Abstract
Tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) can control cancer growth and exist in almost all solid neoplasms. The cells are known to descend from immature monocytic and granulocytic cells, respectively, which are produced in the bone marrow. However, the spleen is also a recently identified reservoir of monocytes, which can play a significant role in the inflammatory response that follows acute injury. Here, we evaluated the role of the splenic reservoir in a genetic mouse model of lung adenocarcinoma driven by activation of oncogenic Kras and inactivation of p53. We found that high numbers of TAM and TAN precursors physically relocated from the spleen to the tumor stroma, and that recruitment of tumor-promoting spleen-derived TAMs required signaling of the chemokine receptor CCR2. Also, removal of the spleen, either before or after tumor initiation, reduced TAM and TAN responses significantly and delayed tumor growth. The mechanism by which the spleen was able to maintain its reservoir capacity throughout tumor progression involved, in part, local accumulation in the splenic red pulp of typically rare extramedullary hematopoietic stem and progenitor cells, notably granulocyte and macrophage progenitors, which produced CD11b+ Ly-6Chi monocytic and CD11b+ Ly-6Ghi granulocytic cells locally. Splenic granulocyte and macrophage progenitors and their descendants were likewise identified in clinical specimens. The present study sheds light on the origins of TAMs and TANs, and positions the spleen as an important extramedullary site, which can continuously supply growing tumors with these cells.
Keywords: cancer immunity, tumor microenvironment
Macrophages and neutrophils participate in defense mechanisms that protect the host against injury and infection. However, extensive animal and clinical studies now indicate that these cells also infiltrate most solid human cancers. Tumor-associated macrophages (TAMs) can stimulate tumor growth (1–4), and their density is associated with adverse outcomes and shorter survival in several cancer types, including breast cancer, Hodgkin lymphoma, and lung adenocarcinoma (5–8). Tumor-associated neutrophils (TANs) can also promote the progression of primary tumors; however, blockade of TGF-β signaling induces a population of antitumor TANs (3). Also, neutrophils in tumor-bearing subjects can act to eliminate disseminated tumor cells, and thus provide antimetastatic protection (9). Although the interactions of TAMs and TANs with neoplastic cells are being unraveled, the origin of TAMs and TANs, and the impact of cancer on these cells’ precursors in vivo, remains less well explored.
Our understanding of the origins of tissue macrophages and neutrophils, going back to self-renewing hematopoietic stem cells (HSCs), is largely based on studies not involving cancer (10–13). These studies have outlined how HSCs located in specialized niches of the bone marrow give rise to progeny that progressively lose self-renewal capacity and commit to certain lineages. Granulocyte/macrophage progenitors (GMPs), for example, are clonogenic bone marrow cells that descend from HSCs and commit to either neutrophils or monocytes. The latter cells are released into circulation and can extravasate in distant tissue (1, 14, 15). The extravasation process is typically concurrent with activation of an irreversible cell differentiation program (5, 13, 16): Circulating monocytes become tissue macrophages (or dendritic cells), whereas circulating neutrophils become activated tissue neutrophils. Extending this to the tumor microenvironment, recent studies have shown that circulating Ly-6Chi “inflammatory” monocytes accumulate in tumors and renew nonproliferating TAM populations (15). TAMs and TANs are also sometimes described as descendants of myeloid-derived suppressor cells (MDSCs). MDSCs in mice are defined as CD11b+ Gr-1+ bone marrow-derived immature cells and are composed of (Ly-6Chi) monocytic and (Ly-6Ghi) granulocytic cells. Ly-6Chi inflammatory monocytes may contribute to monocytic MDSCs associated with tumors (13). In sum, the prevailing paradigm is that TAM and TAN populations derive from monocytic and granulocytic progenitors, which are made in the bone marrow.
However, the spleen has recently been shown to constitute a unique extramedullary reservoir of myeloid cells, specifically monocytes (17), which can be mobilized in response to distant acute inflammation and significantly contribute to the host response. Here, we thought to study the role of the myeloid reservoir in the context of cancer. We performed our animal studies in a conditional genetic mouse model of lung adenocarcinoma (KrasLSL-G12D/+;p53fl/fl; hereafter referred to as KP), which enables generation of autochthonous tumors from a few somatic cells via activation of oncogenic Kras and inactivation of p53 (18). Such genetically engineered mice have shown promise in guiding clinical research because they permit the study of cancers as they develop de novo and as they evolve within their natural environments (18, 19). The lesions of the mice recapitulate the genetic alterations found in the human disease, progress to high-grade tumors, and are infiltrated by TAMs and TANs.
The findings in the KP model indicate that the spleen mobilizes immature myeloid cells and that these cells amplify TAM and TAN responses on recruitment to tumors. Also, HSCs and GMPs accumulate in large numbers within the splenic red pulp of tumor-bearing mice. These cells establish niches of proliferation, produce new CD11b+ Ly-6Chi monocytic and CD11b+ Ly-6Ghi granulocytic cells locally, and participate in the continuous replenishment of the myeloid cell reservoir. The identification of these processes should contribute to our understanding of myeloid responses in cancer and provide new vantage points for therapy.
Results
Spleen Contributes TAMs and TANs.
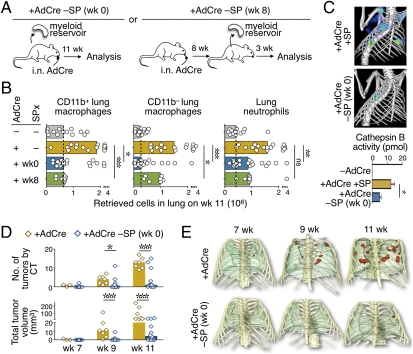
The lung contains different populations of macrophages, which can express CD11b at variable levels (20) and include CD11b− CD11c+ F4/80+ alveolar macrophages. To test whether the spleen participates in macrophage responses during tumor progression, we splenectomized KP mice at week 0 (at tumor initiation) (Fig. 1A) via a procedure that preserves bone marrow and blood cell pools (17). The procedure reduced both CD11b+ and CD11b− lung macrophages (TAMs) by week 11 in a large fraction of animals (Fig. 1B and Fig. S1). Splenectomy also significantly reduced the numbers of CD11b+ Gr1+ Ly-6G+ lung tissue neutrophils (TANs) (Fig. 1B).
Fig. 1.
Spleen removal impairs TAM and TAN responses and tumor growth. (A) Experimental procedures. (B) Total CD11b+ and CD11b− macrophage and neutrophil counts in lungs of mice 11 wk after tumor initiation [+ adenovirus expressing Cre recombinase (AdCre), n = 17] and in mice from which the spleen was removed either immediately before tumor initiation [+AdCre SPx (wk 0), n = 19] or after tumors became apparent [+AdCre SPx (wk 8), n = 7]. Control mice (−AdCre, n = 13) were age-matched. Histograms show median values, and dots represent single mice. (C) In vivo fluorescent-mediated tomography/CT fusion images and quantification of Cathepsin B activity at week 11 after AdCre infection in KP mice with (+AdCre +SP) or without [+AdCre −SP (wk 0)] a spleen (n = 3). Data are presented as the mean ± SEM. (D and E) High-resolution CT analysis of tumor progression at weeks 7, 9, and 11 in mice with (+AdCre, n = 7) or without [+AdCre −SP (wk 0), n = 12] a spleen. (D) Number and volume of tumor nodules in each group. Histograms show median values, and dots represent single mice. (E) Representative 3D volume renderings of tumors for each group. Tumors are shown in red, and lungs are shown in green. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
The experiment was repeated with mice that were splenectomized at week 8 instead of week 0 (Fig. 1A) (i.e., mice with preestablished cancer). These mice likewise showed impaired TAM and TAN responses by week 11 (Fig. 1B). This finding supports the previous notion that TAMs and TANs are continuously replaced during tumor progression (14, 15) and suggests that the spleen acts to contribute these cells at least after week 8. The spleen was also found to be required for full accumulation of TAMs and TANs in two grafted tumor models (Fig. S2).
TAMs produce proteases that degrade ECM proteins and enhance tumor cell invasion and intravasation (21). Ex vivo flow cytometry analysis of KP mice that received a cysteine Cathepsin B-targeted optical sensor revealed high cathepsin activity in TAMs, as expected, but also in splenic monocytes (Fig. S3). We next compared proteolytic activity in vivo by combining the optical sensor with fluorescent-mediated tomography/computed tomography (CT) fusion imaging (2). The lungs of spleen-deficient animals showed significantly reduced proteolytic activity (Fig. 1C), in line with the above findings that splenectomy reduced the TAM and TAN responses. Other tumor-promoting functions have been identified for TAMs (5) and may also be relevant in KP mice.
Spleen Contributes to Tumor Growth.
Given that removal of the spleen prevented full-fledged TAM accumulation and TAM-associated tumor-promoting activity, we investigated whether it also affected tumor progression. To do this, spleen-sufficient and spleen-deficient animals were monitored by CT at weeks 7, 9, and 11 after tumor initiation. The majority of KP mice from which the spleen was removed showed decreased numbers of detectable tumor nodules and reduced total tumor volumes over time (Fig. 1 D and E). Histopathological analysis at week 11 (Fig. S4) confirmed reduced tumor progression in the majority of spleen-deficient mice. This same phenotype was observed regardless of whether mice were splenectomized at week 0 or at week 8. In both cases, splenectomized mice often presented with fewer and smaller tumor nodules, which were either grade 1 adenomatous hyperplasias or grade 2 adenomas compared with grade 2 or grade 3 adenocarcinomas in age-matched control animals.
Splenectomy decreased the TAM response before changes in tumor progression could occur (Fig. S5A), which is in accordance with a causal association between TAMs and tumor progression. Also, in support of the notion that the spleen contributed cells to tumors directly, the number of blood CD11b+ Ly-6Chi monocytic cells decreased shortly after splenectomy in tumor-bearing KP animals (Fig. S5 B and C). Splenectomy likely did not affect bone marrow hematopoiesis because bone marrow leukocyte counts remain normal in steady state (17) and in the presence of growing tumors (Fig. S5D).
Histopathological analysis indicated that the tumor burden at week 11 in spleen-deficient KP mice was similar (i.e., not lower) to that at week 8 in spleen-sufficient mice (Fig. S4); thus, splenectomy delayed tumor progression but did not induce tumor rejection. Antitumor cytotoxic T lymphocytes (CTLs) seemed uninvolved because they remained rare in the tumor stroma of KP mice even in spleen-deficient animals (Fig. S5E). Likewise, the CTLs that did accumulate in tumors did not exhibit enhanced effector functions (Fig. S5F). Splenectomy also did not affect the number of tumor-infiltrating FoxP3+ regulatory T cells (Fig. S5G). In contrast, impaired tumor progression in spleen-deficient KP mice was associated with decreased numbers of TAMs and TANs (Fig. S5 H and I).
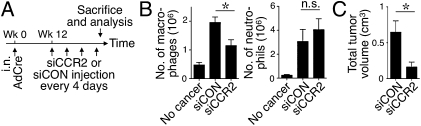
Inflammatory Ly-6Chi monocytes express the chemokine receptor CCR2, which is involved in the amplification of TAM responses (15, 22). To test whether CCR2 controlled spleen-derived TAM accumulation, we used a lipid nanoparticle that contains short interfering (si) CCR2 RNA sequences and that selectively silences CCR2 expression in splenic Ly-6Chi (CCR2+) monocytes (23). KP mice received injections of CCR2-silencing siRNA (siCCR2) or control-silencing (siCON) nanoparticles starting on week 12 after tumor initiation and over 12 d (Fig. 2A). The siCCR2-treated mice showed an approximately twofold reduction of TAMs (Fig. 2B). Splenic granulocytic cells do not express CCR2 and, accordingly, siCCR2 treatment did not alter TAN responses (Fig. 2B). Further evaluation of lung tissue showed reduced tumor progression in mice that received siCCR2 nanoparticles (Fig. 2C and Fig. S6). These data indicate that splenic-derived TAMs were recruited via CCR2 and were causally associated with tumor progression. The apparently unaltered TAN response in siCCR2-treated animals was insufficient to promote tumor growth; thus, further investigation will be needed to define the precise impact of spleen-derived TANs.
Fig. 2.
CCR2 silencing in splenic monocytes impairs TAM responses and tumor growth. (A) Experimental procedures. AdCre, adenovirus expressing Cre recombinase. (B) Number of macrophages and neutrophils in the lungs of KP mice treated with siCCR2 (n = 4) or control-silencing (siCON; n = 4) nanoparticles or in tumor-free mice (no cancer). (C) Volume of tumor nodules in the same KP mice identified by high-resolution CT. *P < 0.05; n.s., not significant.
Splenic Monocytic and Granulocytic Cells Relocate to Cancer Sites.
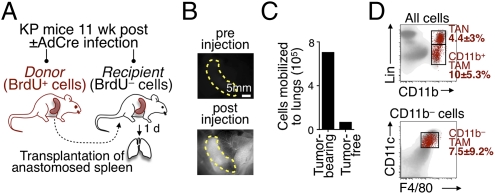
Based on these results, we attempted to define whether reservoir splenocytes physically relocate to tumor sites. We used a method involving transplantation of an anastomosed spleen and tracking of the cells originating from the transplanted spleen (17) (Fig. 3A). Perfusion of the transplant (Fig. 3B) allowed donor splenocytes to enter the recipient's bloodstream directly, and thus to relocate elsewhere. Spleens transplanted into tumor-free animals did not trigger a measurable cell release (17) (Fig. 3C). However, spleens transplanted into tumor-bearing KP mice (11 wk after tumor initiation) triggered relocation of numerous cells to tumors. Namely, 1.9 ± 1.2 × 105 TANs and 5.1 ± 3.2 × 105 TAMs redistributed within 24 h from the donor spleen to the recipient's tumor stroma. A total of 66 ± 4% and 61 ± 17% of splenic-derived TAMs down-regulated Ly-6C and up-regulated F4/80, respectively, in line with the initiation of a differentiation program of these cells on recruitment to tissue. This approach did not permit us to assess the relative contribution of spleen- vs. bone marrow-derived cells; however, it showed that within 24 h, the spleen replaced as many as 4% and 8–10% of the preexisting endogenous TAM and TAN repertoires, respectively (Fig. 3D). Because these repertoires increased, on average, only 2% daily, it is likely that a small fraction of older cells either died locally or exited the tumor stroma, in part, counteracting the contribution of newly arrived splenic cells. Future studies will have to determine whether TAMs and TANs are short-lived or exit the tumor stroma. Either way, these experiments positioned the spleen as a significant contributor of TAM and TAN precursors.
Fig. 3.
Spleen contributes TAMs and TANs directly. (A) Experimental procedure for transplantation of a BrdU-labeled spleen into a splenectomized (unlabeled) KP mouse. Procedural details are provided in SI Methods. (B) Transplanted mice received an i.v. injection of the blood pool agent Angiosense-680 (ViSen Medical–PerkinElmer) to reveal perfusion of the donor organ (spleen outlined by yellow dashed line). Scale bar: 5 mm. (C) The number of BrdU+ cells was detected by flow cytometry from donor spleen mobilized to the lung 24 h after transplantation. The recipient mice were either tumor-bearing (KP mice 11 wk after tumor initiation) or tumor-free (control mice). (D) Dot plots show endogenous cells (gray) and spleen donor-derived cells (red) 24 h posttransplantation in a tumor-bearing mouse (from n = 2). Percentages indicate the contribution of newly arrived splenic cells to the preexisting lung myeloid cell pool. Lin, lineage.
Spleen Produces CD11b+ Ly-6Chi Monocytic and CD11b+ Ly-6Ghi Granulocytic Cells Locally During Cancer Progression.
The spleen may enhance TAM and TAN responses by controlling the quality and/or quantity of immature myeloid precursor/progenitor cells released to tumors. For example, splenic monocytic and granulocytic cells may acquire unique functions locally by receiving microenvironmental instruction signals, which differ from those in bone marrow. Although this mechanism cannot be formally excluded, a comparative analysis of (CD11b+ Ly-6Chi) monocytic and (CD11b+ Ly-6Ghi) granulocytic cells in bone marrow vs. spleen of tumor-bearing hosts (Fig. S7A) and of splenic monocytic and granulocytic cells in tumor-bearing vs. control mice (Fig. S7 B and C) did not reveal notable differences. Phenotypic changes were detected mostly only on cell recruitment to the tumor stroma (Fig. S7C and Table S1).
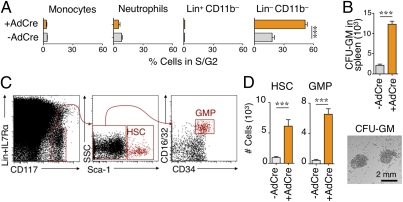
The spleen may also control TAM and TAN responses quantitatively by supplying new monocytic and granulocytic cells continuously during tumor progression. Continuous support of the spleen, however, requires its constant replenishment with new reservoir cells to compensate for the loss of those that are mobilized. Interestingly, the spleen of tumor-bearing mice contained a significantly larger number of cells in the S/G2 phase of the cell cycle, which indicated active cell proliferation within the organ. The proliferating splenocytes exhibited a CD11b− Lin− phenotype (Fig. 4A). Thus, cancer induced the accumulation of proliferating splenocytes that were neither monocytic nor granulocytic but could include their lineage progenitors. Accordingly, in vitro cultures of splenocytes from tumor-bearing mice produced more granulocyte/macrophage colonies than from controls (Fig. 4B).
Fig. 4.
Tumors induce splenic accumulation of HSCs and progenitor cells. (A) Percentage of monocytic [CD11b+ Lin− Ly-6G− (F4/80/CD11c/I-Ab)lo Ly-6C+], granulocytic (CD11b+ Gr-1+ Ly-6G+), Lin+ CD11b− and Lin− CD11b− populations in S/G2 phase in the spleen of control and tumor-bearing mice (n = 3) at week 11 after adenovirus expressing Cre recombinase (AdCre) infection. Lineage (Lin) refers to the following combination: B220/NK1.1/DX5/Ly-6G/CD90.2. (B) (Upper) Number of granulocyte/macrophage colonies in CFU assays (GM-CFU) from splenocytes of tumor-bearing (+AdCre) and control (−AdCre) mice. (Lower) Image of granulocyte/macrophage colonies. Scale bar: 2 mm. (C) Identification of splenic HSCs and GMPs by flow cytometry in KP mice on week 11. (D) Quantification of splenic HSCs and GMPs in tumor-bearing (+AdCre) KP animals (n = 17) and age-matched controls (−AdCre, n = 12). Data are presented as the mean ± SEM. ***P < 0.001.
The established paradigm states the following paths of lineage differentiation for monocytes and neutrophils: HSC→CMP (common myeloid progenitor)→GMP→MDP (macrophage/dendritic cell progenitor)→monocyte (24) and HSC→CMP→GMP→neutrophil (10). The presence of these populations was tested by flow cytometry with a combination of markers used for cell phenotyping in bone marrow (10–12). HSCs, CMPs, GMPs, and MDPs increased largely in the spleen (Fig. 4 C and D and Fig. S8A) in comparison to bone marrow (Fig. S8 B and C) in KP mice at 11 wk after tumor initiation. Similar splenic responses were detected in two other grafted tumor models (Fig. S8D).
Subsequent experiments were restricted to the analysis of HSCs and GMPs. These studies indicated that their expansion in the spleen of tumor-bearing mice was likely the consequence of both their continuous recruitment and their local proliferation. To analyze recruitment, parabiosis was established between two tumor-bearing mice for 16 d until HSC and progenitor cell equilibration. The spleens contained a significant fraction of HSCs (28.5 ± 2%) and GMPs (24 ± 4%) that originated from the other parabiont; thus, circulating HSCs and progenitor cells contributed to the splenic repertoire. These observations are in line with previous evidence that a small fraction of HSCs and progenitor cells exit the bone marrow and traffic through different extramedullary tissues, including the spleen (25, 26), and that in response to inflammatory stimuli, these cells produce tissue-resident cell populations (27–29). To analyze local proliferation, splenic HSCs and GMPs were labeled with a cell cycle marker. Flow cytometry analysis showed that a high fraction of these cells were in the S/G2 phase of the cell cycle, similar to their bone marrow counterparts (Fig. S8E). Splenic HSCs and GMPs were functionally active in vitro, because they formed colonies as efficiently as their bone marrow counterparts.
We performed additional experiments to evaluate the fate of GMPs in vivo. Intravital microscopy 5 d after injection of fluorescently tagged GMPs indicated that the cells gave rise to large numbers of colonies in the splenic red pulp parenchyma next to, but outside, collecting venous sinuses (Fig. 5A). Characterization of the cells by flow cytometry identified that GMP descendants were virtually all monocytic or granulocytic (Fig. 5B). The ability of transferred GMPs to produce myeloid progeny was highest within the spleen of KP animals with cancer. The activity remained relatively low within the bone marrow as well as the liver (another extramedullary site that can exhibit myelopoietic activity) even in splenectomized mice (Fig. 5B and Fig. S8F). We made similar observations on adoptive transfer of HSCs instead of GMPs, although, as expected, the appearance of HSC-derived monocytic and granulocytic cells was delayed by several days. Finally, GMPs had a similar fate in a grafted mouse tumor model (Fig. S8G). The data indicate that splenic HSCs and progenitor cells actively produce Ly-6Chi monocytic and Ly-6Ghi granulocytic cells locally and participate, at least in part, in replenishing the splenic reservoir in tumor-bearing mice.
Fig. 5.
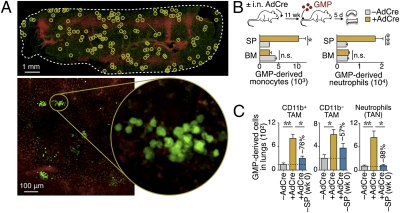
Fate mapping of adoptively transferred GMP cells. (A) (Upper) In situ confocal image of the splenic red pulp from a tumor-bearing mouse 5 d after i.v. injection of (ACTB-eYFP)7AC5Nagy/J (EYFP+) GMPs (green). EYFP+ cell clusters are highlighted in circles, and venus sinuses are shown in red. Scale bar: 1 mm. (Lower) Higher magnification images. Scale bar: 100 μm. (B) Phenotyping and quantification of the progeny of EYFP+ GMP cells in the spleen (SP) and bone marrow (BM) of tumor-bearing [+ adenovirus expressing Cre recombinase (AdCre), n = 12] and tumor-free mice (−AdCre, n = 6). i.n., intranasal. (C) Tracking of EYFP+ GMP cells injected into tumor-free mice (−AdCre, n = 6) or tumor-bearing KP mice with (+AdCre, n = 12) or without [+AdCre −SP (wk 0), n = 4] a spleen. Data are presented as the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
Splenic GMPs Contribute to the TAM and TAN Responses.
Tracking of the GMP progeny in the lungs of KP mice also identified that these cells produced tissue macrophages (TAMs) and neutrophils (TANs) (Fig. 5C). The capacity of the transferred GMPs to generate these cells markedly increased in mice with cancer (11 wk after tumor initiation). To test whether the GMP-derived TAMs and TANs required the spleen, we repeated the GMP adoptive experiments (11 wk after tumor initiation); however, we used spleen-sufficient or spleen-deficient mice as recipients this time. Five days after transfer, GMPs failed to produce full TAM and TAN responses in the spleen-deficient mice (Fig. 5C). This result supports the role of splenic GMPs as TAM and TAN progenitors and positions the spleen as an important site for amplification of these cells.
Human Patients with Cancer Expand Splenic Myeloid Progenitor Cells.
Mouse and human spleens are physiologically very similar (30), and the spleen of patients with chronic inflammatory disorders is capable of producing leukocytes (31–33). However, whether the extramedullary myeloid response seen in animal models also occurs in human patients has not been addressed.
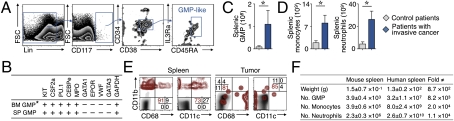
Fresh splenic tissue was collected from seven control patients and seven patients with pathological evidence of invasive cancer. Splenic GMP-like cells were sorted based on the expression of cell surface markers used previously to identify human bone marrow GMPs (11) (i.e., CD117+ CD34+ CD38+ IL-3Ra+ CD45RA+ Lin−/int; Fig. 6A).
Fig. 6.
Splenic myeloid response in human patients with invasive cancer. (A) Identification of GMP-like cells (blue) ex vivo by flow cytometry from single-cell suspensions of spleen tissues obtained from a human patient with invasive cancer. (B) Gene expression signature of purified human splenic GMP-like cells and of bone marrow GMPs, as previously reported (10). (C) Total counts of GMP-like cells in the spleen of patients with (n = 7) or without (n = 7) invasive cancer. (D) Total number of monocytes and neutrophils retrieved from spleens of the same patients. (E) Fate of human Lin− cKit+ splenic progenitor cells 5 d after injection into a nonobese diabetic SCID mouse bearing a lung carcinoma xenograft. Lin, lineage. (F) Table showing spleen weight and total numbers of splenic GMPs and monocyte- and neutrophil-like cells in tumor-bearing mice and in patients with cancer. The fold difference between species is also shown. Data are presented as the mean ± SEM. *P < 0.05.
PCR analysis identified that the splenic GMP-like cells produced the transcription factor PU.1 and CCAAT/enhancer binding protein ε (C/EBPε), the granulocyte colony-stimulating factor (CSF), granulocyte-macrophage (GM)-CSF receptors, and the enzyme myeloperoxidase, in line with the study of Manz et al. (11). The cells did not express GATA binding protein 1, GATA binding protein 3, von Willebrand factor, or IL-7 receptor α (IL-7Ra), all of which are involved in developmental lineages disparate from neutrophils and monocytes (Fig. 6B). In vitro, purified splenic GMP-like cells produced colonies and generated cells that were mostly CD14+ CD115+ CD68+ CD11b+/− (Fig. S9A). Because we could not detect granulocytes, it is possible that the culture conditions skewed maturation toward the mononuclear phagocyte lineage or that the splenic population was already committed to this lineage (and thus may also qualify as human MDPs).
Patients with invasive cancer had significantly higher numbers of splenic GMPs ex vivo compared with controls (P < 0.05; Fig. 6C), and in vitro cultures of splenocytes of patients with cancer (n = 2) produced higher numbers of granulocyte/macrophage colonies than splenocytes of a control patient (Fig. S9B). Also, a flow cytometry protocol established to detect endogenous human splenic monocytes and neutrophils ex vivo (Fig. S9C) showed increased numbers of these cells in patients with cancer (Fig. 6D). These findings suggest that patients with cancer can mount active GMP responses and produce monocytic and granulocytic cells locally.
To address directly whether human splenic GMPs produce monocytic and granulocytic cells in vivo, we purified human splenic progenitor cells obtained ex vivo from a patient with invasive cancer and injected the cells into a nonobese diabetic-SCID mouse bearing an NCI-H226 human non-small cell lung carcinoma xenograft. Within 5 d of the transfer, the GMP progeny accumulated and differentiated mainly into CD11b+ CD68− CD11c− monocyte-like cells in the spleen. Human cells also accumulated at the tumor site, where they acquired a CD68+ macrophage-like phenotype (Fig. 6E).
Together, the human data appear to mirror our observations in the mouse, and they are consistent with the notion that the spleen can support production of monocytic and granulocytic cells, at least in patients with cancer. Aside from the fact that splenic GMP responses develop in both species, it is noteworthy that the magnitude of these responses was comparable, if not more pronounced, in humans (taking scale differences between species into account; Fig. 6F).
Discussion
Here, we show that the spleen significantly amplifies tumor-promoting TAM responses in KP mice and can do so over time during cancer progression. Our findings in favor of this notion are as follows: (i) the impaired TAM response and tumor growth in splenectomized animals, (ii) the impaired TAM response and tumor growth on CCR2 silencing in splenic monocytic cells, (iii) the redistribution of splenic TAM precursors to tumors in the spleen transplant experiments, (iv) the expansion of splenic myeloid progenitors in tumor-bearing mice, and (v) the reduced capacity of these progenitors to produce TAM when transferred into spleen-deficient mice. Future studies should address whether manipulation of the splenic reservoir not only suppresses primary tumor growth but delays metastasis and increases survival. The functions of splenic-derived TANs also remain to be determined.
The constant “demand” for TAMs is met, at least in part, by active and chronic proliferation of splenic HSCs and progenitor cells, which continuously replenish the reservoir with new monocytic cells. The latter cells, produced within the spleen, do not necessarily become local resident cells but are capable of relocating to tumor sites. These findings shed light on the mechanisms of immune cell production and relocation in cancer. They also prompt a number of questions, such as the following: (i) “Why does the bone marrow ‘farm out’ the production of TAM (and TAN) precursors?” (ii) “How do cancers induce the extramedullary response?” (iii) “Does a splenectomy in cancer help or hurt?” (iv) “How does chemotherapy affect splenic myeloid cell production?” and (v) “How can the HSC→TAM(/TAN) axis be modulated to create more efficient anticancer therapies?”
The data presented here agree with the established notion that the bone marrow in the steady state autonomously replaces and maintains the pools of relatively short-lived monocytes and neutrophils (13, 17, 24, 34). However, during chronic inflammation, our findings suggest that the spleen can also continuously contribute Ly-6Chi monocytic and Ly-6Ghi granulocytic cells. Others have shown the liver as a site for extramedullary myelopoiesis in cancer (35) and other inflammatory processes (29). It is likely that the outsourcing process occurs when demand for macrophages/neutrophils is high. It may also be that medullary and extramedullary environments differ significantly enough from one another that they generate cells with divergent functions. Currently, our phenotyping studies suggest that bone marrow- and spleen-derived monocytic cells are similar. However, it is worth noting that CD11b+ Gr-1+ MDSCs, which often accumulate within the spleen of tumor-bearing animals, exhibit a distinct immunosuppressive phenotype (36). GMP-derived splenic monocytic and granulocytic cells are also CD11b+ Gr-1+; thus, at least a fraction of bona fide MDSCs likely derive from splenic (CD11b−) GMPs. Future work should identify the splenic signals that instruct HSCs and progenitor cells and their lineage descendants and also whether these signals differ from those in the bone marrow.
The extramedullary hematopoietic response triggered in cancer could be host-controlled and/or tumor-controlled: The loss of splenic cells on mobilization to tumors may activate host-controlled feedback homeostatic mechanisms to replenish the reservoir, although tumors may also actively secrete long-range signals that trigger/amplify the response. Growth factors, such as GM-CSF (37) and the glycoprotein osteopontin (38), have already been implicated as long-range signals controlling hematopoietic cells in the bone marrow. GM-CSF and other factors control the mobilization of bone marrow HSCs to the periphery and should next be investigated for their roles in extramedullary hematopoietic responses. Interestingly, adenocarcinomas driven by oncogenic Kras only, as opposed to KP cancers, fail to evoke a splenic monocytic/granulocytic response through all stages of tumor progression. Thus, a comparative analysis of KrasLSL-G12D/+ and KP mice may be useful to identify remote cross-talk instigators.
Although this study has indicated that splenectomy can delay tumor growth in KP mice, the consequences of such a procedure in humans remain unclear. Retrospective population-based studies (39–41) have shown that patients whose spleens were removed because of traumatic rupture do not show a reduced risk for developing cancer. However, the spleen is a multicellular organ, capable of accommodating many major functions for the host, including immune protection against pathogens as well as orchestration of healing responses (17). Thus, although removal of the entire spleen may carry positive effects in some circumstances, it would be preferable to identify specific tumor-promoting activity in the organ, against which therapeutic approaches could be designed.
Many chemotherapeutic drugs not only target tumor cells but eliminate proliferating HSCs in the bone marrow. Because TAMs and TANs derive from HSCs, one could imagine that the drugs act to suppress the tumor-promoting immune response. However, chemotherapeutic drugs can also mobilize bone marrow HSCs to the periphery, resulting in an increase in the circulating HSC repertoire (42). It would therefore be useful to determine whether chemotherapies suppress or enhance tumor-promoting extramedullary hematopoietic responses. With accumulating evidence indicating the immune system as critical for tumorigenesis, an emerging concept for fighting established cancer involves identifying drug combinations that not only kill cancer cells but influence the immune system in such a way as to be favorable to the host (43). In the present study, in view of their tumor-promoting effects, we highlight extramedullary stem and progenitor cells as relevant targets for drug therapy.
Methods
Detailed methods are available in SI Methods. KP mice in a 129 background were obtained from Tyler Jacks (Massachusetts Institute of Technology, Cambridge, MA). To induce lung adenocarcinoma, KP mice were infected with an adenovirus expressing Cre recombinase by intranasal instillation (18). Spleen transplants were performed using KP donor and recipient mice 11 wk after AdCre infection.
Supplementary Material
Acknowledgments
We thank T. Jacks (Massachusetts Institute of Technology), R. Weinberg (Whitehead Institute, Cambridge, MA), and E. Meylan and M. Winslow (Massachusetts Institute of Technology) for helpful discussions; K. Hassard, S. Holder, and the Massachusetts General Hospital surgical pathology accessioners for assistance with human tissue collection; J. Sullivan and R. Kohler (Massachusetts General Hospital) for imaging; M. Maier, H. Epstein-Barash, and W. Cantley for medium-scale siRNA synthesis and formulation (Alnylam); and M. Waring (Ragon Institute, Massachusetts General Hospital) for sorting cells. This work was supported, in part, by National Institutes of Health Grants P50-CA86355, R01-AI084880, and R56-AI084880 (to M.J.P.) and National Institutes of Health Grant U54-CA126515 (to R.W.). M.E. was supported by the American Association for Cancer Research Centennial Predoctoral Fellowship and the Boehringer Ingelheim Fonds.
Footnotes
Conflict of interest statement: T.I.N. and V.K. are Alnylam Pharmaceuticals employees, and D.G.A. receives funding from, and is a consultant with, Alnylam Pharmaceuticals.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113744109/-/DCSupplemental.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang BC, et al. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. 2011;28:1447–1452. doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]
- 9.Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 11.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawanobori Y, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 15.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 16.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 21.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 25.Begg SK, et al. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 27.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 31.Snover DC, Frizzera G, Spector BD, Perry GS, 3rd, Kersey JH. Wiskott-Aldrich syndrome: Histopathologic findings in the lymph nodes and spleens of 15 patients. Hum Pathol. 1981;12:821–831. doi: 10.1016/s0046-8177(81)80085-8. [DOI] [PubMed] [Google Scholar]
- 32.O'Keane JC, Wolf BC, Neiman RS. The pathogenesis of splenic extramedullary hematopoiesis in metastatic carcinoma. Cancer. 1989;63:1539–1543. doi: 10.1002/1097-0142(19890415)63:8<1539::aid-cncr2820630814>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Thiele J, Kvasnicka HM, Czieslick C. CD34+ progenitor cells in idiopathic (primary) myelofibrosis: A comparative quantification between spleen and bone marrow tissue. Ann Hematol. 2002;81(2):86–89. doi: 10.1007/s00277-001-0417-4. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 35.Chan IT, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marigo I, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 38.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linet MS, et al. Risk of cancer following splenectomy. Int J Cancer. 1996;66:611–616. doi: 10.1002/(SICI)1097-0215(19960529)66:5<611::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 40.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;75:577–583. doi: 10.1002/1097-0142(19950115)75:2<577::aid-cncr2820750222>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.Robinette CD, Fraumeni JFJ., Jr Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2(8029):127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 42.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 43.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.