Abstract
TCR specific activation is pivotal to dendritic epidermal T cell (DETC) function during cutaneous wound repair. However, DETC TCR ligands are uncharacterized and little is known about their expression patterns and kinetics. Using soluble DETC TCR tetramers, we demonstrate that DETC TCR ligands are not constitutively expressed in healthy tissue, but are rapidly upregulated following wounding on keratinocytes bordering wound edges. Ligand expression is tightly regulated with down-modulation following DETC activation. Early inhibition of TCR-ligand interactions using DETC TCR tetramers delays wound repair in vivo, highlighting DETC as rapid responders to injury. This first visualization of DETC TCR ligand expression provides novel information about how ligand expression impacts early stages of DETC activation and wound repair.
Introduction
Dendritic epidermal T cells (DETC) play key roles in epidermal homeostasis, tumor surveillance, and wound repair (1). While αβ T cell activation is well defined (2), γδ T cell activation is less well understood. DETC do not recognize peptide-MHC (pMHC) complexes, do not express CD4, CD8, or CD28 and use JAML for costimulation (1, 3, 4). DETC proliferate and secrete growth factors, cytokines and extracellular matrix components upon recognition of an uncharacterized Ag expressed on stressed keratinocytes (1).
The invariant Vγ3Vδ1 TCR is required for activation of DETC. Transfection of this TCR into non-reactive T cell lines confers responsiveness to damaged keratinocytes in vitro and Abs specific for the DETC TCR inhibit this response (3). Moreover, mice lacking Vγ3Vδ1 DETC in the epidermis exhibit defective barrier function (5) and delayed healing (6). Dependence upon the Vγ3Vδ1 TCR suggests DETC may recognize a self-Ag expressed on the surface of keratinocytes in response to multiple forms of damage, disease and stress.
To address the hypothesis that DETC recognize self-ligands induced on keratinocytes by tissue damage, soluble Vγ3Vδ1 DETC TCR tetramers (DETC tetramers) were produced. DETC tetramers are a powerful tool to examine the localization and kinetics of ligand expression and expand our knowledge of the early stages of DETC activation following epidermal damage. Here, we show constitutive expression of DETC ligands on transformed keratinocyte cell lines in vitro. In situ, DETC TCR ligands are undetectable in resting tissue, but are rapidly and transiently expressed by keratinocytes in wounded epidermis. Ligand is only detectable near the site of injury, correlating with the localized activation of DETC. Moreover, in vivo application of DETC tetramers to wounded tissue delays wound closure, highlighting the role of early ligand recognition by DETC for efficient wound repair.
Materials and Methods
Mice
C57BL/6J (B6) and TCRδ-/- mice were bred and maintained at The Scripps Research Institute. FVBTac mice were purchased from Taconic Farms. All animal protocols were in accordance with The Scripps Research Institute Institutional Animal Care and Use Policy.
Soluble TCR cloning and protein expression
DETC, isolated as described (3), were used for cloning of Vγ3Vδ1 TCR cDNA. DETC γ and δ cDNAs were PCR amplified and ligated into separate pCR2.1-TOPO vectors (Invitrogen). Truncated gene segments encoding the signal sequence through the C-terminal cysteine bond were isolated from the TOPO cloned Vγ3 and Vδ1 genes and ligated into the Drosophila expression vector, pRMHa-3 (7). All primer sequences are listed in Supplemental Figure 1. Cloning of the soluble G8 TCR was previously described (8). The γ chain of the G8 TCR was modified to contain a biotinylation sequence following the acidic zipper. TCR identity was verified by sequencing.
DETC or G8 TCR were transfected with pHSP70PLpac (9) into Drosophila melanogaster SC2 cells and selected with puromycin (Sigma). Protein expression, purification and biotinylation were conducted as described (7, 8). Tetramers were made by incubating biotinylated soluble TCRs (sTCRs) with PE-labeled (Biosource) or APC-labeled streptavidin (Prozyme) overnight at 4°C.
Antibodies and flow cytometry
Vγ3 (536), 1F4, and Vγ3Vδ1 (17D1) Abs were produced as described (10). All other Abs were purchased from BD Biosciences. Cells were incubated with tetramer for 1 h at room temperature (RT), washed, and fixed. Cells were analyzed on a FACSCalibur or a LSR-II (BD Biosciences), and analyzed with FlowJo (Treestar).
Cells and culture
Cell lines were cultured as described (7). Adherent cells were harvested in 2 mM EDTA for 15 min at 37°C.
Immunoprecipitation and western blotting
Ab bound beads were incubated with DETC sTCRs for 3 h at RT, washed and boiled. Eluted material was run on 10% SDS-PAGE, transferred to nitrocellulose, and visualized with anti-(His)5 Ab (QIAGEN) followed by goat anti-mouse-HRP (Southern Biotech) and Supersignal West Pico Substrate (Pierce).
Wounding procedure
Wounds were made in the ears or backs of mice as described (6). For wound healing studies, 2 μg DETC or G8 tetramer, or streptavidin-PE was immediately applied to each wound. Images were acquired with a Canon Powershot S×100 IS, and wound size monitored using Image J software (NIH). Wound area was normalized to the open surface area 24 h after wounding.
Immunofluorescent staining and microscopy
Epidermal sheets were prepared as described (11) and stained with 4.2 μg/ml PE labeled sTCR tetramers and 1.67 μg/ml anti-CD3ε FITC for 1 h at RT, stained with DAPI (Sigma) for 5 min, washed and mounted in DAKO fluorescent mounting media (DAKOcytomation). Samples were imaged directly after staining with a Nikon Eclipse E800 microscope at 200× magnification. Digital images were collected with an AxioCam HRc camera and AxioCam software (Zeiss). PAM 212 cells grown on coverslips or 8 μm sections of OCT-embedded E13.5 whole embryo or adult thymus were stained with DETC or G8 tetramers and 1.67 μg/ml anti-CD3ε or anti-CD29 FITC overnight at 4°C and fixed with 3% formaldehyde (Polysciences). CD3 was visualized with biotinylated goat-anti-hamster IgG (Jackson Immunoresearch) followed by Alexa-555 conjugated streptavidin. Samples were mounted with Prolong Slowfade (Invitrogen). Samples were imaged on a Bio-Rad Radiance 2100 Rainbow laser scanning confocal microscope (Zeiss) mounted on a TE2000-u (Nikon) microscope using a 60×/1.4NA-oil objective lens and BioRad LaserSharp 2000 software.
Results and discussion
Expression of a soluble DETC TCR
To create a DETC sTCR we used the Drosophila expression system (7, 8, 12). As native TCRs are heterodimeric, integral membrane proteins, several modifications were required to solubilize the DETC TCR (Supplemental Fig. 1). The extracellular domain of the DETC TCR was truncated after the C-terminal cysteines. The transmembrane region was replaced with an acidic zipper on the γ chain (13) followed by a BirA biotinylation site (14), or a basic zipper on the δ chain (13) and a C-terminal His-tag. Following transfection, SC2 cells expressed soluble dimeric DETC sTCR (Supplemental Fig. 1). Purified DETC sTCR was immunoprecipated with anti-TCR Abs (Supplemental Fig. 1), verifying the sTCR is a heterodimeric protein containing both the Vγ3 and Vδ1 gene products. As a negative control, a soluble form of the well-characterized G8 TCR was produced.
DETC tetramers bind cell surface ligands
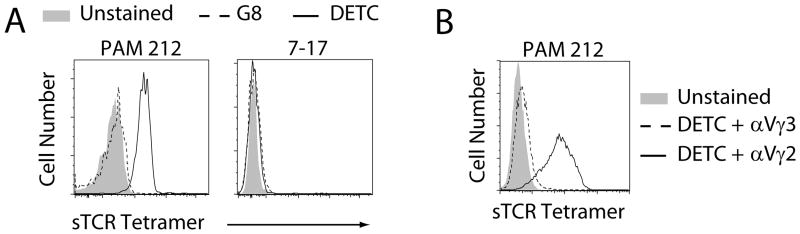
DETC and G8 were produced as tetramers to increase the avidity of the DETC TCR-ligand interaction, as was previously done with soluble MHC molecules (8). The ability of the DETC sTCR to recognize ligands was assessed by flow cytometry. DETC tetramers bound anti-Vγ3Vδ1 (17D1), anti-Vγ3, and anti-Cδ expressing hybridomas, while G8 tetramers only bound the anti-Cδ expressing hybridoma (Supplemental Fig. 1) and a T22-transfected CHO cell line (data not shown [dns]). DETC tetramer binding to 17D1 shows the DETC sTCR has correct Vγ3Vδ1 chain pairing as 17D1 specifically recognizes the Vγ3Vδ1 TCR heterodimer (10). DETC tetramers also bound ligands on the stimulatory transformed keratinocyte cell line, PAM 212, while the G8 tetramers did not (Fig. 1A) and neither DETC nor G8 tetramers bound a DETC cell line (7-17) (Fig. 1A). Pre-incubation with anti-Vγ3 Abs blocked DETC tetramer binding to the PAM 212 cell line (Fig. 1B), demonstrating TCR specificity of DETC tetramer binding. To determine if DETC TCR ligands are expressed exclusively on keratinocytes, cell lines were tested for DETC tetramer binding. DETC tetramers showed some binding to transformed epithelial cells but not to endothelial cells (Supplemental Fig. 1), suggesting DETC ligand expression in vitro is limited to keratinocytes and some other transformed epithelial cells.
Fig. 1. DETC tetramers bind cell surface ligands on keratinocyts.
(A) Tetramer staining of the PAM 212 cell line. (B) TCR specific binding of DETC tetramers. DETC sTCR tetramers were incubated with anti-Vγ2 or anti-Vγ3 Abs prior to cell surface staining. Data are representative of ≥3 experiments.
DETC TCR Ag is upregulated on keratinocytes near wound edges
While the DETC TCR ligand remains unidentified, prior studies show that DETC proximal to wound sites become activated and rounded, while DETC distal to wound sites remain resting and dendritic (6) suggesting DETC ligand expression is spatially restricted to sites of injury. To address this, wounded epidermis was stained with DETC tetramers. Strikingly, 2 h after wounding, punctate DETC tetramer binding was seen only on keratinocytes near the wound edge (Fig. 2), while those located distal to the wound site did not bind DETC tetramers (Fig. 2). No staining was found with G8 tetramers proximal or distal to the wound site (Fig. 2). To address the specificity of DETC tetramer binding in situ, binding to damaged intestinal epithelial cells was assessed. No tetramer binding was evident in undamaged large intestine. Moreover, chemical induction of colitis did not result in upregulation of ligands for the DETC TCR (dns).
Fig. 2. Keratinocytes express DETC TCR ligands at sites of tissue damage.
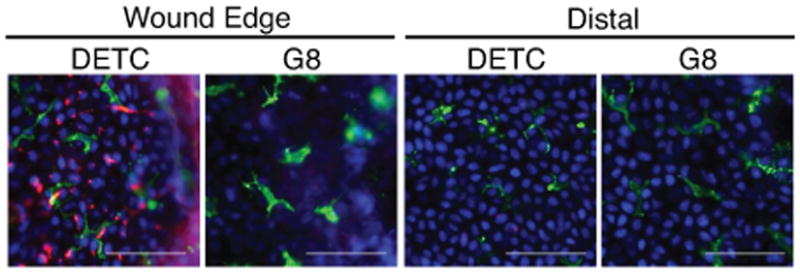
Epidermal sheets isolated from wounded B6 mice stained with DAPI (blue), anti-CD3 (green) and either DETC or G8 tetramer (red). Wound edges delineated by the white dashed line. Scale bar represents 50 μm. Data are representative of ≥3 experiments.
Visualization of Ag expression only at the epidermal wound perimeter correlates well with prior work in which only DETC bordering the wound edge are activated and secrete growth factors that contribute to proper wound healing (6). Activated DETC produce growth factors and cytokines 18 h following tissue damage (6), at which time DETC proximal to the wound edge exhibit a round morphology. At 2 h post wounding, DETC near the wound edge were not fully activated and displayed a largely dendritic morphology (Fig. 2). While it appeared that Ag expression by keratinocytes occurs prior to DETC activation, it is unknown how long Ag expression is maintained and how rapidly DETC are activated.
Keratinocytes rapidly and transiently express DETC TCR ligands
To address the kinetics of Ag expression and the early stages of DETC activation, changes in DETC morphology (Fig. 3A) and keratinocyte binding of DETC tetramers (Fig. 3B-F) were assessed at several times following wounding. Non-wounded epidermis contained highly dendritic (>2 dendrites) DETC (Fig. 3A) and negligible DETC tetramer binding (Fig. 3B), indicating keratinocytes do not constitutively express DETC TCR ligands under homeostatic conditions. 1 h post-wounding, DETC retained a dendritic morphology (Fig. 3A and C) and DETC tetramer binding localized to epithelial cells directly bordering sites of injury (Fig. 3C and G). 2 h after wounding, DETC tetramer binding was detected on keratinocytes within 443.5 ± 102.7 μm of the wound edge (Fig. 3 D and G). At this time, DETC were becoming activated as suggested by the partial retraction of dendrites (Fig. 3 A and D). By 3 h post-wounding, DETC tetramer binding to keratinocytes was reduced (Fig. 3 E and G), and DETC were activated, as indicated by a rounded morphology (Fig. 3 A and E). Only low levels of DETC tetramer binding were observed 19 h after wounding (Fig. 3 F and G), while DETC remained activated and round (Fig. 3 A and F). DETC tetramers did not bind to keratinocytes distal to wound edges at any time points (dns) and control G8 tetramers did not bind proximal or distal to wound sites at any time points (dns). Together, this data suggests that epidermal damage induces local, rapid upregulation of DETC TCR ligands on keratinocytes before DETC activation, and subsequent rapid down-modulation after T cell activation. This rapid and transient ligand expression contrasts the sustained expression of αβ T cell Ags on APC (15, 16). These findings further highlight the role of DETC as rapid responders to injury. Such a rapid response by DETC is supported by the constitutive maintenance of an “activated yet resting” state similar to that seen for NKT cells and marginal zone B cells (17).
Fig. 3. DETC TCR Ag is up-regulated prior to DETC activation, and down-modulated following DETC activation.
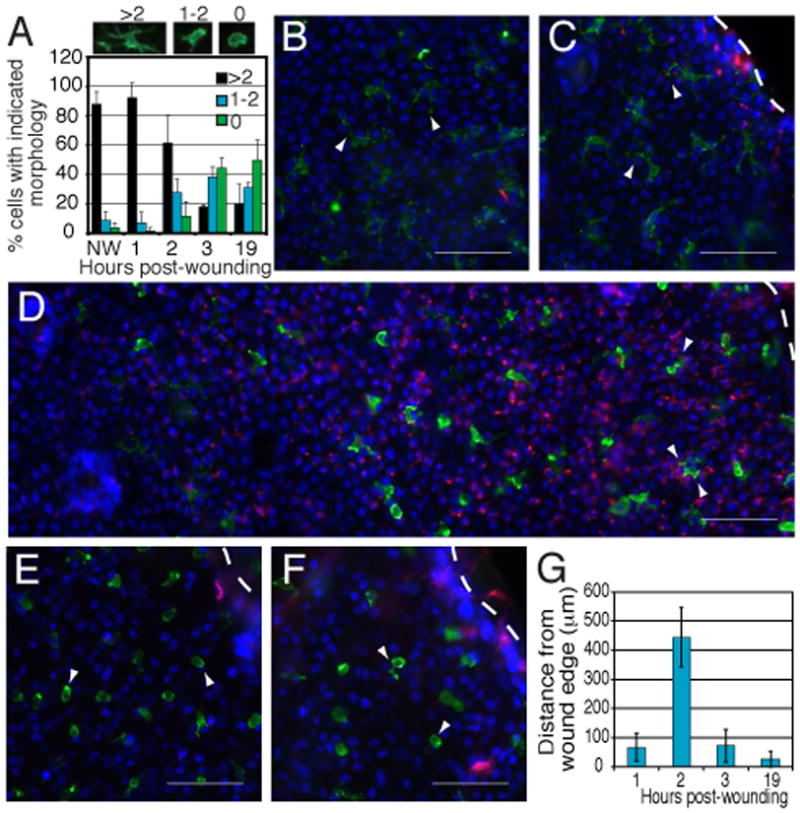
Epidermal sheets were isolated and stained with DAPI (blue), anti-CD3 (green), and DETC TCR tetramers (red) at multiple times following wounding. (A) Morphology of DETC (>2, 1-2, or 0 dendrites) within 300 μm of the wound edge was assessed at each time point. A minimum of 225 cells from ≥3 experiments were counted for each time point. (B) Epidermal sheets from non-wounded skin. Staining of wounded epidermal sheets was observed 1 h (C), 2 h (D), 3 h (E), or 19 h (F) post-wounding. (C-F) Images were acquired at the wound edge, delineated by white dashed lines, and DETC morphology indicated by arrowheads. Scale bar represents 50 μm. (G) Distance of DETC tetramer staining extending from the wound edge was measured at each of the observed time points. Error bars represent SD. Data are representative of ≥3 experiments.
Vγ3Vδ1 deficient mice express DETC TCR Ags
Mice lacking Vγ3Vδ1 DETC have impaired skin homeostasis (18) and barrier function (5, 19) suggestive of an altered epidermal environment under chronic stress that could lead to aberrant keratinocyte expression of DETC Ags. To address this, Ag expression in resting and wounded skin (2 h post-wounding) from animals lacking Vγ3Vδ1 DETC was examined.
TCRδ-/- mice lack all γδ T cells, but have a resident population of αβ T cells in the epidermis (11). FVBTac mice are specifically deficient for Vγ3Vδ1 DETC and have epidermal γδ T cells bearing other Vγ and Vδ chain pairs (19). Despite having altered epidermal homeostasis (18, 19), keratinocytes from TCRδ-/- and FVBTac mice did not express DETC TCR ligands in non-wounded tissue or distal to wound sites (Supplemental Fig. 2). Furthermore, DETC tetramer binding was evident along wound edges indicating TCRδ-/- and FVBTac mice both upregulated ligand on keratinocytes near wound edges similar to wild-type animals (Supplemental Fig. 2). Neither TCRδ-/- nor FVBTac epidermis expressed G8 TCR ligands under these conditions (dns). These findings indicate the absence of Vγ3Vδ1 DETC does not affect Ag expression by keratinocytes under homeostatic conditions or following wounding. Thus, the expression of Vγ3Vδ1 TCR Ags by keratinocytes is a response to physical wounding, and is not induced by the dysregulated keratinocyte environment observed in TCRδ-/- and FVBTac mice (18, 19). The absence of Vγ3Vδ1 DETC in the FVBTac animals is due to a mutation in skint1 (20) required for positive selection of Vγ3Vδ1 DETC (19-21). Although these results raised the possibility that Skint1 may be a ligand for the Vγ3Vδ1 TCR (19), the detection of DETC ligand expression around the wound edges in FVBTac mice demonstrates that DETC TCR binds molecules other than Skint1. In addition, high resolution confocal microscopy of DETC tetramer staining on keratinocytes demonstrates that DETC TCR ligand can be detected at the cell surface (Fig. 4) in contrast to Skint1 (22), further supporting the notion that the DETC TCR ligand expressed on the surface of keratinocytes is not Skint1.
Fig. 4. DETC tetramers bind the cell surface of keratinocytes.
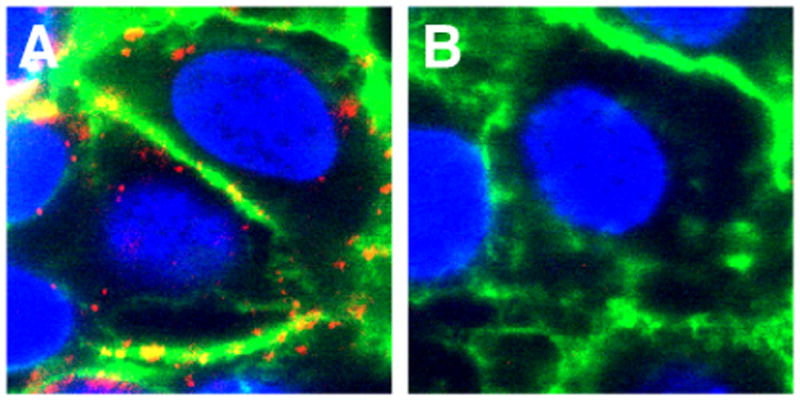
PAM 212 keratinocytes were stained with DETC (A) or G8 (B) tetramers (red), anti-CD29 (green) and DAPI (blue). Data is representative of >3 experiments.
DETC TCR ligands are expressed in the fetal thymus
DETC develop in the fetal thymus between E13-E18 (23). Recent studies suggested that DETC undergo positive selection during development (19, 22) raising the question of whether DETC TCR ligands are expressed by thymic epithelial cells (TEC). Indeed, DETC tetramers bind to E13.5 fetal, but not to adult, TEC (Fig. 5). This staining is TCR-specific as it is blocked by preincubation of the DETC tetramers with an anti-Vγ3 mAb (Fig. 5). These findings provide further support for TCR-based positive selection of DETC during fetal development. As Skint1 is required for DETC development (19, 20, 22), the relationship between DETC TCR ligands and members of the Skint family of molecules requires further investigation.
Fig. 5. DETC tetramers bind E13.5 fetal, but not adult, thymic epithelial cells.
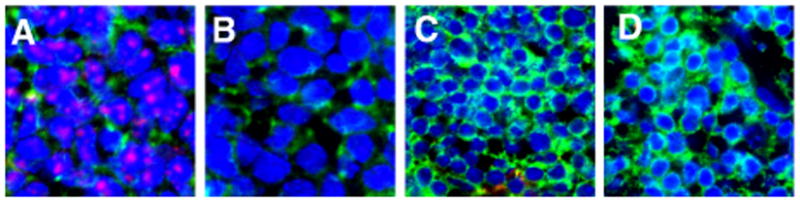
Thymus from E13.5 (A, B) or 5 week old (C, D) animals stained with either DETC (A, C) or anti-Vγ3 blocked DETC (B, D) tetramer (red), anti-CD3 (green), and counterstained with DAPI (blue). Sections were visualized by confocal microscopy. Data are representative of 3 experiments.
DETC tetramers inhibit in vivo wound closure
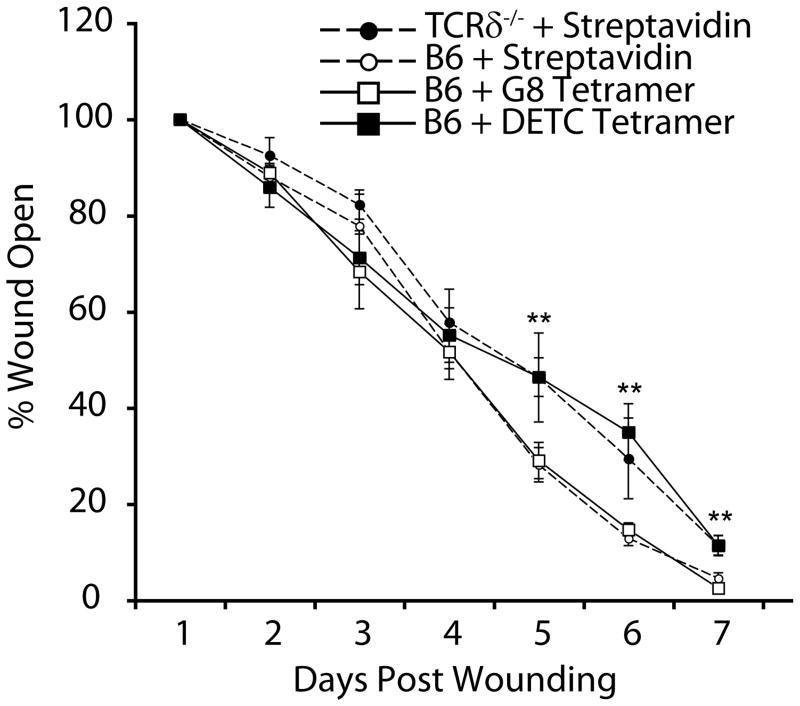
Wound healing is a complex process involving coordination of inflammation, reepithelialization and tissue remodeling (1). Disruption of cell function at any phase of wound repair can alter wound closure. In the absence of DETC, wound closure is delayed (6), indicating DETC participate in the wound repair process. Rapid upregulation of DETC TCR Ag by keratinocytes, and subsequent DETC activation, within hours of wounding places DETC function in the early phases of repair. Thus, we hypothesized that inhibition of DETC activation immediately following wounding would disrupt wound closure. DETC tetramers were applied to wounds in vivo to block TCR interactions with ligand and ensuing DETC activation. A single application of DETC tetramer to wounds delayed wound closure compared to controls (Fig. 6). This delayed wound repair correlates to the wound repair defect seen in TCRδ-/- mice (Fig. 6) (6). Thus, DETC tetramers bound to functional Ags expressed at wound edges and inhibited TCR-mediated DETC activation following injury. The effectiveness of a single dose of DETC tetramer at the time of wounding further demonstrates the importance of rapid TCR-mediated DETC activation in response to physical injury to promote proper wound healing and restoration of epidermal barrier integrity.
Fig. 6. DETC tetramers delay wound closure in vivo.
Full thickness wounds on B6 and TCRδ-/- mice were treated with DETC tetramers, G8 tetramers, or streptavidin and wound closure monitored over time. p values were determined by student t-test, and error bars represent SEM. **p<0.01. Data shown represent the mean of ≥6 wounds from 3 mice per treatment and are representative of 2 experiments.
In summary, creation of a DETC tetramer has provided novel insight into the keratinocyte response to epidermal damage. The rapid and transient expression of DETC Ags by keratinocytes is in sharp contrast to the later and more sustained expression of αβ T cell Ags. Again, it is important to point out the differences in Ag recognition requirements between a polyclonal αβ TCR population and a monoclonal γδ TCR population. An αβ T cell specific for a particular pMHC complex must seek out and recognize the APC expressing as few as 10 pMHC complexes amongst many APC in a secondary lymphoid organ (24). Recognition of pMHC complexes involves transient interaction between T cells and APC lasting 0.5-8 h, followed by a period of stable interactions lasting ∼12 h, and then a return to transient T cell-APC interactions (24). As processing and presentation of peptides in MHC molecules on the cell surface takes 1-3 h (25), full activation of αβ T cells may not occur until ≥12 h after Ag uptake. As DETC express a monoclonal TCR and respond to a ligand expressed by many keratinocytes along wound edges, the process of Ag recognition is much simpler than that for polyclonal αβ T cells, occurring within 3 h of wounding. The constitutive, partially activated phenotype of DETC is consistent with their ability to function as rapid responders to damage or disease in epithelial tissues.
Characterization of the spatial and temporal expression of the DETC TCR ligand, and the ability to bind ligands with the DETC sTCR, opens possibilities for a variety of approaches to identify this enigmatic ligand. As DETC are a prototypic intraepithelial γδ T cell population, characterization of DETC TCR ligands may set a paradigm that will enable identification of other epithelial γδ T cell ligands. Such γδ T cell populations monitor some of the largest organs for damage and transformation (26), and are implicated in multiple diseases (1, 26). Enhanced understanding of γδ T cell activation will enrich development of therapies based upon the protective qualities of these cells. In particular, insight into DETC activation will benefit those suffering from debilitating, chronic, non-healing wounds such as burn victims and diabetics.
Supplementary Material
Acknowledgments
We thank O. Garijo for technical assistance, K. Taylor and A. Toulon for critical reading of the manuscript, S. Hedrick for the T22 construct, P. Verdino and I. Wilson for the G8 TCR template, and J. Lewis and R. Tigelaar for the 17D1 hybridoma.
Supported by the National Institutes of Health (AI064811 and AI36964 to W.L.H, T32AI007244 to R.K., A142267 to L.T.), and a National Science Foundation Graduate Research Fellowship to H.K.K. This is manuscript # 20100 from The Scripps Research Institute.
References
- 1.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–397. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- 3.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 4.Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girardi M, Lewis JM, Filler RB, Hayday AC, Tigelaar RE. Environmentally responsive and reversible regulation of epidermal barrier function by γδ T cells. J Invest Dermatol. 2006;126:808–814. doi: 10.1038/sj.jid.5700120. [DOI] [PubMed] [Google Scholar]
- 6.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 7.Cantu C, 3rd, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of α-galactosylceramide: low affinity, low specificity for CD1d, high affinity for αβ TCRs. J Immunol. 2003;170:4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 8.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. A population of murine γδ T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 9.McLachlin JR, Miller LK. Stable transformation of insect cells to coexpress a rapidly selectable marker gene and an inhibitor of apoptosis. In Vitro Cell Dev Biol Anim. 1997;33:575–579. doi: 10.1007/s11626-997-0101-7. [DOI] [PubMed] [Google Scholar]
- 10.Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science. 1998;279:1729–1733. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 11.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 12.Garcia KC, Tallquist MD, Pease LR, Brunmark A, Scott CA, Degano M, Stura EA, Peterson PA, Wilson IA, Teyton L. αβ T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci U S A. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CA, Garcia KC, Carbone FR, Wilson IA, Teyton L. Role of chain pairing for the production of functional soluble IA major histocompatibility complex class II molecules. J Exp Med. 1996;183:2087–2095. doi: 10.1084/jem.183.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz PJ. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Biotechnology (N Y) 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- 15.Su RC, Miller RG. Stability of surface H-2K(b), H-2D(b), and peptide-receptive H-2K(b) on splenocytes. J Immunol. 2001;167:4869–4877. doi: 10.4049/jimmunol.167.9.4869. [DOI] [PubMed] [Google Scholar]
- 16.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 17.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 20.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 24.Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 26.Nanno M, Shiohara T, Yamamoto H, Kawakami K, Ishikawa H. γδ T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.