Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax oncoprotein actively shuttles between the nucleus, where it interacts with transcriptional and splicing regulatory proteins, and the cytoplasm, where it activates NF-κB. Posttranslational modifications of Tax such as ubiquitination regulate its subcellular localization and hence its function; however, the regulation of Tax trafficking and NF-κB activation by host factors is poorly understood. By screening a deubiquitinating (DUB) enzyme small interfering RNA (siRNA) library, we identified the metalloprotease STAM-binding protein-like 1 (STAMBPL1) as a positive regulator of Tax-mediated NF-κB activation. Overexpression of wild-type STAMBPL1, but not a catalytically inactive mutant, enhanced Tax-mediated NF-κB activation, whereas silencing of STAMBPL1 with siRNA impaired Tax activation of both the canonical and noncanonical NF-κB signaling pathways. STAMBPL1 regulated Tax-induced NF-κB signaling indirectly by controlling Tax nuclear/cytoplasmic transport and was required for DNA damage-induced Tax nuclear export. Together, these results reveal that the deubiquitinase STAMBPL1 is a key regulator of Tax trafficking and function.
INTRODUCTION
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) and the neuroinflammatory disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (15, 56). Pathogenesis associated with HTLV-1 infection is mainly driven by the regulatory protein Tax encoded in the pX region of the viral genome (16). Tax plays an essential role in regulating viral gene expression by interacting with host transcription factors and coactivators such as CREB and CBP/p300 in the viral long terminal repeat (LTR) regions. Tax also functions as an oncoprotein by modulating the activation of host signaling pathways such as nuclear factor κB (NF-κB) and the cell cycle machinery (23, 30).
Because Tax has pleiotropic functions in different compartments in the cell, Tax shuttles between the cytoplasm and nucleus via an amino (N)-terminal nuclear localization sequence (NLS) and a leucine-rich nuclear export sequence (NES) (1, 44). The regulation of Tax subcellular localization and trafficking to different compartments in the cell therefore impinges on the function of Tax (4, 38). Recent studies have shown that posttranslational modifications of Tax regulate its function by governing its subcellular localization, stability, and protein-protein interactions (6, 20, 32). Tax is modified by a number of distinct posttranslational modifications, including ubiquitination, small ubiquitin (Ub)-like modifier sumoylation, phosphorylation, and acetylation (3, 6, 11, 26, 27, 41), all of which regulate Tax function. In particular, ubiquitination and sumoylation of Tax play important roles in Tax trafficking and NF-κB activation.
One of the main signaling pathways activated by Tax that is central to virus-induced transformation is NF-κB. The NF-κB family of transcription factors plays a key role in the regulation of different biological processes, including innate and adaptive immunity and cell survival (53). NF-κB dimers are sequestered in the cytoplasm by physical interaction with ankyrin repeat domain-containing inhibitory proteins known as inhibitor of κB (IκB) (24). In response to proinflammatory cytokines, antigens, or stress signals, intracellular signaling converges at the level of the IκB kinase (IKK) complex containing the catalytic subunits IKKα and IKKβ and the regulatory subunit IKKγ (also known as NEMO) (13). IKKβ phosphorylates IκBα, which triggers its proteasome-dependent degradation, thus liberating active NF-κB dimers, which translocate to the nucleus and regulate the transcription of genes regulating immunity and cell survival (13). In the noncanonical pathway, the IκB family member NF-κB2 (also known as p100) undergoes partial degradation by the proteasome to yield the p52 NF-κB subunit, which dimerizes with RelB and regulates genes involved in lymphoid organogenesis and B-cell survival (46). The noncanonical NF-κB pathway is activated by a more restricted subset of stimuli composed of tumor necrosis factor (TNF) superfamily members, such as CD40L, BAFF, and LT-β, which all activate the mitogen-activated protein type 3 (MAP3) kinase NIK and IKKα (7, 39, 52). Interestingly, Tax is a potent activator of both canonical and noncanonical NF-κB pathways by interacting with IKKγ and triggering the persistent activation of IKKs (10, 18, 36, 51). Tax undergoes Ubc13-dependent lysine 63 (K63)-linked polyubiquitination as part of its mechanism to activate NF-κB and is required for binding to IKKγ (41).
Ubiquitination is a posttranslational modification that regulates a wide array of biological processes, such as protein degradation, DNA repair, signal transduction, and receptor trafficking (14, 21). Ubiquitination is dependent on the sequential action of three enzymes, including a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3) (25). Ubiquitin is first activated by an E1 enzyme in an ATP-dependent manner and is transferred to the active-site cysteine of an E2 enzyme. The E3 enzyme then conjugates ubiquitin onto a lysine residue of a specific substrate by linking the C-terminal glycine of ubiquitin to the ε-amino group of lysine. This process can be repeated several times to assemble polyubiquitin chains, which are linked by any of seven lysine residues in ubiquitin (K6, K11, K27, K29, K33, K48, and K63) (25). The best-studied polyubiquitin chains are K48 or K63 linked, which yield distinct outcomes for the substrate protein. Whereas K48-linked polyubiquitin chains target substrates for proteasomal degradation, K63-linked polyubiquitin chains play an important role in signal transduction, DNA repair, and protein trafficking (35).
Ubiquitination is a reversible process that is negatively regulated by a family of deubiquitinase enzymes (DUBs), of which there are approximately 100 encoded in the human genome (43). DUBs may be divided into five groups: ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), ovarian tumor proteases (OTUs), Machado-Joseph disease proteases, and JAB1/MPN/Mov34 metalloenzymes (JAMMs) (49). Although the majority of DUBs function as cysteine proteases, JAMMs are metalloproteases. AMSH (associated molecule with the SH3 domain of STAM, also known as STAMBP) family members are JAMM DUBs and specifically cleave K63-linked polyubiquitin chains (28, 31, 37). Previous studies have demonstrated that AMSH DUBs coordinate the recycling of receptors to the cell surface by removing K63-linked polyubiquitin chains (31).
The goal of this study was to identify DUBs that may regulate Tax-mediated activation of NF-κB. To this end, we used an unbiased RNA interference (RNAi) approach by screening a small interfering RNA (siRNA) deubiquitinating enzyme library for siRNAs that modulated Tax activation of an NF-κB reporter gene. This screen identified STAMBPL1 (also known as AMSH-LP) as a positive regulator of Tax activation of NF-κB. STAMBPL1 was required for optimal Tax-induced activation of both canonical and noncanonical NF-κB pathways. STAMBPL1 indirectly stabilized Tax by promoting its shuttling from the nucleus to the cytoplasm, thereby protecting Tax from K48-induced ubiquitination and proteasomal degradation in the nucleus. Thus, STAMBPL1 is a DUB that controls Tax trafficking in the cell and is essential for exporting Tax from the nucleus to the cytoplasm, where it triggers IKK and NF-κB activation.
MATERIALS AND METHODS
Biological reagents and antibodies.
Human embryonic kidney cells (HEK 293T) and Jurkat T cells were purchased from ATCC. The HTLV-1-transformed cell line MT-2 was described previously (20). 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM); Jurkat and MT-2 cells were cultured in RPMI medium. Medium was supplemented with fetal bovine serum (10%) and penicillin-streptomycin (1%). Expression vectors encoding pCMV4-Tax, green fluorescent protein (GFP)-Tax and Flag-Tax, hemagglutinin (HA)-Ub, HA-Ub K48 only, κB-luciferase (Luc), pU3R-Luc, pRL-TK (thymidine kinase), and pCMV4-p100 have all been described previously (2, 20, 41). pCDNA-Myc ELKS expression plasmid was a gift from I. Verma (8). Flag/HA-STAMBPL1 was from J. Wade Harper and obtained from Addgene (plasmid 22559) (45). Flag-Tax1 was a gift from U. Bertazzoni (48). The following antibodies were used in this study: anti-STAMBPL1 (ab99172; Abcam), anti-p100/p52 (Santa Cruz Biotechnology), anti-β-actin (AC15; Abcam), anti-IκBα (C-21; Santa Cruz Biotechnology), anti-phospho-IκBα (14D4; Cell Signaling), and anti-TATA-binding protein (anti-TATA-BP) (MAB3658; Millipore). The monoclonal anti-Tax antibody was prepared from a Tax hybridoma (168B17-46-34) that was obtained from the AIDS Research and Reference Program, NIAID, National Institutes of Health. Alexa Fluor 555-conjugated donkey anti-mouse IgG, Alexa Fluor 488-conjugated donkey anti-rabbit IgG, and Alexa Fluor 647-conjugated donkey anti-rabbit IgG were purchased from Molecular Probes/Invitrogen. DAPI(4′,6-diamidino-2-phenylindole) and MG-132 were purchased from EMD Biosciences. Cycloheximide (CHX) was purchased from Sigma. Recombinant TNF-α and interleukin-1β (IL-1β) were purchased from R&D Systems. The SMARTpool siRNA human deubiquitinating enzyme library, control scrambled siRNA, and ELKS and STAMBPL1 SMARTpool siRNAs were purchased from Dharmacon/Thermo Scientific.
Transfections and luciferase assays.
Transient transfections in 293T cells were performed with GenJet (SignaGen) according to the manufacturer's instructions. Jurkat and MT-2 cells were transfected with FuGene HD (Roche) according to the manufacturer's instructions. For siRNA transfections, cells were transfected with 20 or 40 pmol of control or STAMBPL1 siRNA using Lipofectamine 2000 (Invitrogen). For luciferase assays, cells were lysed 24 h after transfection using 1× passive lysis buffer (Promega). Luciferase activity was measured with the dual-luciferase assay system according to the manufacturer's instructions (Promega). Firefly luciferase values were normalized based on the Renilla luciferase internal control values. Luciferase values are presented as “fold induction” relative to the untreated control transfected with empty vector or control siRNA.
qRT-PCR.
Quantitative real-time PCR (qRT-PCR) was performed essentially as described previously (5, 19). Total RNA was isolated from cells using the RNeasy minikit (Qiagen). RNA was converted to cDNA using the First Strand cDNA synthesis kit for RT-PCR (avian myeloblastosis virus [AMV]; Roche). Real-time PCR was performed with a LightCycler instrument (Roche) using LightCycler FastStart DNA MasterPLUS HybProbe (Roche) and gene-specific TaqMan probes (Applied Biosystems). Gene expression for IκBα was normalized to the internal control 18S rRNA.
CHX chase assay.
CHX chase assays were performed as described previously (40). Cells were treated with CHX (100 μg/ml) for various times 2 days after transfection. Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer, and immunoblotting was conducted with anti-Tax.
Immunoblotting.
Immunoblotting was performed essentially as described previously (20, 42). Whole-cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked in 5% milk or bovine serum albumin (BSA) (for phospho-specific antibodies), incubated with the indicated primary and secondary antibodies, and detected with Western Lightning enhanced chemiluminescence reagent (Perkin Elmer).
Immunostaining and confocal microscopy.
Cells were seeded in 6-well plates onto poly-l-lysine-coated coverslips (BD Biosciences) and transfected with either siRNAs or plasmids. After 24 h, the plates were briefly centrifuged, and cells were washed with (phosphate-buffered saline (PBS) and fixed in 1% paraformaldehyde for 10 min at room temperature. The fixed cells were permeabilized with PBS containing 0.2% Triton X-100 (Sigma) and incubated in PBS containing 10% normal donkey serum (Jackson ImmunoResearch) and 0.1% Triton X-100 to prevent nonspecific binding. The primary antibodies were diluted in PBS containing 0.1% Triton X-100 and added to the cells for 1 h, followed by washes in PBS containing 0.1% Triton X-100. Secondary antibodies were added and incubated for 1 h, followed by several washes with PBS containing 0.1% Triton X-100. The coverslips were stained with DAPI to visualize nuclei. Finally, the coverslips were mounted onto slides using AquaPolymount (Polysciences) and analyzed with a Zeiss confocal laser scanning microscope.
Subcellular fractionation.
Cytoplasmic and nuclear extracts were prepared using hypotonic buffer (20 mM HEPES, pH 8.0, 10 mM KCl, 1 mM MgCl2, 0.1% [vol/vol] Triton X-100, 400 mM NaCl) and insoluble buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 1% [vol/vol] NP-40, 10 mM iodacetamide), respectively. The purity of the obtained fractions was confirmed by examining β-actin for cytoplasmic expression and TATA-binding protein (TATA-BP) for nuclear expression. Cells were lysed in hypotonic buffer to obtain the cytoplasmic fractions, and then the insoluble pellets were washed five times with the same buffer. The pellets were then suspended in nuclear buffer to obtain the nuclear fractions.
Ubiquitination assay.
The ubiquitination assay was performed essentially as described previously (41). Briefly, 293T cells were transfected with Flag-Tax, HA-Ub, and HA-Ub K48-only plasmids. Lysates were subjected to immunoprecipitation (IP) with anti-Flag and immunoblotting (IB) with anti-HA to examine ubiquitination of Tax.
Deubiquitination assay.
The deubiquitination assay was performed as described previously (29). 293T cells were transfected with the indicated plasmids and lysed in RIPA buffer after 48 h, and lysates were subjected to immunoprecipitations (IP) with anti-Flag antibody and protein A-agarose beads (Roche). Flag immunoprecipitates were eluted with 3× Flag peptide (Sigma) and incubated with 1 μg immunoaffinity-purified human STAMBPL1 (Origene) at 37°C for 18 h and immediately subjected to SDS-PAGE and immunoblotting. As a positive control for STAMBPL1 DUB activity, STAMBPL1 was incubated with recombinant K63-linked penta-ubiquitin (Ub5) chains (Boston Biochem) for 18 h at 37°C.
Statistical analysis.
All error bars represent the standard deviation (SD) of triplicate samples. Statistical significance was determined by Student's t test. On the figures, * indicates a P value of <0.05 and ** indicates a P value of <0.01.
RESULTS
STAMBPL1 is a positive regulator of Tax-mediated NF-κB signaling.
Polyubiquitination of Tax plays a critical role in IKKγ binding, Tax trafficking, and NF-κB activation (20, 27). However, the regulation of Tax polyubiquitination and NF-κB activation by host proteins is poorly understood. Thus, we used an unbiased approach to identify DUBs that regulate Tax activation of NF-κB signaling. We screened an siRNA library containing 100 siRNAs specific for all known human deubiquitinating enzymes (DUBs). 293T cells were transfected with the siRNA library to evaluate the role of DUBs in Tax-mediated NF-κB activation using a luciferase reporter assay. As expected, a number of negative regulators of Tax-induced NF-κB activation were identified, including the cylindromatosis (CYLD) gene (data not shown) (50). Interestingly, a small number of DUBs were found to be required for Tax activation of NF-κB, including AMSH-LP/STAMBPL1 (STAM binding protein-like 1). STAMBPL1 is a member of the JAMM deubiquitinating enzyme family, which cleaves Lys-63-linked polyubiquitin chains (37). AMSH proteins regulate the recycling of receptors conjugated with K63-linked polyubiquitination chains by opposing ubiquitin-dependent sorting to the lysosome (31). First, we confirmed that siRNA-mediated knockdown of STAMBPL1 indeed impaired Tax-mediated activation of an NF-κB reporter in 293T cells (Fig. 1A).
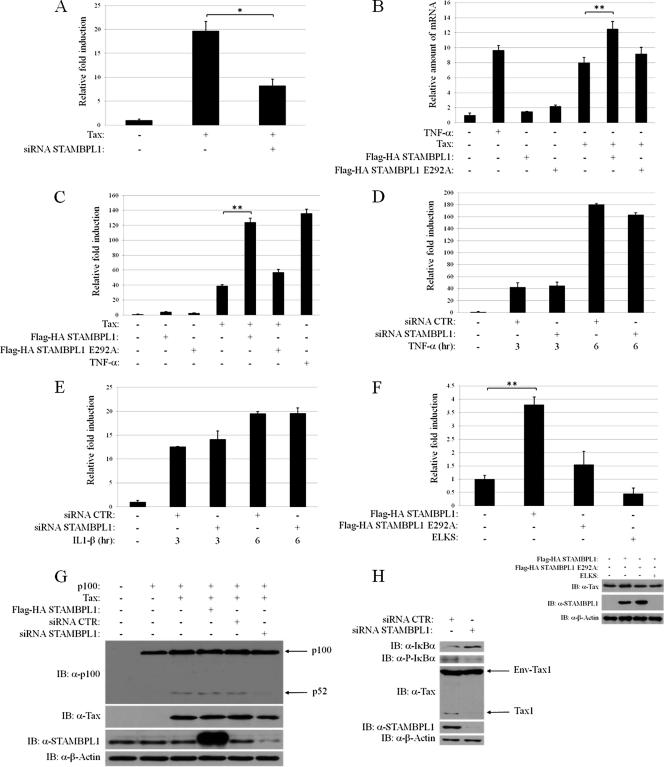
Fig 1.
STAMBPL1 enhances Tax-mediated activation of canonical and noncanonical NF-κB pathways. (A) STAMBPL1 regulates Tax-mediated NF-κB activation. 293T cells were transfected with pCMV4-Tax and either control or STAMBPL1 siRNA, together with a κB-driven firefly luciferase reporter (κB-Luc) and thymidine kinase-driven Renilla luciferase reporter (pRL-TK). The luciferase activity is presented as fold induction compared to vector-transfected cells. (B) STAMBPL1 potentiates the induction of IκBα mRNA by Tax. 293T cells were transfected with the indicated expression vectors. As a positive control, cells were also treated with TNF-α (20 ng/ml) for 4 h. IκBα mRNA was detected by qRT-PCR and normalized according to control 18S rRNA. (C) Positive regulation of Tax-mediated NF-κB activation by STAMBPL1. Jurkat cells were transfected with the indicated expression vectors, together with κB-Luc and pRL-TK reporters. Cells were also treated with TNF-α (20 ng/ml) as a positive control. The luciferase activity is presented as fold induction compared to vector-transfected cells. (D and E) 293T cells were transfected with either control (CTR) or STAMBPL1 siRNA together with κB-Luc and pRL-TK plasmids. After 24 h, cells were treated with either TNF-α or IL-1β (both at 20 ng/ml) for the indicated times. The luciferase activity is presented as fold induction compared to vector-transfected cells. Error bars represent SDs of triplicate samples. (F) Overexpression of wild-type STAMBPL1, but not a catalytically inactive mutant (E292A), in HTLV-1-transformed MT-2 cells enhances the activation of NF-κB. MT-2 cells were transfected with the indicated expression vectors, together with κB-Luc and pRL-TK reporters. The luciferase activity is presented as fold induction compared to vector-transfected cells. The lysates were subjected to immunoblotting with anti-Tax (α-Tax), anti-STAMBPL1 (α-STAMBPL1), and anti-β-actin (α-β-actin). (G) STAMBPL1 is essential for Tax to activate the noncanonical NF-κB pathway. 293T cells were transfected with the indicated expression vectors. The lysates were subjected to immunoblotting with anti-p100 (α-p100), anti-Tax, anti-STAMBPL1, and anti-β-actin. (H) Knockdown of STAMBPL1 in MT-2 cells impairs IκBα phosphorylation and degradation. MT-2 cells were transfected with scrambled control or STAMBPL1 siRNA. After 48 h, the cells were lysed and subjected to SDS-PAGE and immunoblotting using anti-IκBα (α-IκBα), anti-phospho-IκBα (α-P-IκBα), anti-Tax, anti-STAMBPL1, and anti-β-actin. *, P < 0.05; **, P < 0.01.
To further confirm the effect of STAMBPL1 on Tax-induced NF-κB activation, additional NF-κB signaling functional studies were performed. A catalytically inactive mutant of STAMBPL1 was generated in which glutamic acid at position 292 was replaced with alanine (STAMBPL1 E292A) (37). The effects of wild-type STAMBPL1 and the E292A mutant on Tax-induced activation of an NF-κB target gene were evaluated by qRT-PCR (Fig. 1B). Wild-type STAMBPL1, but not the catalytically inactive mutant, enhanced the expression of IκBα mRNA induced by Tax (Fig. 1B). However, overexpression of either STAMBPL1 alone or the STAMBPL1 mutant had no effect on the induction of IκBα mRNA (Fig. 1B). In support of these findings, using an NF-κB reporter assay, wild-type STAMBPL1, but not the catalytically inactive mutant, enhanced Tax-induced NF-κB activation in Jurkat T cells (Fig. 1C). The effects of STAMBPL1 on NF-κB appear to be specific for Tax since siRNA-mediated knockdown of STAMBPL1 had no effect on TNF-α- or IL-1β-induced NF-κB activation (Fig. 1D and E). We next examined the effect of STAMBPL1 overexpression on NF-κB signaling in MT-2 cells, a T-cell line chronically infected with HTLV-1. Wild-type STAMBPL1, but not the E292A mutant, potentiated NF-κB activation in MT-2 cells (Fig. 1F). As a negative control, we overexpressed ELKS (a protein rich in glutamate, leucine, lysine, and serine), an adaptor molecule involved in NF-κB signaling by genotoxic stress, which had no effect on NF-κB activation in MT-2 cells (Fig. 1F). Both wild-type STAMBPL1 and mutant STAMBPL1 were expressed similarly, as detected by Western blotting (Fig. 1F). Taken together, these data suggest that STAMBPL1 is a novel positive regulator of Tax-induced NF-κB activation.
Tax is also a potent activator of the noncanonical NF-κB pathway, which is persistently activated in HTLV-1-transformed cell lines (51), thus prompting us to next examine if STAMBPL1 regulates Tax-induced p100 processing. Silencing of STAMBPL1 with siRNA resulted in an inhibition of Tax-induced p100 processing to p52 (Fig. 1G). Overexpression of STAMBPL1 did not further promote the processing of p100 elicited by Tax (Fig. 1G), perhaps suggesting that Tax activation of the noncanonical pathway had already reached maximal levels. We next examined if STAMBPL1 modulated the phosphorylation and degradation of IκBα in HTLV-1-transformed MT-2 cells. Indeed, knockdown of STAMBPL1 in MT-2 cells reduced IκBα phosphorylation with a concomitant increase in total IκBα (Fig. 1H). Interestingly, knockdown of STAMBPL1 also decreased Tax expression, but not the Env-Tax fusion previously reported in MT-2 cells (Fig. 1H) (47). Collectively, our results strongly suggest that STAMBPL1 positively regulates Tax activation of the canonical and noncanonical NF-κB pathways.
STAMBPL1 stabilizes Tax and inhibits K48-linked polyubiquitination of Tax.
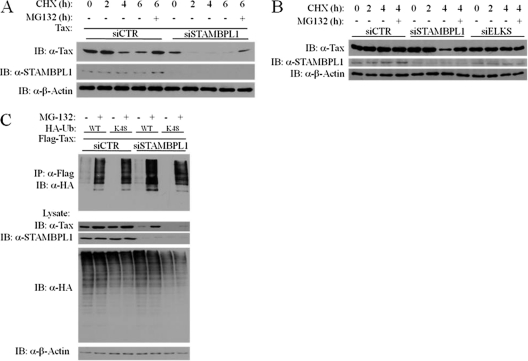
A previous study has demonstrated that Tax undergoes proteasomal degradation in the nucleus, specifically in the nuclear matrix (54). Since STAMBPL1 functions as a deubiquitinating enzyme, we hypothesized that STAMBPL1 may enhance the protein stability of Tax, possibly by removing K48-linked degradative polyubiquitin chains. To test this hypothesis, we conducted cycloheximide (CHX) chase assays with 293T and MT-2 cells. Although ectopic Tax in 293T cells was fairly stable in the presence of control siRNA, STAMBPL1 siRNA greatly accelerated the turnover of Tax (Fig. 2A). STAMBPL1 expression was efficiently silenced by siRNA, as determined by Western blotting (Fig. 2A). The loss of Tax was rescued by the proteasome inhibitor MG-132, indicating that the shorter half-life of Tax in the absence of STAMBPL1 was due to accelerated proteasomal degradation (Fig. 2A). Consistent with these results, knockdown of STAMBPL1 with siRNA in MT-2 cells triggered Tax degradation after 4 h of CHX treatment that was rescued with MG-132 treatment (Fig. 2B). Furthermore, an siRNA specific for an unrelated gene (coding for ELKS) had no effect on Tax stability, thus indicating specificity (Fig. 2B). Therefore, STAMBPL1 plays an important role in the stabilization of Tax protein.
Fig 2.
STAMBPL1 stabilizes Tax protein and inhibits its K48-linked polyubiquitination. (A) Tax is degraded in the absence of STAMBPL1. 293T cells were transfected with pCMV4-Tax and either control (siCTR) or STAMBPL1 (siSTAMBPL1) siRNA. After 24 h cells were treated with CHX and/or MG-132 (10 μM) for the indicated times. Cells were lysed and subjected to SDS-PAGE and immunoblotting using anti-Tax (α-Tax), anti-STAMBPL1 (α-STAMBPL1), and anti-β-actin (α-β-actin). (B) STAMBPL1 stabilizes Tax in HTLV-1-transformed cells. MT-2 cells were transfected with control, STAMBPL1, or ELKS siRNA. After 48 h the cells were treated with CHX and/or MG-132 (10 μM) for the indicated times, lysed, and subjected to SDS-PAGE and immunoblotting using anti-Tax, anti-STAMBPL1, and anti-β-actin. (C) STAMBPL1 inhibits K48-linked polyubiquitination of Tax. 293T cells were transfected with the indicated expression vectors and either control (siCTR) or STAMBPL1 (siSTAMBPL1) siRNA. After 24 h, cells were treated with MG-132 (10 μM) for 3 h. Cells were lysed and subjected to immunoprecipitation with anti-Flag and immunoblotting using anti-HA (α-HA). The lysates were subjected to immunoblotting with anti-Tax, anti-STAMBPL1, anti-HA, and anti-β-actin.
We next examined the effect of STAMBPL1 on Tax ubiquitination using HA-tagged ubiquitin (HA-Ub) and a ubiquitin mutant with all lysines mutated to arginines, except for lysine 48 (HA-Ub K48 only). This ubiquitin mutant only has K48 available for the linkage of polyubiquitin chains and thus only supports K48-linked polyubiquitin instead of the other lysine residues in ubiquitin. 293T cells were transfected with Flag-Tax, wild-type HA-Ub, and K48-only HA-Ub constructs and control or STAMBPL1 siRNA for ubiquitination assays. Tax ubiquitination was observed when Tax and HA-Ub plasmids were coexpressed, but MG-132 treatment greatly increased total as well as K48-linked Tax polyubiquitination (Fig. 2C). STAMBPL1 knockdown resulted in greater Tax polyubiquitination and, in particular, K48-linked polyubiquitination, despite lower levels of Tax expression, as observed by Western blotting (Fig. 2C). These results confirm that STAMBPL1 regulates Tax K48-linked polyubiquitination and Tax stability.
STAMBPL1 enhances the stability of Tax by promoting its nuclear/cytoplasmic shuttling.
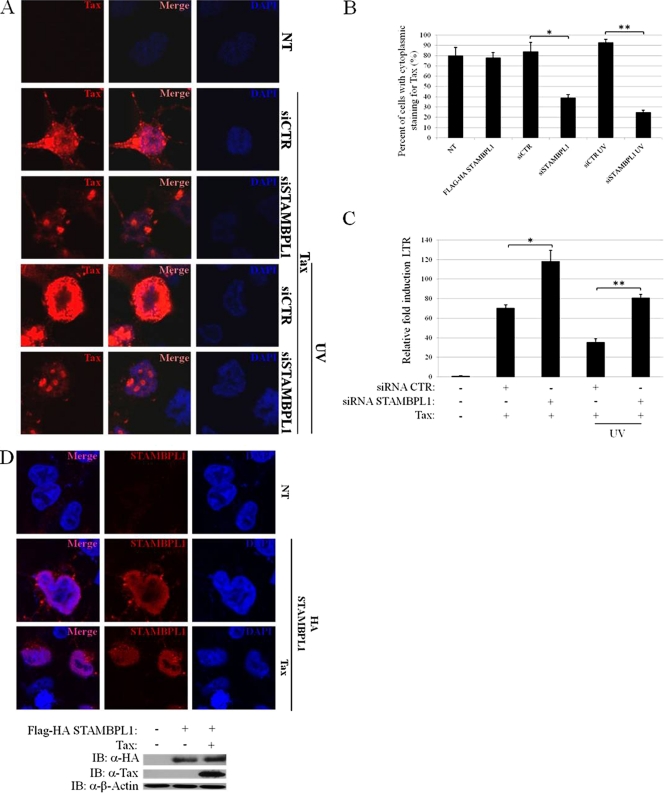
Tax undergoes a number of posttranslational modifications, including ubiquitination and sumoylation, that regulate Tax trafficking in cells (27). Whereas ubiquitinated Tax is predominantly found in the cytoplasm, sumoylated Tax is located primarily in the nucleus. However, nuclear Tax is unstable since Tax is degraded by the proteasome in the nuclear matrix upon K48-linked polyubiquitination (54). Thus, we reasoned that STAMBPL1 might indirectly stabilize Tax by promoting its nuclear export. To examine this hypothesis, we took advantage of previous findings that suggested that nuclear Tax is relocalized to the cytoplasm in a CRM1-dependent manner in response to genotoxic stress and DNA damage (11, 12). Thus, we performed immunofluoresence and confocal microscopy using a Tax expression plasmid and subjected cells to UV irradiation. Consistent with previous studies, Tax localization was heterogeneous with staining in both the cytoplasm and nucleus in 293T cells (Fig. 3A) (48). However, upon siRNA-induced silencing of STAMBPL1, a strongly nuclear distribution of Tax in the Tax speckled structure (TSS) was readily apparent (Fig. 3A). As expected, Tax nuclear staining in the speckles colocalized with the splicing factor SC-35 (data not shown). Upon UV stimulation of cells, Tax was efficiently exported from the nucleus to the cytoplasm, as previously described (Fig. 3A) (12). However, when STAMBPL1 was knocked down with siRNA, UV-induced Tax nuclear export was impaired and Tax was mainly confined to nuclear speckles (Fig. 3A). Similar results were obtained in HTLV-1-transformed MT-2 cells (data not shown). Western blotting of lysates confirmed the knockdown of STAMBPL1 and the destabilization of Tax by UV together with STAMBPL1 siRNA (data not shown). Quantitation of the confocal results revealed that Tax expression in the cytoplasm (either completely cytoplasmic or cytoplasmic and nuclear) decreased from 84% to 39% upon silencing of STAMBPL1 (Fig. 3B). Tax expression in the cytoplasm decreased from 93% to 25% in UV-stimulated cells transfected with control or STAMBPL1 siRNA, respectively (Fig. 3B). The effect of STAMBPL1 appears to be specific for Tax trafficking since knockdown or overexpression of STAMBPL1 did not influence p53 localization (data not shown). Together, these studies suggest that STAMBPL1 is essential for the shuttling of Tax from the nucleus to the cytoplasm, thus allowing Tax to elude proteasome-dependent degradation in the nucleus.
Fig 3.
STAMBPL1 is required for the nuclear export of Tax. (A) STAMBPL1 regulates the nuclear export of Tax. 293T cells were seeded on poly-l-lysine-coated coverslips and transfected with Flag-Tax and either control (siCTR) or STAMBPL1 (siSTAMBPL1) siRNA. Cells were irradiated with UV light (30 J/m2) for 30 min and immunostained with anti-Tax. The coverslips were subjected to confocal microscopy to visualize Tax (red) and nuclei using DAPI (blue). (B) Quantitation of Tax immunostaining experiments. Cytoplasmic Tax (either completely cytoplasmic or cytoplasmic/nuclear) was quantitated by enumerating 100 cells for each of the indicated experimental conditions, and is presented as the percentage of cytoplasmic Tax. (C) STAMBPL1 inhibits Tax-induced HTLV-1–LTR transcriptional activity. 293T cells were transfected with pCMV4-Tax and either control or STAMBPL1 siRNA together with an HTLV-1–LTR-driven firefly luciferase reporter (pU3R-Luc) and pRL-TK. Cells were also treated with UV light (30 J/m2) as indicated. The luciferase activity is presented as fold induction compared to vector-transfected cells. (D) STAMBPL1 is predominantly localized in the nucleus. 293T cells were seeded on poly-l-lysine-coated coverslips and transfected with Flag-Tax and Flag-HA-STAMBPL1. Cells were immunostained with anti-HA (α-HA). The coverslips were subjected to confocal microscopy to visualize STAMBPL1 (red) and nuclei (blue) using DAPI. Cell lysates were also subjected to immunoblotting using anti-HA, anti-Tax (α-Tax), and anti-β-actin (α-β-actin). NT, not transfected. *, P < 0.05; **, P < 0.01.
Given that STAMBPL1 plays an essential role in the shuttling of Tax from the nucleus to the cytoplasm, we next examined Tax activation of the HTLV-1 LTR, an event that occurs in the nucleus. In contrast to its effects on NF-κB activation, knockdown of STAMBPL1 enhanced Tax activation of the HTLV-1 LTR reporter (Fig. 3C). The effects of STAMBPL1 loss were even more significant when cells were exposed to UV irradiation (Fig. 3C). These results are consistent with our hypothesis that STAMBPL1 regulates Tax nuclear export; thus, when STAMBPL1 is knocked down, there is more abundant Tax in the nucleus, which correlates with greater LTR activation.
We next examined the localization of STAMBPL1 in cells by confocal microscopy to determine where STAMBPL1 may potentially be regulating Tax. STAMBPL1 was predominantly expressed in the nucleus, and Tax had no effect on the localization of STAMBPL1 (Fig. 3D). Western blotting confirmed the expression of both STAMBPL1 and Tax (Fig. 3D). Therefore, STAMBPL1 is a nuclear protein that is essential for Tax nuclear export.
STAMBPL1 promotes Tax relocalization to the cis-Golgi.
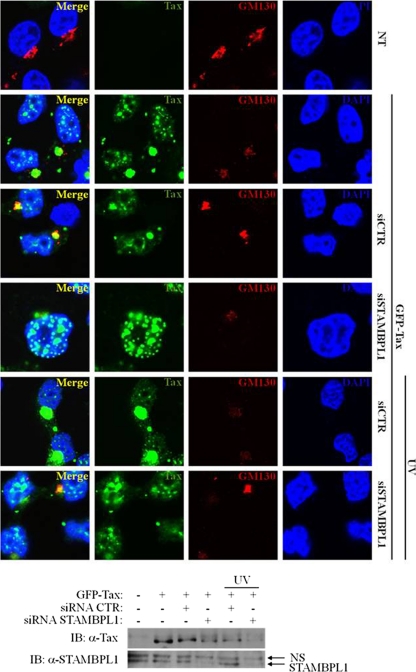
We have previously demonstrated that Tax promotes the relocalization of IKKγ to perinuclear “hot spots” in the proximity of the Golgi apparatus (20). The relocalization of IKK to the cis-Golgi by Tax is an important mechanism of Tax-induced activation of NF-κB. Since STAMBPL1 enhanced Tax-induced NF-κB activation, we next performed confocal studies to determine if STAMBPL1 promoted Tax colocalization with GM-130 (a cis-Golgi matrix protein). 293T cells were transfected with GFP-Tax and either control or STAMBPL1 siRNA, and cells were irradiated with UV light. As seen in Fig. 4, Tax colocalized with GM-130 in the presence of control siRNA, but not STAMBPL1 siRNA, which resulted in mostly nuclear Tax expression. Upon UV stimulation, Tax colocalization with GM-130 was also readily observed, although Tax expression was reduced and was predominantly nuclear when STAMBPL1 was silenced with siRNA (Fig. 4). Thus, STAMBPL1 facilitates Tax nuclear export and trafficking to the cis-Golgi, where it activates IKK and NF-κB. In the absence of STAMBPL1, Tax is trapped in the nucleus and undergoes proteasomal degradation.
Fig 4.
Tax localization in the cis-Golgi is dependent on STAMBPL1. 293T cells were seeded on poly-l-lysine-coated coverslips and transfected with GFP-Tax and either control or STAMBPL1 siRNA. Cells were irradiated with UV light (30 J/m2) for 30 min and subjected to immunostaining using anti-GM-130. Coverslips were analyzed by confocal microscopy to visualize GFP-Tax (green), GM-130 (red), and nuclei (blue) using DAPI. Cell lysates were subjected to immunoblotting using anti-STAMBPL1 (α-STAMBPL1) and anti-Tax (α-Tax). NT, not transfected; NS, nonspecific band.
Tax is degraded by the proteasome in the nucleus in the absence of STAMBPL1.
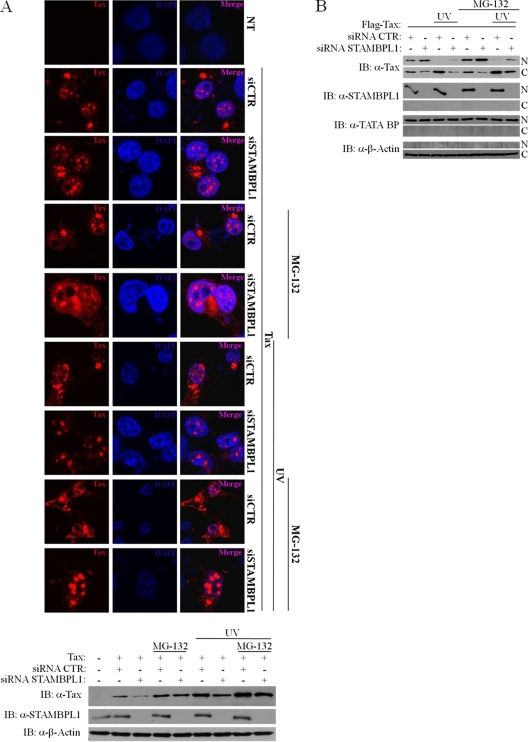
In order to further test the hypothesis that STAMBPL1 stabilizes Tax expression by promoting nuclear export, we performed additional immunostaining and confocal microscopy studies to determine if a proteasome inhibitor rescues Tax nuclear expression in the absence of STAMBPL1. 293T cells were transfected with Tax and either control or STAMBPL1 siRNA, and cells were treated with MG-132 and UV light (Fig. 5A). As demonstrated earlier, Tax was localized to perinuclear “hot spots” when transfected with control siRNA, but was predominantly nuclear in the presence of STAMBPL1 siRNA (Fig. 5A). Furthermore, MG-132 stabilized nuclear Tax that otherwise would have been degraded in cells expressing STAMBPL1 siRNA (Fig. 5A). Western blotting confirmed the diminished Tax expression by STAMBPL1 knockdown, and the stabilization of Tax by MG-132 treatment (Fig. 5A). Again, the effects of STAMBPL1 siRNA on Tax trafficking were more pronounced upon UV stimulation of cells. Strong nuclear focal Tax staining was observed in UV- and MG-132-treated cells knocked down for STAMBPL1 (Fig. 5A).
Fig 5.
A proteasome inhibitor rescues Tax degradation in the nucleus elicited by STAMBPL1 knockdown. (A) A proteasome inhibitor rescues degradation of nuclear Tax. 293T cells were seeded on poly-l-lysine-coated coverslips and transfected with pCMV4-Tax and either control (CTR) or STAMBPL1 siRNA. Cells were then irradiated with UV light (30 J/m2) and/or treated with MG-132 (10 μM) and then processed for immunostaining using anti-Tax (α-Tax). Coverslips were analyzed by confocal microscopy to visualize Tax (red) and nuclei (blue) using DAPI. Cell lysates were subjected to immunoblotting using anti-STAMBPL1, anti-Tax and anti-β-actin (α-β-actin). (B) Knockdown of STAMBPL1 with siRNA inhibits Tax nuclear/cytoplasmic transport. 293T cells were transfected with the indicated expression vectors and either control or STAMBPL1 siRNA, and after 24 h, cells were irradiated with UV light (30 J/m2) and/or treated with MG-132 (10 μM). Cells were lysed and subjected to nuclear (N) and cytoplasmic (C) subcellular fractionation and immunoblotting using anti-Tax, anti-STAMBPL1 (α-STAMBPL1), anti-TATA-binding protein (α-TATA BP), and anti-β-actin. NT, not transfected.
To confirm the confocal results using an independent assay, we also examined Tax localization by biochemical fractionation. We performed subcellular fractionation to separate cytoplasmic and nuclear fractions and examined Tax localization by Western blotting. Detection of TATA-binding protein and β-actin in the nuclear and cytoplasmic compartments, respectively, confirmed the purity of the fractions (Fig. 5B). Consistent with the confocal studies, Tax was expressed in both the cytoplasm and nucleus, but after UV irradiation, Tax was almost exclusively expressed in the cytoplasm (Fig. 5B). However, STAMBPL1 knockdown impaired Tax shuttling to the cytoplasm and Tax was more evenly distributed between the cytoplasmic and nuclear compartments (Fig. 5B). This effect on Tax was also observed upon treatment of cells with both UV and MG-132 (Fig. 5B). Thus, both biochemical fractionation and confocal analysis confirm that STAMBPL1 is essential for the nuclear export of Tax in response to UV stimulation.
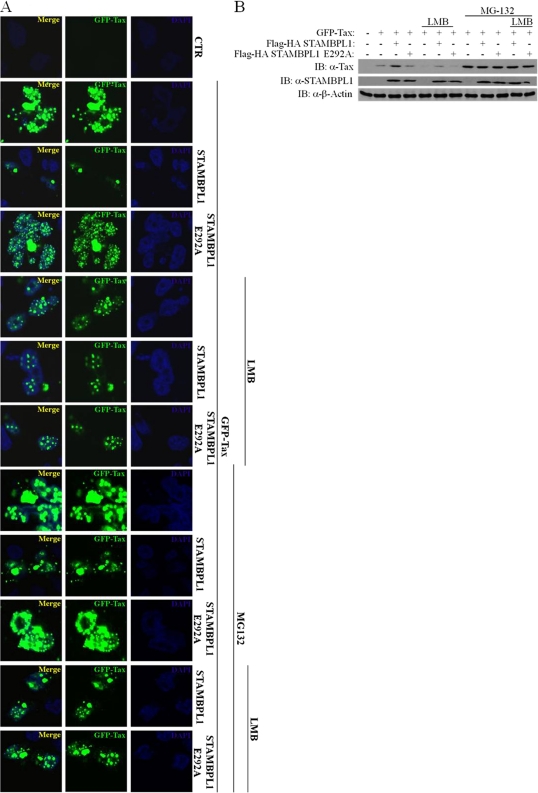
Together, these results suggest that STAMBPL1 indirectly stabilizes Tax by promoting its nuclear export and preventing its proteasomal degradation in the nucleus. To further evaluate the role of STAMBPL1 in regulating Tax nuclear export, we used leptomycin B (LMB), a drug originally discovered as an antibiotic that blocks the nuclear export of proteins dependent on the nuclear export receptor CRM-1 (34). Wild-type STAMBPL1 and the catalytically inactive mutant E292A, together with Tax, were transfected into cells and treated with MG-132 and/or LMB. When STAMBPL1 was transfected together with Tax, the localization of Tax was almost completely cytoplasmic, forming a perinuclear aggregate (Fig. 6). However, this effect was dependent on STAMBPL1 catalytic activity since Tax was mainly confined to the nucleus in the presence of the STAMBPL1 mutant (Fig. 6). Upon treatment of cells with LMB, Tax expression was diminished and was localized predominantly in the nucleus; however, MG-132 stabilized nuclear Tax that was prevented from shuttling by LMB and thus was strongly expressed in the TSS (Fig. 6). These results were also corroborated by Western blotting, which demonstrated that LMB destabilized Tax but was rescued with MG-132 treatment (Fig. 6). Collectively, these results indicate that STAMBPL1 inhibits Tax proteasomal degradation by controlling Tax nuclear/cytoplasmic transport.
Fig 6.
STAMBPL1 indirectly stabilizes Tax by promoting its nuclear export. LMB blocks the shuttling of Tax to the cytoplasm mediated by wild-type STAMBPL1, but not by the mutant STAMBPL1 E292A. 293T cells were seeded on poly-l-lysine-coated coverslips and transfected with GFP-Tax, STAMBPL1, or STAMBPL1 E292A. Cells were then irradiated with UV light (30 J/m2) and/or treated with MG-132 (10 μM) and LMB (40 ng/ml) and then processed for immunostaining. Coverslips were analyzed by confocal microscopy to visualize Tax (green) and nuclei (blue) using DAPI. Cell lysates were subjected to immunoblotting using anti-STAMBPL1 (α-STAMBPL1), anti-Tax (α-Tax), and anti-β-actin (α-β-actin).
STAMBPL1 does not directly deubiquitinate Tax.
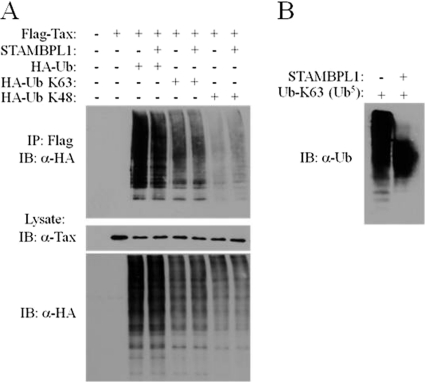
The previous experiments using the STAMBPL1 DUB mutant strongly suggest that the positive regulation of STAMBPL1 on Tax-induced NF-κB activation is driven by the deubiquitinating activity of this protein. Furthermore, STAMBPL1 knockdown enhances the K48-linked polyubiquitination of Tax (Fig. 2C). However, it is unclear if STAMBPL1 directly disassembles polyubiquitin chains from Tax or another target that regulates Tax trafficking. Thus far, we have been unable to detect an interaction between Tax and STAMBPL1 by co-IP (data not shown). Nevertheless, we performed an in vitro DUB assay using immunoaffinity-purified STAMBPL1. 293T cells were transfected with Flag-Tax together with wild-type HA-Ub, K63-only HA-Ub, or K48-only HA-Ub. Tax was immunoprecipitated from lysates using Flag antibody and eluted with Flag peptide. The ubiquitinated Tax proteins (either wild type, K48 only, or K63 only) were incubated with STAMBPL1 for a DUB assay. STAMBPL1 had little, if any, deubiquitination activity on either wild-type, K48-, or K63-linked polyubiquitin chains on Tax in vitro (Fig. 7A). However, STAMBPL1 effectively hydrolyzed recombinant K63-linked penta-ubiquitin (Ub5) chains in a DUB assay (Fig. 7B). Therefore, STAMBPL1 does not directly deubiquitinate Tax in vitro, suggesting that it likely regulates Tax nuclear export via an indirect mechanism.
Fig 7.
STAMBPL1 does not directly deubiquitinate Tax. (A) STAMBPL1 is not a DUB for Tax in vitro. 293T cells were transfected with pCMV4-Tax and HA-ubiquitin (Ub), K63-only HA-Ub, or K48-only HA-Ub. The lysates were subjected to immunoprecipitation using anti-Flag (α-Flag). The immunoprecipitated proteins were eluted with 3× Flag peptide and incubated with immunoaffinity-purified human STAMBPL1 (1 μg) for 18 h at 37°C in DUB buffer. Proteins were resolved by SDS-PAGE and subjected to immunoblotting using anti-HA (α-HA) and anti-Tax (α-Tax). (B) STAMBPL1 is a DUB for K63-linked polyubiquitin chains. Purified STAMBPL1 (1 μg) was incubated with K63-linked penta-ubiquitin chains (Ub5) at 37°C for 18 h. Proteins were resolved by SDS-PAGE and subjected to immunoblotting using anti-K63-specific Ub (α-Ub).
DISCUSSION
HTLV-1 Tax trafficking in cells is dynamic and is controlled by posttranslational modifications, most notably ubiquitination and sumoylation (32). Whereas K63-linked polyubiquitination of Tax promotes cytoplasmic and Golgi localization of Tax, sumoylation of Tax promotes its nuclear localization (27). Tax shuttles between distinct subcellular compartments, such as nuclear bodies and the cis-Golgi, to carry out different functions. Whereas Tax predominantly activates NF-κB in the cytoplasm and Golgi apparatus, it activates LTR-dependent viral gene expression in the nucleus. However, the host factors that regulate NF-κB activation and Tax trafficking have not yet been identified. To this end, we used an RNAi approach by screening a DUB siRNA library to identify DUBs that regulate Tax activation of NF-κB. Although a number of negative regulators were identified, STAMBPL1 was one of the few positive regulators of Tax-mediated NF-κB activation. STAMBPL1, a JAMM family member, functions as a metalloprotease with specificity for K63-linked polyubiquitination chains (37).
Our results indicate that STAMBPL1 potentiates Tax-mediated NF-κB activation indirectly by controlling its nuclear export. The effects of STAMBPL1 are specific for Tax since STAMBPL1 knockdown had no effect on TNF-α- or IL-1-induced NF-κB activation, and overexpression of STAMBPL1 by itself did not activate NF-κB. We have also found that knockdown of STAMBPL1 greatly diminished the stability of Tax, as observed by CHX chase assays. Tax is subject to proteasomal degradation in the nucleus, specifically in the nuclear matrix, upon catalysis of K48-linked polyubiquitin chains by the E3 ubiquitin ligase PDLIM2 (54). Since STAMBPL1 is required to export Tax from the nucleus, it is likely that in the absence of STAMBPL1, Tax is destabilized due to PDLIM2 targeting Tax for degradation in the nucleus. Indeed, we have demonstrated increased K48-linked polyubiquitination of Tax upon knockdown of STAMBPL1. Furthermore, the proteasome inhibitor MG-132 rescued the degradation of nuclear Tax that was more pronounced in the absence of STAMBPL1. As such, the amount of Tax in the nucleus was significantly increased upon proteasome inhibition, particularly when LMB was used to prevent Tax nuclear export.
Previous studies have demonstrated that genotoxic stress and DNA damage promote the nuclear export of Tax (11, 12). Tax contains a leucine-rich NES that is normally masked since Tax nucleocytoplasmic trafficking is insensitive to LMB treatment unless cells are exposed to stress, such as UV or ionizing radiation (1, 12). Therefore, stress may trigger a conformational change in Tax which unmasks the NES to facilitate nuclear export. UV stimulation is known to cause the monoubiquitination of Tax at K280 and K284, which promotes nuclear export via a CRM1-dependent pathway (11). Our results are consistent with these studies in that we have observed dramatic relocalization of Tax from the TSS in the nucleus to the cytoplasm upon UV stimulation. The requirement of STAMBPL1 for Tax nuclear export is readily observed when cells are exposed to UV light. A recent study has demonstrated that the SUMO-targeted E3 ubiquitin ligase RNF4 also regulates Tax trafficking and nuclear export in response to genotoxic stress (9). However, RNF4 directly ubiquitinates Tax to trigger its nuclear export. It will be interesting to determine if there is any cross talk between STAMBPL1 and RNF4 in the regulation of Tax nuclear/cytoplasmic transport upon genotoxic stress.
Knockdown of STAMBPL1 with siRNA decreases the colocalization of Tax with the cis-Golgi marker GM-130. We have previously demonstrated that Tax relocalizes the IKK complex to the cis-Golgi as part of its mechanism to activate IKK and NF-κB persistently (20, 41). Tax K63-linked polyubiquitination, which is dependent on the E2 ubiquitin-conjugating enzyme Ubc13, is critical for interactions with IKKγ and the subsequent relocalization of IKK to the cis-Golgi (20, 22, 33) or centrosome (26). In addition, a subset of Tax in cells may be detected in the cis-Golgi compartment (33). STAMBPL1 regulates Tax activation of NF-κB by shuttling Tax from the nucleus to the cis-Golgi, where IKK is persistently activated (26).
Since STAMBPL1 regulates the stability of Tax and its K48-linked polyubiquitination, we initially hypothesized that STAMBPL1 directly removed K48-linked polyubiquitin chains from Tax. In this regard, recent studies have demonstrated that USP20 and CYLD deubiquitinases hydrolyze K63-linked polyubiquitin chains from Tax and therefore suppress Tax-induced NF-κB activation (50, 55). However, STAMBPL1 was incapable of hydrolyzing K48- and K63-linked polyubiquitin chains from Tax in vitro, although it effectively cleaved recombinant K63-linked penta-ubiquitin chains. Furthermore, we were unable to observe an interaction between STAMBPL1 and Tax by co-IP (data not shown) or colocalization using confocal microscopy, although STAMBPL1 is predominantly a nuclear protein (Fig. 3D). Taken together, these results indicate that STAMBPL1 may not directly act as a DUB for Tax, but may indirectly regulate Tax via a distinct factor that regulates Tax nuclear export. A potential candidate for STAMBPL1 regulation is the nuclear export factor CRM1, which interacts with and facilitates the nuclear export of Tax in response to genotoxic stress (12). Future studies will determine if STAMBPL1 regulates CRM1 ubiquitination and genotoxic stress-induced interactions with Tax. Furthermore, the effect of STAMBPL1 appears to be specific for Tax trafficking because knockdown of STAMBPL1 had no effect on the nuclear export of p53 (data not shown). Another possibility, although less likely, is that STAMBPL1 may indeed function as a DUB for Tax, but may require cofactors or adaptor molecules that are lacking in the in vitro DUB assay. For example, the DUBs A20 and CYLD require adaptor molecules to confer target specificity in vivo (17). Additional studies are needed to determine the precise target(s) of STAMBPL1 that regulates Tax nuclear export.
ACKNOWLEDGMENTS
We thank S. C. Sun, B. Wigdahl, I. Verma, J. Wade Harper, and U. Bertazzoni for plasmids, G. McNamara for assistance with confocal microscopy, and S. Charoenthongtrakul for critical reading of the manuscript.
These studies were supported by NIH grant RO1CA135362 awarded to E.W.H.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Alefantis T, Barmak K, Harhaj EW, Grant C, Wigdahl B. 2003. Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J. Biol. Chem. 278:21814–21822 [DOI] [PubMed] [Google Scholar]
- 2. Alefantis T, et al. 2005. Secretion of the human T cell leukemia virus type I transactivator protein tax. J. Biol. Chem. 280:17353–17362 [DOI] [PubMed] [Google Scholar]
- 3. Bex F, Murphy K, Wattiez R, Burny A, Gaynor RB. 1999. Phosphorylation of the human T-cell leukemia virus type 1 transactivator Tax on adjacent serine residues is critical for Tax activation. J. Virol. 73:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burton M, Upadhyaya CD, Maier B, Hope TJ, Semmes OJ. 2000. Human T-cell leukemia virus type 1 Tax shuttles between functionally discrete subcellular targets. J. Virol. 74:2351–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charoenthongtrakul S, Zhou Q, Shembade N, Harhaj NS, Harhaj EW. 2011. HTLV-I Tax inhibits innate antiviral signaling via NF-κB-dependent induction of SOCS1. J. Virol. 85:6955–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiari E, et al. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 Tax is required for proteasome binding. J. Virol. 78:11823–11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Claudio E, Brown K, Park S, Wang H, Siebenlist U. 2002. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat. Immunol. 3:958–965 [DOI] [PubMed] [Google Scholar]
- 8. Ducut Sigala JL, et al. 2004. Activation of transcription factor NF-κB requires ELKS, an IκB kinase regulatory subunit. Science 304:1963–1967 [DOI] [PubMed] [Google Scholar]
- 9. Fryrear KA, Guo X, Kerscher O, Semmes OJ. 21 November 2011, posting date The SUMO-targeted ubiquitin ligase RNF4 regulates the localization and function of the HTLV-1 oncoprotein Tax. Blood. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu J, et al. 2011. The tumor suppressor gene WWOX links the canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 117:1652–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gatza ML, Dayaram T, Marriott SJ. 2007. Ubiquitination of HTLV-I Tax in response to DNA damage regulates nuclear complex formation and nuclear export. Retrovirology 4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gatza ML, Marriott SJ. 2006. Genotoxic stress and cellular stress alter the subcellular distribution of human T-cell leukemia virus type 1 Tax through a CRM1-dependent mechanism. J. Virol. 80:6657–6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh S, Hayden MS. 2008. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 8:837–848 [DOI] [PubMed] [Google Scholar]
- 14. Grabbe C, Husnjak K, Dikic I. 2011. The spatial and temporal organization of ubiquitin networks. Nat. Rev. Mol. Cell Biol. 12:295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant C, et al. 2002. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell Physiol. 190:133–159 [DOI] [PubMed] [Google Scholar]
- 16. Grassmann R, Aboud M, Jeang KT. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 24:5976–5985 [DOI] [PubMed] [Google Scholar]
- 17. Harhaj EW, Dixit VM. 2011. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 21:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harhaj EW, Sun SC. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911–22914 [DOI] [PubMed] [Google Scholar]
- 19. Harhaj NS, Janic B, Ramos JC, Harrington WJ, Jr, Harhaj EW. 2007. Deregulated expression of CD40 ligand in HTLV-I infection: distinct mechanisms of downregulation in HTLV-I-transformed cell lines and ATL patients. Virology 362:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harhaj NS, Sun SC, Harhaj EW. 2007. Activation of NF-κB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IκB kinase. J. Biol. Chem. 282:4185–4192 [DOI] [PubMed] [Google Scholar]
- 21. Hicke L, Dunn R. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141–172 [DOI] [PubMed] [Google Scholar]
- 22. Huang J, Ren T, Guan H, Jiang Y, Cheng H. 2009. HTLV-1 Tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-κB. J. Biol. Chem. 284:6208–6217 [DOI] [PubMed] [Google Scholar]
- 23. Jeang KT, Giam CZ, Majone F, Aboud M. 2004. Life, death, and tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 279:31991–31994 [DOI] [PubMed] [Google Scholar]
- 24. Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663 [DOI] [PubMed] [Google Scholar]
- 25. Kerscher O, Felberbaum R, Hochstrasser M. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22:159–180 [DOI] [PubMed] [Google Scholar]
- 26. Kfoury Y, et al. 2008. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene 27:1665–1676 [DOI] [PubMed] [Google Scholar]
- 27. Kfoury Y, et al. 2011. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 117:190–199 [DOI] [PubMed] [Google Scholar]
- 28. Kim MS, Kim JA, Song HK, Jeon H. 2006. STAM-AMSH interaction facilitates the deubiquitination activity in the C-terminal AMSH. Biochem. Biophys. Res. Commun. 351:612–618 [DOI] [PubMed] [Google Scholar]
- 29. Liang J, et al. 2010. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κΒ signaling. J. Exp. Med. 207:2959–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marriott SJ, Semmes OJ. 2005. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 24:5986–5995 [DOI] [PubMed] [Google Scholar]
- 31. McCullough J, Clague MJ, Urbe S. 2004. AMSH is an endosome-associated ubiquitin isopeptidase. J. Cell Biol. 166:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nasr R, et al. 2006. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-κB activation. Blood 107:4021–4029 [DOI] [PubMed] [Google Scholar]
- 33. Nejmeddine M, Barnard AL, Tanaka Y, Taylor GP, Bangham CR. 2005. Human T-lymphotropic virus, type 1, Tax protein triggers microtubule reorientation in the virological synapse. J. Biol. Chem. 280:29653–29660 [DOI] [PubMed] [Google Scholar]
- 34. Ossareh-Nazari B, Bachelerie F, Dargemont C. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141–144 [DOI] [PubMed] [Google Scholar]
- 35. Pickart CM, Eddins MJ. 2004. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695:55–72 [DOI] [PubMed] [Google Scholar]
- 36. Qu Z, Xiao G. 2011. Human T-cell lymphotropic virus: a model of NF-κB-associated tumorigenesis. Viruses 3:714–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato Y, et al. 2008. Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature 455:358–362 [DOI] [PubMed] [Google Scholar]
- 38. Semmes OJ, Jeang KT. 1996. Localization of human T-cell leukemia virus type 1 Tax to subnuclear compartments that overlap with interchromatin speckles. J. Virol. 70:6347–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senftleben U, et al. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495–1499 [DOI] [PubMed] [Google Scholar]
- 40. Shembade N, et al. 2008. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 9:254–262 [DOI] [PubMed] [Google Scholar]
- 41. Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. 2007. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-κB activation. J. Virol. 81:13735–13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shembade N, Ma A, Harhaj EW. 2010. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327:1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skaug B, Jiang X, Chen ZJ. 2009. The role of ubiquitin in NF-κB regulatory pathways. Annu. Rev. Biochem. 78:769–796 [DOI] [PubMed] [Google Scholar]
- 44. Smith MR, Greene WC. 1992. Characterization of a novel nuclear localization signal in the HTLV-I Tax transactivator protein. Virology 187:316–320 [DOI] [PubMed] [Google Scholar]
- 45. Sowa ME, Bennett EJ, Gygi SP, Harper JW. 2009. Defining the human deubiquitinating enzyme interaction landscape. Cell 138:389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun SC. 2011. Non-canonical NF-κB signaling pathway. Cell Res. 21:71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeuchi K, Kobayashi N, Nam SH, Yamamoto N, Hatanaka M. 1985. Molecular cloning of cDNA encoding gp68 of adult T-cell leukaemia-associated antigen: evidence for expression of the pX IV region of human T-cell leukaemia virus. J. Gen. Virol. 66:1825–1829 [DOI] [PubMed] [Google Scholar]
- 48. Turci M, et al. 2009. HTLV-2B Tax oncoprotein is modified by ubiquitination and sumoylation and displays intracellular localization similar to its homologue HTLV-1 Tax. Virology 386:6–11 [DOI] [PubMed] [Google Scholar]
- 49. Wilkinson KD. 2009. DUBs at a glance. J. Cell Sci. 122:2325–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu X, Zhang M, Sun SC. 2011. Mutual regulation between deubiquitinase CYLD and retroviral oncoprotein Tax. Cell Biosci. 1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiao G, et al. 2001. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J. 20:6805–6815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiao G, Harhaj EW, Sun SC. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7:401–409 [DOI] [PubMed] [Google Scholar]
- 53. Xiao G, Rabson AB, Young W, Qing G, Qu Z. 2006. Alternative pathways of NF-κB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 17:281–293 [DOI] [PubMed] [Google Scholar]
- 54. Yan P, et al. 2009. PDLIM2 suppresses human T-cell leukemia virus type I Tax-mediated tumorigenesis by targeting Tax into the nuclear matrix for proteasomal degradation. Blood 113:4370–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yasunaga J, Lin FC, Lu X, Jeang KT. 2011. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 Tax to negatively regulate NF-κB signaling. J. Virol. 85:6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoshida M, Miyoshi I, Hinuma Y. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. U. S. A. 79:2031–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]