Abstract
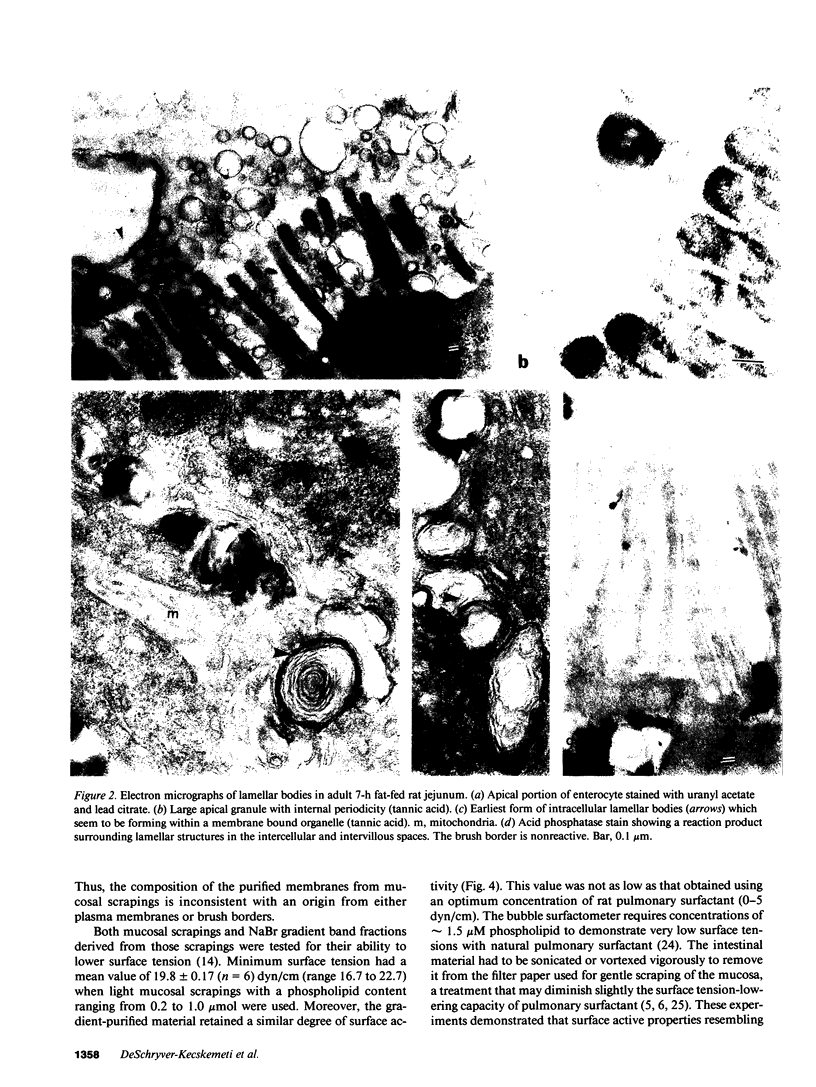
Surface-active phospholipid-containing particles are traditionally considered to be the product of type II pneumocytes. We now demonstrate membrane-bound lamellar cytoplasmic organelles in adult and suckling rat enterocytes that are densely reactive with phospholipid-staining reagents. These structures were seen in the basolateral space, within the intercellular junctions, and unraveling on the lumenal surface, and were more abundant after fat feeding. Light scrapings of intestinal mucosa and lumenal washings that contained these bodies, as evidenced by morphology and biochemical analysis, lowered surface tension in a pulsating bubble assay. Production by normal enterocytes of material with surfactant-like appearance and properties demonstrates that these structures are present in extrapulmonary epithelia, and extends the possible range of their function beyond gaseous exchange, e.g., solute exchange or lubrication on membrane surfaces.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Black B. L., Yoneyama Y., Moog F. Microvillous membrane vesicle accumulation in media during culture of intestine of chick embryo. Biochim Biophys Acta. 1980 Sep 18;601(2):343–348. doi: 10.1016/0005-2736(80)90538-6. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Yeh K. Y., Holt P. R., Schachter D. Lipid fluidity and composition of intestinal microvillus membranes isolated from rats of different ages. Biochim Biophys Acta. 1984 Dec 5;778(2):341–348. doi: 10.1016/0005-2736(84)90378-x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst D., Lathe G. H., Aparicio S. R. Serum alkaline phosphatase, nucleotide pyrophosphatase, 5'-nucleotide and lipoprotein-X in cholestasis. Clin Chim Acta. 1976 Mar 15;67(3):269–279. doi: 10.1016/0009-8981(76)90335-1. [DOI] [PubMed] [Google Scholar]
- Butler B. D., Lichtenberger L. M., Hills B. A. Distribution of surfactants in the canine gastrointestinal tract and their ability to lubricate. Am J Physiol. 1983 Jun;244(6):G645–G651. doi: 10.1152/ajpgi.1983.244.6.G645. [DOI] [PubMed] [Google Scholar]
- De Broe M. E., Roels F., Nouwen E. J., Claeys L., Wieme R. J. Liver plasma membrane: the source of high molecular weight alkaline phosphatase in human serum. Hepatology. 1985 Jan-Feb;5(1):118–128. doi: 10.1002/hep.1840050124. [DOI] [PubMed] [Google Scholar]
- De Broe M. E., Wieme R. J., Logghe G. N., Roels F. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977 Dec 15;81(3):237–245. doi: 10.1016/0009-8981(77)90054-7. [DOI] [PubMed] [Google Scholar]
- Ehle H., Bublitz R., Horn A. Intralumenal alkaline phosphatase of the calf intestine. Biomed Biochim Acta. 1985;44(2):223–233. [PubMed] [Google Scholar]
- Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- Gil J. Histological preservation and ultrastructure of alveolar surfactant. Annu Rev Physiol. 1985;47:753–763. doi: 10.1146/annurev.ph.47.030185.003541. [DOI] [PubMed] [Google Scholar]
- Groniowski J. A. Fine structural basis of pulmonary surfactant. Int Rev Exp Pathol. 1983;25:183–238. [PubMed] [Google Scholar]
- Harwood J. L., Richards R. J. Lung surfactant. Mol Aspects Med. 1985;8(5):423–514. doi: 10.1016/0098-2997(85)90012-3. [DOI] [PubMed] [Google Scholar]
- Hauser H., Howell K., Dawson R. M., Bowyer D. E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta. 1980 Nov 18;602(3):567–577. doi: 10.1016/0005-2736(80)90335-1. [DOI] [PubMed] [Google Scholar]
- Hills B. A., Butler B. D., Lichtenberger L. M. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983 May;244(5):G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R. Biochemical and ultrastructural characterization of the molecular topography of the rat intestinal microvillous membrane. Asymmetric distribution of hydrophilic groups and anionic binding sites. Gastroenterology. 1983 Jul;85(1):46–54. [PubMed] [Google Scholar]
- Kalina M., Pease D. C. The preservation of ultrastructure in saturated phosphatidyl cholines by tannic acid in model systems and type II pneumocytes. J Cell Biol. 1977 Sep;74(3):726–741. doi: 10.1083/jcb.74.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y. C., Lichtenberger L. M. Localization of phospholipid-rich zones in rat gastric mucosa: possible origin of a protective hydrophobic luminal lining. J Histochem Cytochem. 1987 Nov;35(11):1285–1298. doi: 10.1177/35.11.2443559. [DOI] [PubMed] [Google Scholar]
- Kawai K., Fujita M., Nakao M. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim Biophys Acta. 1974 Nov 18;369(2):222–233. [PubMed] [Google Scholar]
- Komoda T., Koyama I., Nagata A., Sakagishi Y., DeSchryver-Kecskemeti K., Alpers D. H. Ontogenic and phylogenic studies of intestinal, hepatic, and placental alkaline phosphatases. Evidence that intestinal alkaline phosphatase is a late evolutionary development. Gastroenterology. 1986 Aug;91(2):277–286. doi: 10.1016/0016-5085(86)90558-5. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd Cytochemistry of pulmonary alveolar epithelial cells. Am J Pathol. 1968 Nov;53(5):809–833. [PMC free article] [PubMed] [Google Scholar]
- Lavker R. M. Membrane coating granules: the fate of the discharged lamellae. J Ultrastruct Res. 1976 Apr;55(1):79–86. doi: 10.1016/s0022-5320(76)80083-4. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Pappenheimer J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Misch D. W., Giebel P. E., Faust R. G. Intestinal microvilli: responses to feeding and fasting. Eur J Cell Biol. 1980 Aug;21(3):269–279. [PubMed] [Google Scholar]
- Neutra M. R., Ciechanover A., Owen L. S., Lodish H. F. Intracellular transport of transferrin- and asialoorosomucoid-colloidal gold conjugates to lysosomes after receptor-mediated endocytosis. J Histochem Cytochem. 1985 Nov;33(11):1134–1144. doi: 10.1177/33.11.2997327. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Taeusch H. W., Jr, Benson B., Hawgood S. An electrophoretic and immunochemical characterization of human surfactant-associated proteins. Biochim Biophys Acta. 1984 Dec 7;791(2):226–238. doi: 10.1016/0167-4838(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Sanders R. L., Longmore W. J. Phosphatidyglycerol in rat lung. II. Comparison of occurrence, composition, and metabolism in surfactant and residual lung fractions. Biochemistry. 1975 Feb 25;14(4):835–840. doi: 10.1021/bi00675a030. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Possmayer F. Calcium interactions in pulmonary surfactant. Biochim Biophys Acta. 1984 Oct 24;796(1):83–91. doi: 10.1016/0005-2760(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Ultrastructure of tubular myelin and lamellar bodies in fast-frozen adult rat lung. Exp Lung Res. 1982 Dec;4(1):37–46. doi: 10.3109/01902148209039248. [DOI] [PubMed] [Google Scholar]