Several companies are working to develop antagonists that will selectively block some modes of activation of TRPV1 while sparing others, and these may provide useful solutions to the problems faced by antagonists that block all modes with approximately equal affinity. This search is currently proceeding in a heuristic manner, but it could be assisted by gaining a better understanding at a fundamental level of how the different modes of activation lead to channel opening. Do all modes of activation act simply as triggers of a single pathway leading to channel opening or do the different pathways of activation converge on channel opening at a late stage? Recent evidence suggests that it is possible to separate activation of TRPV1 by heat, which produces structural changes in the extracellular domain adjacent to the pore region, from activation by agonists that activate the channel without producing these structural changes (Grandl et al., 2008; Yang et al., 2010).
Antagonists for other thermo-TRPs are at earlier stages of development. The most promising target is likely to be TRPA1, which is exclusively expressed in a subset of nociceptive nerve endings. This thermo-TRP is a highly sensitive detector of environmental irritants, such as are present in smog, and of cold, and its activation probably contributes to the brochospasm that many asthmatics experience as a particularly distressing symptom on cold, smoggy mornings. TRPA1 antagonists, either oral or inhaled along with antiasthmatic pharmaceuticals, may provide effective relief.
Direct roles for other thermo-TRPs in pain are less well established. The cool receptor TRPM8 is typically expressed in non-nociceptive sensory neurons and its activation has an analgesic action, as is exploited by many folk and non-prescription remedies. In some sensory neurons (for instance in the cornea) it is co-expressed with TRPV1 and, therefore, is likely to serve a nociceptive function. Antagonists may therefore have value as analgesics in (for instance) eye drops to treat the dry eye, which is often an after effect of corneal surgery. TRPV3, in a similar way, is activated by non-noxious warm temperatures and seems largely to have an analgesic function, and is exploited by traditional anti-inflammatory preparations. There have been some preliminary indications that TRPV3 may also contribute to inflammatory heat pain, and if this is confirmed, then antagonists may be of value. Little is known about whether the other thermo-TRPs play important roles in inflammatory pain, though knockout studies and development of antagonists are currently being reported, and the picture will no doubt become clearer in the near future.
Acknowledgments
We thank Dr Michael Fischer for his comments on the manuscript. Work in the authors' lab was supported by grants from the BBSRC, the Human Frontiers Science Program, the Newton Trust, the Helmholtz Foundation, the European Union and the Spanish Ministry of Science and Innovation. PAM thanks the BBVA Foundation, Spain for funding a professorship, and the Professor C. Belmonte and the Instituto de Neurociencias, Alicante, Spain for hospitality during the writing of this review.
Glossary
- 2-APB
2-aminoethoxydiphenylborate
- AKAP
A-kinase anchoring protein
- CaMK
calcium/calmodulin-dependent kinase
- CFA
complete Freund's adjuvant
- c-Kit
receptor for SCF
- DRG
dorsal root ganglia
- GDNF
glial cell line-derived nerve growth factor
- IGF
insulin-like growth factor
- NGF
nerve growth factor
- PGE2
prostaglandin E2
- PIP2
phosphatidylinositol 4 5-bisphosphate
- RET
‘rearranged during transfection’ (GDNF receptor)
- SCF
stem cell factor
- Ser
serine
- Src (‘sarcoma’)
a non-receptor tyrosine kinase
- Thr
threonine
- TM
transmembrane
- TrkA
tyrosine receptor kinase type 1 (NGF receptor)
- TRP
transient receptor potential
- TRPA
transient receptor potential ankyrin
- TRPC
transient receptor potential canonical
- TRPP
transient receptor potential polycystic
- TRPM
transient receptor potential melastatin
- TRPML
transient receptor potential melastatin-like
- TRPN
transient receptor potential NOMPC
- TRPV
transient receptor potential vanilloid
- Tyr
tyrosine
- VR1
vanilloid receptor 1
Conflict of interest
None of the authors has any conflict of interest.
References
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. A novel type of cold-sensitive neuron in rat dorsal root ganglia with rapid adaptation to cooling stimuli. Eur J Neurosci. 2006;24:691–698. doi: 10.1111/j.1460-9568.2006.04941.x. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bavencoffe A, Gkika D, Kondratskyi A, Beck B, Borowiec AS, Bidaux G, et al. The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J Biol Chem. 2010;285:9410–9419. doi: 10.1074/jbc.M109.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Brock JA, Viana F. Converting cold into pain. Exp Brain Res. 2009;196:13–30. doi: 10.1007/s00221-009-1797-2. [DOI] [PubMed] [Google Scholar]
- Bender FL, Mederos YS, Li Y, Ji A, Weihe E, Gudermann T, et al. The temperature-sensitive ion channel TRPV2 is endogenously expressed and functional in the primary sensory cell line F-11. Cell Physiol Biochem. 2005;15:183–194. doi: 10.1159/000083651. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boels K, Glassmeier G, Herrmann D, Riedel IB, Hampe W, Kojima I, et al. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114:3599–3606. doi: 10.1242/jcs.114.20.3599. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprubi-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009;23:3722–3733. doi: 10.1096/fj.09-134346. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton PA. A novel heat-activated current in nociceptive neurons, and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877–1885. doi: 10.1002/ptr.3335. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Colsoul B, Nilius B, Vennekens R. On the putative role of transient receptor potential cation channels in asthma. Clin Exp Allergy. 2009;39:1456–1466. doi: 10.1111/j.1365-2222.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Crutchlow M. Pharmacologic inhibition of TRPV1 impairs sensation of potentially injurious heat in healthy subjects. 2009. ASCPT 2009 annual meeting, 69.
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Perkins MN. Substance P and capsaicin-induced mechanical hyperalgesia in the rat knee joint; the involvement of bradykinin B1 and B2 receptors. Br J Pharmacol. 1996;118:2206–2212. doi: 10.1111/j.1476-5381.1996.tb15664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- De la Pena E, Malkia A, Cabedo H, Belmonte C, Viana F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol. 2005;567:415–426. doi: 10.1113/jphysiol.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Doherty EM, Fotsch C, Bannon AW, Bo Y, Chen N, Dominguez C, et al. Novel vanilloid receptor-1 antagonists: 2. Structure-activity relationships of 4-oxopyrimidines leading to the selection of a clinical candidate. J Med Chem. 2007;50:3515–3527. doi: 10.1021/jm070190p. [DOI] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci. 2008;28:7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I. Neurotrophin actions during the development of the peripheral nervous system. Microsc Res Tech. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Proudfoot CW, Garry EM, Allchorne A, Vinuela-Fernandez I, Mitchell R. Cold comfort pharm. Trends Pharmacol Sci. 2007;28:621–628. doi: 10.1016/j.tips.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Frederick J, Buck ME, Matson DJ, Cortright DN. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem Biophys Res Commun. 2007;358:1058–1064. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Gaudet R. Divide and conquer: high resolution structural information on TRP channel fragments. J Gen Physiol. 2009;133:231–237. doi: 10.1085/jgp.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomtsyan A, Faltynek CR. Vanilloid Receptor TRPV1 in Drug Discovery. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- Gomtsyan A, Bayburt EK, Schmidt RG, Surowy CS, Honore P, Marsh KC, et al. Identification of (R)-1-(5-tert-butyl-2, 3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)urea (ABT-102) as a potent TRPV1 antagonist for pain management. J Med Chem. 2008;51:392–395. doi: 10.1021/jm701007g. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandl J, Hu H, Bandell M, Bursulaya B, Schmidt M, Petrus M, et al. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautkappe M, Roizen MF, Toledano A, Roth S, Jeffries JA, Ostermeier AM. Review of the effectiveness of capsaicin for painful cutaneous disorders and neural dysfunction. Clin J Pain. 1998;14:97–106. doi: 10.1097/00002508-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes. 2009;58:174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, et al. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142:27–35. doi: 10.1016/j.pain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, et al. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Chen P, Hwang J, Yaksh TL. Antinociception induced by civamide, an orally active capsaicin analogue. Pain. 1997;71:313–322. doi: 10.1016/s0304-3959(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang X, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol. 2006;17:638–645. doi: 10.1016/j.semcdb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, et al. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain. 2009;146:301–307. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jung J, Sun WH, Kwak J, Lee S-Y, Kang C-J, Won BK, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4, 5-bisphosphate. Am J Physiol Cell Physiol. 2008;295:C92–C99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koerber HR, McIlwrath SL, Lawson JJ, Malin SA, Anderson CE, Jankowski MP, et al. Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TPV1 containing C-heat fibers. Mol Pain. 2010;6:58. doi: 10.1186/1744-8069-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JY, Jung JY, Hwang SW, Lee WT, Oh U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in the rat: evidence for an endogenous capsaicin-like substance. Neuroscience. 1998;86:619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kym PR, Kort ME, Hutchins CW. Analgesic potential of TRPV1 antagonists. Biochem Pharmacol. 2009;78:211–216. doi: 10.1016/j.bcp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Frenzel H, Wang R, Lewin GR. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO J. 2009;28:1479–1491. doi: 10.1038/emboj.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(tri fluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326:218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. European. J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res. 2007;178:89–98. doi: 10.1007/s00221-006-0712-3. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkia A, Madrid R, Meseguer V, De La PE, Valero M, Belmonte C, et al. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol. 2007;581:155–174. doi: 10.1113/jphysiol.2006.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, et al. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron. 2007;56:893–906. doi: 10.1016/j.neuron.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Moiseenkova-Bell VY, Wensel TG. Hot on the trail of TRP channel structure. J Gen Physiol. 2009;133:239–244. doi: 10.1085/jgp.200810123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272 doi: 10.1126/stke.2722005re3. re3. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns C, Alqatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Nagy I, Rang H. Noxious heat activates all capsaicin-sensitive and also a sub-population of capsaicin-insensitive dorsal root ganglion neurons. Neuroscience. 1999a;88:995–997. doi: 10.1016/s0306-4522(98)00535-1. [DOI] [PubMed] [Google Scholar]
- Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J Neurosci. 1999b;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Novakova-Tousova K, Vyklicky L, Susankova K, Benedikt J, Samad A, Teisinger J, et al. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149:144–154. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Sun WH, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa M, Inoue W, Hori A, Hosokawa H, Matsumura K, Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci Lett. 2004;359:33–36. doi: 10.1016/j.neulet.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Park U, Vastani N, Guan Y, Srinivasa NR, Koltzenburg M, Caterina MJ. TRP Vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Penna A, Juvin V, Chemin J, Compan V, Monet M, Rassendren FA. PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium. 2006;39:495–507. doi: 10.1016/j.ceca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, et al. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain. 2007;3:35. doi: 10.1186/1744-8069-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, et al. PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol. 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson D, Binshtok A, Blasl F, Bean B, Woolf C. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol. 2011;164:48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- Shimosato G, Amaya F, Ueda M, Tanaka Y, Decosterd I, Tanaka M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain. 2005;119:225–232. doi: 10.1016/j.pain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Kim J, Ahn S, Xiao K, Shenoy SK, Liedtke W, et al. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem. 2010;285:30115–30125. doi: 10.1074/jbc.M110.141549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- Sowa NA, Street SE, Vihko P, Zylka MJ. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30:10282–10293. doi: 10.1523/JNEUROSCI.2162-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf S, Franck MC, Marmigere F, Mattsson JP, Ernfors P. Dynamic expression of the TRPM subgroup of ion channels in developing mouse sensory neurons. Gene Expr Patterns. 2010;10:65–74. doi: 10.1016/j.gep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-Kinase Binds to TRPV1 and Mediates NGF-stimulated TRPV1 Trafficking to the Plasma Membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med. 2004;200:137–147. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545:43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, McNaughton PA. Modulation of single-channel properties of TRPV1 by phosphorylation. J Physiol. 2010;588:3743–3756. doi: 10.1113/jphysiol.2010.190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, et al. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Ferrer-Montiel A. TRPA1 modulators in preclinical development. Expert Opin Ther Pat. 2009;19:1787–1799. doi: 10.1517/13543770903393771. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002a;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002b;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wegierski T, Lewandrowski U, Muller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wood JN, Perl ER. Pain. Curr Opin Genet Dev. 1999;9:328–332. doi: 10.1016/s0959-437x(99)80049-5. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Xu F, Satoh E, Iijima T. Protein kinase C-mediated Ca2+ entry in HEK 293 cells transiently expressing human TRPV4. Br J Pharmacol. 2003a;140:413–421. doi: 10.1038/sj.bjp.0705443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem. 2003b;278:11520–11527. doi: 10.1074/jbc.M211061200. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci USA. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Zhu MX. Understanding the role of voltage gating of polymodal TRP channels. J Physiol. 2007;585:321–322. doi: 10.1113/jphysiol.2007.147082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Fischer MM, Messlinger K, Nau C, Reeh PW. The TRPV1/2/3 activator 2-aminoethoxydiphenyl borate sensitizes native nociceptive neurons to heat in wildtype but not TRPV1 deficient mice. Neuroscience. 2005;135:1277–1284. doi: 10.1016/j.neuroscience.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]


