Abstract
A large hexanucleotide (GGGGCC) repeat expansion in the first intron of C9ORF72, a gene located on chromosome 9p21, has been recently reported to be responsible for ∼40% of familial amyotrophic lateral sclerosis cases of European ancestry. The aim of the current article was to describe the phenotype of amyotrophic lateral sclerosis cases carrying the expansion by providing a detailed clinical description of affected cases from representative multi-generational kindreds, and by analysing the age of onset, gender ratio and survival in a large cohort of patients with familial amyotrophic lateral sclerosis. We collected DNA and analysed phenotype data for 141 index Italian familial amyotrophic lateral sclerosis cases (21 of Sardinian ancestry) and 41 German index familial amyotrophic lateral sclerosis cases. Pathogenic repeat expansions were detected in 45 (37.5%) patients from mainland Italy, 12 (57.1%) patients of Sardinian ancestry and nine (22.0%) of the 41 German index familial amyotrophic lateral sclerosis cases. The disease was maternally transmitted in 27 (49.1%) pedigrees and paternally transmitted in 28 (50.9%) pedigrees (P = non-significant). On average, children developed disease 7.0 years earlier than their parents [children: 55.8 years (standard deviation 7.9), parents: 62.8 (standard deviation 10.9); P = 0.003]. Parental phenotype influenced the type of clinical symptoms manifested by the child: of the 13 cases where the affected parent had an amyotrophic lateral sclerosis–frontotemporal dementia or frontotemporal dementia, the affected child also developed amyotrophic lateral sclerosis–frontotemporal dementia in nine cases. When compared with patients carrying mutations of other amyotrophic lateral sclerosis-related genes, those with C9ORF72 expansion had commonly a bulbar onset (42.2% compared with 25.0% among non-C9ORF72 expansion cases, P = 0.03) and cognitive impairment (46.7% compared with 9.1% among non-C9ORF72 expansion cases, P = 0.0001). Median survival from symptom onset among cases carrying C9ORF72 repeat expansion was 3.2 years lower than that of patients carrying TARDBP mutations (5.0 years; 95% confidence interval: 3.6–7.2) and longer than those with FUS mutations (1.9 years; 95% confidence interval: 1.7–2.1). We conclude that C9ORF72 hexanucleotide repeat expansions were the most frequent mutation in our large cohort of patients with familial amyotrophic lateral sclerosis of Italian, Sardinian and German ancestry. Together with mutation of SOD1, TARDBP and FUS, mutations of C9ORF72 account for ∼60% of familial amyotrophic lateral sclerosis in Italy. Patients with C9ORF72 hexanucleotide repeat expansions present some phenotypic differences compared with patients with mutations of other genes or with unknown mutations, namely a high incidence of bulbar-onset disease and comorbidity with frontotemporal dementia. Their pedigrees typically display a high frequency of cases with pure frontotemporal dementia, widening the concept of familial amyotrophic lateral sclerosis.
Keywords: amyotrophic lateral sclerosis; familial ALS, C9ORF72 gene; phenotype–genotype correlation
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder of adult life characterized by a progressive loss of upper and lower motorneurons at spinal and bulbar level. Its course is invariably fatal within 3–5 years from symptom onset, and there are currently no effective therapies (Kiernan et al., 2010). Approximately 5% of the cases are familial in nature, mostly inherited in an autosomal dominant manner (Chiò et al., 2008).
Recently, we described a large hexanucleotide (GGGGCC) repeat expansion in the first intron of C9ORF72, a gene located on the short arm of chromosome 9 and responsible for ∼40% of familial ALS cases of European ancestry (Dejesus-Hernandez et al., 2011; Renton et al., 2011). Although this expansion accounted for an unprecedented large proportion of patients with familial ALS, little clinical data have been reported about the phenotypic characteristics of patients with ALS carrying this type of mutation.
The aim of the current article was to address this gap in our knowledge by describing the phenotype of ALS cases carrying the expansion by providing a detailed clinical description of affected cases from representative multi-generational kindreds, and by analysing the age of onset, gender ratio and survival in a large cohort of patients with familial ALS. As the underlying genetic lesion was a repeat expansion, we also examined effects of the parental gender transmitting the mutant allele on the manifestation of disease in their children and analysed variation of the age of onset in parent–child couples looking for evidence of anticipation.
Patients and methods
Patients and controls
We collected DNA and analysed phenotype data for 141 index Italian familial ALS cases. These cases were collected thorough the Italian ALS Genetic Consortium (ITALSGEN), which is a collaborative effort involving 15 Italian ALS centres throughout the Italian peninsula (n = 120 patients with familial ALS) and the Mediterranean island of Sardinia (n = 21 patients with familial ALS). Of note, we previously reported the C9ORF72 repeat expansion status for 29 of these patients in our original article (Renton et al., 2011). A separate cohort of 41 German index cases of familial ALS, which had been collected at the ALS centre of the Würzburg University, was also assessed. Genetic analyses of this cohort were also included in our original article (Renton et al., 2011). Familial ALS was defined as the presence of at least one relative (even more distant than first- or second-degree) affected by ALS in the pedigree (Byrne et al., 2011).
Controls consisted of 353 neurologically healthy subjects of mainland Italian ancestry, 166 of Sardinian ancestry and 309 of German ancestry.
All Italian cases included in the present study were negative for mutations in major ALS genes [superoxide dismutase 1 (SOD1), TAR DNA-binding protein (TARDBP), fused in sarcoma (FUS), optineurin and valosin containing protein]. The same genes, with the exception of optineurin and valosin containing protein, were assessed in German cases. All Italian familial ALS and most German familial ALS underwent formal neurocognitive testing of executive function, memory/learning, attention/concentration, language and visual–spatial functions (Strong et al., 2008), though different batteries were used at the participating centres. Formal tests for anxiety and depression were also used. When applicable, test results were corrected for age and educational level.
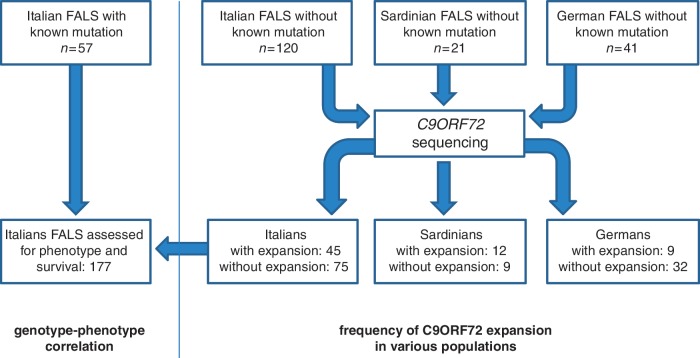
To establish a baseline cohort for comparison with the patients with familial ALS with the C9ORF72 pathological repeat expansion, we identified unrelated familial ALS cases that carried mutations of the SOD1 (n = 38), TARDBP (n = 13) or FUS (n = 6) genes. These patients were collected in the same manner as the 141 familial ALS samples described above, namely consecutively ascertained from the 15 centres participating in the ITALSGEN Consortium. Cases of Sardinian ancestry were excluded from this analysis due to the particularly high presence of the p.A382T missense mutation of the TARDBP gene in this population related to a founder effect (Chiò et al., 2011a). Patients with optineurin mutations (n = 1) and valosin containing protein mutations (n = 1) were similarly excluded from the analyses due to their low numbers. A flow diagram reporting the numbers of patients in the various part of the study is shown in Fig. 1.
Figure 1.
Flow diagram reporting the number of patients included in the various part of the study. FALS = familial ALS.
Screening of hexanucleotide repeat expansion of the C9ORF72 gene
A repeat-primed polymerase chain reaction assay was used to screen the presence of the GGGGCC hexanucleotide expansion in the first intron of C9ORF72 as described in the original article (Renton et al., 2011). This assay rapidly and robustly determined whether a sample carries the repeat expansion.
Statistical analysis
Differences between groups were analysed using t-tests for continuous variables (such as age at symptom onset) and χ2 for discrete variables [such as gender, site of onset and presence of frontotemporal dementia (FTD)]. Comparison between a series of means was performed with ANOVA. Survival was calculated using Kaplan–Meier curves and the log-rank test was used to compare survival across groups. The last day of follow-up was 1 October 2011, and none of the patients were lost to follow-up. A P < 0.05 was considered significant, and all tests were two-sided. All calculations were made using SPSS software (IBM corporation, version 17). The ethical committees of the participating centres approved the study, and all patients signed a written informed consent.
Results
We screened 120 unrelated familial ALS cases from mainland Italy and 21 unrelated familial ALS cases from Sardinia for C9ORF72 hexanucleotide repeat expansions using a repeat-primed polymerase chain reaction assay. Pathogenic repeat expansions were detected in 45 (37.5%) patients from mainland Italy and 12 (57.1%) patients of Sardinian ancestry. Nine (22.0%) of the 41 German index familial ALS cases were previously reported to carry the repeat expansion (Renton et al., 2011).
The clinical characteristics of patients with familial ALS carrying the C9ORF72 expansion with different ethnic origin (Italians versus Sardinians versus Germans) are reported in Table 1. No statistically significant differences were found in age at onset, gender, site of onset and frequency of FTD between these cohorts.
Table 1.
Comparison of clinical characteristics of Italian, Sardinian and German familial ALS cases carrying the C9ORF72 hexanucleotide repeat expansion
| Variable | Italian familial ALS n = 45 | Sardinian familial ALS n = 12 | German familial ALS n = 9 | Overall n = 66 |
|---|---|---|---|---|
| Gender (female) (%) | 23 (51.1) | 4 (33.3) | 6 (66.7) | 33 (50.0) |
| Site of onset (bulbar) (%) | 19 (42.2) | 4 (33.3) | 5 (55.6) | 28 (42.4) |
| Presence of FTD (%) | 21 (46.7) | 7 (58.3) | 2 (22.2) | 30 (45.5) |
| Age at onset, mean (SD) (years) | 57.0 (9.5) | 60.4 (7.4) | 56.4 (11.5) | 57.6 (9.4) |
Nearly half of the patients with ALS carrying the pathogenic repeat expansion developed comorbid cognitive impairment during the course of their illness (44.2%, n = 30 of 68 patients). All of these cases presented with behavioural variant FTD. More specifically, all patients assessed with the Frontal Systems Behaviour Scale (Family Rating form) (Grace and Malloy, 2001) (n = 23) had impairment in the apathy and executive domains (median T-scores: apathy 79, range 66–91, executive dysfunction 83, range 70–94; normal value < 65), while the disinhibition domain was pathological in only three patients. Patients with ALS with C9ORF72 mutations and comorbid FTD also had pathological scores in tests for executive function, such as Stroop Colour-Word Interference Test, letter and category fluency test and Trail Making A and B. In contrast, tests for memory function, such as Rey Figure and Digit Span, were generally normal. Three index cases displayed prominent delusions and hallucinations, whereas one case presented with an acute-onset obsessive–compulsive disorder.
Representative pedigrees carrying the GGGGCC hexanucleotide repeat expansion of C9ORF72
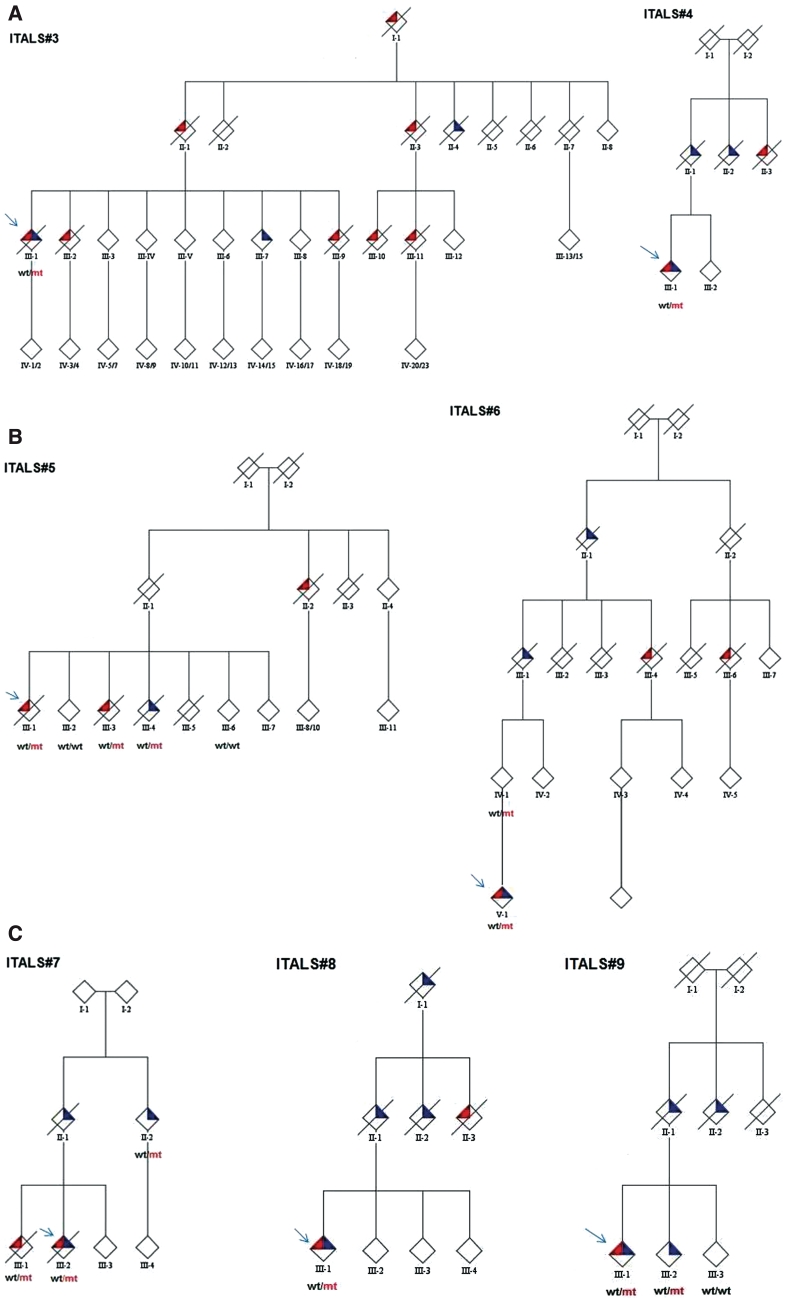
Figure 2 shows pedigrees that are most representative of the phenotype and course of disease associated with the hexanucleotide repeat expansion. Co-occurrence of ALS and FTD within each kindred is noteworthy.
Figure 2.
Pedigrees of ALS families of patients carrying the C9ORF72 GGGGCC hexanucleotide repeat expansion of the C9ORF72 gene (ITALS#3 to ITALS#9). Mutant alleles are shown by mt, whereas wild-type alleles are indicated by wt. Red triangles represent a diagnosis of ALS, blue triangles represent FTD. Probands are indicated by arrows. Gender of the pedigree members is obscured to protect privacy.
ITALS#3 pedigree
The proband (III-1) developed spinal ALS at 64 years of age and behavioural FTD one year later. She also had sensorineural hearing loss. She died 33 months after the onset of ALS at the age of 66 years. She carried the repeat expansion of the C9ORF72 gene. Patient III-2 developed spinal ALS at 48 years of age. She died 24 months later, and was cognitively normal at the time of her death. Patient III-7 presented with behavioural variant FTD, and remains alive at the time of last follow-up, 48 months after the onset of FTD. Patient III-9 developed spinal ALS at the age of 42 years and died 2 years later at the age of 44 years. Patient III-10 presented with spinal ALS at the age of 52 years and died at 54 years. Patient III-11 developed spinal ALS at the age of 55 years and died at 57 years. Patient II-1 died from spinal ALS at 79 years; disease duration is uncertain. Patient II-3 died from ALS at 57 years; disease duration is unknown. Patient II-4 had FTD at 66 years; disease duration is unknown. Patient I-1 had a progressive motor palsy, without features of FTD. He died at 65 years; disease duration is unknown.
ITALS#4 pedigree
The proband (III-1) developed spinal ALS at the age of 33 years and a prominent behavioural FTD 6 months later, with vivid hallucinations and delusions. He underwent elective tracheostomy 2 years after the onset of ALS for respiratory failure; he was still alive 38 months after the onset of ALS. He carries the hexanucleotide repeat expansion of the C9ORF72 gene. The patient's father (II-1) developed FTD at the age of 67 years and died 3 years later. A paternal uncle of the proband (II-2) had FTD at the age of 65 years and a paternal aunt had spinal ALS at the age of 61 years.
ITALS#5 pedigree
The proband (III-1) developed spinal ALS at the age of 63 years and died 27 months later. He carried the hexanucleotide repeat expansion of the C9ORF72 gene. His sister (III-3) had a spinal onset ALS at 62 years and died 37 months later. Another sister (III-4) had FTD at the age of 62 years and died 3 years later. The mother of the proband (II-1) died at 80 years, without signs of ALS or FTD. A maternal aunt of the proband (II-2) had bulbar ALS at 74 and died 12 months later.
ITALS#6 pedigree
The proband (IV-1) developed spinal onset ALS with a prominent upper motor involvement at the age of 36 years, comorbid with FTD. She was still alive, 8 months after the onset of ALS. She carries the hexanucleotide repeat expansion of the C9ORF72 gene. The proband's mother (III-1) is alive without signs of ALS or FTD. The proband's maternal grandfather (II-1) had FTD at the age of 72 years and died 3 years later. One of his sisters (III-4) had bulbar ALS at the age of 67 years. The great-grandfather (II-1) had FTD and died at 81 years. A nephew of the great-grandfather (III-6) had bulbar ALS at the age of 59 years and died 38 months later.
ITALS#7 pedigree
The proband (III-2) developed spinal ALS at the age of 45 years with comorbid behavioural FTD; he also developed visual hallucinations; he did not adapt to non-invasive ventilation and died 11 months after the onset of ALS. He carried the repeat expansion of the C9ORF72 gene. His brother (III-1), who also carried the repeat expansion, developed flail leg ALS at the age of 59 years, without FTD and died 4 years later. The proband's mother (II-1) had FTD at the age of 80 years and died at 83 years. The maternal aunt (II-2) developed FTD at the age of 78 years and is alive after 4 years. She carries the C9ORF72 repeat expansion. Her son (III-4) is currently 44 years of age and was diagnosed with schizophrenia at the age of 22. It was not possible to test this subject for C9ORF72.
ITALS#8 pedigree
The proband (III-1) developed bulbar ALS at the age of 59 years and behavioural FTD 6 months later. He carries a pathological repeat of the C9ORF72 gene. He was still alive 16 months after the onset of ALS. The proband's father (II-1) had FTD at the age of 71 years and died 3 years later. A proband's aunt (II-2) developed bulbar ALS at the age of 66 years and died 2 years later, and another proband's aunt (II-3) developed FTD at the age of 69 years and died 4 years later. The proband's grandfather (III-1) developed FTD at the age of 71 years and died 3 years later.
ITALS#9 pedigree
The proband (III-1) developed bulbar ALS comorbid with behavioural FTD at the age of 59 years. She is alive, 40 months after the onset of ALS–FTD. She carries the C9ORF72 repeat expansion. Her brother (III-2) developed FTD at the age of 62 years and was still alive 30 months later. He carries the C9ORF72 repeat expansion. The proband's father had FTD at the age of 62 years and died 5 years later. A proband's aunt died from FTD at the age of 65 years. The duration of the disease is unknown.
Effect of parental gender and phenotype
The gender of the parent transmitting the mutant allele is known to influence the phenotype of an affected child in certain repeat expansion diseases (Machuca-Tzili et al., 2005; Reiner et al., 2011). To determine if such an effect existed for the C9ORF72 hexanucleotide repeat expansion, we compared the age at onset of index cases with the age at onset of the affected mother or father in 66 years ALS pedigrees for which data was available. Of these, the transmitting parent had ALS in 33 years (50.0%) kindreds, ALS–FTD in four (6.1%) kindreds and FTD in nine (13.4%) kindreds; in one of these pedigrees, the parents of the index case were consanguineous. In the remaining 10 (15.2%) pedigrees, both parents of the index case died without developing symptoms, and it was not possible to identify the transmitting parent as there were no known cases of ALS or FTD in the earlier generations.
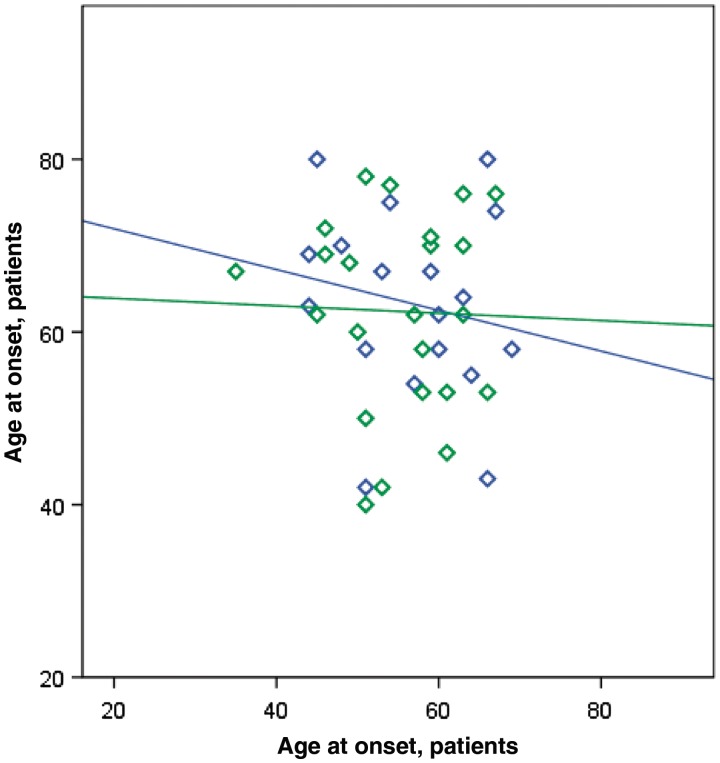
Considering the 55 pedigrees in which the line of parental transmission was established, the disease was maternally transmitted in 27 (49.1%) pedigrees and paternally transmitted in 28 pedigrees (50.9%) (P = non-significant). On average, children developed disease 7.0 years earlier than their parents [children: 55.8 years (SD 7.9), parents: 62.8 (SD 10.9); P = 0.003]. Maternal or paternal transmission had no apparent effect on age at onset of the children (Fig. 3). In contrast, parental phenotype influenced the type of clinical symptoms manifested by the child: of the 13 cases where the affected parent had an ALS–FTD or FTD phenotype, the affected child also developed ALS–FTD in nine cases. This relationship was not exclusive, as the affected parent of four index cases diagnosed with ALS–FTD did not ostensibly have cognitive defects at the time of examination.
Figure 3.
Scatter plot of age at onset of index cases versus their parents. Male transmitting parent is indicated by green diamonds and maternal transmission by blue diamonds. Regression lines for paternal and maternal transmission data are also shown (maternal line, r2 = 0.03, P-value = non-significant; paternal line, r2 = 0.01, P-value = non-significant). On average, children developed disease 7.0 years earlier than their parents (see main text). Maternal or paternal transmission had no apparent effect on age at onset of the children.
Phenotype of patients carrying the C9ORF72 expansion
The clinical characteristics of patients with familial ALS carrying the C9ORF72 expansion and those with mutations of other ALS-related genes are reported in Table 2. Bulbar-onset disease was more common among patients with ALS with C9ORF72 hexanucleotide repeat expansions compared with patients with ALS with mutations involving other genes (42.2% compared with 25.0% among non-C9ORF72 expansion cases, P = 0.03). Mean age at symptom onset was similar among patients carrying the C9ORF72 hexanucleotide repeat expansion [median 59.0 years, interquartile range (IQR) 50.6–62.9], SOD1 mutations (50.0 years, IQR 42.8–62.6) and TARDBP mutations (66.0 years, IQR 58.0–70.6). In contrast, patients with FUS mutations typically manifested symptoms at a much younger age (median 35.3 years, IQR 30.4–39.6).
Table 2.
Gender, site of onset, frequency of cognitive impairment and median age at onset (IQR) of index mainland Italian patients with familial ALS with different gene mutations
| Gene | Number of cases | Gender (female) (%) | Bulbar onset (%) | Cognitive impairment (%) | Median age at onset (IQR) |
|---|---|---|---|---|---|
| C9ORF72 | 45 | 23 (51.1) | 19 (42.2) | 21 (46.7) | 59.0 (50.6–62.9) |
| FUS | 6 | 2 (33.3) | 1 (16.7) | 0 | 35.3 (30.4–39.6) |
| SOD1 | 38 | 20 (52.6) | 3 (7.9) | 1 (2.6) | 50.0 (42.8–62.6) |
| TARDBP | 13 | 4 (30.8) | 4 (30.8) | 4 (30.8) | 66.0 (58.0-70.6) |
| Unknown gene | 75 | 32 (42.7) | 25 (33.3) | 7 (9.3) | 60.7 (53.0–68.9) |
| Overall | 177a | 81 (45.8) | 52 (29.4) | 32 (18.1) | 58.0 (47.7–67.5) |
| P-value | – | 0.54 | 0.011 | 0.0001 | 0.0001 |
a Two index cases were not included: one with optineurin missense mutation and one with valosin containing protein missense mutation.
Overall, cognitive impairment was much more common in patients with C9ORF72 hexanucleotide repeat expansion (46.7% of cases) compared with individuals carrying mutations in other genes or unknown mutation (9.1% of cases) (P = 0.0001). Furthermore, psychotic symptoms (delusions and hallucinations) were reported more commonly among patients carrying the chromosome 9p21 repeat expansion compared with non-expanded cases. Two index familial ALS cases with unknown mutation had a semantic dementia variant of FTD, whereas this form of FTD was not observed among any of the cases carrying the pathogenic repeat expansion. Interestingly, the presence of comorbid FTD was associated with a significantly older mean age at symptom onset in index cases [60.6 years (SD 8.9) among patients with ALS–FTD versus 55.2 (SD 9.0) among patients with pure ALS phenotype, P = 0.02]. A similar pattern was observed in the transmitting parents [72.1 years (8.9) among parents with ALS–FTD or FTD versus 61.8 years (9.9) among parents with pure ALS phenotype, P = 0.003]. Finally, patients with comorbid FTD had more frequent bulbar-onset disease [17 (58.6%) versus 12 (41.4%), P = 0.05].
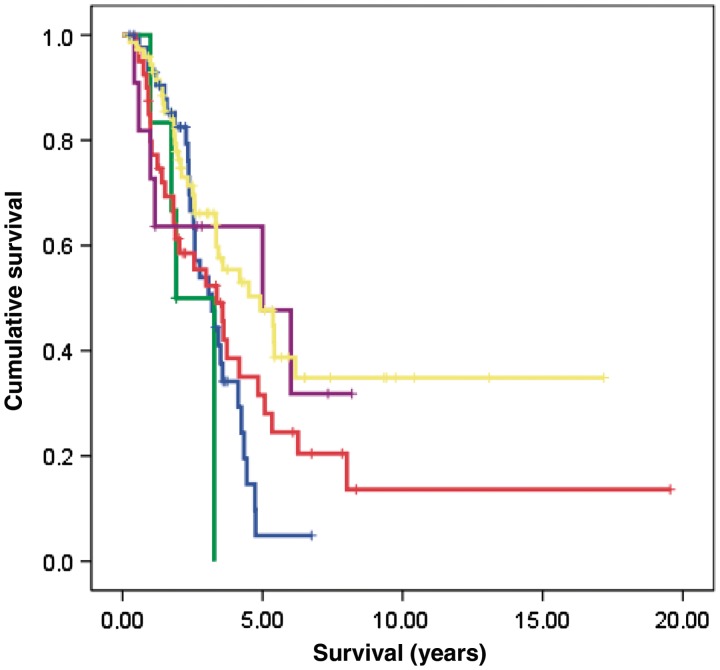
Median survival from symptom onset among cases carrying C9ORF72 repeat expansion was 3.2 years [95% confidence interval (CI): 2.9–3.4]. This was similar to the survival observed among SOD1 cases (3.8 years; 95% CI 3.1–6.5) (Fig. 4). In contrast, FUS-positive ALS had the shortest median survival (1.9 years; 95% CI 1.7–2.1), whereas TARDBP-positive disease was associated with relatively prolonged survival (5.0 years; 95% CI 3.6–7.2).
Figure 4.
Cumulative survival probability from time of disease onset. Comparison between patients with familial ALS with SOD1, TARDBP and FUS genes missense mutations, hexanucleotide repeat expansion of the C9ORF72 gene or unknown genetic mutation (comparison of survival curves by log-rank text, χ2 = 28.05, df 4, P = 0.0001). Green = FUS; blue = C9ORF72; red = SOD1; violet = TARDBP; yellow = unknown gene. Marks are censored patients.
Discussion
We found that the GGGGCC hexanucleotide repeat expansion of the C9ORF72 gene is the most common mutation in our large cohort of patients with familial ALS of Italian, Sardinian and German ancestry. This confirmed our previous findings and demonstrated the importance of this gene in the pathogenesis of the familial form of ALS (Renton et al., 2011). Overall, the discovery of the repeat expansion means that the genetic defect has now been elucidated for 102 (57.6%) of our consecutive series of 177 Italian familial ALS index cases collected across the Italian mainland. Furthermore, the high rate of this mutation in the isolated population of Sardinia, together with the previously described prevalence of TARDBP mutations in the same population (Chiò et al., 2011a), means that the cause of nearly two-thirds of all familial ALS on this island is now explained.
The frequency of familial ALS carrying the C9ORF72 hexanucleotide repeat expansions in Italy and Germany is lower than that reported in the Finnish population (Renton et al., 2011) and similar to that of the American series (Dejesuz-Hernandez et al., 2011; Renton et al., 2011), supporting the notion of the possible north-European origin of the mutation.
Patients with C9ORF72 hexanucleotide repeat expansions display phenotypic differences compared with patients with mutations of other genes or with unknown mutations. The most notable difference is a significantly higher frequency of cognitive impairment both within the index patients and within the extended kindreds (Fig. 2). Mutations in other genes, particularly TARDBP and valosin containing protein, have been occasionally associated with FTD syndromes (Borroni et al., 2010; Chiò et al., 2010; Johnson et al., 2010). However, the high frequency of cognitive impairment associated with the GGGGCC pathogenic repeat expansion supports the hypothesis that the overlap between ALS and FTD phenotypes is largely driven by the chromosome 9p21 genetic lesion (Renton et al., 2011). In this way, our genetic findings confirm what was already known about the neuropathology of ALS and FTD: these clinically disparate syndromes are characterized by the mislocalization and cytoplasmic accumulation of TDP-43 leading this group of disorders to be reclassified as TDP43-proteinopathies (Geser et al., 2009). Indeed, the neuropathological features of chromosome 9p-linked ALS/FTD include a TDP43 proteinopathy, with the peculiar involvement of hippocampal pyramidal neurons (Boxer et al., 2011).
In our cohort, FTD in C9ORF72 mutated cases almost invariably presents with behavioural symptoms, and there was an absence of cases presenting with semantic dementia or progressive non-fluent aphasia. Moreover, we identified psychotic-like symptoms, such as delusions and hallucinations both in three index cases with ALS–FTD and in several relatives within the extended kindreds (see, for example, Pedigree ITALS#7). These findings are consistent with previous observations of families linked to chromosome 9.21, where behavioural symptoms were reported to be prominent (Morita et al., 2006; Boxer et al., 2011; Pearson et al., 2011). The neuroanatomical substrate underlying these psychotic symptoms is not clear, though it is noteworthy that pathological involvement of the hippocampus is prominent in cases carrying the C9ORF72 repeat expansion, a CNS region that is thought to have a role in the production of psychotic-like symptoms (Goto and Grace, 2008).
Studies on family aggregations of neurodegenerative disorders are relatively scarce in ALS, but seem to confirm the co-occurrence of ALS and dementia in the same families. In an article based on a retrospective analysis of a series of 197 Irish patients with ALS and 235 general neurology controls, there was a significantly higher risk of Parkinson's disease and dementia in first-degree relatives of patients with ALS compared with controls (Fallis and Hardiman, 2009). Similarly, a case control study on 635 patients with ALS and 1616 controls in the Netherlands found a mild increase of dementia among siblings and parents of patients with sporadic ALS (Huisman et al., 2011). However, in both papers no distinction between the different types of dementia (in particular, Alzheimer's disease versus FTD) was made, making it difficult to interpret their findings in light of our new understanding of the genetics of chromosome 9p21-linked ALS/FTD.
The reason why C9ORF72 hexanucleotide repeat expansions manifest as disparate ALS and FTD clinical syndromes is unknown. It could be related to variation in the size of the GGGGCC expansion, to the effect of unknown regulatory genes, or to environmental causes. However, an affected child was more likely to manifest symptoms of cognitive dysfunction if the affected parent had a FTD or ALS–FTD phenotype (see, for example, Pedigrees ITALS#4, ITALS#7, ITALS#8 and ITALS#9). This strongly suggests that phenotype is being influenced by a heritable factor, rather than by a pure stochastic event.
Familial patients with ALS with C9ORF72 gene repeat expansions had nearly double the frequency of bulbar-onset disease compared with those with mutations of other genes. This finding is in line with the observation that bulbar onset is more frequent in Scandinavian and north-European populations (Logroscino et al., 2010), which have been reported to have the highest frequency of C9ORF72 mutations (Laaksovirta et al., 2010; Mok et al., 2011).
On average, patients carrying the C9ORF72 expansion manifested disease in their fifth decade of life. A similar pattern has been observed for patients with SOD1 and TARDBP mutations. The biological substrate underlying this late-adult onset is a matter of speculation at present. Normal cellular ageing, environmental exposures, other genetic variants or a combination of these factors may play a role in determining clinical onset. However, we have observed in our cohort that patients with ALS manifesting cognitive impairment had a significantly higher age at onset. One possible explanation for this observation is that the clinical phenotype is, at least in part, driven by the age at onset. A strong influence of the age at onset of ALS phenotype has been already found in sporadic ALS, with a prevalence of bulbar onset in older patients and a prominent upper motor neuron (pyramidal) phenotype in younger patients (Chiò et al., 2011b).
In our series, there was a trend towards an earlier age at onset in younger generations. This 7-year difference in age of onset between parents and children may represent anticipation, a phenomenon known to occur in repeat expansion diseases, such as Huntington's disease and myotonic dystrophy, and may reflect increase in the expansion size with each meiosis. Care is required in interpreting these data, because of the relatively small number of the cohort, and because the individual kindreds were not sufficiently detailed to detect an effect beyond two generations (e.g. grandparent and great-grandparent generation). Accurate measurements of the repeat expansions size by Southern blot analysis will be required to confirm if there truly is repeat expansion instability from generation to generation: such analysis could not be performed in the current cohort due to lack of sufficient quantities of DNA required for such genomic analysis.
The likelihood of detecting the C9ORF72 repeat expansion mutation was not related to the strength of family history of ALS. In fact, in several pedigrees the relative affected by ALS was more distant than a first- or second-degree relative (see, for example, Pedigree ITALS#6). In fact, careful examination of most pedigrees showed that a first- or second-degree relative was more likely to be affected by FTD, strongly supporting the motion of the ALS–FTD continuum of disease. It also highlights the importance (and difficulties) of obtaining a detailed family history of all neurodegenerative disease from patients with ALS and argues against current efforts to limit the definition of what is familial ALS (Byrne et al., 2011).
The current European Federation of Neurological Society (EFNS) guidelines clinical DNA analysis for gene mutations should only be performed in cases with a known family history of ALS, and in sporadic ALS cases with the characteristic phenotype of the recessive D90A mutation (EFNS Task Force, 2011). The complexity of the phenotype of pedigrees related to C9ORF72 mutations, which include cases with pure ALS, ALS–FTD and pure FTD, challenge the current notion of familial ALS, expanding its boundaries to families with a single patients with ALS phenotype and relatives with pure FTD, as exemplified by pedigree ITALS#9 of our series.
In conclusion, C9ORF72 hexanucleotide repeat expansions represent the most frequent genetic mutation among Italian familial ALS. Together with mutation of SOD1, TARDBP and FUS, mutations of C9ORF72 account for ∼60% of familial ALS in Italy. A large number of ALS cases with C9ORF72 mutations present a comorbid behavioural variant of FTD, sometimes associated with hallucinations and delusions. The identification of specific phenotypes related to the different ALS genes is still in its beginnings, but early indications are already emerging that can aid the clinical decision-making. In particular, patients with ALS carrying C9ORF72 repeats are characterized by a high incidence of bulbar-onset disease and comorbidity with FTD, and their pedigrees typically display a high frequency of cases with pure FTD.
Funding
Federazione Italiana Giuoco Calcio (Grant 2010 #1 to M.S. and A.C.); Fondazione Vialli e Mauro per la Sclerosi Laterale Amiotrofica onlus (to A.C.); Ministero della Salute (Ricerca Sanitaria Finalizzata, 2007) (to A.C. and G.R.); European Community's Health Seventh Framework Programme (FP7/2007-2013) under grant agreement 259867 (to A.C.); The Intramural Research Programmes of the National Institutes of Health (NIH); National Institute on Aging (Z01-AG000949-02); and National Institute on Neurological Disorders and Stroke (NINDS); The Packard Centre for ALS Research at Hopkins (to B.J.T.); the ALS Association (to B.J.T. and A.C.); Microsoft Research (to B.J.T.); the Deutsche Forschungsgemeinschaft (SFB 581 and TP B1 to C.D. and M.S.).
Acknowledgements
The authors thank patients with ALS and their families for their invaluable support.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- C9ORF72
chromosome 9 open reading frame 72
- FTD
frontotemporal dementia
- FUS
fused in sarcoma
- ITALSGEN
Italian ALS genetic consortium
- SOD1
superoxide dismutase 1
- TARDBP
TAR DNA-binding protein
Appendix 1
Members of the ITALSGEN consortium: Cristina Moglia, MD, Stefania Cammarosano, MD, Giuseppe Fuda, Antonio Canosa, MD, Sara Gallo, MD (Department of Neuroscience, University of Turin, Italy), Laura Papetti, PharmD (Salvatore Maugeri Foundation, IRCCS, Scientific Institute of Milan, Italy), Marco Luigetti, MD (Neurological Institute, Catholic University and I.CO.M.M. Association for ALS Research, Rome, Italy), Serena Lattante, BS, Giuseppe Marangi, MD (Molecular Genetics Laboratory, Catholic University of Rome, Italy), Tiziana Colletti, MD (ALS Clinical Research Center, Bio.Ne.C., University of Palermo, Italy), Claudia Ricci, MD (Department of Neuroscience, Neurology Section, University of Siena, Italy), Paola Origone, PhD (Department of Neuroscience, Ophthalmology and Genetics, University of Genoa, Italy), Gianluca Floris, MD, Antonino Cannas, MD, Valeria Piras, MD, (Azienda Universitaria-Ospedaliera di Cagliari, and University of Cagliari, Italy), Leslie D. Parish, MD (Department of Neuroscience, University of Sassari, Italy), Giuliana Solinas, PhD, Lucia Ulgheri PhD (Department of Biomedical Science, University of Sassari, Italy), Anna Ticca, MD (AO San Francesco, Nuoro, Italy), Francesco Izzo, MD, Anna Laiola, MD and Francesca Trojsi, MD (Department of Neurological Sciences, Second University of Naples, Naples, Italy).
References
- Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) - revised report of an EFNS task force. Eur J Neurol. 2011 doi: 10.1111/j.1468-1331.2011.03501.x. doi:10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Del Bo R, Papetti A, Buratti E, Bonvicini C, et al. TARDBP mutations in frontotemporal lobar degeneration: frequency, clinical features, and disease course. Rejuvenation Res. 2010;13:509–17. doi: 10.1089/rej.2010.1017. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, Feldman H, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82:196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S, Bede P, Elamin M, Kenna K, Lynch C, McLaughlin R, et al. Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:157–9. doi: 10.3109/17482968.2010.545420. [DOI] [PubMed] [Google Scholar]
- Chiò A, Borghero G, Pugliatti M, Ticca A, Calvo A, Moglia C, et al. Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch Neurol. 2011a;68:594–8. doi: 10.1001/archneurol.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Calvo A, Moglia C, Restagno G, Ossola I, Brunetti M, et al. Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations. Arch Neurol. 2010;67:1002–9. doi: 10.1001/archneurol.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Calvo A, Moglia C, Mazzini L, Mora G PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011b;82:740–6. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- Chiò A, Traynor BJ, Lombardo F, Fimognari M, Calvo A, Ghiglione P, et al. Prevalence of SOD1 mutations in the Italian ALS population. Neurology. 2008;70:533–7. doi: 10.1212/01.wnl.0000299187.90432.3f. [DOI] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallis BA, Hardiman O. Aggregation of neurodegenerative disease in ALS kindreds. Amyotroph Lateral Scler. 2009;10:95–8. doi: 10.1080/17482960802209664. [DOI] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–9. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–8. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace J, Malloy P. Lutz, Fla: Psychological Assessment Resources; 2001. Frontal Systems Behavior Scale (FrSBe): Professional Manual. [Google Scholar]
- Huisman MH, de Jong SW, Verwijs MC, Schelhaas HJ, van der Kooi AJ, de Visser M, et al. Family history of neurodegenerative and vascular diseases in ALS: a population-based study. Neurology. 2011;77:1363–9. doi: 10.1212/WNL.0b013e318231530b. [DOI] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–64. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai SL, Myllykangas L, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9:978–85. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81:385–90. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuca-Tzili L, Brook D, Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve. 2005;32:1–18. doi: 10.1002/mus.20301. [DOI] [PubMed] [Google Scholar]
- Mok K, Traynor BJ, Schymick J, Tienari PJ, Laaksovirta H, Peuralinna T, et al. The chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol Aging. 2012;33:209.e3–8. doi: 10.1016/j.neurobiolaging.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–44. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, et al. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011;258:647–55. doi: 10.1007/s00415-010-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington's disease. Int Rev Neurobiol. 2011;98:325–72. doi: 10.1016/B978-0-12-381328-2.00014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndromes in amyotrophic lateral Sclerosis. Amyotrophic Lateral Sclerosis. 2009;10:131–46. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]