Abstract
Adoptively transferred tumor-specific T cells offer the potential for non–cross-resistant therapy and long-term immunoprotection. Strategies to enhance in vivo persistence of transferred T cells can lead to improved antitumor efficacy. However, the extrinsic (patient conditioning) and intrinsic (effector cell) factors contributing to long-term in vivo persistence are not well-defined. As a means to enhance persistence of infused T cells in vivo and limit toxicity, 11 patients with refractory, progressive metastatic melanoma received cyclophosphamide alone as conditioning before the infusion of peripheral blood mononuclear cell-derived, antigen-specific, CD8+ cytotoxic T-lymphocyte (CTL) clones followed by low-dose or high-dose IL-2. No life-threatening toxicities occurred with low-dose IL-2. Five of 10 evaluable patients had stable disease at 8 wk, and 1 of 11 had a complete remission that continued for longer than 3 y. On-target autoimmune events with the early appearance of skin rashes were observed in patients with stable disease or complete remission at 4 wk or longer. In vivo tracking revealed that the conditioning regimen provided a favorable milieu that enabled CTL proliferation early after transfer and localization to nonvascular compartments, such as skin and lymph nodes. CTL clones, on infusion, were characterized by an effector memory phenotype, and CTL that persisted long term acquired phenotypic and/or functional qualities of central memory type CTLs in vivo. The use of a T-cell product composed of a clonal population of antigen-specific CTLs afforded the opportunity to demonstrate phenotypic and/or functional conversion to a central memory type with the potential for sustained clinical benefit.
Keywords: adoptive transfer, immunotherapy
Strategies aimed at increasing the number and quality of autologous T cells targeting melanoma-associated antigens have been effective in reducing tumor burden in a limited number of patients, and clinical responses have been correlated with the duration of in vivo persistence of transferred T cells (1–4). Adoptive transfer of ex vivo-expanded melanoma-specific cytotoxic T-lymphocyte (CTL) clones can increase the in vivo frequency of melanoma-reactive T cells in a setting in which the elicited response can be tracked and the transferred cells characterized (5, 6). Improving the benefit of infused T cells has involved either extrinsic modulation of the host environment or intrinsic manipulation of the infused product to promote ultimate in vivo survival of T cells (7).
Nonmyeloablative chemotherapy conditioning regimens [high-dose (HD) cyclophosphamide (CY) and fludarabine] or ablative doses of total body irradiation (TBI; 1,200 rad) administered before T-cell infusions have been shown to facilitate the in vivo engraftment and expansion of adoptively transferred cells at the cost of serious and potentially life-threatening toxicities (8). We hypothesized that administration of HD CY given alone may induce a transient lymphopenia sufficient to allow engraftment and persistence of transferred T cells without incurring prolonged immunosuppression (8–10). Concurrent HD IL-2 (600,000–720,000 U/kg thrice daily) may contribute to increased toxicity (8), and although low-dose (LD) IL-2 (250,000 U/m2 s.c. twice daily) can enhance T-cell survival by several days, it is not sufficient to prolong the clinical efficacy of adoptively transferred cells significantly (3). We sought to determine in a phase I/II clinical trial whether HD CY (4 g/m2) followed by LD or HD IL-2 was sufficient to promote survival of melanoma-specific T-cell clones and induce measurable clinical responses.
Previous studies in mice and nonhuman primates have shown that infusion of T cells derived from a central memory (Tcm) population exhibit greater replicative potential in response to antigen and prolonged in vivo persistence compared with those derived from an effector memory (Tem) source (11, 12), and may likely be poised to eliminate tumor more effectively. However, it remains unclear if cytotoxic T cells can be established from a Tcm pool in patients with high tumor burden, wherein the majority of endogenous circulating T cells are likely Tem; thus, T-cell clones generated from these patients in vitro are likely to be Tem or naive T cell-derived. In this study, particular attention was given to the analysis of the phenotype and function of T-cell clones that had prolonged in vivo survival because studies in the nonhuman primate model demonstrated that after in vitro expansion, T cells derived from Tcm or Tem share a Tem phenotype but their behavior is ultimately representative of their source, reverting to their original phenotype in vivo (11).
Here, we show that conversion from a Tem to Tcm phenotype occurred in the setting of minimal, nonablative, lymphodepleting conditioning in two patients, wherein long-term in vivo persistence of transferred CTLs was observed. Furthermore, in this limited number study, HD CY alone accompanied by LD IL-2 postinfusion allowed for a transient expansion of the transferred cells, on-target autoimmune skin toxicity, disease stabilization in at least half of the patients at 8 wk, and a continuous complete response in one patient.
Results
Adoptive Transfer of Melanoma-Specific CTL Clones Preceded by HD CY and Followed by LD IL-2 Is Well-Tolerated and Safe.
Eleven patients with bulky metastatic melanoma who had failed previous therapy (Table 1) received CTL clones specific for Mart1/A2, Tyr/A2, gp100/A2, gp100/A3, and Tyr/B44. Characteristics of the infused melanoma-specific CTL clones are described in Fig. S1, Tables S1, S2, and the treatment plan in Fig. S2. Of the patients who received LD IL-2 following CY and T cells (cohort 1), all experienced transient (<96 h) grade 4 neutropenia, lymphopenia, and thrombocytopenia. Of the patients who received HD IL-2 (cohort 2), administration of IL-2 was interrupted after 13, 6, and 4 doses, respectively, because of toxicity. All experienced cytopenias (grade 4), neutropenic fever, rigors, tachycardia, and hypoxia. One patient had significant pulmonary infiltrates as a likely result of the cytokine release syndrome. All side effects, other than hematological toxicities, resolved within 48 h of the interruption of HD IL-2. After 3 patients had been treated, the protocol Data Safety Monitoring Board concluded the toxicities associated with HD IL-2 in this protocol were substantial and terminated recruitment in cohort 2. Although this study is underpowered to detect therapeutic differences between LD and HD IL-2, LD IL-2 administered following CY and CTL infusions is generally well-tolerated and safe, but HD IL-2 administered following the same regimen yielded unacceptable toxicities.
Table 1.
Patient characteristics
| Patient no. | Age, y, (gender) | Previous treatments | Disease site |
| LD IL-2 | |||
| 1 | 57 (M) | Surgery, radiation, HD IL-2, anti–CTLA-4 | Mesenteric LN, scapular, s.c. |
| 2 | 38 (F) | Surgery, radiation, carboplatin, and paclitaxel | Cervical, supraclavicular LN, chest wall, breast, pulmonary nodes |
| 3 | 65 (M) | Surgery, radiation, MEK inhibitor | Lung, mediastinal and mesenteric LN |
| 4* | 60 (M) | Ocular enucleation, surgery, Mel48 vaccine | Kidney, pancreas, liver, muscle |
| 5 | 68 (M) | Surgery, radiation, gamma-knife, temozolomide | Mediastinal LN, pulmonary nodes |
| 6 | 40 (F) | Surgery, radiation, anti–CTLA-4, peptide-based vaccine | Mediastinal, supraclavicular, mammary chain, periportal, portacaval nodes. |
| 7 | 61 (F) | Surgery, radiation, bevacizumab, paclitaxel | Right and left kidneys, adrenal, liver |
| 8 | 50 (M) | Surgery, radiation, IFN-α, HD IL-2, paclitaxel | Pulmonary, inguinal, s.c. |
| HD IL-2 | |||
| 9 | 75 (M) | HD IL-2 | Liver, peritoneum, bone |
| 10* | 35 (M) | Brachytherapy, lipiodol, 90Y microsphere liver embolization | Liver, mesenteric LN, sacrum |
| 11* | 55 (M) | Surgery, radiation, cisplatin, vinblastine, dacarbazine, IFN-α HD IL-2 | Liver, mesenteric LN, sacrum |
F, female; LN, lymph node; M, male.
*Choroidal melanoma.
Assessment of Clinical Responses.
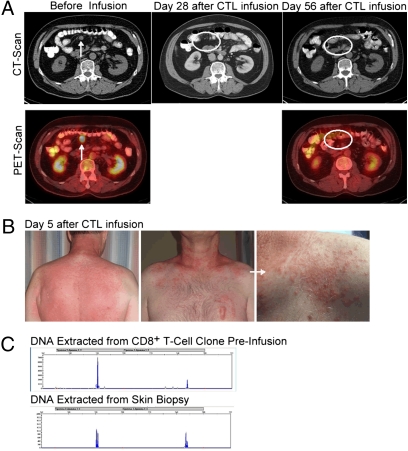
Clinical response to treatment was not the primary end point of this study because of the small number of patients enrolled. However, 1 of 10 evaluable patients achieved confirmed complete remission (CR) and 5 of 10 patients experienced stable disease (SD) at 8 wk (Table 2). Patient 1 presented with a sizable abdominal lymph node that regressed entirely by 4 wk (Fig. 1A), and the patient remains in confirmed CR >36 mo after CTL infusion without further intervention. In patient 6, there was a 42% decrease in all target lesions 19 wk after infusion (a growing paraspinal lesion was surgically removed before imaging was performed). Except for patient 1 (CR), all patients eventually progressed by 12–19 wk. Patients who experienced stabilization or regression of disease ≥4 wk after infusions experienced skin rashes within 7 d of CTL infusion. Patient 1's rash (Fig. 1B) was biopsied at day 5, and the T-cell receptor-γ (TCR-γ) rearrangement found in DNA extracted from the skin biopsy was identical to the infused CTL clone (Fig. 1C), demonstrating that infused CTLs were present in the skin and were the likely cause of the inflammatory rash. Although not all rashes were biopsied, these results suggest that infused CTLs have the potential to migrate to the skin consistent with our previous findings (13).
Table 2.
Assessment of clinical response
| On study |
Follow-up |
|||||||||
| Patient no. | Cohort | T-cell specificity | HLA type | 4 wk | 8 wk | 12 wk | 16 wk | 19 wk | 24 wk | Rash |
| 1 | 1 | Mart1 | A2 | CR (−100%) | CR (−100%) | CR (−100%) | CR (−100%) | Y | ||
| 2 | 1 | Tyr | B44 | ND | SD (−13%) | PD (NT) | PD (new) | Y | ||
| 3 | 1 | gp100 | A2 | SD (+0.6%) | ND* | PD* (+52%) | Y | |||
| 4 | 1 | gp100 | A3 | SD (+0.6%) | SD (+8%) | PD (+24%) | Y | |||
| 5 | 1 | Tyr | A2 | SD (+10%) | PD (new) | Y | ||||
| 6 | 1 | Tyr | A2 | SD (−7%) | SD (−2%) | NE† | Y | |||
| 7 | 1 | Tyr | B44 | SD (+6.3%) | PD (+28%) | PD (+61%) | Y | |||
| 8 | 1 | Mart1 | A2 | PD (+37%) | PD (+47%) | PD (+64%) | N | |||
| 9 | 2 | Mart1 | A2 | SD (+13%) | SD (+15%) | PD (new) | Y | |||
| 10 | 2 | Mart1 | A2 | SD (−9.1%) | SD (−4.6%) | SD (+9%) | PD (+21%) | Y | ||
| 11 | 2 | Mart1 | A2 | PD (+36%) | PD (+31%) | PD (+51%) | N | |||
N, no; ND, not done; new, new metastasis; NT, nontarget lesion progression; Y, yes.
*Patient initiated alternate treatment modality.
†Not evaluable: 42% reduction in all lesions except growing paraspinal mass surgically removed before scans.
Fig. 1.
Complete response associated with a skin rash infiltrated with the adoptively transferred CTL clone. (A) Abdominal computed tomography (CT) (Upper) and PET scan combined with low-resolution noncontrast CT (Lower) before infusion (Left) and 28 d (Center) and 56 d after infusion (Right). Arrows indicate radiological image and active fluorodeoxyglucose (FDG) uptake before treatment, and circled areas show absence of detectable image and FDG uptake. (B) Back (Left), front chest (Center), and close-up of the front chest lesion (Right) of patient 1's rash 5 d after CTL infusion. (C) Superposition of TCR-γ length analysis of DNA extracted from the CTL clone preinfusion (Upper) and paraffin-embedded skin biopsy (Lower).
Melanoma-Specific CTL Clones Isolated from Patients with Metastatic Melanoma Can Persist in Vivo Following Transfer.
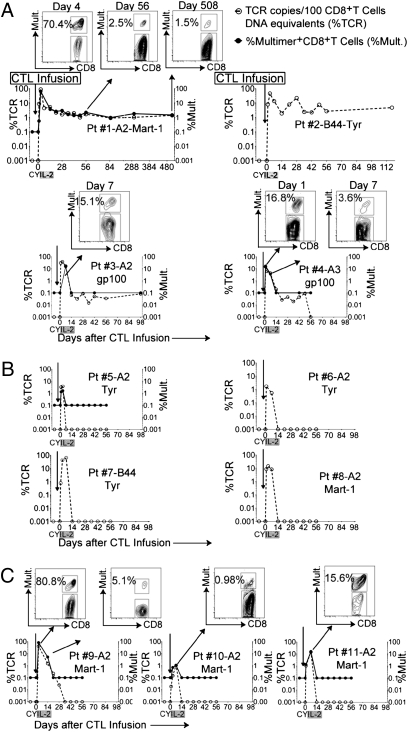
Tracking of the infused cells in vivo was performed by clone-specific TCR Vβ quantitative PCR and, where possible, corresponding multimer stains (3) (Fig. 2). These methods have different limits of detection (1 of 105 cells for quantitative TCR-specific PCR and 0.1% for multimer stains), and although they have previously been individually used to quantitate in vivo frequencies of infused CTL clones (1, 14), they were compared for patient 1 (Fig. 2A). The frequency of multimer-positive CTLs reached 70.4%, 2.2%, and 1.5% compared with 86.9%, 2.6%, and 1.4% by TCR Vβ-specific PCR at days 4, 56, and 508, respectively, thus yielding near-coincident results for frequencies ≥0.1% of CD8+ T cells and validating the use of either method for tracking purposes. Patient 2 was infused with an HLA-B44/Tyr CTL clone for which an MHC multimer could not be synthesized. Clone frequency by TCR Vβ-specific PCR revealed frequencies of 2.6% and 4.8% at days 56 and 117, respectively. Clones for Patients 3 and 4 persisted at levels below multimer but above quantitative PCR detection levels for 49 d and ≥98 d, respectively. Overall, for patients in cohort 1, persistence beyond 42 d was observed in 4 of 8 patients (Fig. 2 A and B). Infused CTL could not be detected beyond 28 d in cohort 2 (Fig. 2C). The frequency of specific CTL reached peak levels at 7 ± 3 d (range: 0.49–95%, mean of 23.8% for cohort 1; range: 0.98–80%, mean of 32.5% for cohort 2). No correlation could be established between the phenotype, avidity of infused clones to cognate antigen (Fig. S1), and in vivo persistence or clinical response.
Fig. 2.
In vivo persistence of melanoma-specific CTL clones. (A) TCR copies per 100 CD8+ T-cell DNA equivalents (Left, x axis) and percentage of multimer-positive (%Mult.) CD8+ T cells (Right, x axis) in PBMCs collected 7 d (±2 d) before and after infusions for four of eight patients in cohort 1 whose clones showed persistence (≥48 d). Inset dot plots above the graph for patient 1 show percentages of multimer-positive CD8+ T cells at days 4, 56, and 508. (B) Same analysis performed for four of eight patients in cohort 1 whose clones demonstrated persistence for <7 d. (C) Plots for three patients in cohort 2. Inset dot plots show the percentages of multimer-positive CD8+ T cells in PBMCs at the time point peak frequencies observed. Pt, patient.
CTL Clones That Persist Sustain Function in Peripheral Blood and Tissues.
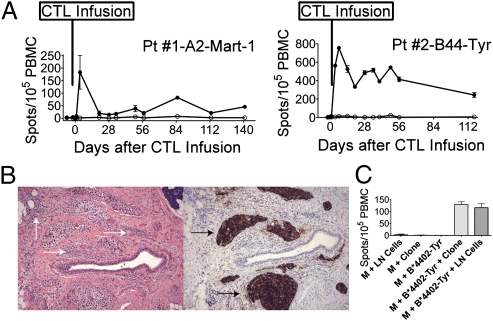
Enzyme-linked immunospot (ELISpot) assays were performed by stimulating whole peripheral blood mononuclear cells (PBMCs) obtained before and after infusions with patient-specific peptide-pulsed autologous monocytes (Table S1). IFN-γ was detected (Fig. 3A) at the same time points the infused clones reached frequencies >0.1% in vivo by multimer stains (patient 1) and/or TCR PCR (patients 1 and 2) (Fig. 2A). Patient 2 developed enlargement of a lymph node in the neck 51 d after T-cell infusion. The node was excised for diagnostic purposes. Tumor microinvasion with an associated lymphocytic infiltrate was identified within connective tissue (Fig. 3B, Left), and expression of tyrosinase was confirmed on the melanoma tumor cells (Fig. 3B, Right). Specific IFN-γ secretion in response to peptide-pulsed autologous monocytes was detected by ELISpot in fresh cells isolated from the lymph node (Fig. 3C). Taken together, this suggests the infused clones from patients 1 and 2 had the capacity to extravasate from peripheral blood and to sustain functional capacity in vivo in peripheral tissue, including skin (patient 1) and tumor-infiltrated lymph nodes (patient 2).
Fig. 3.
Adoptively transferred melanoma-specific CTL clones show sustained functional capacity in peripheral blood and tumor-infiltrated lymph nodes. (A) IFN-γ spot-forming cells per 105 PBMCs for patient (Pt) 1 (Left) and patient 2 (Right) after stimulation with unpulsed (empty circles) or HLA-A*0201-Mart1- or HLA-B*4402-tyrosinase-pulsed monocytes (filled circles), respectively, over time (y axis). (B) Stain of a cervical lymph node from patient 2 51 d after infusion stained with H&E (Left) and tyrosinase (Right). White arrows indicate lymphocyte infiltrates, and black arrows indicate invasive tyrosinase-positive melanoma. Images were collected with an objective with a magnification of 10×. (C) IFN-γ spot-forming cells per 105 cells (y axis). (Left to Right) Cells isolated from patient 2's infiltrated lymph node (LN) with unpulsed autologous monocytes (M); unpulsed M and infused clone cells; HLA-B*4402-tyrosinase-pulsed M only (negative controls); HLA-B*4402-tyrosinase-pulsed M and infused clone (positive control); and HLA-B*4402-tyrosinase-pulsed M and LN.
Transferred CTLs Acquire Phenotypic Characteristics of Tcm in Vivo with Complete Tumor Regression.
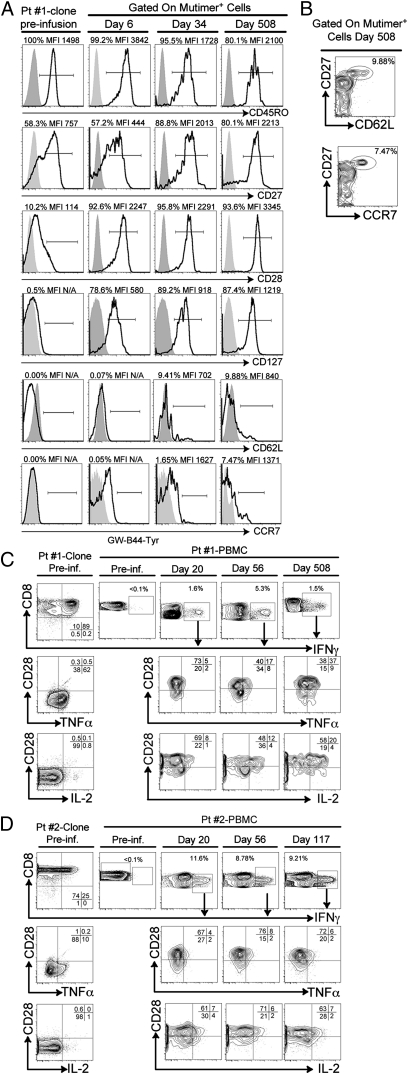
Immediately before infusion, patient 1's clone expressed CD45RO (100%), moderate levels of CD27 [53.8%, median fluorescence intensity (MFI) = 757], but relatively low or absent levels of CD28 (10.2%, MFI = 114), CD127 (0.5%), CD62L (0.0%), and CCR7 (0.0%) (Fig. 4A, Left). MFI was not calculated for markers with a surface expression of ≤1% compared with isotype controls. However, the phenotype of the infused clone based on the gating of multimer-positive CD8+ T cells at days 6, 34, and 508 demonstrated that the infused clone maintained CD45RO; further accumulated expression of CD27 consistent with previous studies (15) (Fig. 4A, Upper); and, additionally, accumulated expression of CD28, CD127, CD62L, and CCR7 in vivo over time to reach 93.6%, 87.4%, 9.88%, and 7.47%, respectively, at day 508 (Fig. 4A, Lower). CD62L- and CCR7-expressing cells were comprised within a CD27hi subset of the infused CTLs (Fig. 4B). A similar analysis could not be performed for patient 2 because no multimer was available (Table S1). Although an expansion of an endogenous CTL contributing to the Tcm phenotype observed cannot formally be excluded, concurrent detection of the clone-specific TCR renders this alternative unlikely and suggests that a subset of infused cells for patient 1 acquired a Tcm phenotype in vivo.
Fig. 4.
Adoptively transferred melanoma-specific CTLs show phenotypic and functional characteristics shared with CD8+ Tcm in vivo. (A) Patient (Pt) 1: Expression of CD45RO, CD27, CD28, CD127 CD62L, and CCR7 (bold lines) on the HLA*0201-Mart1-specific CTL clone preinfusion (Pre-inf.; Left), and on days 6, 34, and 508 after transfer (Right). MFIs are shown for surface markers with >1% positivity compared with isotype controls (gray areas). (B) Expression of CD27 (y axis), CD62L (x axis) (Upper), and CCR7 (Lower) gated on multimer-positive CTLs 508 d after transfer. Expression of CD28; secretion of TNF-α and IL-2 within IFN-γ+ cells on infused clones; and PBMCs preinfusion and on days 20, 56, and 508 (C; patient 1) and days 20, 56, and 117 (D; patient 2).
CTL Clones That Persist Express Functional Characteristics Shared with Tcm in Vivo.
Further determination of the functional profile of both infused CTL clones that persisted and remained detectable by multimer in the peripheral blood could be assessed by gating on IFN-γ+–reactive cells. Clones for patient 1 (CR) and patient 2 (progressive disease by 19 wk) secreted IFN-γ and variable TNF-α but no IL-2 in response to peptide-pulsed, HLA-matched, B-lymphoblastoid cell lines, and expressed little or no CD28 (Fig. 4 C and D, Left). Consistent with a low frequency of melanoma-reactive cells, IFN-γ secretion was below detection levels before infusions (0.1%) (Fig. 4 C and D, second column). However, CD8+ IFN-γ+–reactive cells obtained on days 20, 56, and 508 (patient 1) or 117 (patient 2) after CTL infusion demonstrated acquisition of a polyfunctional phenotype characterized by concurrent expression of IFN-γ, TNF-α, and IL-2 (Fig. 4 C and D, Right). IL-2–secreting cells were contained within the CD28+ fraction of antigen-reactive IFN-γ+ cells (Fig. 4 C and D, Lower), compatible with the associated functional capacity of CD28+ cells to secrete IL-2 in response to cognate antigen (16, 17). Together, these data suggest that on the basis of both functional (patients 1 and 2) and phenotypic (patient 1) criteria, infused cells that persisted long term expressed features associated with CD8+ Tcm. No evidence of persistence by multimer analysis or antigen-specific IFN-γ secretion was observed in the peripheral blood of the remaining patients.
CY Conditioning Followed by Exogenous IL-2 Fosters a Favorable Environment for Replication of Infused CTLs.
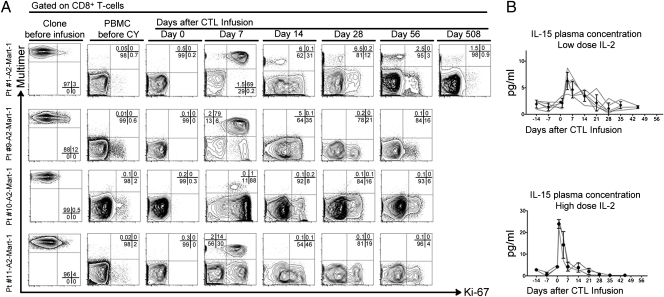
Because cells that possess the potential to divide or replicate are likely better equipped to persist, we investigated whether infused CTL clones expressed Ki-67 in vivo (18). Before infusion, clones exhibited a resting state (3–12% Ki-67+) for the most part. However, a majority of infused multimer-positive CTLs (>90%) entered the cell cycle early after transfer (day 7). By day 14, the number of detectable CTLs in the peripheral blood contracted and either persisted in a Ki-67− quiescent state (patient 1) or had disappeared from peripheral blood (patients 9, 10, and 11) (Fig. 5A). Localization of transferred CTLs to nonvascular compartments (i.e., lymph node, tumor sites) cannot be excluded. Proliferation of infused cells was distinct from the recovery of endogenous CD8+ T cells after lymphodepletion because the peak percentage of replicating endogenous cells occurred later (day 14) and involved a lower fraction of total CD8+ T cells (mean of 51%). Plasma IL-15 levels (Fig. 5B, Upper) were increased in patients postinfusion. Significantly higher levels were observed in patients who had received HD IL-2 (Fig. 5B, Lower). However, there was no correlation between peak levels or cumulative levels (area under the curve) of IL-15 and clone persistence. Similarly, plasma levels of other cytokines tested in vivo (Fig. S3) did not correlate with clone persistence or survival. Regulatory T-cell (T-reg) dynamics were also monitored because murine and human studies suggested a role for CY in reducing the circulating T-reg populations (19, 20). After a transient decrease in absolute numbers of T-regs coinciding with lymphodepletion induced by CY, both percentages and absolute numbers of T-regs peaked 14 d after infusions; they then declined and reached pretreatment levels at 21 and 49 d, respectively (Fig. S4 A and B). Ki-67 expression by T-regs was significantly higher before and after treatment compared with CD4+ T-effector cells, suggesting a replicative advantage of T-regs compared with CD4+ T-effector cells (Fig. S4C). Overall, we observed that CY lymphodepletion followed by LD or HD IL-2 fostered an environment that enabled infused cells to proliferate early after transfer. However, neither early proliferation detected in the peripheral blood or plasma IL-15 nor the dynamics of T-regs translated into long-term peripheral in vivo persistence.
Fig. 5.
CY conditioning followed by exogenous IL-2 fosters a favorable environment for replication of infused CTLs. (A) Intranuclear Ki-67 expression on clones (Left) and CD8+ multimer-positive or -negative cells within PBMCs of patients 1, 9, 10, and 11 (rows) before CY and on days 0, 7, 14, 28, 56, and 508 (patient 1 only) after T-cell infusion (columns). Multimer (y axis) and Ki-67 (x axis). (B) IL-15 plasma levels (pg/mL) (y axis) plotted over time (x axis). Gray lines represent individual patients, and filled circles show mean and SD. Patients in cohort 1 (Upper) and cohort 2 (Lower).
Discussion
The optimal host conditions and effector cells favoring the establishment of a long-lived, transferred, tumor-specific population have not been determined. In this study, we set out to evaluate prospectively the effects of HD CY followed by LD or HD IL-2 on the persistence of transferred, antigen-specific CTLs in patients with progressive metastatic melanoma. Adoptive therapy using antigen-specific CTL clones for patients receiving HD CY conditioning alone followed by LD s.c. IL-2 is well-tolerated, nontoxic, and sufficient to render a favorable milieu enabling infused T cells to proliferate early after transfer. CTL persistence beyond 42 d was observed in 4 of 11 patients, of whom 2 presented with prolonged T-cell persistence and establishment of an antigen-specific Tcm clonal T-cell population, with 1 of these 2 patients experiencing objective sustained CR.
Evidence supporting the critical role of Tcm comes from murine lymphocytic choriomeningitis virus infection models, where protective immunity provided by Tcm was more robust compared with Tem after in vivo challenge. The greater effectiveness of Tcm in this setting likely reflects the distinct ability of Tcm to secrete and use IL-2 as an autocrine growth factor, rendering these cells “helper-independent” (12). Furthermore, studies in nonhuman primates have demonstrated that cells derived from Tcm, differentiated to Tem ex vivo, can reacquire inherent features shared with Tcm in vivo (11). In this study, analysis of the transferred CTLs in two patients (patients 1 and 2) demonstrated long-term persistence, up-regulation of phenotypic markers associated with Tcm, and/or acquisition of helper independence (antigen-driven IL-2 production), suggesting that these melanoma-specific CTL clones may have originated from a Tcm population. Although the infused CTLs were not genetically marked, the established specific Vβ clonal identity argues almost unequivocally in favor of phenotypic reexpression of markers shared with Tcm and against the outgrowth of a Tcm subset in the infused population as might be conceivable when infusing polyclonal T cells. For patient 1, CTL persistence coincided with tumor regression by 4 wk and continuous remission for 3+ years. Patient 2 demonstrated extended in vivo persistence of the transferred CTLs but eventually progressed by 19 wk.
Studies performed by the Surgery Branch at the National Cancer Institute (NCI) have also demonstrated the significant impact of lymphodepletion conditioning on in vivo persistence of adoptively transferred tumor-infiltrating lymphocytes (TILs); in these seminal studies, CR rates as high as 16% were observed using myeloablative regimens combining HD CY, fludarabine, and 12-Gy TBI, followed by HD IL-2 postinfusion (8, 9, 21). However, there are marked differences in the configuration and toxicity profile of these NCI trials: A surgical procedure was required to acquire TILs, TILs were propagated in vitro with high doses of IL-2, and the regimens led to acute morbidity and potentially life-threatening toxicities, thereby limiting the pool of eligible patients. In the present study, preinfusion conditioning with a regimen of HD CY alone when used in conjunction with postinfusion LD IL-2 is safe and well-tolerated and represents a clinically feasible alternative with a broader patient eligibility profile. HD CY alone achieved a transient lymphodepleted condition that induced measurable circulating levels of the “prosurvival” cytokine IL-15 consistent with previous studies (8, 10) and enabled transferred cells to undergo early cell division as demonstrated by Ki-67 expression (22). On-target toxicities (skin rashes) appeared within 1 wk of infusion in patients who achieved SD or CR at ≥4 wk, suggesting that the environment fostered by HD CY enabled transferred T cells to reach peripheral tissue. In our study, the administration of HD IL-2 after T-cell infusion yielded unacceptable toxicities and added uncertain benefit.
The use of CTL clones in this study provided very strong evidence that transferred CTLs that persist in vivo (a reported predictor of response) acquire characteristics of Tcm. In contrast to a previous study using a transferred polyclonal T-cell product, the use of CTL clones eliminates the possibility of an outgrowth of a preexisting Tcm subpopulation (4). However, the selection of persistent T-cell clones used for patients 1 and 2 was stochastic. A means to generate Tcm-like effector cells for adoptive therapy prospectively would be desirable. More precise control over a T-cell product with a Tcm phenotype may be achieved through the use of in vitro cytokine modulation (23, 24), Tcm selection and TCR gene transfer, or infusion of a polyclonal T-cell population with an increased statistical probability of comprising a Tcm population. Such Tcm-derived effectors become highly responsive to homeostatic cytokines, such as IL-15, which is up-regulated in vivo following CY lymphodepletion. In future studies, it is conceivable that the combination of adoptively transferred Tcm effectors, together with a source of LD exogenous cytokines, may eliminate the requirement for a conditioning regimen altogether while providing long-term antitumor control.
Methods
All clinical investigations were conducted according to the Declaration of Helsinki principles. Protocol 2140 was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board and the Food and Drug Administration. All patients provided written informed consent, had metastatic melanoma (Table 1), and had an historical estimated survival of less than 40% at 1 y (25).
Detailed descriptions of the materials and methods used, including selection of targets, treatment plan, cytotoxicity assays, T-cell tracking assays, and flow cytometry, are provided in SI Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Cancer Institute Grant R21 CA128283. A.G.C. was supported by the Oncology Fellowship T32 Grant of the University of Washington Medical Center. C.Y. is supported by a Burroughs Wellcome Fund Translational Scientist Award TS60637.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113748109/-/DCSupplemental.
References
- 1.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler MO, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011 doi: 10.1126/scitranslmed.3002207. 3:80ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddell SR, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 6.Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009;21:224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallen H, et al. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS ONE. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 13.Yee C, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: Direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee C, Riddell SR, Greenberg PD. In vivo tracking of tumor-specific T cells. Curr Opin Immunol. 2001;13:141–146. doi: 10.1016/s0952-7915(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 15.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topp MS, et al. Restoration of CD28 expression in CD28- CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Michel L, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiringhelli F, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SH, et al. High-dose cyclophosphamide-mediated anti-tumor effects by the superior expansion of CD44(high) cells after their selective depletion. Immunobiology. 2010;215(3):182–193. doi: 10.1016/j.imbio.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 25.Balch CM, et al. New TNM melanoma staging system: Linking biology and natural history to clinical outcomes. Semin Surg Oncol. 2003;21(1):43–52. doi: 10.1002/ssu.10020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.