Abstract
The aim of this work was to investigate starch granule numbers in Arabidopsis (Arabidopsis thaliana) leaves. Lack of quantitative information on the extent of genetic, temporal, developmental, and environmental variation in granule numbers is an important limitation in understanding control of starch degradation and the mechanism of granule initiation. Two methods were developed for reliable estimation of numbers of granules per chloroplast. First, direct measurements were made on large series of consecutive sections of mesophyll tissue obtained by focused ion beam-scanning electron microscopy. Second, average numbers were calculated from the starch contents of leaves and chloroplasts and estimates of granule mass based on granule dimensions. Examination of wild-type plants and accumulation and regulation of chloroplast (arc) mutants with few, large chloroplasts provided the following new insights. There is wide variation in chloroplast volumes in cells of wild-type leaves. Granule numbers per chloroplast are correlated with chloroplast volume, i.e. large chloroplasts have more granules than small chloroplasts. Mature leaves of wild-type plants and arc mutants have approximately the same number of granules per unit volume of stroma, regardless of the size and number of chloroplasts per cell. Granule numbers per unit volume of stroma are also relatively constant in immature leaves but are greater than in mature leaves. Granule initiation occurs as chloroplasts divide in immature leaves, but relatively little initiation occurs in mature leaves. Changes in leaf starch content over the diurnal cycle are largely brought about by changes in the volume of a fixed number of granules.
Chloroplasts in Arabidopsis (Arabidopsis thaliana) mesophyll cells are generally stated to contain about five starch granules at the end of the light period (Zeeman et al., 2002, 2007), but nothing is known about how this number is determined or the extent of genetic, temporal, developmental, and environmental variation in the number. This is an important limitation in understanding control of starch degradation at night, a process essential for the normal growth of the plant (Gibon et al., 2006; Smith and Stitt, 2007; Stitt et al., 2007; Usadel et al., 2008). The surface area and volume of starch granules in the chloroplast are relevant to the control of starch degradation in two ways. First, surface area can potentially limit the rate of degradation during the night. This limitation is not a major determinant of the rate of degradation in wild-type Arabidopsis leaves in controlled conditions; degradation is near linear through most of the night and consumes almost all of the starch reserves by dawn. If degradation were limited by surface area, the rate would decline with time through the night. However, reductions in starch granule numbers may result in limitation of starch degradation. The starch synthase4 (ss4) mutant of Arabidopsis has only one starch granule per chloroplast. It has a low rate of starch degradation and a low growth rate, consistent with the idea that the reduced surface area provided by one rather than five starch granules restricts the rate of degradation and hence the availability of carbon for growth at night (Roldán et al., 2007).
Second, recent experiments implicate starch granules in the mechanism that sets the rate of starch degradation at the start of the night. When Arabidopsis plants grown in 12 h of light and 12 h of dark were subjected to darkness after only 8 h of light, the rate of starch degradation was one-half that on previous nights, so that starch reserves were not exhausted until dawn (Graf et al., 2010). The capacity to adjust the rate in this precise way requires measurement of both the time until dawn (probably provided by the circadian clock) and the amount of starch in the leaf at the onset of darkness. Although the way in which starch content is measured is not known, the presence of starch granules is essential for correct adjustment of the rate of starch degradation. In mutants that accumulate a soluble form of starch (phytoglycogen) in place of starch granules, reserves are degraded in an exponential manner and are exhausted well before dawn (Delatte et al., 2005).
Understanding variation in starch granule numbers in mesophyll chloroplasts is also important in the context of starch granule initiation. Almost nothing is known about how granules are initiated. It is reasonable to assume that initiation accompanies the three rounds of chloroplast division that occur during the expansion phase of mesophyll cell development, and it may also accompany the very large increase in starch content that typically occurs during each light period in mature leaves. However, no quantitative information is available on the times and locations of granule initiation in mesophyll cells. Such information would provide new possibilities for studying the nature and control of initiation.
To shed light on the control of starch granule number in Arabidopsis leaves, we developed two new methods for robust and reproducible estimation of the numbers of granules per chloroplast. The first involves sequential sectioning and imaging of leaves embedded in resin blocks by focused ion beam-scanning electron microscopy (FIB-SEM). The second involves measurement of the starch content of whole leaves and of known numbers of isolated chloroplasts, plus estimation of granule volumes from SEM images. We applied these methods to leaves at different points in the day-night cycle, of different ages, under different growth conditions, and with different chloroplast sizes and numbers per cell.
RESULTS
Choice of Methods for Estimating Starch Granule Numbers per Chloroplast
Most observations and measurements of starch granules in leaves have been made on a transmission electron microscope (TEM) or light microscope images of sections through mesophyll cells. These methods are not suitable for accurate estimation of granule numbers per chloroplast. A single section through a chloroplast is likely to underestimate granule numbers. Serial sectioning through multiple chloroplasts followed by three-dimensional (3D) image reconstruction would reveal the exact number of granules per chloroplast, but this is a time-consuming and technically challenging process unsuitable for straightforward comparison of multiple replicates and conditions.
Flow cytometry has been used to estimate the number of starch granules per chloroplast in beans (Phaseolus spp.; Yang et al., 2002). Suspensions of ruptured chloroplasts separated into two peaks of particles, assumed to represent chloroplast membranes and starch granules. However, no direct evidence was provided for these peak assignations. In other studies, intact chloroplasts, ruptured chloroplasts, and isolated thylakoids migrated as three separate peaks (Ashcroft et al., 1986; Petit et al., 1989; Bergounioux et al., 1992; Schröder and Petit, 1992). Because rupture of a significant fraction of originally intact chloroplasts is almost inevitable during flow cytometry, the assumptions about peak identity made by Yang et al. (2002) may be incorrect. In addition, the small size of leaf starch granules (1–2 μm in diameter) is likely to prevent clean separation from other small particulate matter in chloroplast preparations.
We developed two methods for estimating starch granule numbers per chloroplast. In method 1, good resolution of granules within chloroplasts is obtained by imaging the surface of a resin block containing leaf material in a SEM. Removal of successive thin sections (typically approximately 50 nm) from the surface of the block with an FIB then allows a 3D image of the leaf tissue to be built up (FIB-SEM; see Armer et al., 2009; Bushby et al., 2011). Typical single images for individual chloroplasts are shown in Figure 1, and example images from which data were derived are shown in Supplemental Figure S1. The method has major advantages of speed, accuracy, and convenience over conventional serial sectioning and TEM. Because the block face is imaged rather than the removed material, there is no distortion of the image surface and alignment of the images is relatively simple. The risk of missing or damaged sections is eliminated, making it possible to collect large numbers of sequential images through a volume of many cubic micrometers. The process is automated, and data collection and processing for multiple samples, each containing numerous chloroplasts, can be performed in a few days. The processed 3D image enables accurate measurements of granule and chloroplast volume as well as granule numbers and analysis of the biological variability of all of these parameters within a cell or a region of the leaf. Major disadvantages are the requirement for specialized and expensive equipment and computing time, the fact that the small tissue samples may not be representative of the whole leaf and the fact that differential shrinkage of cell compartments can occur during sample preparation. We typically imaged blocks of tissue of approximately 50 × 103 μm3. This volume is comparable with that of a spongy mesophyll cell in a fully expanded Arabidopsis leaf (Wuyts et al., 2010). Loss of cell volume during sample preparation for microscopy is primarily because of vacuolar shrinkage, but chloroplast volume may decrease by about 30% (Winter et al., 1993, 1994). This is not a major problem when making comparisons between chloroplasts, but it means that estimates of the relationship between starch volume and chloroplast volume contain systematic errors.
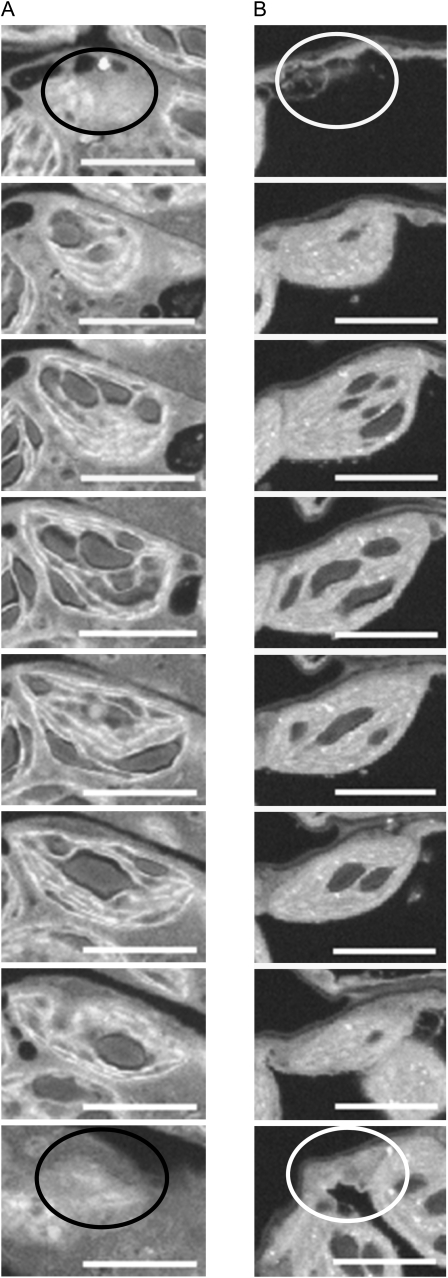
Figure 1.
Typical FIB-SEM images. Images are sections through a single chloroplast of an immature leaf (A) and a mature leaf (B) from 25-d-old plants at the end of a 12-h-light period. Every 15th or 16th section (A) or every 23rd or 24th section (B) is shown. Slice depth was 49 nm; thus, sections in A are at intervals of approximately 0.75 μm through the chloroplast, and those in B are at intervals of approximately 1.15 μm. Circles on the first and last images of each series show the position at which the chloroplast appeared in the next or previous section. Bars, 5 μm. The chloroplast in A contained a total of 17 starch granules and that in B contained eight starch granules.
Method 2 involves preparation of intact chloroplasts from a leaf sample, measurement of the starch content of a known number of chloroplasts, then estimation of the average volume of starch granules in preparations of starch from replicate leaf samples. These data are used to calculate the weight of starch per chloroplast and thus the number of starch granules, using published values for starch density. Calculation of granule volumes employs measurements of granule thickness made on SEM images of granules cracked by freezing a starch slurry in liquid nitrogen then grinding it in a mortar, and a method of estimating granule radii from measurements made on SEM images of intact granules (Supplemental Fig. S2). Method 2 does not require specialized equipment other than a standard SEM, and it can be applied rapidly to many samples. It provides values averaged over numerous leaves. However, it is much less direct than method 1, increasing the possibility of systematic errors, and it assumes that granule density is constant over the day-night cycle. It provides no information about chloroplast volumes or variation in granule numbers per chloroplast within leaves or cells, or differences between mature and developing leaves.
One potential concern for both methods is whether the full range of granule sizes is sampled without bias. In method 1, resolution may be insufficient to detect the smallest granules, leading to underestimation of granule numbers at the end of the night. In method 2, granules may be isolated from tissues other than the mesophyll, and there may be losses of particularly small or large granules. We compared the range of granule sizes detected by the two methods, using replicate samples from a single batch of plants (Table I). Both methods showed that granules isolated at the end of the day were more than 2-fold greater in diameter than those isolated at the end of the night. There was no statistically significant difference between the end-of-night values obtained by the two methods. The end-of-day value obtained by method 1 was 13% higher than that obtained by method 2, and the variance was also greater. Supplemental Figure S3 compares these data with values obtained from measurements on thin sections imaged by light microscopy. Light microscopy gave values comparable to methods 1 and 2 for granules at the end of the night but lower values for granules at the end of the day.
Table I. Comparison of measurements of granule size and number per chloroplast made by methods 1 and 2.
The methods were both applied to a single batch of mature, nonflowering rosettes.
| End of Day | End of Night | |
| Average granule diameter (μm)a | ||
| Method 1 | 1.69 ± 0.05b | 0.75 ± 0.02 |
| Method 2 | 1.93 ± 0.04 | 0.79 ± 0.04 |
| Average number of granules per chloroplast | ||
| Method 1c | 6.8 ± 0.2 | 4.0 ± 0.1d |
| Method 2 | 5.5 ± 0.3 | 5.8 ± 0.3 |
Values are means ± sem of measurements on 222 granules.
End-of-day values obtained by the two methods are statistically significantly different (Student’s t test, P < 0.05).
Measurements were made on 50 chloroplasts from each of three leaves.
End-of-night values obtained by the two methods are statistically significantly different
Granule Numbers over the Light-Dark Cycle
Methods 1 and 2 were used to determine the average number of granules per chloroplast in mature, nonflowering rosettes at the end of the day when starch content is maximal and at the end of the night when starch content is minimal (Table I). At the end of the day, method 1 gave an average value of 6.8, and method 2 gave an average value of 5.5. Given the major differences between the methods themselves and the samples to which they are applied, the agreement between the methods is good. At the end of the night, method 1 gave an average value of 4.0 and method 2 gave an average value of 5.8. Thus, values from method 1 are consistent with a decline in total granule number per chloroplast overnight (from 6.8 to 4.0), whereas values from method 2 suggest that there is no change in granule number per chloroplast overnight. This discrepancy may be in part because of a failure to detect all of the very small granules present at the end of the night in the sections viewed in method 1.
Relationship between Starch Granule Number and Leaf Starch Content
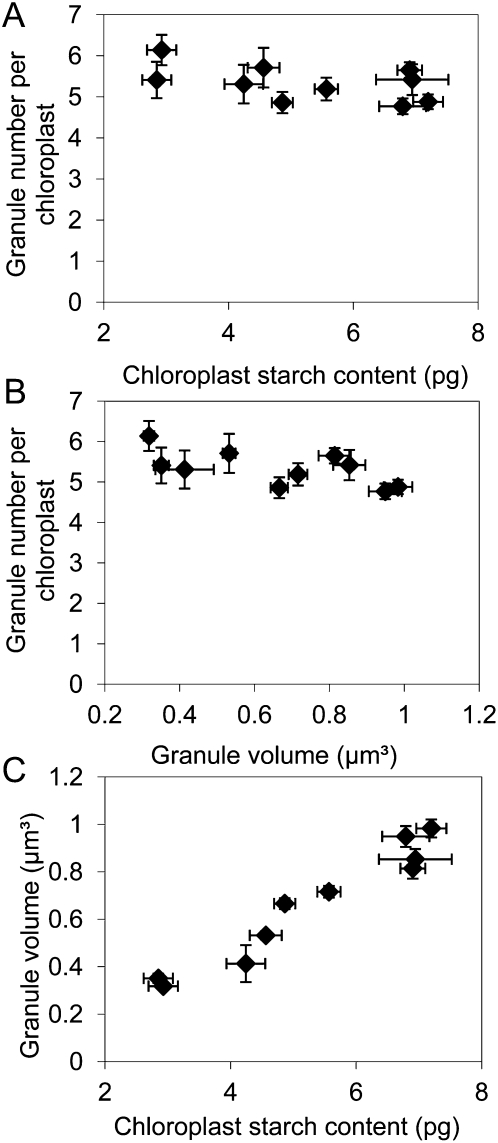
To examine the relationship between granule numbers and leaf starch content, method 2 was applied to leaves with a wide range of starch contents, obtained by harvesting mature, nonflowering rosettes at different times of day and from different light and daylength regimes. Starch content in plants grown with 12 h of light and 12 h of dark increased about 40-fold over the light period (Table II). However, there was no statistically significant change in the number of starch granules per chloroplast over this period. Chloroplasts at the start, middle, and end of the light period contained on average between five and six starch granules. The starch contents of plants harvested in the middle of the light period from different daylength and light intensity regimes showed a 2-fold range of variation, but average granule number per chloroplast varied by only 12% across all of the treatments (Table II). When data from time of day, daylength, and light intensity treatments were considered together, it was apparent that average granule number per chloroplast was essentially constant over a wide range of values of leaf starch content (Table II). This was reflected in a lack of correlation between the granule number per chloroplast and either chloroplast starch content or granule volume (Fig. 2, A and B) but a strong, positive relationship between granule volume and chloroplast starch content (Fig. 2C). Thus, changes in starch content through the day and in response to different light intensities and daylengths appear to be brought about primarily by changes in the volumes of a constant number of starch granules in each chloroplast, rather than changes in the numbers of granules per chloroplast.
Table II. Calculations of the number of starch granules per chloroplast using method 2.
| Average No. of Starch Granules per Chloroplast | Starch Content of Leaf | ||
| mg g−1 fresh wt | |||
| Experiment 1: time of daya | End of night | 5.8 ± 0.3 | 0.21 ± 0.08 |
| Middle of day | 5.1 ± 0.3 | 4.98 ± 0.74 | |
| End of day | 5.5 ± 0.3 | 8.40 ± 0.33 | |
| Experiment 2: light levelb | 180 μmol quanta m−2 s−1 | 4.9 ± 0.2 | 5.64 ± 1.09 |
| Transfer to 245 μmol quanta m−2 s−1 before harvestc | 4.8 ± 0.2 | 5.35 ± 0.65 | |
| 245 μmol quanta m−2 s−1 | 4.9 ± 0.3 | 2.85 ± 0.35 | |
| Experiment 3: daylengthd | Middle of day, 16 h light | 5.5 ± 0.5 | 5.73 ± 0.44 |
Plants were grown in 12 h of light, 12 h of dark. For end of night, values are means ± se of eight measurements on four chloroplast preparations from each of two separately grown batches of plants. For middle of day and end of day, values are means of 12 measurements on four chloroplast preparations from each of three separately grown batches of plants.
Plants were harvested in the middle of the light period (6 h of light). Values are means ± se of measurements on four chloroplast preparations.
Plants were grown at 180 μmol quanta m−2 s−1 and transferred to the higher light level 24 h before harvest; they still experienced the 12-h night as normal.
Plants were grown in 16 h of light, 8 h of dark and harvested in the middle of the light period. Values are means ± se of measurements on four chloroplast preparations.
Figure 2.
Relationship among chloroplast starch content, starch granule number, and starch granule volume. Data were obtained using method 2 and are from 10 independent experiments shown in Table II. Seven different combinations of harvest time and light regime are represented (Table II). For each experiment, values of granule number per chloroplast and chloroplast starch content are means ± se from four independent samples. Values for mean granule volumes are from measurements on between 378 and 878 granules. A, Relationship between the number of starch granules per chloroplast and chloroplast starch content. B, Relationship between the number of starch granules per chloroplast and granule volume. C, Relationship between the volume of individual starch granules and chloroplast starch content.
Relationship between Starch Granule Size and Number and Chloroplast Volume in Wild-Type Leaves
Using method 1, we examined the extent of variation in chloroplast volumes and starch granule numbers per chloroplast within individual cells, in both mature (fully expanded) and immature leaves at the end of the day. For mature leaves, we harvested leaf 6 from 25-d-old plants grown with 12 h of light and 12 h of dark (growth stage 1.14; Boyes et al., 2001), where leaf 1 is the first true leaf formed. For immature leaves, we harvested leaves 14, 15, and 16 from the same plants. The extent of starch turnover in mature and immature leaves was similar. In a typical experiment, starch content decreased overnight by 12 mg g−1 fresh weight in a pool of mature leaves (leaves 5–8) and by 9 mg g−1 fresh weight in a pool of immature leaves (leaves 13–16; data not shown).
There was a huge range of variation in chloroplast size in both immature and mature leaves. Measurements of 50 chloroplasts in mesophyll cells from three different plants gave volumes between 38 and 240 μm−3 for mature leaves and volumes between 11 and 129 μm−3 for immature leaves. Within plants, the variation in chloroplast volume was 3.5-, 6.1-, and 2.7-fold for mature leaves and 3.8-, 8.1-, and 6.7-fold for immature leaves. There was also a very large variation in numbers of starch granules per chloroplast. There were between three and 14 granules per chloroplast in mature leaves and between four and 21 granules per chloroplast in immature leaves.
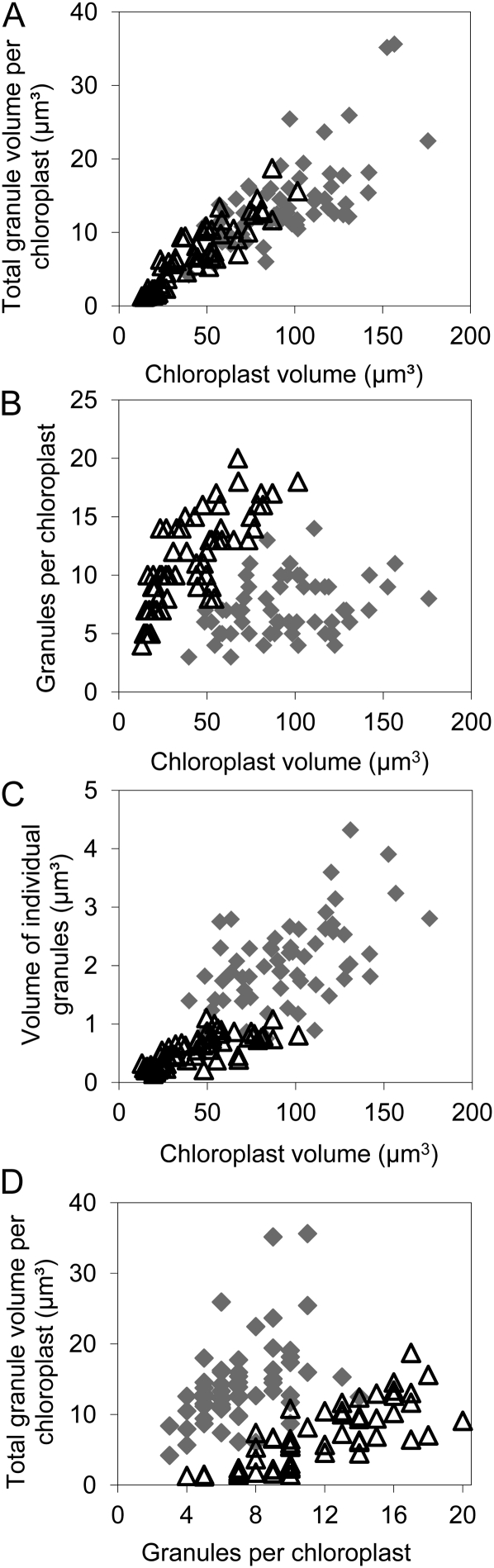
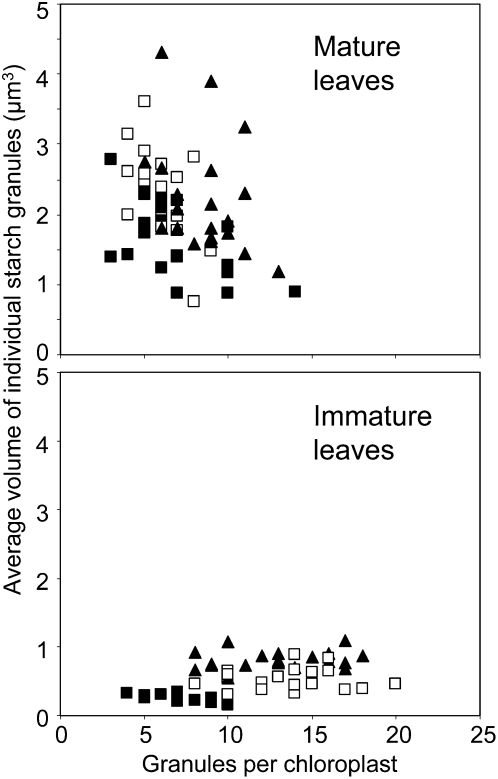
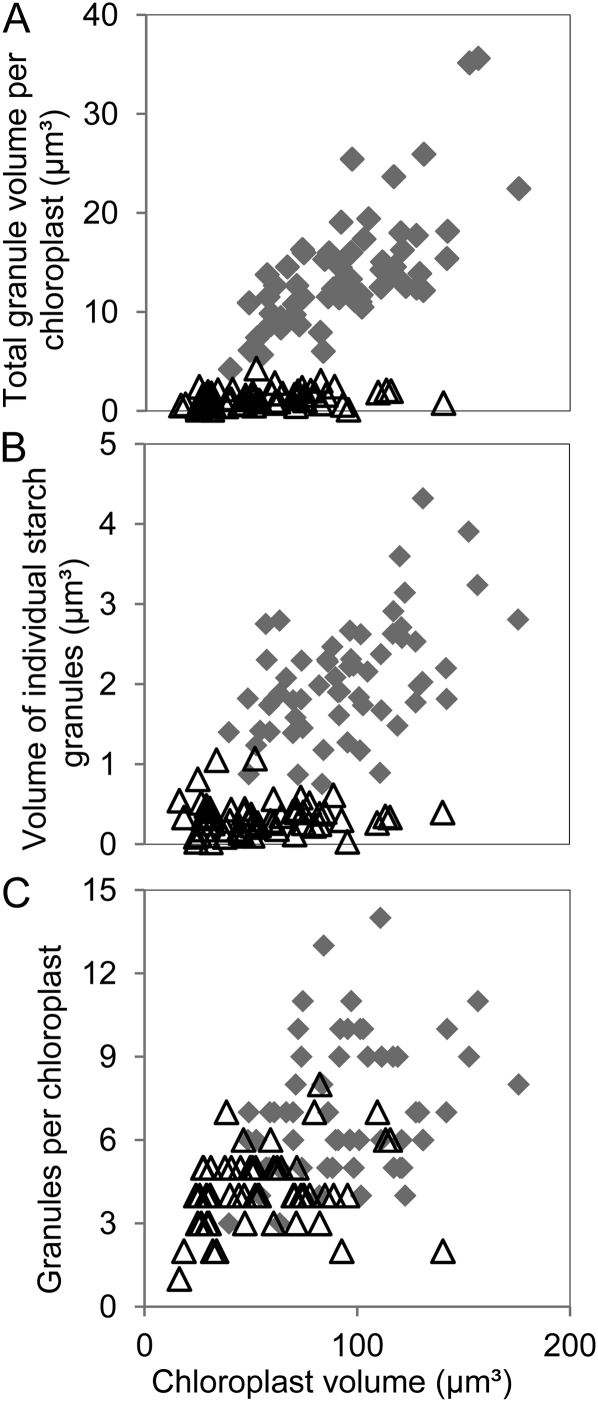
For both mature and immature leaves at the end of the day, chloroplast volume was positively correlated with the number of starch granules and the total volume of starch in the chloroplast (Fig. 3, A and B). Immature and mature leaves differed considerably with respect to the relationship between chloroplast volume and number of starch granules (Fig. 3B) and chloroplast volume and volume of individual granules (Fig. 3C). For a given chloroplast volume, immature leaves had more and smaller starch granules than mature leaves (Fig. 3). On average, immature leaves had more granules per chloroplast, smaller granules (Figs. 3, C and D, and 4), and less stromal volume per starch granule (Fig. 3B) than mature leaves. There was no strong relationship between granule number per chloroplast and the average volume of individual granules for either mature or immature leaves. (Fig. 4). However, the two ages of leaf had similar relationships between chloroplast volume and the total volume of starch per chloroplast (Fig. 3A). In both cases, starch occupied an average of 15% of the chloroplast volume.
Figure 3.
Relationships between chloroplast volume and starch parameters in mature and immature leaves. Measurements were made using method 1 on 20 chloroplasts from each of three mature leaves (gray diamonds) or immature leaves (white triangles) harvested at the end of the day. Graphs show the following relationships. A, Total granule volume per chloroplast and chloroplast volume. B, Number of granules per chloroplast and chloroplast volume. C, Volume of individual starch granules and chloroplast volume. D, Total granule volume per chloroplast and number of starch granules per chloroplast.
Figure 4.
Relationship between the volumes of individual starch granules and the numbers of granules per chloroplast. Data were obtained using method 1. They represent measurements made on 20 chloroplasts from each of three leaves. Values from an individual leaf are all represented by the same symbol. The average volume of the individual granules in each chloroplast is plotted against the number of granules in that chloroplast.
For mature leaves, we examined differences between the end of the day and the end of the night in the relationship between chloroplast volume and starch granule numbers and volumes. Average chloroplast volume declined by about 45% during the night. About one-third of this decline was accounted for by starch degradation. The general relationship between chloroplast volume and starch granule volume seen at the end of the day was lost, so that starch contents and the volumes of individual granules were similar in large and small chloroplasts at the end of the night (Fig. 5, A and B). These data imply that volumes of individual granules underwent larger changes over the day-night cycle in large chloroplasts than in small chloroplasts. The average percentage of chloroplast volume occupied by starch was much lower at the end of the night than at the end of the day (Fig. 5A). However, the general relationship between chloroplast volume and the number of starch granules per chloroplast was maintained during the night (Fig. 5C).
Figure 5.
Effect of time of day on the relationship between chloroplast volume and starch parameters. Data were obtained using method 1 on mature leaves. They represent measurements made on 20 chloroplasts from each of three leaves (i.e. 60 chloroplasts) harvested at the end of the night (white triangles) or the end of the day (gray diamonds). Graphs show the relationship between chloroplast volume and the following starch parameters. A, Total granule volume per chloroplast. B, Average volume of individual starch granules in each chloroplast. C, Number of granules per chloroplast.
Relationship between Starch Granule Number and Chloroplast Volume in Chloroplast Division Mutants
To shed further light on the control of starch granule numbers, we examined starch in six Arabidopsis chloroplast division mutants that have fewer, larger chloroplasts per mesophyll cell than wild-type plants. Whereas wild-type plants have 80 to 120 chloroplasts per mesophyll cell, the accumulation and regulation of chloroplast (arc) mutants used in this study have between one and about 30 chloroplasts per mesophyll cell (Table III). The ARC genes identified thus far encode components of the chloroplast division apparatus; failure of chloroplasts to divide at the normal rate during mesophyll cell expansion in the mutants results in fewer chloroplasts per cell in mature leaves. There has been no systematic study of the effect of arc mutations on starch content or starch granule numbers. However, there are indications that some of the ARC proteins may influence starch accumulation. Arabidopsis arc3, arc5, arc6, and arc10 mutants were reported to have increased starch accumulation, whereas plants overexpressing FtsZ1 (ARC10) have no starch granules (Austin and Webber, 2005; El-Kafafi et al., 2008). Overexpression of FtsZ in potato (Solanum tuberosum) tubers resulted in smaller plastids apparently containing fewer, larger starch granules (de Pater et al., 2006) and rice (Oryza sativa) endosperm misexpressing FtsZ proteins contains granules of abnormal sizes and shapes (Yun and Kawagoe, 2010). Some other mutant and transgenic plants defective in chloroplast division are also reported to have altered starch granule numbers (e.g. giant chloroplast1; Maple et al., 2004).
Table III. Plastid division mutants used in this study.
| Gene | Name(s) | Mutant Alleles Used in This Study | Chloroplasts per Mesophyll Cell |
| At1g75010 | ARC3 | arc3 (Ler)a: EMSb, Pyke and Leech (1992)c | 13–18 |
| arc3-2 (Col): T-DNA insertion line SALK_057144d, Shimada et al. (2004) | |||
| arc3-3 (Col): T-DNA insertion line SALK_012892 | |||
| At3g19720 | ARC5 | arc5 (Ler): EMS, Pyke and Leech (1992) | 3–15 |
| arc5-2 (Col): T-DNA insertion line SAIL_71_D11, Miyagishima et al. (2006) | |||
| At5g42480 | ARC6 | arc6 (Ws): T-DNA insertion line, Pyke et al. (1994) | 1–2 |
| arc6-5 (Col): T-DNA insertion line SAIL_693_G04, Glynn et al. (2008) | |||
| At5g55280 | ARC10 (AtFtsZ1) | arc10 (Ler): EMS, Rutherford (1996) | 23e |
| arc10-2 (Col): T-DNA insertion line SALK_073878, Yoder et al. (2007) (AtftsZ1-1-Δ1) | |||
| At5g24020 | ARC11 (AtMinD1) | arc11 (Ler): point mutation, Marrison et al. (1999), Fujiwara et al. (2004) | 29–33f |
| At1g69390 | ARC12 (AtMinE1) | arc12 (Ler): 1196GC to A–, Pyke et al. (1994); Glynn et al. (2007) | 1–2 |
Mutant alleles are in Ler, Col, or Ws.
Mutation arose through mutagenesis with ethyl methanesulfonate.
References are to previous descriptions of the mutant alleles.
Details of the T-DNA insertion sites are in Supplemental Figure S4.
Mutants have one greatly enlarged chloroplast and some smaller chloroplasts in each cell.
Mutants have a heterogeneous population of chloroplasts, 40% to 50% wild-type size, 50% to 60% larger than wild type.
To minimize any effects of genetic background on starch content and granule numbers, we compared the original arc mutants with their ecotype Landsberg erecta (Ler) or Wassilewskija (Ws) parental lines, and for four of the six ARC genes we also compared T-DNA insertion mutant and wild-type plants in the Columbia (Col) background (for details, see Table III and Supplemental Fig. S4). We checked that each mutant line had the expected reduction in chloroplast number per cell (Supplemental Fig. S5).
There were no statistically significant and consistent differences in starch content between mature nonflowering rosettes of wild-type plants and any of the arc mutants (Fig. 6A) at the end of either the day or the night. There were small differences in starch content between the arc mutants and wild-type plants at the end of the night, but no one genotype was statistically significantly different from all the others (data not shown). Significant differences were in some cases dependent on the genetic background. For example, although arc3 and Ler wild-type had significantly different starch levels at the end of night, there was no difference between arc3-2 and Col wild type at this point. There were also no consistent differences in starch granule size between wild-type plants and any of the arc mutants (Supplemental Fig. S6). The granule shape of all the arc mutants was similar to that of wild-type plants (Supplemental Fig. S5).
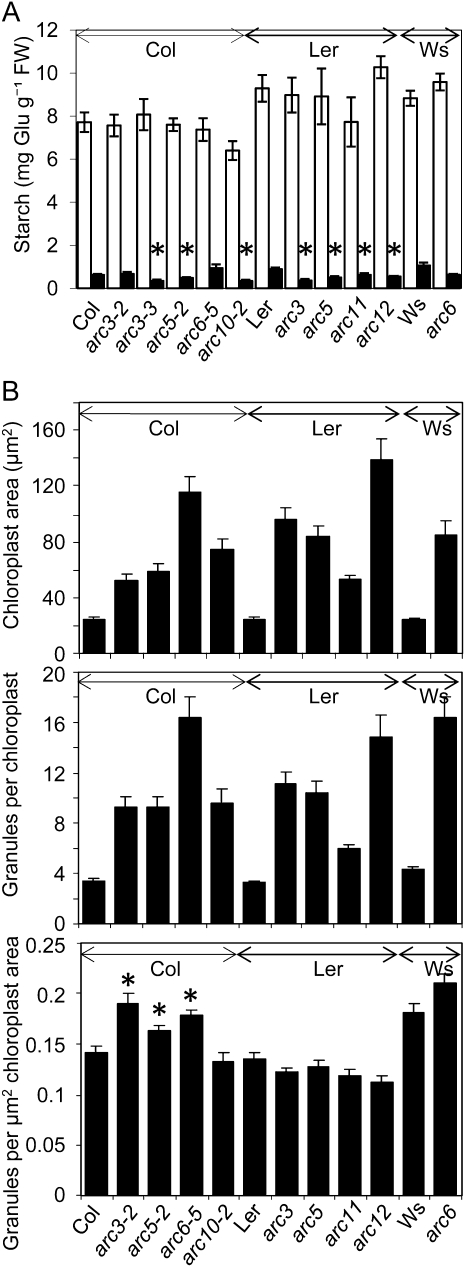
Figure 6.
Comparison of starch and chloroplast parameters in wild-type and arc mutant plants. Arrows above graphs indicate data sets from the same genetic background (Col, Ler, or Ws). A, Starch contents of rosettes at the end of the day (white bars) and the end of the night (black bars). Plants in Col and Ler backgrounds were harvested at 25 d; because of earlier bolting, plants in the Ws background were harvested at 21 d. Values are means ± se of measurements on eight plants except for arc10-2 end of day (seven plants), arc6-5 end of night (six plants), and arc5 end of night (four plants). There were no statistically significant differences at the end of the day between Col and arc mutants in the Col background, between Ler and arc mutants in the Ler background, and between Ws and the arc mutant in the Ws background. Asterisked values for mutants are statistically significantly different from the wild-type values for the end of night (Student’s t test, P < 0.05). B, Comparison of arc mutants and their wild-type backgrounds with respect to chloroplast area, (top), number of granules per chloroplast section (middle), and number of granules per μm2 of chloroplast area (bottom; obtained by division of chloroplast sectional area by the number of starch granules per chloroplast section). Measurements were made using imaging software on photos of iodine-stained sections (0.9 μm) of blocks prepared as for FIB-SEM. Values are means ± se of measurements on 40 chloroplasts in five sections per genotype, representing material from two different rosettes (growth stage 3.90; Boyes et al., 2001). Leaves were harvested 9 h into a 12-h light period. For the bottom graph, asterisked values for mutants are statistically significantly different from the wild-type values (Student’s t test, P < 0.05).
As expected, all of the arc mutants had greater chloroplast cross-sectional areas than wild-type plants. They also had greater numbers of granules per chloroplast. Mutants with fewer, larger chloroplasts had more starch granules per chloroplast than mutants with more, smaller chloroplasts (Fig. 6B). However, there was a remarkable conservation in the stromal area per granule across all of the arc mutants and wild-type lines; there were no consistent, statistically significant differences between mutant and wild-type plants in number of granules per μm2 of chloroplast cross-section, regardless of chloroplast size or number per cell (Fig. 6B).
DISCUSSION
Comparison of Methods for Estimation of Granule Numbers
We have developed two new techniques that between them provide radically new information about the dynamics of starch granule numbers in leaves. Sectioning by FIB-SEM allows rapid and accurate imaging of whole chloroplasts and the starch granules they contain. FIB-SEM can be applied specifically to photosynthetic mesophyll cells and to defined regions of the leaf. Method 2, involving isolation of chloroplasts and starch granules, provides an average figure for starch granule number per chloroplast in the rosette as a whole. Both methods also have drawbacks, but their strengths are complementary.
Our results show that previous estimates of granule size and number made on single sections of leaves are likely to have been inaccurate and may have given rise to misleading conclusions. For example, based on TEM images, granule number was proposed to be about two per chloroplast in Arabidopsis leaves grown in 370 μL L−1 CO2 and about three per chloroplast in leaves grown in 700 μL L−1 CO2 (Teng et al., 2009). It is likely that these values are serious underestimates; hence, no firm conclusions can be drawn about the effects of elevated CO2 on granule numbers.
We summarize our principal findings below and discuss their impact on our understanding of the control of starch degradation and starch granule initiation.
Remarkable Heterogeneity of Chloroplast Size
We observed a very wide range of chloroplast volumes in the mesophyll cells of wild-type plants. For mature and immature leaves, FIB-SEM revealed an average 4- and 6-fold variation, respectively, in chloroplast volume within a tissue volume equivalent to a single mature mesophyll cell. We are not aware of previous reports of this remarkable local heterogeneity of chloroplast volume in wild-type leaves, although some chloroplast division mutants have heterogeneous populations of chloroplasts because of unequal chloroplast division (e.g. Maple et al., 2002; Fujiwara et al., 2004).
There was also a marked increase in chloroplast size through leaf development. The three immature leaves we examined by FIB-SEM had statistically significantly different average chloroplast volumes (Fig. 4; data not shown), indicating that chloroplast volume is changing rapidly through this developmental stage.
The origins of chloroplast heterogeneity in wild-type plants are unclear. Possibilities include nonsynchronous division of chloroplasts, so that different chloroplasts are at different stages of expansion after division, or irregularities of the division plane so that daughter chloroplasts are not of identical size. Resolution of this question will require deeper understanding of signals that trigger chloroplast division (Okazaki et al., 2009) and further analyses of changes in chloroplast numbers and volumes through mesophyll cell development. FIB-SEM offers new opportunities for robust, quantitative analyses of this sort.
Starch Metabolism Is Independent of Chloroplast Size
In general, starch metabolism is independent of chloroplast size for a given leaf age. The relationship among chloroplast volume, the volume of starch per chloroplast, and the size of starch granules at the end of the day was approximately the same for large and small chloroplasts. Strikingly, all of the arc mutants were very similar to wild-type plants with respect to patterns of starch turnover and starch granule sizes, even though they differed hugely from wild-type plants and from each other in chloroplast volume.
The relationship between chloroplast size and starch metabolism changes radically as mesophyll cells mature. Although starch occupied approximately 15% of the volume of chloroplasts of both mature and immature leaves at the end of the day, and daily starch turnover was comparable, the chloroplasts of immature leaves contained more, smaller granules than those of mature leaves. Thus, starch granules in immature leaves present a larger surface for synthesis and degradation than in mature leaves, and the rate of deposition/loss of material is slower per unit surface area.
Granule Number per Chloroplast Is Strongly Related to Chloroplast Volume
There is a strong, positive relationship between chloroplast volume and numbers of granules per chloroplast. The number of granules per unit of chloroplast stroma was similar across the very large range of chloroplast sizes found in mature wild-type and arc mutant leaves. This finding implies that the frequency of granule initiation may depend on the concentrations of factors in the stroma that are themselves rather invariant. The possible nature of such factors is discussed further below. The precise relationship between chloroplast volume and numbers of starch granules per chloroplast changes as the leaf matures. There are more granules per unit of stroma in immature than in mature leaves even though the amount of starch per unit of stroma is very similar in the two sorts of leaf.
The discovery that granule number is related to chloroplast volume for a given leaf age arose from measurements on many individual chloroplasts by FIB-SEM. This insight could not have been obtained by microscopy on individual sections or by our method 2. Previous microscopy studies and results from method 2 both indicate that granule number per chloroplast is relatively invariant. Results from method 2 showed a strong conservation of average granule number per chloroplast across a wide range of leaf starch contents and granule sizes. One might thus conclude that each chloroplast has the same, fixed number of granule initiation sites or primers (see also “Discussion” below). However, method 1 shows that such a conclusion would be incorrect. It reveals considerable variation in numbers of granules per chloroplast, dependent on chloroplast volume.
Granule Initiation Is Infrequent in Mature Leaves
Taken as a whole, our data indicate that the diurnal change in starch content per chloroplast in mature leaves is brought about primarily by the growth during the day and shrinkage at night of a fixed number of granules, regardless of the rate of starch accumulation. Thus, loss of granules at night and appearance of new granules during the day are both infrequent events. It seems likely that individual granules are long lived and that initiation of new granules is rare. The relationship between granule surface area and starch content is simpler than would be the case were new granules initiated as chloroplast starch content increases. This relationship may be of significance in the unknown mechanism by which starch content is measured and used to set an appropriate rate of degradation at the onset of darkness. Elucidation of the precise nature of the relationship will require more detailed analyses of the changes in shape of individual granules through the day-night cycle.
Our conclusions are at odds with a previous proposal about diurnal patterns of change in granule size and number in Arabidopsis leaves. Based on measurements of leaf starch content and size of isolated granules (using a laser diffraction particle size analyzer), Howitt et al. (2006) reported that the distribution of starch granule sizes was the same at the end of the day and the end of the night. They concluded that some granules are quickly and completely degraded during the night, but others are not subject to degradation. This is not the case in our plant material. Most granules underwent a large reduction in volume during the night, and there was relatively little loss of individual granules.
In contrast to the situation in mature leaves, it is clear that granule initiation must occur throughout the period of expansion as the leaf matures. In Arabidopsis seedlings, starch granules appear in chloroplasts at the tip of the first true leaf between 6 and 8 d after germination (Sakamoto et al., 2009). We observed that chloroplasts in immature leaves, early in the expansion phase, contained on average only 1.5 times more starch granules than the chloroplasts of mature leaves. Because chloroplasts undergo at least three rounds of division during expansion of mesophyll cells (Marrison et al., 1999), division must therefore be accompanied by initiation of new granules. Further work will be required to establish whether the initiation of new granules is specifically and exclusively linked to chloroplast division events. Initiation of new granules may be triggered by chloroplast expansion, hence an increased stromal volume, following divisions. Alternatively, granule loss and initiation may simply be more frequent events on a daily basis in developing than in mature leaves.
How Are Granules Initiated?
Two different types of mechanisms have been proposed to explain how granules are initiated. The first requires a specific, biologically generated primer. In the second, the process is spontaneous, without a specific primer requirement. Suggestions for specific primers originally centered on reversibly glucosylated proteins, thought to be analogous to the glycogen-priming glycogenins of yeasts and animals (for discussion, see Langeveld et al., 2002). However, it is more likely that reversibly glucosylated proteins are involved in cell wall synthesis (e.g. D’Hulst and Mérida, 2010; Mortimer et al., 2010; Rautengarten et al., 2011). A recent proposal for a priming mechanism involves the soluble starch synthase SS4, which is necessary for normal numbers of starch granules per chloroplast; Arabidopsis ss4 mutants have only one granule per chloroplast (Roldán et al., 2007; Szydlowski et al., 2009; D’Hulst and Mérida, 2010). Structural components of the chloroplast division apparatus might also act as primers or nucleation loci for starch granules (but see below).
The view that starch granule initiation may be a spontaneous event, dependent on biological and physicochemical conditions in the chloroplast, comes from consideration of the behavior of solutions of glucans and proteins in vitro. Under appropriate conditions, glucans can undergo phase separation followed by nucleation and the growth of a crystalline structure (Lorén et al., 2001; Ziegler et al., 2005; Biliaderis, 2009). The rate and frequency of formation of stable crystalline nuclei in the chloroplast stroma would depend on factors including glucan concentration, size, and structure as well as background conditions such as the nature and concentration of proteins. Although granule initiation via this mechanism would not require a specific, biologically generated primer, its frequency would be biologically determined and would thus be subject to genetic and developmental variation.
We could not confirm earlier reports of altered starch accumulation and granule number in arc3, arc5, arc6, and arc10 mutants (Austin and Webber, 2005; El-Kafafi et al., 2008); thus, there is at present no good evidence that components of the chloroplast division apparatus act as primers for granule initiation. Our observations do not allow us to distinguish between other possible priming mechanisms. However, efforts to discover this mechanism will be informed by our observation of the remarkable constancy of granule number per unit volume of stroma and facilitated by our methods for estimation of granule numbers.
MATERIALS AND METHODS
Plant Material
Experiments were conducted on plants of Arabidopsis (Arabidopsis thaliana) accessions Col-0 (N1093), Ws (N1602), and Ler (N1686), originally from the Nottingham Arabidopsis Stock Centre (University of Nottingham). The arc mutants arc1, arc3, arc5, arc6, arc11, and arc12 were kindly provided by Dr. Kevin Pyke (University of Nottingham).
Segregating populations of seeds carrying T-DNA insertions were obtained from the Nottingham Arabidopsis Stock Centre: SALK_057144 and SALK_012892 in At1g75010, SAIL_71_D11 in At3g19720, SAIL_693_G04 in At5g42480, and SALK_073878 in At5g55280. Oligonucleotide primers and methods used to select plants homozygous for the insertions are shown in Supplemental Table S1.
Unless otherwise stated, plants were grown in compost in a controlled-environment room at 20°C and 75% relative humidity, with a 12-h photoperiod at 180 μmol quanta m−2 s−1 throughout the growing period.
Chloroplast Isolation
Intact chloroplasts were isolated mechanically at 4°C using the Sigma Chloroplast Isolation Kit. Approximately 4 g of leaves (whole rosettes, typically 25 d old) was washed, cut into 1-cm2 pieces, and macerated in a blender (Multi-Moulinette) with 6 mL g−1 chloroplast isolation buffer (CIB) plus 0.1% (w/v) bovine serum albumin (BSA) using four 1-s bursts. After filtration (100-μm mesh), the chloroplast suspension was centrifuged at 200g for 3 min. The supernatant was centrifuged at 1,000g for 7 min, and the resulting pellet was resuspended in 2 mL of CIB plus BSA and centrifuged on a two-step Percoll gradient (80% and 40% Percoll in CIB plus BSA) at 1,500g for 15 min. Intact chloroplasts were collected from between the Percoll layers. Chloroplast intactness was estimated from assay of NADP-dependent glyceraldehyde-3-phosphate dehydrogenase activity before and after rupturing the chloroplasts by osmotic shock (Batz et al., 1995).
Measurement of Starch Content and Iodine Staining
Leaves were harvested directly into liquid nitrogen and ground to a powder in a ball mill. Extraction in dilute perchloric acid, starch solubilization, enzymatic hydrolysis, and assay of Glc were as described previously (Critchley et al., 2001; Smith and Zeeman, 2006).
For iodine staining, leaves were heated at 80°C in 80% (v/v) aqueous ethanol until fully decolorized, drained, and immersed in iodine/K iodide solution (50% [v/v] aqueous Lugol’s solution, Sigma-Aldrich).
Purification of Starch Granules
Whole rosettes were harvested directly into liquid nitrogen at the end of the light period. Further processes were at 4°C. Frozen material was ground in a mortar in 4 volumes of isolation medium: 100 mm MOPS, pH 7.2, 5 mm EDTA, 2.6 mm Na metabisulfite, 0.05% (v/v) Triton X-100, and 5 mm dithiothreitol. The homogenate was filtered through two layers of Miracloth, and the filtrate was centrifuged at 20,000g for 10 min. The pellet was resuspended in 5 mL of isolation medium and centrifuged into 20 mL 90% of Percoll, 10% isolation medium in a 30-mL Corex tube at 5,000g for 10 min. The pellet was resuspended in 2 mL of isolation medium and centrifuged as above but into 10 mL of Percoll solution in a 15-mL tube. The pellet was washed three times with water by resuspension and centrifugation, then resuspended in 1.5 mL of acetone and centrifuged for 5 min at 20,000g at 4°C. The pellet was air dried for 4 h and stored at −20°C.
SEM
Starch granules were sprinkled onto double-sided, carbonated, sticky stills (Leit-tabs) attached to SEM stubs (both from Agar Scientific) then sputter coated with gold for 75 s at 10 mA in argon and imaged at 3 kV in a FEI XL30 FEG SEM (FEI). Measurements of granule radii were made with ImageJ software (http://rsbweb.nih.gov/ij/). Equations used to calculate the radius and thus derive the granule volume are in Supplemental Figure S2.
FIB-SEM: Method 1
Small pieces (2 mm2) were rapidly cut with a razor blade from the lamina close to the middle of a leaf and immediately fixed in 2.5% (v/v) glutaraldehyde in 0.05 m Na cacodylate, pH 7.3. Samples were vacuum-infiltrated and left overnight in fresh fixative at 4°C, washed in 0.05 m Na cacodylate, and postfixed with 1% (w/v) osmium tetroxide in 0.05 m Na cacodylate for 1 h at 4°C. Samples were dehydrated in an ethanol series and infiltrated with LR White resin by successive changes of resin/ethanol mixes over 3 d at room temperature, with the last infiltration overnight in 100% resin. The resin was polymerized at 60°C for 16 h. FIB-SEM was performed on an FEI Quanta 3D FEG SEM by small modifications of the method of Bushby et al. (2011). Backscattered electron imaging was typically at an accelerating voltage of 5 keV with a 1,000-μm aperture, a current of 4 nA, a dwell time of 30 μs, an acquisition time per image of 28 s, and a resolution of 1,024 × 884 pixels at 50 nm pixel−1. Milling was typically at 30 kV and 7 nA, with a slice thickness of 49 nm and a milling time per slice of 7 to 12 s. A typical volume of tissue 50 × 30 × 30 μm was imaged in a single run. Total run time for each block was 6 to 11 h.
Granule Number Estimation: Method 2
Method 2 consisted of the following steps. First, a suspension of intact chloroplasts was isolated, and an estimation of chloroplast number per unit volume was conducted using a double-celled modified Fuchs-Rosenthal hemocytometer (Agar Scientific Ltd.) according to Kouri et al. (2003). Starch content per unit volume of the chloroplast suspension was measured. After these steps, the average mass of starch per chloroplast was calculated. Isolation and SEM imaging of starch granules from a representative sample of leaves were conducted. Granule radii were estimated using imaging software. Starch granule volumes were calculated (see Supplemental Fig. S2). The mass of individual starch granules was calculated by multiplying the volume by starch density (1.5 g cm−3; Dengate et al., 1978; Munck et al., 1988). The number of starch granules per chloroplast was calculated by dividing the mass of starch per chloroplast by the mass of individual starch granules.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Seventeen sequential sections through a mature leaf at the end of the day, obtained by FIB-SEM.
Supplemental Figure S2. Estimation of granule volume.
Supplemental Figure S3. Starch granule size distributions obtained by different methods of measurement.
Supplemental Figure S4. Analysis of T-DNA insertion lines.
Supplemental Figure S5. Phenotypes of the arc mutants and their wild types.
Supplemental Figure S6. Starch granule size in the arc mutants.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Kevin Pyke (Plant and Crop Sciences, University of Nottingham) for the kind gift of arc mutants, Kim Findlay and Sue Bunnewell (John Innes Centre) for advice and help with sample preparation for microscopy, and Alastair Skeffington (John Innes Centre) for helpful comments on the manuscript.
References
- Armer HEJ, Mariggi G, Png KMY, Genoud C, Monteith AG, Bushby AJ, Gerhardt H, Collinson LM. (2009) Imaging transient blood vessel fusion events in zebrafish by correlative volume electron microscopy. PLoS ONE 4: e7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft RG, Preston C, Cleland RE, Critchley C. (1986) Flow-cytometry of isolated chloroplasts and thylakoids. Photobiochem Photobiophys 13: 1–14 [Google Scholar]
- Austin JA, Webber AN. (2005) Photosynthesis in Arabidopsis thaliana mutants with reduced chloroplast number. Photosynth Res 85: 373–384 [DOI] [PubMed] [Google Scholar]
- Batz O, Scheibe R, Neuhaus HE. (1995) Purification of chloroplasts from fruits of green pepper (Capsicum annuum L.) and characterization of starch synthesis. Evidence for a functional chloroplastic hexose-phosphate translocator. Planta 196: 50–57 [Google Scholar]
- Bergounioux C, Brown SC, Petit PX. (1992) Flow-cytometry and plant protoplast cell biology. Physiol Plant 85: 374–386 [Google Scholar]
- Biliaderis C. (2009) Structural transitions and related physical properties of starch. In J BeMiller, R Whistler, eds, Starch: Chemistry and Technology, Ed 3. Elsevier, Amsterdam, pp 293–372 [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby AJ, P’ng KMY, Young RD, Pinali C, Knupp C, Quantock AJ. (2011) Imaging three-dimensional tissue architectures by focused ion beam scanning electron microscopy. Nat Protoc 6: 845–858 [DOI] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM. (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Delatte T, Trevisan M, Parker ML, Zeeman SC. (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J 41: 815–830 [DOI] [PubMed] [Google Scholar]
- Dengate HN, Baruch DW, Meredith P. (1978) The density of wheat starch granules: a tracer dilution procedure for determining the density of an immiscible dispersed phase. Starch-Stärke 30: 80–84 [Google Scholar]
- de Pater S, Caspers M, Kottenhagen M, Meima H, ter Stege R, de Vetten N. (2006) Manipulation of starch granule size distribution in potato tubers by modulation of plastid division. Plant Biotechnol J 4: 123–134 [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Mérida A. (2010) The priming of storage glucan synthesis from bacteria to plants: current knowledge and new developments. New Phytol 188: 13–21 [DOI] [PubMed] [Google Scholar]
- El-Kafafi S, Karamoko M, Pignot-Paintrand I, Grunwald D, Mandaron P, Lerbs-Mache S, Falconet D. (2008) Developmentally regulated association of plastid division protein FtsZ1 with thylakoid membranes in Arabidopsis thaliana. Biochem J 409: 87–94 [DOI] [PubMed] [Google Scholar]
- Fujiwara MT, Nakamura A, Itoh R, Shimada Y, Yoshida S, Møller SG. (2004) Chloroplast division site placement requires dimerization of the ARC11/AtMinD1 protein in Arabidopsis. J Cell Sci 117: 2399–2410 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M. (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JM, Froehlich JE, Osteryoung KW. (2008) Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 20: 2460–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JM, Miyagishima S-Y, Yoder DW, Osteryoung KW, Vitha S. (2007) Chloroplast division. Traffic 8: 451–461 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM. (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt CA, Rahman S, Morell MK. (2006) Expression of bacterial starch-binding domains in Arabidopsis increases starch granule size. Funct Plant Biol 33: 257–266 [DOI] [PubMed] [Google Scholar]
- Kouri T, Gyory A, Rowan RM, ISLH Urinalysis Task Force (2003) ISLH recommended reference procedure for the enumeration of particles in urine. Lab Hematol 9: 58–63 [PubMed] [Google Scholar]
- Langeveld SMJ, Vennik M, Kottenhagen M, Van Wijk R, Buijk A, Kijne JW, de Pater S. (2002) Glucosylation activity and complex formation of two classes of reversibly glycosylated polypeptides. Plant Physiol 129: 278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorén N, Altskär A, Hermansson AM. (2001) Structure evolution during gelation at later stages of spinodal decomposition in gelatin/maltodextrin mixtures. Macromolecules 34: 8117–8127 [Google Scholar]
- Maple J, Chua N-H, Møller SG. (2002) The topological specificity factor AtMinE1 is essential for correct plastid division site placement in Arabidopsis. Plant J 31: 269–277 [DOI] [PubMed] [Google Scholar]
- Maple J, Fujiwara MT, Kitahata N, Lawson T, Baker NR, Yoshida S, Møller SG. (2004) GIANT CHLOROPLAST 1 is essential for correct plastid division in Arabidopsis. Curr Biol 14: 776–781 [DOI] [PubMed] [Google Scholar]
- Marrison JL, Rutherford SM, Robertson EJ, Lister C, Dean C, Leech RM. (1999) The distinctive roles of five different ARC genes in the chloroplast division process in Arabidopsis. Plant J 18: 651–662 [DOI] [PubMed] [Google Scholar]
- Miyagishima SY, Froehlich JE, Osteryoung KW. (2006) PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 18: 2517–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, Yu X, Seffen KA, Stephens E, Turner SR, et al. (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA 107: 17409–17414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck L, Rexen F, Haastrup L. (1988) Cereal starches within the European Community. Agricultural production, dry and wet milling and potential use in industry. Starch-Stärke 40: 81–87 [Google Scholar]
- Okazaki K, Kabeya Y, Suzuki K, Mori T, Ichikawa T, Matsui M, Nakanishi H, Miyagishima SY. (2009) The PLASTID DIVISION1 and 2 components of the chloroplast division machinery determine the rate of chloroplast division in land plant cell differentiation. Plant Cell 21: 1769–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit PX, Diolez P, de Kouchkovsky Y. (1989) Flow cytometric analysis of energy transducing organelles: mitochondria and chloroplasts. In A Yen, ed, Flow Cytometry: Advanced Research and Clinical Applications, Vol 1. CRC Press, Boca Raton, FL, pp 271–303 [Google Scholar]
- Pyke KA, Leech RM. (1992) Chloroplast division and expansion is radically altered by nuclear mutations in Arabidopsis thaliana. Plant Physiol 99: 1005–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Rutherford SM, Robertson EJ, Leech RM. (1994) arc6, a fertile Arabidopsis mutant with only two mesophyll cell chloroplasts. Plant Physiol 106: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Ebert B, Herter T, Petzold CJ, Ishii T, Mukhopadhyay A, Usadel B, Scheller HV. (2011) The interconversion of UDP-arabinopyranose and UDP-arabinofuranose is indispensable for plant development in Arabidopsis. Plant Cell 23: 1373–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán I, Wattebled F, Mercedes Lucas M, Delvallé D, Planchot V, Jiménez S, Pérez R, Ball S, D’Hulst C, Mérida A. (2007) The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J 49: 492–504 [DOI] [PubMed] [Google Scholar]
- Rutherford SM. (1996) The genetic and physical analysis of mutants of chloroplast number and size in Arabidopsis thaliana. PhD thesis. University of York, York, UK [Google Scholar]
- Sakamoto W, Uno Y, Zhang Q, Miura E, Kato Y, Sodmergen (2009) Arrested differentiation of proplastids into chloroplasts in variegated leaves characterized by plastid ultrastructure and nucleoid morphology. Plant Cell Physiol 50: 2069–2083 [DOI] [PubMed] [Google Scholar]
- Schröder WP, Petit PX. (1992) Flow cytometry of spinach chloroplasts: determination of intactness and lectin-binding properties of the envelope and the thylakoid membranes. Plant Physiol 100: 1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Koizumi M, Kuroki K, Mochizuki M, Fujimoto H, Ohta H, Masuda T, Takamiya K. (2004) ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant Cell Physiol 45: 960–967 [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. (2006) Quantification of starch in plant tissues. Nat Protoc 1: 1342–1345 [DOI] [PubMed] [Google Scholar]
- Stitt M, Gibon Y, Lunn JE, Piques M. (2007) Multilevel genomics analysis of carbon signalling during low carbon availability: coordinating the supply and utilisation of carbon in a fluctuating environment. Funct Plant Biol 34: 526–549 [DOI] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21: 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N, Jin B, Wang Q, Hao H, Ceulemans R, Kuang T, Lin J. (2009) No detectable maternal effects of elevated CO(2) on Arabidopsis thaliana over 15 generations. PLoS ONE 4: e6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191: 180–190 [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Wuyts N, Palauqui JC, Conejero G, Verdeil JL, Granier C, Massonnet C. (2010) High-contrast three-dimensional imaging of the Arabidopsis leaf enables the analysis of cell dimensions in the epidermis and mesophyll. Plant Methods 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Juang YS, Hsu BD. (2002) A quick method for assessing chloroplastic starch granules by flow cytometry. J Plant Physiol 159: 103–106 [Google Scholar]
- Yoder DW, Kadirjan-Kalbach D, Olson BJ, Miyagishima SY, Deblasio SL, Hangarter RP, Osteryoung KW. (2007) Effects of mutations in Arabidopsis FtsZ1 on plastid division, FtsZ ring formation and positioning, and FtsZ filament morphology in vivo. Plant Cell Physiol 48: 775–791 [DOI] [PubMed] [Google Scholar]
- Yun MS, Kawagoe Y. (2010) Septum formation in amyloplasts produces compound granules in the rice endosperm and is regulated by plastid division proteins. Plant Cell Physiol 51: 1469–1479 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. (2007) The diurnal metabolism of leaf starch. Biochem J 401: 13–28 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM. (2002) Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol 129: 516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler GR, Creek JA, Runt J. (2005) Spherulitic crystallization in starch as a model for starch granule initiation. Biomacromolecules 6: 1547–1554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.