Abstract
We recently described the effect of the constitutively expressed chaperone, Hsc70 protein, on α‑Synuclein aggregation, a phenomenon associated with Parkinson disease. In vitro, Hsc70 binds to soluble α‑Syn and slows down its assembly into fibrils. Hsc70 also binds fibrillar α‑Syn, 5-fold tighter than soluble α‑Syn. This interaction reduces the cytotoxicity associated with naked α‑Syn fibrils. Herein, we discuss the feasibility of engineering a “minichaperone” which could be used against α‑Syn assembly propagation in Parkinson disease: taking what is necessary and sufficient within Hsc70 to protect against the damaging repercussions of high molecular weight α‑Syn species’ passage from one neuron to another in the brain.
Keywords: alpha synuclein, chaperones, heat shock protein, Hsc70, Parkinson’s disease
Intracellular Lewy bodies (LBs) and Lewy neurites (LNs) which contain a fibrillar form of the synaptic terminal protein α‑Synuclein (α‑Syn) as their principal component are neuropathological markers of Parkinson disease (PD).1 Although extensive research has previously shown various members of the human molecular chaperone family to hinder or promote the progression of soluble α‑Syn into fibrils (Hsp70, Hsp27, αB‑crystallin or Hsp90 respectively; for a review see refs. 2–3), no one had yet to investigate the effect of the constitutively expressed human heat shock protein, Hsc70. This was surprising as not only have Hsp70 and Hsc70 been shown to have different cellular roles and different effects on aggregated luciferase and polyQ protein but being constitutively expressed, Hsc70 is the first molecular chaperone to encounter assembling α‑Syn in a cellular context.4,5
We showed that Hsc70 inhibits the assembly of α‑Syn into fibrils, by binding with high affinity to the soluble form of α‑Syn. We also showed that Hsc70 binds fibrillar α‑Syn, with a dissociation constant 5-fold lower than that we measured for soluble α‑Syn. Hsc70 binding to fibrils has a cytoprotective effect, as it renders α‑Syn fibrils less toxic to mammalian cultured cells.6
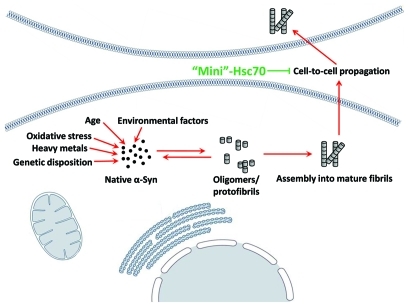
The point mutations within α‑Syn at the origin of familial early onset PD suggest that the stochastic aggregation of α‑Syn within cells is an important factor which contributes to the onset of PD.7,8 A second recently discovered and documented factor, namely the propagation of aggregates from one cell to another accounts for the neurodegenerative patterns described, which play a critical role in PD.9,10 Indeed, exogenous α‑Syn fibrils enter cells and recruit soluble endogenous α‑Syn, which results in synaptic dysfunction and neuronal cell death.11,12 As our work demonstrated that Hsc70 reduces the toxicity of fibrils most likely because binding alters their physicochemical properties, there is a possibility that Hsc70 could be effective as a therapeutic agent in PD, by abolishing the cell to cell transfer of α‑Syn fibrils, as schematised in Figure 1.
Figure 1.
Possible protective mechanism of Hsc70 as a therapeutic agent. A minichaperone based on Hsc70 could be used to reduce the toxicity of α‑Syn fibrils by binding to extracellular fibrils, altering their physicochemical properties, and preventing cell-to-cell propagation.
Furthermore, we demonstrated that Hsc70 is capable of binding fibrillar α‑Syn in a nucleotide independent manner and in the absence of co-chaperones.6 This suggests that Hsc70s α‑Syn binding site, or a subset of this site may be used as a therapeutic tool to modify fibrillar α‑Syn properties and as a consequence their binding to and uptake by neurons. Thus further characterization of Hsc70 residues that interact specifically with fibrillar α‑Syn using a cross-linking and mass spectrometry approach will lead, as in the case of the GroEL apical domain,13-15 to the design of an Hsc70 derived minichaperone.
However, there is a chance that basing a therapeutic agent on Hsc70, an endogenous protein, may evoke an autoimmune response when introduced to the body. An exogenous engineered polypeptide which has a similar if not the same effect on α‑Syn toxicity and crosses the blood-brain barrier to reach its target may be more successful as an eventual therapeutic agent – does the answer to PD lie in a bacterial or yeast homolog of Hsc70?
Glossary
Abbreviations:
- α‑Syn
alpha synuclein
- Hsc
constitutive heat shock protein
- LB
Lewy body
- LN
Lewy neurite
- PD
Parkinson’s disease
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18483
References
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson's disease: emerging role of molecular chaperones. Trends Mol Med. 2010;16:27–36. doi: 10.1016/j.molmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Arawaka S, Machiya Y, Kato T. Heat shock proteins as suppressors of accumulation of toxic prefibrillar intermediates and misfolded proteins in neurodegenerative diseases. Curr Pharm Biotechnol. 2010;11:158–66. doi: 10.2174/138920110790909713. [DOI] [PubMed] [Google Scholar]
- 4.Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, et al. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci USA. 2006;103:5817–22. doi: 10.1073/pnas.0507903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J. 2011;435:127–42. doi: 10.1042/BJ20101247. [DOI] [PubMed] [Google Scholar]
- 6.Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. Hsc70 Protein Interaction with Soluble and Fibrillar {alpha}-Synuclein. J Biol Chem. 2011;286:34690–9. doi: 10.1074/jbc.M111.261321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, et al. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–18. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Uversky VN, Fink AL. Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–13. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 10.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatellier J, Hill F, Lund PA, Fersht AR. In vivo activities of GroEL minichaperones. Proc Natl Acad Sci USA. 1998;95:9861–6. doi: 10.1073/pnas.95.17.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golbik R, Zahn R, Harding SE, Fersht AR. Thermodynamic stability and folding of GroEL minichaperones. J Mol Biol. 1998;276:505–15. doi: 10.1006/jmbi.1997.1538. [DOI] [PubMed] [Google Scholar]
- 15.Zahn R, Buckle AM, Perrett S, Johnson CM, Corrales FJ, Golbik R, et al. Chaperone activity and structure of monomeric polypeptide binding domains of GroEL. Proc Natl Acad Sci USA. 1996;93:15024–9. doi: 10.1073/pnas.93.26.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]