Abstract
Helminths induce potent Th2-type immune responses that can mediate worm expulsion but the importance of this response in controlling acute tissue damage caused by migrating multi-cellular parasites through vital tissues remains uncertain. We used a helminth infection model where parasitic nematode larvae migrate transiently through the lung causing damage resulting in hemorrhage and inflammation. Our findings showed initial elevations in IL-17 contributed to inflammation and lung damage while subsequent IL-4R signaling controlled IL-17 elevations, enhanced expression of insulin-like growth factor 1 and IL-10 and stimulated development of M2 cells, each of which contributed to rapid resolution of tissue damage. These studies indicate an essential role for the Th2-type immune response in mediating acute wound healing during helminth infection.
Keywords: wound healing, Th2, Parasitic helminth
Introduction
The Th2-type immune response is characterized by elevated IL-4, IL-13 and other Th2 cytokines that can mediate host protective responses against helminth parasites. These protective responses can impair larval development1,2 and adult worm feeding3,4 leading to worm expulsion. Helminth parasites, trafficking through host tissues, can cause extensive damage5–7 and it has been postulated that the Th2-type immune response also evolved to promote localized wound healing to rapidly repair and regenerate damaged tissue8–10.
Inflammation is considered an important component of the wound healing process, contributing to both tissue degradation and formation. The role of immune cell populations and cytokines in promoting tissue repair or damage remains unclear11. Both neutrophils and macrophages can rapidly infiltrate sites of tissue damage. These innate immune cell populations may initially be beneficial in removing infectious microbes, clearing debris, and expressing factors that promote wound healing. As the healing process continues, however, control of inflammation may be essential for effective tissue repair11. Recent studies have indicated an important role for macrophages in resolution of tissue damage during viral infection12. The finding that IL-17 mediates neutrophil recruitment and activation13,14 suggests that this cytokine may also contribute to early stages of the tissue repair process, although other studies have suggested that prolonged IL-17 production may contribute to both inflammation and tissue damage15–18. Several studies have suggested that IL-4 and IL-13, both elevated in Th2-type immune responses to helminths, may promote wound healing, stimulating innate and adaptive immune cells to secrete cytokines, growth factors and angiogenic factors that can promote fibroplasia and angiogenesis and inhibit classical inflammation10,19. IL-10 may also play a role in controlling inflammation at the site of tissue damage20,21.
A number of different helminth parasites can cause host tissue damage. Schistosome cercariae and some Strongyloides and hookworms typically penetrate the skin as larvae, migrate into the lungs, causing lung inflammation, and ultimately reach other tissue sites for adult development22,23. Generally, these parasites trigger potent Th2-type immune responses in vivo24 and previous studies have indicated a role for helminth-induced Th2-type responses in controlling pathologic Th1-type responses25–27. A well-studied murine model for intestinal nematode parasite infection is Nippostrongylus brasiliensis (Nb). Following subcutaneous inoculation, third stage larvae (L3) migrate to the lung, where they reside for two days after which they traffic through airways to the esophagus and then to the small intestine28. In the lung, these parasites trigger a potent and highly polarized Th2-type response29–31. Previous studies have demonstrated that at later stages after infection Th2-type responses can promote allergy-associated inflammation and pathology including fibrosis32.
In the studies reported herein, we examined whether the Th2-type response controlled acute lung injury (ALI) shortly after infection. Our findings indicate an important role for the immune response in controlling ALI during in vivo trafficking of helminth parasites.
Results
Kinetics of Nb larvae-induced lung hemorrhaging and inflammation
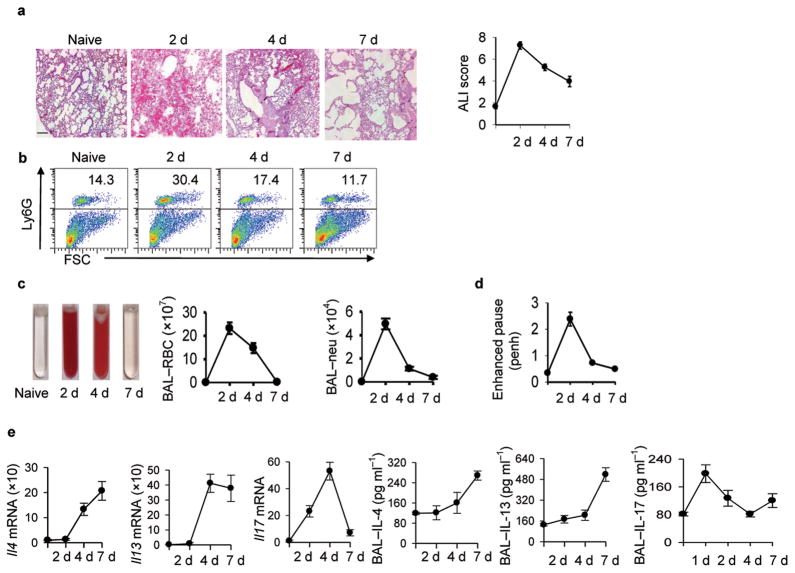
To examine the association between lung damage and the innate immune response, BALB/c mice were inoculated with 500 Nb infective L3 treated with antibiotics to remove residual bacteria. Larvae enter the lung between 24 and 48 hours after inoculation, reside in the lung for 36–48 hours, and migrate to the small intestine by day 328. At days 2, 4, and 7 after inoculation, lung tissue and bronchial-alveolar lavage (BAL) fluid were collected. Increased RBCs, reflecting extensive hemorrhaging, were observed at day 2 after inoculation, but by day 4 hemorrhaging was reduced and by day 7 undetectable (Fig. 1a). Histologic quantitation of lung inflammation, as assessed using the acute lung injury (ALI) scoring method33,34, showed ALI by day 2, which was resolved by day 7. Inflammation, primarily by neutrophils, was also pronounced at day 2, reduced by day 4, and at baseline by day 7 (Fig. 1b). Analysis of BAL also showed increased RBCs by day 2 after inoculation, which were reduced by day 7 (Fig. 1c). The correlation of RBCs in the BAL with overall increases in RBCs in lung tissue samples provided a useful quantitative indicator for assessing lung hemorrhaging by counting BAL RBCs. The BAL neutrophil count was also closely correlated with lung neutrophil inflammation (see Fig. 1b). To assess lung function, airway resistance was measured in conscious, spontaneously breathing mice using a whole-body plethysmography system. Enhanced pause was increased at day two, but returned to near normal levels at days 4 and 7 (Fig. 1d). Thus, shortly after Nb inoculation ALI occurs, associated with hemorrhaging, inflammation, and decreased lung function, and the lung injury is quickly resolved.
Figure 1. Kinetics of N. brasiliensis-induced lung damage.
Mice (five/treatment group) were inoculated with Nippostrongylus brasiliensis (Nb) and 2, 4, and 7 days later airway responsiveness was determined or bronchial alveolar lavage (BAL) and lung tissue was collected. (a) Representative H&E staining of lung tissue (Scale bar, 50 μm) showing peak hemorrhage on day 2 and influx of leukocytes by day 4; acute lung injury scores (ALI) showing increased lung damage at day 2 which was largely resolved by day 7. (b) FACS analysis of neutrophils (Ly6G+) vs. forward scatter (FSC) (c) Bronchial alveolar lavage (BAL) fluid with red color caused by increased RBCs; BAL- red blood cell (RBC) count, and BAL-neutrophil (Neu) count. (d) airway hyperresponsiveness (baseline measurement) indicated as penh (pause enhanced) values. (e) Il4, Il13, and Il17 mRNA and corresponding BAL cytokine protein levels. BAL protein levels were measured by ELISA. Mean and SEM is shown and all results are representative of at least two independent experiments.
Lung tissue Il4 and Il13 mRNA were increased by day 4 after inoculation and remained high at day 7 (Fig. 1e). Unexpectedly, Il17 mRNA was also elevated by day 2, reaching peak levels at day 4 and returning to baseline by day 7. Elevations in Ifng were not detected (data not shown). Elevated BAL IL-4, IL-13, and IL-17 protein were also detected. Gene expression of a panel of factors associated with wound healing showed elevations in Arginase 1 (Arg1), insulin-like growth factor 1 (Igf1), and collagen as early as 2 days, while matrix metalloprotease 13 (Mmp13) elevations were detected by day 7 (Supplementary Fig. 1). Th2-type innate cytokines were also examined with elevations in Tslp and Il33, but not Il25 mRNA, by day 2 after inoculation (Supplementary Fig. 1).
Mice were also inoculated with different doses of Nb and decreases in BAL-RBCs, BAL and lung neutrophils, and ALI were observed at lower doses (Supplementary Fig. 2). However, increases in Il13, Arg1, and Il17 mRNA were generally sustained, indicating that parasites, probably through tissue damage to lung tissue during migration, were contributing directly to lung damage.
IL-17 dependent neutrophil recruitment to the lung contributed to acute hemorrhaging
To examine whether lung infiltration of neutrophils enhanced hemorrhaging, neutrophils were depleted by in vivo administration of Ly6G-specific Ab., which reduced RBCs (Fig. 2a) and ALI (Supplementary Fig. 3a). However, neutrophil depletion had no effect on bleeding time indicating that blood coagulation was not decreased (Supplementary Fig. 4 d), as observed in other model systems35. As expected, neutrophils, identified morphologically, were also depleted in the BAL following Ly6G-specific Ab treatment (Fig. 2a) and in the lung as determined by FACS (Supplementary Fig. 4a).
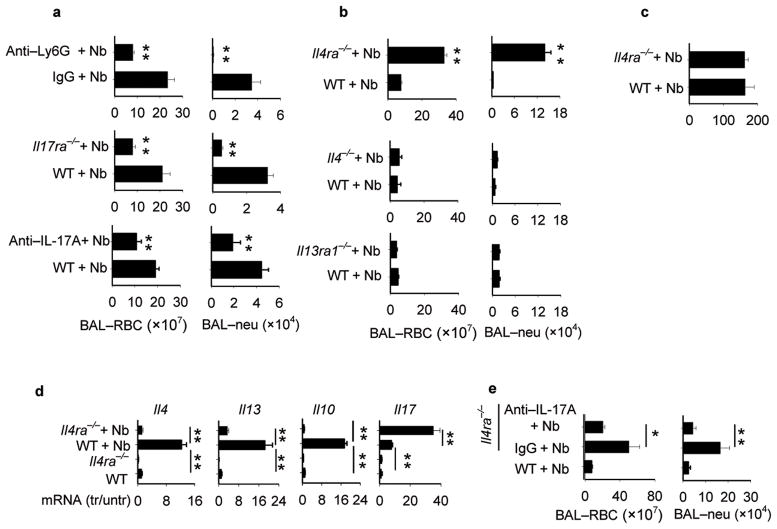
Figure 2. IL-4R signaling controls acute lung hemorrhaging and IL-17 dependent neutrophil infiltration.
(a) WT mice administered Ly6G-specific antibody or isotype control were inoculated with N. brasiliensis (Nb) and on day 2 BAL was collected and RBCs and neutrophils counted. In separate experiments, Il17ra−/−, WT controls and WT mice administered IL-17A-specific antibody or isotype control were inoculated with Nb and at day 2 BAL was collected and RBCs and neutrophils were counted. (b) Il4ra−/−, Il4−/−, and Il13ra1−/− mice and corresponding WT controls were inoculated with Nb and on day 4 BAL was collected and RBCs and neutrophils were counted. (c) Worms were counted in intestine at day 4 after Nb inoculation of Il4ra−/− and WT mice. (d) Lung tissue from WT and Il4ra−/− mice was collected on day 4 after inoculation with N. brasiliensis (Nb) L3. Gene expression of Il4, Il13, Il10 and Il17 were determined using RTPCR. (e) Il4ra−/− mice were inoculated with N. brasiliensis and administered IL-17A specific antibody or Isotype control. At day 2 after inoculation BAL RBCs and neutrophils were enumerated. Results are reported as mean and SEM from three to five individual mice per group and are representative of two independent experiments. *p< 0.05, ** p< 0.01.
As IL-17 was rapidly elevated after inoculation and as IL-17 can mediate neutrophil recruitment13,14, we hypothesized that IL-17 may contribute to lung damage through neutrophil recruitment. Lung hemorrhaging and neutrophil recruitment were markedly decreased in BAL of Il17ra−/− mice compared to WT mice at day 2 after inoculation (Fig. 2a) and the ALI score was significantly reduced (Supplementary Fig. 3b). As IL-17RA is required for IL-25 (IL-17E) as well as IL-17A signaling36, we also used blocking IL-17A-specific antibody. BAL neutrophils and RBCs were significantly decreased (Fig. 2a), as was ALI (Supplementary Fig. 3c) and lung neutrophils (Supplementary Fig. 4c) in Nb-inoculated mice administered IL-17A-specific antibody.
IL-4Rα signaling enhanced resolution of lung damage and promoted factors mediating wound healing and control of inflammation
To assess whether Nb-induced hemorrhaging and influx of neutrophils to the lung were influenced by the Th2-type response, BALB/c Il4ra−/− and WT mice were inoculated with Nb. Increased BAL RBCs and neutrophil infiltration were observed at day 4 after inoculation of Il4ra−/− mice (Fig. 2b). Similarly, blockade of IL-4Rα signaling resulted in increased ALI and enhanced airway resistance (Supplementary Fig. 3 d, f). Nb-inoculated Il4−/− and Il13ra1−/− mice exhibited no increase in either hemorrhaging or inflammation (Fig. 2b), suggesting that either IL-4 or IL-13 alone can mediate control of hemorrhaging and inflammation through IL-4Rα signaling. No difference was detected in the larvae intestinal count between Il4ra−/− mice and WT controls at day 4 after Nb inoculation (Fig. 2c), indicating that larval migration was not affected by IL-4Rα signaling blockade. Il4, Il13, and Il10 gene expression were also markedly reduced in BALB/c Il4ra−/− mice compared to BALB/c WT controls at day 4 after inoculation, while IL-17 was markedly elevated (Fig. 2d).
The increased IL-17 in Nb-inoculated Il4ra−/− mice suggested that elevated IL-17 contributed to the increased lung injury. Nb-inoculated Il4ra−/− mice were administered blocking IL-17A-specific antibody, resulting in markedly reduced BAL hemorrhaging and BAL inflammation (Fig. 2e), and ALI (Supplementary Fig. 3e). These findings indicate that IL-4Rα signaling controls lung injury at least partly through down modulating IL-17 activity.
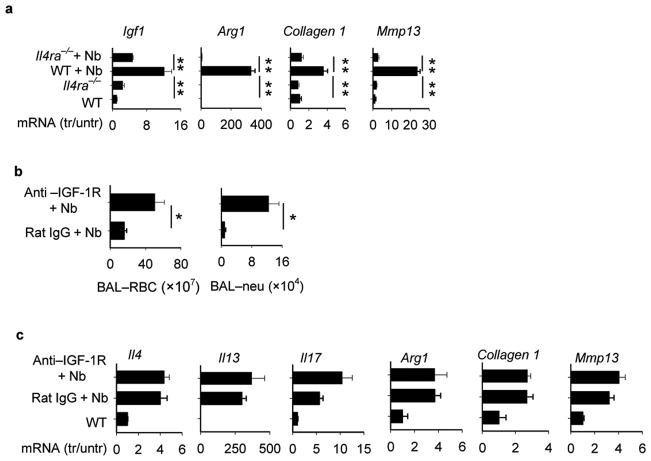
Rapid resolution of Nb-induced hemorrhaging in WT mice suggested that the Th2-type response may promote factors that mediate wound healing. IGF-1, a member of the insulin family of growth hormones, has been shown to be expressed by a number of cell types including T cells, macrophages, and epithelial cells, and recent in vitro studies suggested that it may contribute to localized wound healing effects 37–39 Arg1 may also be expressed by macrophages and can promote collagen formation and cellular proliferation40. Elevations in Igf1 and Arg1 gene expression were blocked in Il4ra−/− mice at day 4 after Nb inoculation, consistent with their expression being dependent on the Th2-type response (Fig. 3a). In addition, elevations in Collagen1 and Mmp13, both important in regulation of tissue regeneration, were inhibited in Nb-inoculated Il4ra−/− mice.
Figure 3. Blockade of IL-4R-dependent IGF-1 signaling inhibits control of inflammation and hemorrhaging in N. brasiliensis- inoculated mice.
(a) Lung tissue from WT and Il4ra−/− mice was collected on day 4 after inoculation with N. brasiliensis (Nb). Gene expresson of Insulin-like growth factor (Igf1), arginase 1 (Arg1), Collagen1, and matrix metalloproteinase 13 (Mmp13) were determined by RT-PCR (b) Mice were administered IGF-1R antibody prior to inoculation with Nb and at day 4 BAL was collected and RBCs and neutrophils counted. (c) Lung tissue from Nb-inoculated mice administered IGF-1R-specific antibody or isotype control antibody were examined for changes in gene expression at day 4 after inoculation using RT-PCR. In all experiments, results are reported as mean and SEM from three to five individual mice per group and are representative of two independent experiments.* p< 0.05, ** p< 0.01.
Blocking IGF-1Rspecific Ab treatment increased both BAL RBCs and neutrophils (Fig. 3b) and increased ALI (Supplementary Fig. 5a) on day 4 after inoculation with Nb, suggesting that IGF-1 contributed to control of both hemorrhaging and inflammation. Increases in Il4 and Il13 were sustained in Nb-inoculated mice administered IGF-1R-specific Abs, indicating that IGF-1 does not affect expression of Th2 cytokines, while Il17 levels were not significantly elevated (Fig. 3c). Other wound healing factors were not affected by IGF-1R-specific antibody treatment (Fig. 3c). Taken together, these findings suggest that IGF-1 is promoting repair of lung damage in vivo through mechanisms downstream of Th2 cytokine expression.
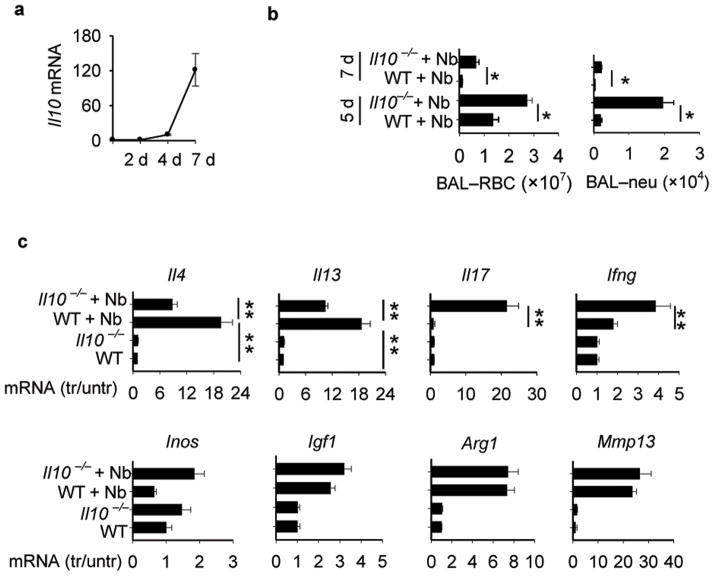
To examine whether the late increase in IL-10, relative to the earlier IL-4 and IL-13 elevations, also contributed to resolution of lung damage, Il10−/− were inoculated with Nb. At both days 5 and 7 after inoculation, time points when IL-10 is elevated, significantly more hemorrhaging, neutrophil infiltration, and ALI was detected in Il10 −/− mice compared to WT controls (Fig. 4b and Supplementary Fig. 5b). Both Il4 and Il13 were partially suppressed at these later time points in Il10 deficient mice (Fig. 4c), suggesting that although IL-10 can enhance expression of these Th2 cytokines at days 5 and 7, IL-10-independent increases still occurred. In contrast, Il17, which had returned to baseline at these later time points in Nb-inoculated WT mice, was markedly increased in Nb-inoculated Il10 −/− mice. Modest elevations in Ifng also occurred, while Inos expression was not affected. Furthermore, elevations in wound healing factors, including Igf1, were not affected by Il10 deficiency. These data suggested that initial increases in IL-4R signaling triggers IL-10 expression, which plays an important role in resolving lung damage, by controlling lung inflammation. Unlike Il4ra deficiency, however, Il10 deficiency does not markedly affect expression of factors important in wound healing.
Figure 4. IL-10 down regulates lung inflammation and IL-17 elevations in N. brasiliensis-inoculated mice.
(a) Il10 gene expression at days 2, 4, and 7 after inoculation with N. brasiliensis (Nb). (b) Il10−/− mice and corresponding WT controls were inoculated with Nb. At day 5 and day 7 BAL was collected and RBCs and neutrophils were counted. (c) At day 7, gene expression of lung Il4, Il13, Il17, Ifng, Inos, Igf1, Arg1, and Mmp13 was determined by RT-PCR. Data are the mean and SEM of five mice per group and are representative of two independent experiments. * p< 0.05, ** p< 0.01.
Macrophages control acute hemorrhaging and inflammation
Macrophages are potential sources of IL-10, IGF-1, MMP-13, and Arg1, all of which were elevated in the lung during the acute wound healing process. Macrophages in BAL (Supplementary Fig. 6a) and lung tissue (Fig. 5a), although present in untreated groups, were markedly increased by day 4 after Nb inoculation. Histological staining revealed numerous RBCs engulfed by individual macrophages at day 4 after inoculation suggesting an important role in RBC clearance. Macrophages were sorted from lung tissue at days 0, 3, and 7 after inoculation with Nb. Although IL-10 was not elevated, increases in Arg1, Igf1, and Mmp13 were detected (Supplementary Fig. 6b). Further cell sorting analyses revealed that IL-10 was elevated in CD4+ T cells (data not shown).
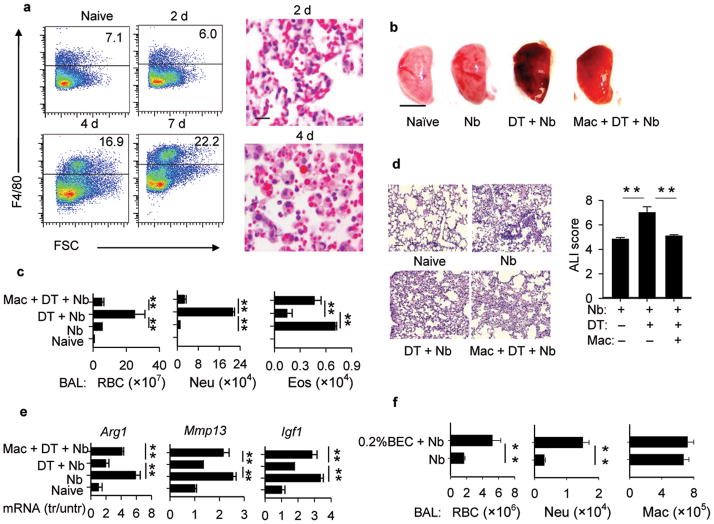
Figure 5. Macrophages control both lung hemorrhaging and inflammation during infection with N. brasiliensis.
(a) Lung tissues were collected at day 0, 2, 4, and 7 after inoculation with N. brasiliensis (Nb), and flow cytometric analysis showed increases in lung macrophages (F4/80+). lung tissue, as detected by H&E staining, showed RBCs engulfed by macrophages especially at day 4 (Scale bar, 5μm). (b e) CD11bDTR mice were inoculated with Nb and administered DT to delete macrophages in vivo. In some groups WT lung-derived macrophages were transferred to DT treated CD11bDTR mice and all mice were examined at day 3 after inoculation. (b) left lobe of lung was collected and photographed (Scale bar, 5mm); (c) BAL was collected and RBCs, neutrophils, and eosinophils were counted at day 3 after inoculation; (d) H&E histological staining (Scale bar, 50μm) and ALI score of lung tissue; (e) Gene expression of lung Arg1, Mmp13, and Igf1 was determined by RT-PCR. (g) WT mice were administrated BEC and inoculated with Nb. On day 4, BAL was collected and RBCs, neutrophils, and macrophages were counted. All images are representative examples of experiments with five mice/group and all data are expressed as the mean and SEM of five mice/group. Experiments were repeated two times with similar results. * p< 0.05, ** p< 0.01.
To examine whether macrophages were required for the rapid wound healing effect of the Th2-type response, transgenic mice expressing the human diptheria toxin receptor (DTR) gene under the control of the CD11b regulatory sequence were used to conditionally ablate macrophages in vivo through DT administration41,42. Nb inoculated, CD11bDTR mice administered DT showed increased lung hemorrhaging and inflammation, while transfer of WT macrophages restored control of both hemorrhaging and inflammation, although some lung damage was still observed in whole lung samples (Fig. 5b–e). BAL and lung eosinophils were also depleted following DT administration, while add-back of WT macrophages restored eosinophil levels (Fig. 5c, Supplementary Fig. 6c) indicating that eosinophil recruitment was blocked by macrophage depletion not by nonspecific effects of DT treatment. Macrophage add-back also largely restored lung macrophages to levels of control groups not administered DT (Supplementary Fig. 6d). Arg1, Mmp13, and Igf1 mRNA were all reduced by DT administration of Nb inoculated CD11bDTR mice; macrophage add back largely restored these levels (Fig. 5e). To examine whether arginase contributed to the resolution of lung damage, the arginase 1 antagonist, BEC, was administered to Nb-inoculated WT mice, using doses previously described to block Arg1 activity in vivo1,43. BEC administration reduced lung arginase activity (Supplementary Fig. 5d) and resulted in increased BAL RBCs and neutrophils (Fig. 5f) and also ALI (Supplementary Fig. 5c), at day 4 after Nb inoculation. Liposome- encapsulated dichloro-methylene-diphosphonate (clodronate) liposomes were used as an alternative approach to inactivate macrophages. Increases in BAL RBCs, BAL neutrophils and ALI, and decreased expression of factors associated with wound healing, were also observed in Nb-inoculated chlodronate liposome-treated mice (Supplementary Fig. 7).
Discussion
These studies demonstrate an essential role for the Th2-type response in controlling acute tissue damage that would otherwise impair lung function during nematode parasite infection. Separate components of this immune response coordinated the control of tissue damage and inflammation. It has been previously suggested that an important host protective effect of the Th2-type response to migrating multi-cellular parasites may include enhanced acute wound healing as an adaptation to the considerable damage caused by these large pathogens migrating through tissues8,10,44. Our report now provides evidence supporting this possibility.
Our finding that control of both IL-17 gene expression and neutrophil infiltration were dependent on IL-4R signaling, either through IL-4 or IL-13, indicated that Th2 cytokines played an important role in down regulating helminth-induced lung inflammation. Our finding that blocking IL-17A prevented increased lung inflammation in Nb-inoculated Il4ra −/− mice further demonstrated a protective role for IL-4R signaling, through controlling IL-17A elevations. IL-10 increased after IL-4 and IL-13 elevations and was dependent on IL-4Rα signaling, consistent with recent observations that IL-4 can promote IL-10 expression45. As Nb-inoculated Il10 −/− mice also showed increased inflammation, our studies suggested that inflammation is controlled by IL-4R signaling at least partly though its induction of increased IL-10.
Several other factors, potentially important in wound healing, were also elevated. IGF-1 elevations were blocked in Il4ra−/− but not in Il10−/− mice. IGF-1 has been implicated in fibroblast proliferation, collagen matrix synthesis, and control of apoptosis37,38,46, all important factors in tissue regeneration. Recent in vitro studies have also suggested that T cells produce IGF-1 and that T cell-derived IGF-1 plays an important role in wound closure39. Our findings provide the first in vivo evidence that production of IGF-1 during the helminth-induced Th2-type immune response plays an essential role in the acute wound healing response. Arg1 can contribute to both cell proliferation and collagen deposition through production of polyamines and proline, respectively47,48. Our findings now indicate that Arg1 activity contributes to the control of helminth-induced lung damage.
Macrophages expressed elevated Arg1 and IGF-1 suggesting that they were either directly contributing to the process of wound healing and/or more indirectly involved by suppressing the pro-inflammatory cytokine response, as recently attributed to Arg1-expressing macrophages43,49. The observation that macrophages did not express elevated IL-10 is consistent with recent paradigms where IL-4 induces wound healing macrophages, which, in contrast to regulatory macrophages, do not express IL-1050. Depletion of macrophages resulted in marked increases in hemorrhaging and inflammation, and decreased expression of factors important in wound healing. The importance of M2 macrophages, and more specifically Arg1, was further confirmed by the increased tissue damage and inflammation observed following inhibition of Arg1 activity in vivo. It is also possible that these macrophages contributed to clearance of RBCs and debris. Recent in vitro studies suggested that M2 macrophages had impaired phagocytosis51, although other studies have suggested that they may be important in neutrophil clearance in vivo10. Macrophages may also have more indirect effects on wound healing through recruitment of additional cell populations. Depletion of lung macrophages in DT-treated CD11bDTR mice reduced lung eosinophils, which were restored by adoptive transfer of WT macrophages. Eosinophils have been previously implicated in wound healing responses52.
The damage and associated hemorrhaging that follows Nb larval transit through the lung may partly result from rapid neutrophil infiltration. Previous studies indicate that neutrophil infiltration at wound sites is not required for tissue repair and may inhibit tissue repair processes, contributing to tissue necrosis and hemorrhaging53, possibly due to direct cellular damage through secretion of neutral proteases and oxygen metabolites or through the release of signals that inhibit cell growth and differentiation54,55. In some cases neutrophils may play an important role in clearing bacteria associated with nematodes56. However, treatment of Nb L3 with anti-microbial antibiotics prior to inoculation made it unlikely that this was a major function or that their infiltration was in response to microbes. As it takes 24–48 hours for the larvae to migrate to the lung, the neutrophils infiltrated the lungs shortly after larval invasion. Our findings demonstrated that the rapid recruitment of neutrophils to the lung and airways was largely dependent on IL-17A signaling.
Taken together, these studies suggest that different components of the helminth-induced Th2-type response interact to mediate acute wound healing and control of inflammation that would otherwise result from migration of these multicellular pathogens through tissues (Fig. 6). The potent and highly polarized Th2-type response induced by infection with parasitic nematodes may have characteristics that could potentially be harnessed in the development of future therapies to enhance acute wound healing responses including treatment of acute lung injury. It should be noted, however, that chronic Th2-type immune responses can be associated with fibrosis and associated pathology, which may need to be subsequently controlled.
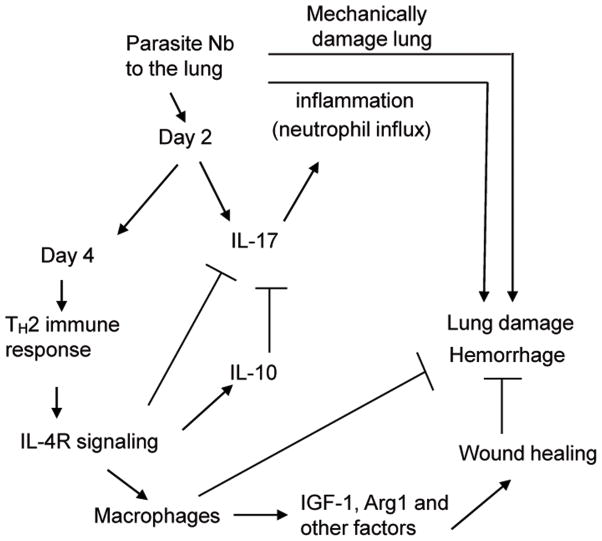
Figure 6. Model of acute resolution of lung damage mediated by a helminth-induced Th2-type response.
Invasion of lung tissue by N. brasiliensis (Nb) larvae and associated tissue damage initially triggers up regulation of IL-17 resulting in pronounced neutrophil infiltration within 2 days after inoculation. By 4 days IL-4 and IL-13 are markedly up regulated and together they inhibit IL-17 expression and inflammation. IL-4 and IL-13 stimulate subsequent expression of IL-10, which further contributes to control of inflammation. Macrophages are also activated to express IGF-1, Arg1, and other wound healing factors that directly mediate tissue regeneration and remodeling and macrophages also likely contribute to clearance of debris and erythrocytes in tissue.
Materials and Methods
Mice
Female 5–8 weeks old BALB/c mice were purchased from NCI. Il4ra−/−, Il4−/− and Il10−/− BALB/c mice, and CD11bDTR C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Il13ra1−/− BALB/c mice, were provided by Dr. Thomas Wynn (NIAID, Bethesda, MD). Il17ra−/− BALB/c mice were from Amgen (Thousand Oaks, CA). All mice were maintained in a specific pathogen-free, virus Ab-free facility during the experiments. The experiments herein were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Animal Resources, National Research Council, Department of Health, Education and Welfare (US National Institutes of Health).
Parasite infection, antibody, and inhibitor administration
Nb L3 were incubated with 400U penicillin, 400 μg ml−1 streptomycin plus 400 μg ml−1 Neomycin (GIBCO, Rockville, MD) for 2hrs at room temperature, and then washed with sterile PBS56. For neutrophil depletion, Ly6G-specific-antibody (BioXcell, West Lebanon, NH) or isotype control IgG was administered to mice both i.p. (0.5 mg in 0.2 ml) and intratracheally (i.t.) (0.2 mg in 0.05 ml) one day before parasite inoculation, as described previously57. For in vivo IL-17A neutralization, 17A-specific antibody (R&D systems, CA) was administered to mice both i.t.(30 μg) and i.v.(30 μg) on days −2, −1, 0, and i.v. 1 day after inoculation. IGF-1R-specific antibody (clone: IMC-A12, generously provided by ImClone Systems, NY) was administered to mice i.p. (1 mg in 0.2 ml) one day before inoculation with Nb. For arginase blockade, mice were provided with drinking water that contained 0.2% S-(2--boronoethyl)-L-cysteine (BEC) (Enzo Life Sciences, Farmingdale, NY) one day before parasite infection. Arginase bioactivity was measured using the Arginase Assay Kit (DARG-200) (BioAssay Systems, Hayward, CA). Clodronate-liposomes and PBS-liposomes were administered to mice both i.t. (0.1 ml) and i.p.(0.1 ml) on days −2, 0, +2, after Nb inoculation to deplete macrophages, as previously described1,58.
Gene Expression by realtime PCR
RNA was extracted from lung tissue and then reverse transcribed to cDNA, as previously described2. Quantitative fluorogenic Real-Time PCR was performed using Taqman® (Applied Biosystems, Foster City, CA) kits and the Applied Biosystems 7700 sequence detector. All data were normalized to 18s ribosomal values and quantification of differences between treatment groups was calculated according to the manufacturer’s instructions. Gene expression is presented as the fold increase over naïve WT controls.
Bronchial-alveolar lavage (BAL)
BAL was collected from anesthetized mice and cell suspensions were prepared as previously described59. RBCs were lysed with ACK lysing buffer (Lonza, MD) and differential counts performed on Giemsa-Wright (CAMCO) stained cytospin slides.
Airway responsiveness
Airway responsiveness (baseline measurement) was measured in mice using a whole-body plethysmography system (BuxcoResearch Systems, Wilmington, NC), as previously described59. Readings of breathing parameters were taken for 2 min after nebulization during which Penh values were determined. The chamber-pressure-time wave was continuously measured via a transducer connected to a computer data-acquisition system.
Histology
Lungs were formalin-fixed, embedded in paraffin and 5μm sections stained with hematoxylin and eosin (H&E). Using previously described methods60, ALI was scored, based on four parameters: alveolar capillary congestion; hemorrhage; infiltration or aggregation of neutrophils in the airspace or the vessel wall; thickness of the alveolar wall. Each parameter was graded from 0–4 based on the damage level (0, no or little damage; 1, less than 25% damage; 2, 25%–50% damage; 3, 50%–75% damage; 4, more than 75% damage). The degree of ALI was assessed by sum of scores of 4 parameters, and the average sum of each was compared among groups.
Flow cytometry
Lung tissue was minced and incubated with stirring at 37 °C for 30 min in Hank’s balanced salt solution (HBSS) with 1.3 mM EDTA (Invitrogen), followed by treatment at 37 °C for 1 hour with collagenase (1 mg ml−1; 170 18–037, Gibco-BRL) in RPMI with 5% fetal calf serum and with 100 μg ml−1 of DNase for 10 min. Cells were lysed with ACK to remove erythrocytes, and 0.1×106 cells were blocked with Fc Block (BD PharMingen), directly stained with FITC- or PE- Ly6G-specific antibody (1A8), Gr-1 (RB6-8C5), or F4/80 (BD Biosciences), and analyzed by flow cytometry1.
Adoptive transfer of macrophages into CD11bDTR mice
Cell suspensions were prepared from whole lungs of WT naïve mice as described above. Positive selection of macrophage cells (F4/80+) was conducted by magnetic cell sorting (MiltenyiBiotec). 2–5×106 cells (in 100 μl of PBS) were transferred into CD11bDTR recipients through i.v. injection. After 2 days, CD11bDTR recipients and control groups received DT (25 mg kg−1) i.v. and were inoculated with parasites the next day.
Statistical analysis
Data were analyzed using SigmaPlot 11 (Systat Software, San Jose, CA, USA) and are reported as means ± SEM. Differences between multiple groups were assessed by one way ANOVA and individual comparisons were analyzed using Holm-sidak test. Differences of p < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health Grants AI031678.
References
- 1.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Q, et al. B Cells Have Distinct Roles in Host Protection against Different Nematode Parasites. J Immunol. 2010 doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbert DR, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao A, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 5.Enobe CS, et al. Early stages of Ascaris suum induce airway inflammation and hyperreactivity in a mouse model. Parasite Immunol. 2006;28:453–461. doi: 10.1111/j.1365-3024.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T, Gutierrez C. Pulmonary complications of cystic echinococcosis in children in Uruguay. Pathol Int. 2005;55:497–503. doi: 10.1111/j.1440-1827.2005.01859.x. [DOI] [PubMed] [Google Scholar]
- 7.Girod N, Brown A, Pritchard DI, Billett EE. Successful vaccination of BALB/c mice against human hookworm (Necator americanus): the immunological phenotype of the protective response. Int J Parasitol. 2003;33:71–80. doi: 10.1016/s0020-7519(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil KS, Knox DP, Proudfoot L. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 2002;24:15–22. doi: 10.1046/j.0141-9838.2001.00428.x. [DOI] [PubMed] [Google Scholar]
- 10.Loke P, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 11.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Shirey KA, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995–986. [DOI] [PubMed] [Google Scholar]
- 14.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 15.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper AM. IL-17 and anti-bacterial immunity: protection versus tissue damage. Eur J Immunol. 2009;39:649–652. doi: 10.1002/eji.200839090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgerton C, et al. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peranteau WH, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 21.Bliss SK, Alcaraz A, Appleton JA. IL-10 prevents liver necrosis during murine infection with Trichinella spiralis. J Immunol. 2003;171:3142–3147. doi: 10.4049/jimmunol.171.6.3142. [DOI] [PubMed] [Google Scholar]
- 22.Apewokin S, Steciuk M, Griffin S, Jhala D. Strongyloides hyperinfection diagnosed by bronchoalveolar lavage in an immunocompromized host. Cytopathology. 2010;21:345–347. doi: 10.1111/j.1365-2303.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- 23.Kolosionek E, Crosby A, Harhay MO, Morrell N, Butrous G. Pulmonary vascular disease associated with schistosomiasis. Expert Rev Anti Infect Ther. 2010;8:1467–1473. doi: 10.1586/eri.10.124. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Kreider T, Urban JF, Jr, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbert DR, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 26.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 27.Brunet LR, Dunne DW, Pearce EJ. Cytokine Interaction and Immune Responses during Schistosoma mansoni Infection. Parasitology today. 1998;14:422–427. doi: 10.1016/s0169-4758(98)01317-9. [DOI] [PubMed] [Google Scholar]
- 28.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 29.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 30.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsland BJ, Kurrer M, Reissmann R, Harris NL, Kopf M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol. 2008;38:479–488. doi: 10.1002/eji.200737827. [DOI] [PubMed] [Google Scholar]
- 33.Li B, et al. Pulmonary epithelial CCR3 promotes LPS-induced lung inflammation by mediating release of IL-8. Journal of cellular physiology. 2011;226:2398–2405. doi: 10.1002/jcp.22577. [DOI] [PubMed] [Google Scholar]
- 34.Krzyzaniak M, et al. Burn-induced acute lung injury requires a functional toll-like receptor 4. Shock. 2011;36:24–29. doi: 10.1097/SHK.0b013e318212276b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massberg S, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 36.Rickel EA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 37.Rom WN, et al. Alveolar macrophages release an insulin-like growth factor I-type molecule. J Clin Invest. 1988;82:1685–1693. doi: 10.1172/JCI113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynes MW, Frankel SK, Riches DW. IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol. 2004;76:1019–1027. doi: 10.1189/jlb.0504288. [DOI] [PubMed] [Google Scholar]
- 39.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cailhier JF, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 43.Herbert DR, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 45.Balic A, Harcus YM, Taylor MD, Brombacher F, Maizels RM. IL-4R signaling is required to induce IL-10 for the establishment of T(h)2 dominance. Int Immunol. 2006;18:1421–1431. doi: 10.1093/intimm/dxl075. [DOI] [PubMed] [Google Scholar]
- 46.Gillery P, Leperre A, Maquart FX, Borel JP. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J Cell Physiol. 1992;152:389–396. doi: 10.1002/jcp.1041520221. [DOI] [PubMed] [Google Scholar]
- 47.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 48.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 49.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varin A, Mukhopadhyay S, Herbein G, Gordon S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood. 2010;115:353–362. doi: 10.1182/blood-2009-08-236711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 53.Asti C, et al. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000;13:61–69. doi: 10.1006/pupt.2000.0231. [DOI] [PubMed] [Google Scholar]
- 54.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 55.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pesce JT, et al. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2-type response. J Immunol. 2008;180:464–474. doi: 10.4049/jimmunol.180.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 58.Pribul PK, et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J Virol. 2008;82:4441–4448. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mionnet C, et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16:1305–1312. doi: 10.1038/nm.2253. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. American journal of physiology Lung cellular and molecular physiology. 2006;291:L580–587. doi: 10.1152/ajplung.00270.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.