ABSTRACT
Drastic reorganization of the nucleus is a hallmark of herpesvirus replication. This reorganization includes the formation of viral replication compartments, the subnuclear structures in which the viral DNA genome is replicated. The architecture of replication compartments is poorly understood. However, recent work with human cytomegalovirus (HCMV) showed that the viral DNA polymerase subunit UL44 concentrates and viral DNA synthesis occurs at the periphery of these compartments. Any cellular factors involved in replication compartment architecture are largely unknown. Previously, we found that nucleolin, a major protein component of nucleoli, associates with HCMV UL44 in infected cells and is required for efficient viral DNA synthesis. Here, we show that nucleolin binds to purified UL44. Confocal immunofluorescence analysis demonstrated colocalization of nucleolin with UL44 at the periphery of replication compartments. Pharmacological inhibition of viral DNA synthesis prevented the formation of replication compartments but did not abrogate association of UL44 and nucleolin. Thus, association of UL44 and nucleolin is unlikely to be a nonspecific effect related to development of replication compartments. No detectable colocalization of 5-ethynyl-2′-deoxyuridine (EdU)-labeled viral DNA with nucleolin was observed, suggesting that nucleolin is not directly involved in viral DNA synthesis. Small interfering RNA (siRNA)-mediated knockdown of nucleolin caused improper localization of UL44 and a defect in EdU incorporation into viral DNA. We propose a model in which nucleolin anchors UL44 at the periphery of replication compartments to maintain their architecture and promote viral DNA synthesis.
IMPORTANCE
Human cytomegalovirus (HCMV) is an important human pathogen. HCMV infection causes considerable rearrangement of the structure of the nucleus, largely due to the formation of viral replication compartments within the nucleus. Within these compartments, the virus replicates its DNA genome. We previously demonstrated that nucleolin is required for efficient viral DNA synthesis and now find that the nucleolar protein nucleolin interacts with a subunit of the viral DNA polymerase, UL44, specifically at the periphery of replication compartments. Moreover, we find that nucleolin is required to properly localize UL44 at this region. Nucleolin is, therefore, involved in the organization of proteins within replication compartments. This, to our knowledge, is the first report identifying a cellular protein required for maintaining replication compartment architecture.
Introduction
Viral replication requires the ordered association of proteins with the viral genome and the coordination of progressive steps of the viral replication cycle. Many viruses form discrete compartments within the infected cell in order to concentrate factors and processes required for virus replication. In cells infected with herpesviruses, including human cytomegalovirus (HCMV), viral replication compartments form within the nucleus (1–12). Formation and growth of these compartments result in drastic and dynamic changes to the nuclear architecture, including the partitioning of host cell chromatin and rearrangement of cellular nuclear proteins (5, 7, 8). It is unknown what cellular proteins, if any, are required for the formation and maintenance of these compartments.
Recently, we have found that the architecture of HCMV replication compartments is complex (12). In particular, we found that DNA synthesis occurs at the periphery of the compartments and that replicated DNA subsequently localizes to the interior of compartments. Of note, the presumptive viral DNA polymerase processivity subunit UL44 (also known as ICP36) concentrates at the periphery of replication compartments where DNA synthesis occurs. UL44 can bind DNA, and it associates with a number of other proteins (13–21). Thus, this protein may have a role in the organization of proteins and the viral genome within viral replication compartments.
One protein with which UL44 associates throughout infection is nucleolin, a major protein component of nucleoli (21, 22). Nucleolin is a DNA and RNA binding phosphoprotein with many reported protein interaction partners (22). It is thought that nucleolin has multiple functions in ribosome biogenesis, for example, ribosomal DNA (rDNA) transcription, rRNA maturation, and ribosome assembly (reviewed in reference 22). Knockdown of nucleolin mRNA with small interfering RNA (siRNA) results in a specific defect in HCMV DNA synthesis but does not affect levels of UL44 in the infected cell (21). Immunofluorescence (IF) microscopy indicated that nucleolin and UL44 appear to colocalize at replication compartments (21), although precisely where these proteins colocalize was not analyzed.
What role nucleolin plays in viral DNA synthesis is unknown. To better understand the architecture of HCMV replication compartments and nucleolin’s role in virus replication, we examined the interaction of nucleolin with UL44 and the organization of nucleolin, UL44, and viral DNA synthesis within replication compartments.
RESULTS
Interaction of UL44 and nucleolin in vitro.
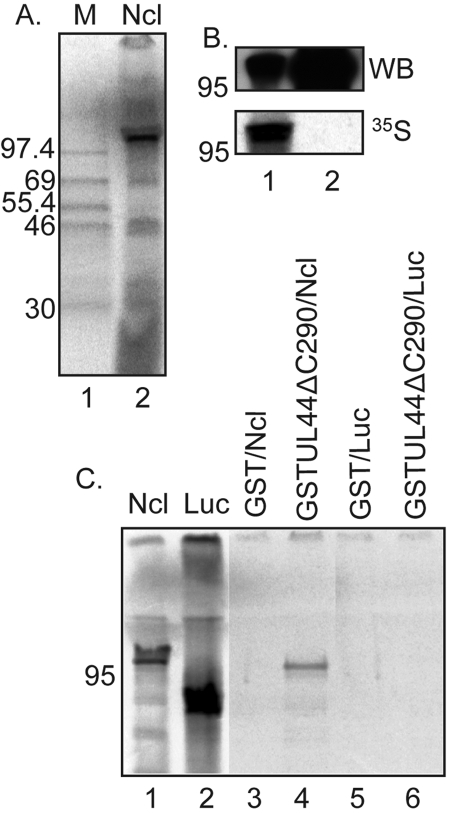
We first sought to investigate if the association of UL44 and nucleolin observed in infected cell lysate (21) could be recapitulated in vitro. Using glutathione S-transferase (GST) pulldown assays, we assayed the binding of radiolabeled nucleolin, expressed by in vitro transcription-translation, to bacterially purified UL44. Nucleolin in HCMV-infected and uninfected cells is predicted to have a molecular mass of 77 kDa but exhibits a molecular mass of greater than 100 kDa in the cell, presumably due to posttranslational modification (21, 22). We found that nucleolin expressed in vitro also exhibited a molecular mass of greater than 100 kDa (Fig. 1A), comigrating with nucleolin from infected cell lysate (Fig. 1B). The carboxyl terminus of UL44 undergoes extensive proteolytic cleavage in bacteria (23). In our GST pulldown assays, we therefore used a UL44 mutant lacking the proteins’ carboxyl terminus, UL44ΔC290 (24). In vitro-expressed nucleolin bound to GSTUL44ΔC290 (Fig. 1C, lane 4) but not to GST (Fig. 1C, lane 3), indicating that nucleolin associates with the UL44 component of the GSTUL44ΔC290 fusion protein. Thus, the carboxyl-terminal segment of UL44 is not essential for interaction of UL44 with nucleolin. No association of luciferase with either GST or GSTUL44ΔC290 was observed (Fig. 1C, lanes 5 and 6), indicating that GSTUL44ΔC290 does not merely interact nonspecifically with any radiolabeled protein generated by in vitro transcription-translation. Thus, UL44 and nucleolin can associate in the absence of any other viral protein.
FIG 1 .
Binding of UL44 and nucleolin in vitro. (A) Radiolabeled nucleolin (Ncl) was produced by in vitro transcription-translation (lane 2), and its mobility in SDS-PAGE was compared to those of radiolabeled proteins of known molecular masses (lane 1). The molecular masses (kDa) of the proteins in lane 1 are noted to the left of the panel. (B) The mobility of radiolabeled nucleolin (lane 1) was compared to the mobility of nucleolin in infected cell lysate (HFF cells infected for 72 h with AD169 at an MOI of 3) and analyzed by Western blotting (WB) using polyclonal rabbit antisera recognizing nucleolin (top panel). Signal from radiolabeled protein (35S) detected on a phosphorimager screen is shown in the bottom panel. The position of a molecular mass marker (kDa) is noted to the left of each panel. (C) GST or GSTUL44ΔC290 was incubated with radiolabeled nucleolin (Ncl) or radiolabeled luciferase (Luc), passed over a glutathione column, and analyzed by SDS-PAGE and autoradiography. The GST and radiolabeled proteins used in each reaction are noted above the figure. The nucleolin and luciferase proteins used in each reaction are shown in lanes 1 and 2. The radiolabeled proteins eluted from each reaction are shown in lanes 3 to 6. The position of a molecular mass marker (kDa) is noted to the left of the panel.
Localization of UL44 and nucleolin in infected cells.
We previously examined the localization of nucleolin and UL44 in HCMV-infected cells using immunofluorescence (IF) and spinning disk confocal microscopy (21). In our previous study, images were produced by acquiring and merging sequential optical planes through the cell to provide a projection of the entire volume (21). In these images, nucleolin was observed both within replication compartments, where it colocalized with UL44 in a speckled distribution, and outside replication compartments, distributed throughout the nucleus (21). Recently, analyzing individual optical planes, we found that UL44 concentrates at the periphery of replication compartments (12). To better understand the distribution of UL44 and nucleolin relative to each other in replication compartments, we conducted further experiments using spinning disk confocal microscopy.
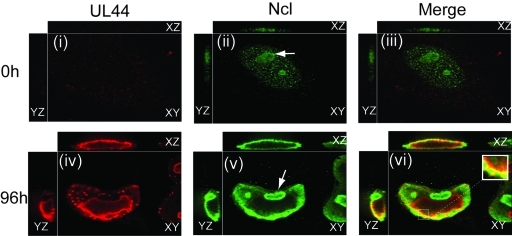
We infected human foreskin fibroblast (HFF) cells for 96 h with HCMV strain AD169. Cells were stained with a polyclonal antibody recognizing nucleolin and a monoclonal antibody (MAb) against UL44 (MAb αICP36) (Fig. 2). (Our laboratory has confirmed that MAb αICP36 specifically recognizes UL44 [25].) In mock-infected (0 h postinfection [h.p.i.]) cells, we detected no UL44 staining, while nucleolin localized to discrete structures—the nucleoli. In infected cells, UL44 accumulated at the periphery of viral replication compartments, with some faint staining within compartments and elsewhere throughout the nucleus (Fig. 2, panel iv). Consistent with previous observations from our laboratory (10, 12), nucleolin staining could be observed diffusely distributed throughout the infected cell nucleus (Fig. 2, panel v). Nucleolin concentrated around replication compartments and within nucleoli (indicated with an arrow in Fig. 2, panel v). From cell to cell, there was variation in the size and shape of the nucleoli observed in uninfected and infected cells.
FIG 2 .
Localization of UL44 and nucleolin in HCMV-infected cells. HFF cells were infected at an MOI of 3 with HCMV strain AD169. Cells were prepared for IF analysis by spinning disk confocal microscopy at the indicated time points. Cells were stained with a MAb recognizing UL44 (αICP36) or nucleolin (Ncl). UL44 and nucleolin were detected using secondary antibodies conjugated to red and green fluorophores, respectively. Panels i to vi show the images obtained by acquiring sequential optical planes in the z axis. Each panel shows the xz and yz axis of the cells and a single focal plane from the z axis (xy). The proteins detected by IF in each experiment are noted above the panels in the left and middle columns. Panels in the right column show the images in the left and middle columns merged. The time points assayed are noted to the left of the figure. The arrow in panels ii and v indicates nucleoli in uninfected and infected cells. The white box in panel vi represents a magnified area.
Consistent with our previous observations (21) that nucleolin levels were greater in infected cells than in uninfected cells, the intensity of nucleolin staining in HCMV-infected cells was greater than that in uninfected cells (compare Fig. 2, panel ii, and Fig. 2, panel v) and greater levels of nucleolin accumulated in the nucleus than in the cytoplasm (Fig. 3A).
FIG 3 .
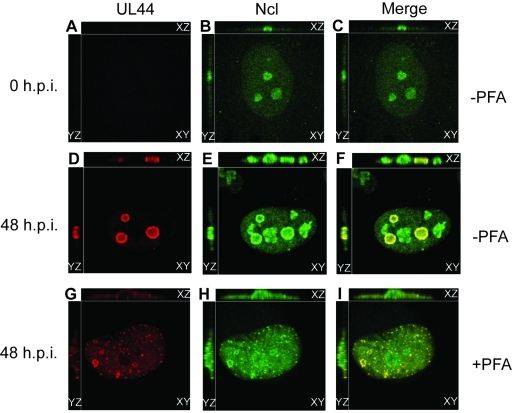
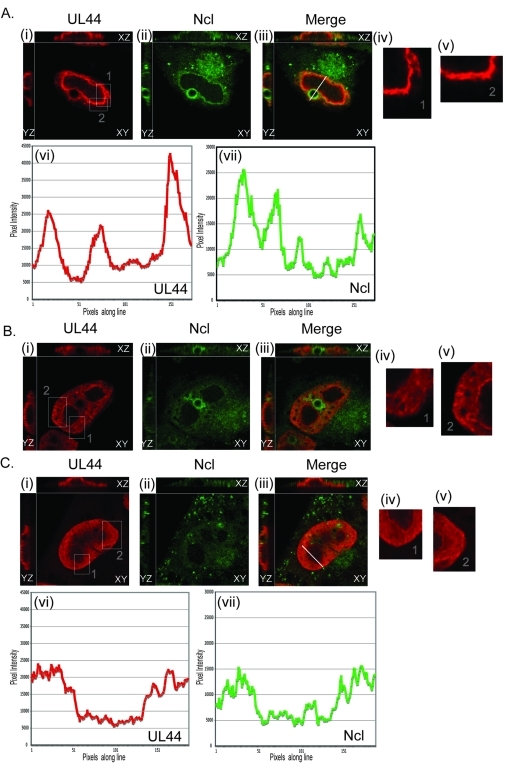
Localization of UL44 and nucleolin in the presence of an inhibitor of viral DNA synthesis. HFF cells were infected with AD169 at an MOI of 3 in the presence or absence of phosphonoformic acid (PFA) (520 µM). Forty-eight hours postinfection, infected cells were analyzed by IF staining with antibodies recognizing UL44 (αICP36 MAb) and nucleolin (Ncl). UL44 and nucleolin were detected using secondary antibodies conjugated to red and green fluorophores, respectively. The images in each panel were obtained by acquiring sequential optical planes in the z axis. Each panel shows the xz and yz axis of the cells and a single focal plane from the z axis (xy). Panels in the left column show UL44 staining, and the middle column shows nucleolin staining. Panels in the right column show the images in the left and middle columns merged. (A to C) Uninfected (0 h.p.i.) cells. (D to F) Infected cells 48 h.p.i. (G to I) Infected cells 48 h.p.i. in the presence of PFA.
Comparing UL44 and nucleolin staining, although no appreciable UL44 was found in nucleoli, nucleolin colocalized with UL44 at the periphery of replication compartments (Fig. 2, panels iv and v, respectively, and merged in Fig. 2, panel vi). In particular, a layer of nucleolin surrounded the layer of UL44 at the periphery of the compartments, with colocalization of the two proteins at the borders of the layers and some interpenetration of the layers. The most intense nucleolin staining colocalized with the most concentrated layer of UL44. We observed similar results in cells at 48 h.p.i. (data not shown; also see below). Thus, nucleolin is not found within HCMV replication compartments. Rather, it localizes to the periphery of replication compartments where it localizes on the outside of the region defined by UL44 staining.
Colocalization of UL44 and nucleolin in the presence of an inhibitor of viral DNA synthesis.
To determine if viral DNA synthesis affects the association of UL44 and nucleolin, we analyzed the localization of nucleolin relative to UL44 by IF in the presence or absence of the viral DNA synthesis inhibitor phosphonoformic acid (PFA) (Fig. 3). In the absence of PFA, nucleolin colocalized with UL44 at the periphery of replication compartments (Fig. 3D to F), as in Fig. 2. In the presence of PFA, no obvious replication compartments could be observed in infected cells, akin to observations made by Penfold and Mocarski (9). Rather, we observed punctate staining of UL44 in infected cell nuclei with faint UL44 staining throughout the nucleus (Fig. 3G). Similarly, we observed punctate nucleolin staining (Fig. 3H) and notable colocalization of punctate UL44 and nucleolin (Fig. 3G). Efficient viral DNA replication is, therefore, not required for the association of UL44 and nucleolin. Moreover, as UL44 and nucleolin colocalize in the absence of fully formed replication compartments, the association of nucleolin with UL44 at the periphery of replication compartments is unlikely to be a nonspecific effect related to the development of replication compartments in infected cell nuclei.
Localization of nucleolin relative to replicating viral DNA.
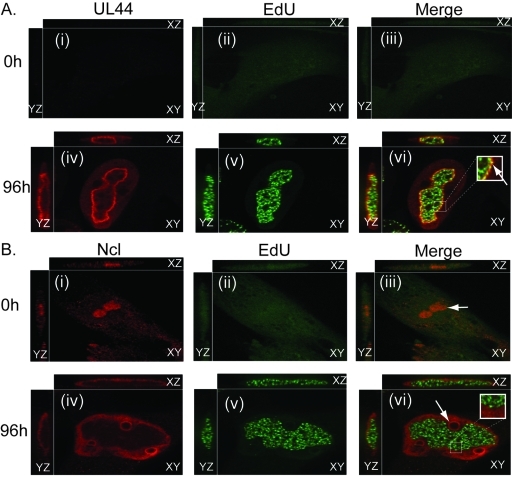
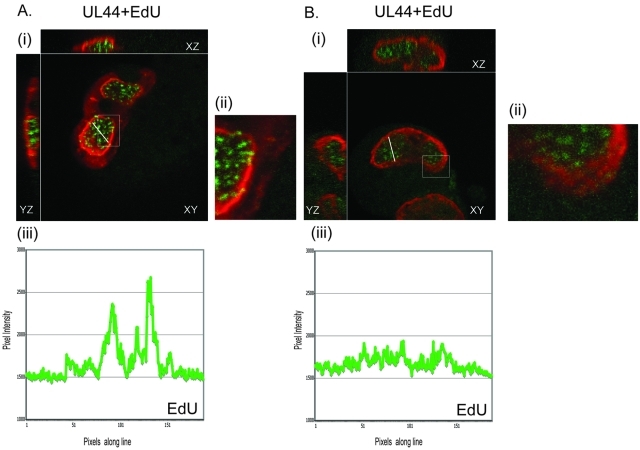
We also examined the localization of UL44 and nucleolin relative to that of viral DNA synthesis by preparing cells for IF after incubation in the presence or absence of 5-ethynyl-2′-deoxyuridine (EdU) (Fig. 4A and B). As described elsewhere (26), EdU incorporated into replicating DNA can be visualized using a fluorescent azide that reacts with the EdU via a Cu(I)-catalyzed [3 + 2] cycloaddition reaction (“click” chemistry). In cells stained with UL44 MAb αICP36 and incubated with EdU for 1 h (Fig. 4A), UL44 again concentrated at the periphery of viral replication compartments (Fig. 4A, panel iv). As we have previously observed (12), punctate staining of EdU-labeled viral DNA was distributed throughout the replication compartment (Fig. 4A, panel v) and colocalized with UL44 only at the periphery of replication compartments (an example is indicated with an arrow in Fig. 4A, panel vi). Elsewhere, we have shown that the DNA synthesis occurs within the UL44 layer at the periphery of replication compartments (12).
FIG 4 .
Localization of UL44, nucleolin, and EdU-labeled viral DNA in HCMV-infected cells. HFF cells were infected at an MOI of 3 with HCMV strain AD169. At 96 h.p.i., infected cells were incubated in the presence or absence of EdU for 60 minutes. Cells were prepared for IF analysis by spinning disk confocal microscopy. Cells were treated using a green fluorescent azide to detect EdU incorporated into DNA and stained with a MAb recognizing UL44 (αICP36) (A) or nucleolin (Ncl) (B). UL44 and nucleolin were detected using secondary antibodies conjugated to red fluorophores. Panels i to vi of panels A and B show the images obtained by acquiring sequential optical planes in the z axis. Each panel shows the xz and yz axis of the cells and a single focal plane from the z axis (xy). EdU or the protein detected by IF in each experiment is noted above the panels in the left and middle columns. Panels in the right column show the images in the left and middle columns merged. The time points assayed are noted to the left of the figure. The arrows in panels iii and vi of panel B indicate nucleoli in uninfected and infected cells. The white boxes in panel vi of panels A and B represent magnified areas. The arrow within the box in panel A indicates colocalization of UL44 with punctate EdU staining at the periphery of the replication compartment.
In cells stained with a polyclonal antibody recognizing nucleolin and treated with EdU for 1 h (Fig. 4B), we observed localizations of nucleolin and EdU similar to those in Fig. 4A and Fig. 2 (Fig. 4B, panels iv and v, respectively). However, we found no detectable colocalization of nucleolin with EdU-labeled DNA (Fig. 4B, panel vi). Instead, all of the EdU staining was internal to the layer of nucleolin. We observed similar results in cells at 48 h.p.i. (data not shown; also see below). Therefore, nucleolin concentrated at the periphery of viral replication compartments where it colocalized with UL44 protein detected by MAb αICP36 but not with the sites of viral DNA synthesis.
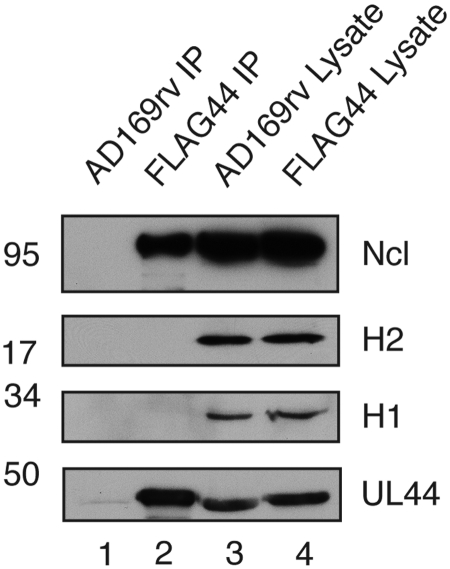
Lack of nucleolin association with histones.
We had initially hypothesized that nucleolin is present at the viral replication fork to participate in chromatin remodeling (21), as nucleolin has been reported to interact directly with histone H2-H3 dimers (27) and histone H1 (28). We therefore investigated the association of UL44, nucleolin, and histone proteins in infected cell lysate immunoprecipitation. Protein was immunoprecipitated from lysates of cells infected with a virus expressing FLAG-tagged UL44 (HCMV-FLAG44) (21) or wild-type virus AD169rv (29) using a monoclonal antibody recognizing FLAG and examined by Western blotting (Fig. 5). Nucleolin was immunoprecipitated with FLAG-tagged UL44 from lysates of cells infected with HCMV-FLAG44 (Fig. 5, lane 2), but FLAG-tagged UL44 and nucleolin were not observed in protein immunoprecipitated from lysates of cells infected with AD169rv (Fig. 5, lane 1). Neither histone H1 nor histone H2 proteins were found in protein immunoprecipitated from either infected cell lysate (Fig. 5, lanes 1 and 2). Similar results were observed in analyzing samples for the presence of histone H3 (data not shown). Therefore, it is unlikely that the purpose of the association of nucleolin with UL44 in the infected cell is to remodel nucleosome proteins during viral DNA synthesis. Together with the data presented in Fig. 4 that show no colocalization of nucleolin with replicating DNA, our results make it unlikely that nucleolin plays a direct role in viral DNA synthesis.
FIG 5 .
Analysis of viral and cellular proteins in infected cells by immunoprecipitation. Lysates from AD169rv (lane 3)- and HCMV-FLAG44 (lane 4)-infected cells and proteins immunoprecipitated using an anti-FLAG antibody from those lysates (lanes 1 and 2, respectively) were separated on a 10% polyacrylamide gel. Proteins in each lane were examined by Western blotting for the presence of nucleolin (Ncl), histone H1 (H1), histone H2 (H2), or UL44 using antibodies recognizing these proteins, as indicated to the right of the figure. The positions of molecular mass markers (kDa) are indicated to the left of the figure.
Localization of UL44 and EdU-labeled DNA in nucleolin-depleted cells.
Based on our results, we hypothesized that the role of nucleolin in viral DNA synthesis (12) might be to maintain the architecture of replication compartments, particularly the localization of UL44 at the periphery of these compartments. To address this hypothesis, cells were treated with either control siRNA or siRNA targeting nucleolin mRNA (Ncl siRNA) to knock down nucleolin protein levels and were infected with AD169. Consistent with our previous observations (21), treatment of cells with Ncl siRNA resulted in an approximately 10-fold decrease in virus yield compared to that produced from cells treated with control siRNA (data not shown). Cells were stained with MAb recognizing UL44 (αICP36) and a polyclonal antibody recognizing nucleolin (Fig. 6). In infected cells treated with control siRNA (Fig. 6A), we observed staining patterns similar to those seen in Fig. 2 and 4. We observed bright staining of nucleolin in the nucleoli of infected cells. UL44 and UL44-nucleolin colocalization concentrated at the periphery of replication compartments. In cells treated with Ncl siRNA (Fig. 6B), we observed heterogeneous levels of nucleolin staining in infected cells. In cells with high levels of nucleolin staining, presumably due to inefficient knockdown, nucleolin colocalized with intensely stained UL44 at the periphery of replication compartments. In cells where we observed a more complete knockdown of nucleolin, however, UL44 staining within infected cell nuclei was considerably more dull and diffuse than was UL44 staining in cells containing high levels of nucleolin, and there were “holes” in the UL44 staining (examples of these cells are indicated with arrows in Fig. 6B). We measured the mean pixel intensities of both UL44 and nucleolin staining in the nuclei of cells shown in Fig. 6B. We plotted these values for each cell analyzed and found a highly statistically significant correlation between the intensity of UL44 and nucleolin staining (Fig. 6C). Thus, with a decreased level of nucleolin in the infected cell, the concentration of UL44 staining at the periphery of replication compartments decreased. The staining patterns of infected cells treated with Ncl siRNA are markedly different from those observed in infected cells treated with the inhibitor of viral DNA synthesis PFA (Fig. 3). Thus, the staining patterns caused by Ncl siRNA are not due merely to inhibition of viral DNA synthesis. Moreover, we have assayed the localization of UL44 throughout infection (21) and have not observed staining patterns similar to those found in infected cells treated with Ncl siRNA. The localization of UL44 observed in Ncl siRNA-treated infected cells is therefore not likely to be due to delayed virus replication.
FIG 6 .
Localization of UL44 in Ncl siRNA-treated cells. (A and B) HFF cells transfected with control or nucleolin siRNA were infected at an MOI of 3 with HCMV strain AD169. Ninety-six hours postinfection, infected cells were analyzed by IF staining with UL44 MAb αICP36 or antibody recognizing nucleolin (Ncl). UL44 and nucleolin were detected using secondary antibodies conjugated to red and green fluorophores, respectively. The images in each panel were obtained by acquiring sequential optical planes in the z axis. Each panel shows a single focal plane from the z axis. Panels in the right column show the images in the left and middle columns merged. Cells shown in panels i to ix of panel B are from the same experiment. Arrows show “holes” in staining. (C) The mean pixel intensity of UL44 and nucleolin (Ncl) staining in the nucleus of each cell in panel B was measured and plotted with respect to those of the other cells. Each data point represents a single cell. The r2 value and P value (t test for r) of the trend line shown in the figure are noted.
We sought to provide a more detailed analysis of the heterogeneity of nucleolin and UL44 staining in infected cells treated with Ncl siRNA. Infected cells treated with Ncl siRNA were prepared for analysis as in Fig. 6. Again, treatment of cells with Ncl siRNA resulted in an approximately 10-fold decrease in virus yield compared to that produced from cells treated with control siRNA (data not shown), and again we found heterogeneous levels of nucleolin protein in different infected cells treated with Ncl siRNA (Fig. 7). In some cells, we observed higher levels of nucleolin staining than others (compare the levels of nucleolin staining in Fig. 7A with those observed in Fig. 7B and 7C.) We measured the UL44 and nucleolin staining pixel intensity along the region indicated with a white line in Fig. 7A, panel iii. In cells where nucleolin staining was high, well-defined peaks of pixel intensity corresponding to the accumulation of UL44 at the periphery of nucleoli and replication compartments were evident (Fig. 7A, panel vi), as were peaks corresponding to the concentration of nucleolin at the periphery of nucleoli (Fig. 7A, panel vii). In Ncl siRNA-treated infected cells where knockdown appeared to be efficient (Fig. 7B, panels i to iii, and Fig. 7C, panels i to iii), we observed low levels of nucleolin (Fig. 7B, panel ii, and Fig. 7C, panel ii). In these cells, replication compartments could be observed; however, UL44 staining at the periphery of compartments was less defined and broader (see enlarged areas shown in Fig. 7B and 7C, panels iv and v). When we examined the pixel intensity of UL44 and nucleolin staining along the region indicated with a white line in Fig. 7C, panel iii, no well-defined peaks of pixel intensity such as those seen in Fig. 7A, panel vi, could be found. Instead, there were found poorly defined maxima of intensity corresponding to diffuse UL44 staining around the periphery of replication compartments (Fig. 7C, panel vi). A similar distribution of pixel intensity from nucleolin staining was seen (Fig. 7C, panel vii). Our data, therefore, argue that high levels of nucleolin are not required for the formation of replication compartments but are required to ensure the proper localization and accumulation of UL44 at the periphery of compartments.
FIG 7 .
Localization of UL44 in Ncl siRNA-treated cells. HFF cells transfected with nucleolin siRNA (A to C) were infected at an MOI of 3 with HCMV strain AD169. Ninety-six hours postinfection, infected cells were analyzed by IF staining with UL44 MAb αICP36 or antibody recognizing nucleolin (Ncl). UL44 and nucleolin were detected using secondary antibodies conjugated to red and green fluorophores, respectively. The images in each panel were obtained by acquiring sequential optical planes in the z axis. Each panel shows the xz and yz axis of the cells and a single focal plane from the z axis (xy). Panels A, B, and C show three different cells from the same experiment that contain different levels of nucleolin. (i to iii) Panels in the left column show UL44 staining, and those in the middle column show nucleolin staining. Panels in the right column show the images in the left and middle columns merged. (iv) Enlarged area from box 1 in panel i. (v) Enlarged area from box 2 in panel i. (vi and vii) Pixel intensities of UL44 (red) and nucleolin (green) staining, respectively, from along the white lines in panels iii of panels A and C. Pixel intensity from the bottom to the top of each line is shown.
We also analyzed incorporation of EdU into viral DNA and MAb αICP36 staining in siRNA-treated infected cells (Fig. 8). Treatment of cells with control siRNA had no obvious effect on the localization of UL44 or nucleolin or incorporation of EdU in infected cells (data not shown). Treatment with Ncl siRNA again resulted in heterogeneous profiles of UL44 accumulation at the periphery of replication compartments. In Ncl siRNA-treated cells where we found a well-defined concentration of UL44 localized at the periphery of replication compartments, akin to the staining pattern of cells containing high levels of nucleolin shown in Fig. 6 and 7, we observed bright punctate staining of EdU incorporated into viral DNA (Fig. 8A, panels i and ii). This is evident from several peaks of pixel intensity from a region along the white line indicated in Fig. 8A, panel i (Fig. 8A, panel iii). Conversely, in cells where we found diffuse staining of UL44 around the periphery of replication compartments, as in cells where nucleolin was efficiently depleted (Fig. 6 and 7), we observed only a faint signal from EdU incorporated into viral DNA (Fig. 8B, panels i and ii). Consistent with this, we observed no obvious peaks of pixel intensity from a region along the white line indicated in Fig. 8B, panel i (Fig. 8B, panel iii). Similar results were observed in EdU-treated infected cells stained with polyclonal antibody to detect nucleolin: only in cells displaying high levels of nucleolin could high levels of EdU incorporation be found (data not shown). Thus, consistent with our previous observations (21), depressing nucleolin levels in infected cells with siRNA results in decreased viral DNA synthesis. The data from Fig. 6 to 8 indicate that nucleolin is required for the proper architecture of UL44 at the periphery of replication compartments, which, in turn, promotes viral DNA synthesis.
FIG 8 .
EdU incorporation into viral DNA in siRNA-treated cells. (i) HFF cells transfected with nucleolin siRNA were infected at an MOI of 3 with HCMV virus AD169. Ninety-six hours postinfection, infected cells were incubated with EdU (60 min) and then analyzed by IF. Cells were stained with a MAb recognizing UL44 (αICP36 MAb) and treated with a green fluorescent azide to detect EdU incorporated into DNA. UL44 was detected using a secondary antibody conjugated to a red fluorophore. Each panel shows the xz and yz axis of the cells and a single focal plane from the z axis (xy). Each panel shows the merged signals from the red and green fluorophores. Cells shown are from the same experiment. (ii) Enlarged areas from the boxes indicated in panel i. (iii) Pixel intensities of EdU (green) staining from along the white lines in panels A and B. Pixel intensity from the bottom to the top of each line is shown.
DISCUSSION
The formation of viral replication compartments in herpesvirus-infected cells allows viral and cellular factors to be concentrated and organized so that virus replication can proceed efficiently. The factors and events involved in formation, maintenance, and organization of HCMV replication compartments are not well understood. Here, we provide insights into the organization of HCMV replication compartments, demonstrating that the organization of UL44 and viral DNA synthesis at the periphery of replication compartments is dependent upon the presence of nucleolin. To our knowledge, this is the first report identifying a cellular protein required for the maintenance of herpesvirus replication compartment architecture.
We have considered the possibility that depletion of nucleolin only indirectly impacts replication compartment architecture and thus DNA synthesis. We cannot exclude this possibility, but several lines of evidence argue against it. Depletion of nucleolin reduced viral DNA synthesis without effects on earlier stages of HCMV replication and had no meaningful effect on cellular viability or the replication of an unrelated virus (21). Depletion of nucleolin did not otherwise affect the morphology of the nucleus in HCMV-infected cells and did not exert effects on replication compartment shape, only on UL44 and EdU staining patterns. Instead, the localization of UL44 and nucleolin at the periphery of replication compartments and the effects of nucleolin depletion on UL44 distribution suggest a hitherto-unforeseen role for these proteins in organizing and maintaining the structure of replication compartments. We therefore propose a model in which nucleolin maintains proper replication compartment architecture and viral DNA synthesis by anchoring a layer of UL44, in which viral DNA synthesis occurs, at the periphery of the replication compartment. In this model, some molecules of UL44 would interact with nucleolin while other UL44 molecules interact with viral DNA. Thus, while nucleolin does not appear to be required at the viral replication fork, it does help to organize the compartments where viral DNA synthesis occurs. This role would explain the requirement for nucleolin in viral DNA synthesis (reference 21 and this report).
The association of UL44 and nucleolin does not require any other viral protein. Although we cannot exclude the possibility that the interaction of UL44 with nucleolin expressed in rabbit reticulocyte lysate is mediated by nucleic acid or reticulocyte proteins, we have previously observed this interaction in infected cell lysate treated with nuclease (21), are organized and reticulocytes lack nuclei. Thus, the interaction is likely direct. It is unclear what surfaces of the two proteins mediate the interaction and if interaction with UL44 is required to recruit nucleolin to replication compartments. It is interesting that nucleolin decorates both the periphery of replication compartments and nucleoli in infected cells. Further analysis is required to determine whether the striking reorganization of nucleoli and nucleolar factors is required for the presence of nucleolin at the periphery of replication compartments.
The formation and organization of herpes simplex virus (HSV) replication compartments have been extensively studied (1–6, 8). There are notable similarities and differences in how HCMV and HSV replication compartments are organized (12). There are also notable similarities and differences in the roles of nucleolin in HCMV and HSV replication. Nucleolin is required for efficient HSV-1 replication (30), much as it is for HCMV replication (21). However, in contrast to what we have found for HCMV, infection of cells with HSV-1 stimulates dispersal of nucleolin from nucleoli without any obvious association with replication compartments (31; B. L. Strang and D. M. Coen, unpublished data). Recent data indicate that nucleolin is required for the proper localization of the multifunctional HSV protein US11 (32). Thus, while nucleolin is important for the replication of different herpesviruses, it may be used for different purposes.
DNA and RNA viruses employ diverse strategies to form and maintain compartments and structures required for viral genome replication. For example, changes in lipid metabolism and proteins involved in maintaining endoplasmic reticulum architecture are involved in producing cytoplasmic lipid-containing vesicles on which positive-sense RNA virus replication takes place (e.g., references 33 and 34). In contrast, our data support a model in which a nucleolar protein is recruited to help form proteinaceous structures in the nucleus within which replication of a herpesvirus DNA genome takes place. The data that we present here will form the basis of further study exploring the formation and maintenance of HCMV replication compartments and compartments formed by other herpesviruses. Continued study of nucleolar proteins and nucleoli is required to fully appreciate how they are utilized in virus replication and pathogenesis.
MATERIALS AND METHODS
Generation of nucleolin expression vectors.
A plasmid containing the full-length nucleolin cDNA (35) was a kind gift from Les Hanakahi (University of Illinois) The full-length nucleolin cDNA sequence was amplified by PCR (5′ GCGCGGGATCCATGGTGAAGCTCGCGAAGGCAGG 3′ and 5′ CGCGCGAATTCCTATTCAAACTTCGTCTTCTTTCCTTGTGGCTTGTGGTCACCTCC 3′) and cloned into the BamI/XhoI sites of pREST-A (Invitrogen) to create plasmid pRSET-Ncl(fl).
GST pulldown assays.
In vitro transcription-translation of the pRSET-Ncl(Fl) plasmids described above or a luciferase expression vector (Promega) was performed using the TNT T7 coupled transcription-translation system (Promega) in the presence of [35S]methionine (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. GST or GSTUL44ΔC290 proteins (2.7 nmol), generated as described elsewhere (36), were incubated in a final volume of 450 µl with 40 µl of in vitro transcription-translation reaction mixture (2 h on ice) in binding buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 0.1 mM EDTA, 2 mM dithiothreitol [DTT]) containing 50 U of benzonase (Sigma) and then loaded onto 0.2-ml glutathione columns. The columns were washed 3 times with 5 ml of wash buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 0.1 mM EDTA, 2 mM DTT, 0.5% NP-40, and 0.5% Triton X-100). Bound proteins were then eluted with wash buffer containing 15 mM glutathione. Proteins eluted from columns were visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. Molecular weight markers were obtained from Perkin-Elmer.
Cells and viruses.
Human foreskin fibroblast (HFF) cells (American Type Culture Collection no. CRL-1684) were used in all experiments. HCMV laboratory strain AD169 was used. Virus expressing FLAG-tagged UL44 is described elsewhere (21).
Immunofluorescence.
HFF cells (5 × 104) were plated on glass coverslips. Cells were mock infected or infected in the presence or absence of phosphonoformic acid (520 µM) with AD169 or HCMV-FLAG44 (21) (multiplicity of infection [MOI], 3). Cells were fixed at room temperature with 4% formaldehyde in Dulbecco’s phosphate-buffered saline (DPBS) at time points indicated above. Where indicated, cells were incubated prior to fixation with 10 µM 5-ethynyl-2′-deoxyuridine (EdU). After washing in DPBS, cells were permeabilized at room temperature (RT) for 10 min with 0.5% Triton X-100 dissolved in DPBS. Where indicated, EdU incorporated into DNA was detected using “click chemistry” (26) with a fluorescent azide (Alexa Fluor 488) per the manufacturer’s instructions (Invitrogen). Once washed again with DPBS, cells were incubated in 0.5% bovine serum albumin (BSA) dissolved in DPBS for 20 min at RT. Primary antibodies in 0.5% BSA dissolved in DPBS were applied and incubated for 1 h at 37°C. Antiserum was removed by washing cells once in 0.5% Tween dissolved in DPBS once and twice with DPBS, each time for 5 min with rocking. This procedure was repeated for the secondary antibodies. Coverslips were mounted on microscope slides with ProLong Antifade (Invitrogen-Molecular Probes).
Images were acquired using an inverted spinning disk confocal microscope based on an Axiovert 200M inverted microscope (Carl Zeiss, Inc., Thornwood, NY), a CSU-X1 spinning disk confocal unit (Yokogawa Electric Corporation, Tokyo, Japan), a spherical aberration correction (SAC) device (Infinity Photo-Optical, Boulder, CO), and a 63× objective lens (Plan-Apochromat; numerical aperture [NA] 1.4; Carl Zeiss). Images shown were obtained by acquiring sequential optical planes of the entire cell spaced by 0.15 µm in the z axis and projecting those planes using Slidebook 4.2 (Intelligent Imaging). When necessary, Slidebook 4.2 was used to analyze pixel intensity of staining in the nucleus by drawing a line around the nucleus as defined by nucleolin staining.
Antibody recognizing UL44 (αICP36 [Virusys; CA006]), was used at a dilution of 1:100. Rabbit antinucleolin polyclonal antibody (Abcam; ab50279) was also used at a dilution of 1:100, as was murine MAb recognizing FLAG (Sigma). All fluorescently labeled secondary antibodies (Alexa Fluor 488 and Alexa Fluor 594) were obtained from Molecular Probes and used at a dilution of 1:1,000.
In each experiment, 3 to 5 cells representative of the phenotypes observed throughout each coverslip (5 × 104 cells) were imaged and analyzed. Each experiment was performed at least twice.
Transfection of siRNA into HFF cells.
Transfection of HFF cells with siRNA was carried out essentially as described elsewhere (21, 37). Briefly, 1 × 105 HFFs per well were seeded in 12-well plates 24 h before transfection. siControl Nontargeting siRNA pool or On-Targetplus SMARTpool human Ncl (both from Dharmacon) was used. Per well, 3 µl 20 µM siRNA and 3 µl Lipofectamine (Invitrogen) were diluted in 50 µl and 12 µl Opti-MEM (Invitrogen), respectively. After 5 min at room temperature, the two solutions were combined. After 20 min, 400 µl prewarmed Opti-MEM was added to each transfection reaction mixture. The cell culture medium was removed, and transfection mixture was added. Six hours posttransfection, siRNA-containing medium was removed and cells were washed twice with 1 ml of prewarmed complete Dulbecco’s modification Eagle’s medium (DMEM) containing 5% fetal bovine serum (FBS). Transfected cells were then incubated in 1 ml of complete DMEM containing 5% FBS, and transfection was repeated twice more at 24-h intervals. Twenty-four hours after the third round of transfection, cells were plated onto glass coverslips for infection and IF analysis.
ACKNOWLEDGMENTS
We thank Lynne Chang and David Knipe (Harvard Medical School) for helpful discussions, comments on the manuscript, and provision of microscopy facilities and technical assistance in preliminary experiments. We gratefully acknowledge Les Hanakahi (University of Illinois) for providing reagents and all members of the Coen laboratory for their support, especially Jean Pesola for assistance with statistical analysis.
This work was supported by NIH grants AI19838 and AI26077 to D.M.C. and grants GM 075252 (NIH) and U54 AI057159 (New England Regional Center of Excellence in Biodefense and Emerging Infectious Disease, Core Imaging Facility) to T.K.
Footnotes
Citation Strang BL, Boulant S, Kirchhausen T, Coen DM. 2012. Host cell nucleolin is required to maintain the architecture of human cytomegalovirus replication compartments. mBio 3(1):e00301-11. doi:10.1128/mBio.00301-11.
REFERENCES
- 1. Quinlan MP, Chen LB, Knipe DM. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857–868 [DOI] [PubMed] [Google Scholar]
- 2. Zhong L, Hayward GS. 1997. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J. Virol. 71:3146–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lukonis CJ, Burkham J, Weller SK. 1997. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J. Virol. 71:4771–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lukonis CJ, Weller SK. 1996. Characterization of nuclear structures in cells infected with herpes simplex virus type 1 in the absence of viral DNA replication. J. Virol. 70:1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lukonis CJ, Weller SK. 1997. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J. Virol. 71:2390–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liptak LM, Uprichard SL, Knipe DM. 1996. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J. Virol. 70:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson-Holley M, Colgrove RC, Nalepa G, Harper JW, Knipe DM. 2005. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J. Virol. 79:12840–12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monier K, Armas JC, Etteldorf S, Ghazal P, Sullivan KF. 2000. Annexation of the interchromosomal space during viral infection. Nat. Cell Biol. 2:661–665 [DOI] [PubMed] [Google Scholar]
- 9. Penfold ME, Mocarski ES. 1997. Formation of cytomegalovirus DNA replication compartments defined by localization of viral proteins and DNA synthesis. Virology 239:46–61 [DOI] [PubMed] [Google Scholar]
- 10. Hamirally S, et al. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahn JH, Jang WJ, Hayward GS. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458–10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strang BL, et al. Human cytomegalovirus UL44 concentrates at the periphery of replication compartments, the site of viral DNA synthesis. J. Virol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Komazin-Meredith G, et al. 2008. The human cytomegalovirus UL44 C clamp wraps around DNA. Structure 16:1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cihlar T, Fuller MD, Cherrington JM. 1997. Expression of the catalytic subunit (UL54) and the accessory protein (UL44) of human cytomegalovirus DNA polymerase in a coupled in vitro transcription/translation system. Protein Expr. Purif. 11:209–218 [DOI] [PubMed] [Google Scholar]
- 15. Krosky PM, et al. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marschall M, et al. 2003. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 311:60–71 [DOI] [PubMed] [Google Scholar]
- 17. Gao Y, Colletti K, Pari GS. 2008. Identification of human cytomegalovirus UL84 virus- and cell-encoded binding partners by using proteomics analysis. J. Virol. 82:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strang BL, Geballe AP, Coen DM. 2010. Association of the human cytomegalovirus DNA polymerase subunit UL44 with the viral proteins IRS1 and TRS1. J. Gen. Virol. 91:2167–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strang BL, Sinigalia E, Silva LA, Coen DM, Loregian A. 2009. Analysis of the association of the human cytomegalovirus DNA polymerase subunit UL44 with the viral DNA replication factor UL84. J. Virol. 83:7581–7589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim YE, Ahn JH. 2010. Role of the specific interaction of UL112-113 p84 with UL44 DNA polymerase processivity factor in promoting DNA replication of human cytomegalovirus. J. Virol. 84:8409–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strang BL, Boulant S, Coen DM. 2010. Nucleolin can associate with the human cytomegalovirus DNA polymerase accessory subunit UL44 and is necessary for viral replication. J. Virol. 84:1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ginisty H, Sicard H, Roger B, Bouvet P. 1999. Structure and functions of nucleolin. J. Cell Sci. 112:761–772 [DOI] [PubMed] [Google Scholar]
- 23. Loregian A, Appleton BA, Hogle JM, Coen DM. 2004. Specific residues in the connector loop of the human cytomegalovirus DNA polymerase accessory protein UL44 are crucial for interaction with the UL54 catalytic subunit. J. Virol. 78:9084–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Appleton BA, et al. 2006. Crystal structure of the cytomegalovirus DNA polymerase subunit UL44 in complex with the C terminus from the catalytic subunit. Differences in structure and function relative to unliganded UL44. J. Biol. Chem. 281:5224–5232 [DOI] [PubMed] [Google Scholar]
- 25. Silva LA, Strang BL, Lin EW, Kamil JP, Coen DM. 2011. Sites and roles of phosphorylation of the human cytomegalovirus DNA polymerase subunit UL44. Virology 417:268–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salic A, Mitchison TJ. 2008. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:2415–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godfrey JE, Baxevanis AD, Moudrianakis EN. 1990. Spectropolarimetric analysis of the core histone octamer and its subunits. Biochemistry 29:965–972 [DOI] [PubMed] [Google Scholar]
- 28. Erard MS, Belenguer P, Caizergues-Ferrer M, Pantaloni A, Amalric F. 1988. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur. J. Biochem. 175:525–530 [DOI] [PubMed] [Google Scholar]
- 29. Hobom U, Brune W, Messerle M, Hahn G, Koszinowski UH. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720–7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Callé A, et al. 2008. Nucleolin is required for an efficient herpes simplex virus type 1 infection. J. Virol. 82:4762–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lymberopoulos MH, Pearson A. 2007. Involvement of UL24 in herpes-simplex-virus-1-induced dispersal of nucleolin. Virology 363:397–409 [DOI] [PubMed] [Google Scholar]
- 32. Greco A, et al. 2012. Nucleolin interacts with US11 protein of herpes simplex virus type 1 and is involved in its trafficking. J. Virol. 86:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boulant S, et al. 2008. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic 9:1268–1282 [DOI] [PubMed] [Google Scholar]
- 34. Diaz A, Wang X, Ahlquist P. 2010. Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function. Proc. Natl. Acad. Sci. U. S. A. 107:16291–16296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Srivastava M, Fleming PJ, Pollard HB, Burns AL. 1989. Cloning and sequencing of the human nucleolin cDNA. FEBS Lett. 250:99–105 [DOI] [PubMed] [Google Scholar]
- 36. Appleton BA, Loregian A, Filman DJ, Coen DM, Hogle JM. 2004. The cytomegalovirus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol. Cell 15:233–244 [DOI] [PubMed] [Google Scholar]
- 37. Wiebusch L, Truss M, Hagemeier C. 2004. Inhibition of human cytomegalovirus replication by small interfering RNAs. J. Gen. Virol. 85:179–184 [DOI] [PubMed] [Google Scholar]