Background: Inactivating mutations of the V2 receptor (V2R) cause nephrogenic diabetes insipidus (NDI).
Results: Two V2R mutants discovered in partial NDI show partial defects, and V2R antagonists rescued them.
Conclusion: V2R antagonists operate as pharmacochaperones for defective mutants, whereas they operate as inverse agonists for normal receptors.
Significance: V2R antagonists can act as protean agonists, potentially underlying their dual effects.
Keywords: Drug Action, G-protein-coupled Receptors (GPCR), Membrane Trafficking, Molecular Pharmacology, Protein Misfolding, Receptor Regulation, Inverse Agonist, Pharmacochaperone, Protean Agonism, Vasopressin Receptor
Abstract
Inactivating mutations of the V2 vasopressin receptor (V2R) cause cross-linked congenital nephrogenic diabetes insipidus (NDI), resulting in renal resistance to the antidiuretic hormone AVP. In two families showing partial NDI, characterized by an apparently normal response to diagnostic tests and an increase in the basal ADH levels suggesting AVP resistance, we have identified two V2R mutations, Ser-333del and Y128S. Both mutant V2Rs, when expressed in COS-7 cells, show partial defects in vasopressin-stimulated cAMP accumulation and intracellular localization. The inhibition of internalization does not rescue their localization. In contrast, the non-peptide V2R antagonists OPC41061 and OPC31260 partially rescue the membrane localization and basal function of these V2R mutants, whereas they inhibit the basal activity of the wild-type V2R. These results indicate that a partial loss of function of Ser-333del and Y128S mutant V2Rs results from defective membrane trafficking. These findings further indicate that V2R antagonists can act as protean agonists, serving as pharmacological chaperones for inactivating V2R mutants and also as inverse agonists of wild-type receptors. We speculate that this protean agonism could underlie the possible dual beneficial effects of the V2R antagonist: improvement of hyponatremia with heart failure or polycystic kidney disease and potential rescue of NDI.
Introduction
Loss of function mutations of G-protein-coupled receptors (GPCRs)5 cause many endocrine and other diseases (1, 2). Although many mutations cause the complete loss of expression of GPCR proteins, some mutations cause loss of function because of defects in protein localization, protein-protein interactions, and so on, and may therefore provide new insights into the physiological operations of the GPCRs. Partial loss of function mutations are good candidates in this regard because they show at least some protein expression.
Nephrogenic diabetes insipidus (NDI) is caused by renal resistance to the antidiuretic effects of arginine vasopressin (AVP) and is associated with elevated levels of this hormone (3, 4). Inactivating mutations of V2 receptor (V2R) are responsible for about 90% of congenital NDI cases and are transmitted in a cross-linked manner, thus manifesting as a complete loss of function phenotype in most instances. Among the more than 200 V2R mutations identified to date, however, only seven have been reported to cause a partial congenital NDI phenotype in clinical cases (5–11).
The classic mode of GPCR activation is the two-state model (12–14), in which the receptor exists in equilibrium between an inactive (R) and active (R*) conformation. Recently, however, accumulating evidence has suggested that GPCRs can adopt multiple conformational states (12–15). In this multistate model, the receptor is proposed to alternate spontaneously between multiple active and inactive conformations, and each ligand is postulated to stabilize a specific conformation of the GPCR, which then leads to a set of specific biological effects. This type of unique ligand behavior has been termed functional selectivity or biased agonism (15, 16). Strikingly, some β2 adrenergic receptor ligands, such as ICI 118551 and propranolol, are inverse agonists of G protein-mediated signaling and thereby decrease cAMP levels while at the same time functioning as partial agonists of the MAPK cascade (17). Furthermore, our recent identification of a unique autoantibody against calcium-sensing receptor in an acquired hypocalciuric hypercalcemia patient (18, 19) suggests that the allosteric modulation and functional selectivity of GPCRs are a pathophysiological mode of operation and may possibly, therefore, be physiological processes also.
A special form of GPCR-based functional selectivity is “protean” agonism, in which certain agonists may change or even reverse their effects depending on the states or systems adopted (13, 15). These agonists are thus termed protean agonists. Kenakin (20) was the first to describe this phenomenon, in which it was demonstrated that although some β2-adrenergic receptor ligands produce positive agonism in whole cells, the same ligands produce inverse agonism when the cAMP assay is performed for cell membranes. In this model, a partial agonist of a GPCR that is mildly activating a GPCR in a quiescent system, may operate as an inverse agonist in a constitutively active system, for example in the presence of a constitutive mutant of the same GPCR (13, 15). Thus, the agonist, inverse agonist, or neutral antagonist activities of each GPCR ligand do not seem to be intrinsically decided by the characteristic of each ligand but by the system adopted.
In this study, we have identified two mutations in the V2R gene (AVPR2) in patients with partial NDI who had both been initially diagnosed with psychogenic polydipsia. One is a novel mutation, Ser-333del, which causes a serine deletion at the 333 position of the C terminus of the V2R. The other is a previously reported mutation Y128S (21–23), but an association with partial NDI has not been identified for this amino acid substitution. We further analyzed the functional properties of these two mutations. To clarify the mechanisms underlying the loss of function of V2R in each case, we also evaluated other V2R mutants: R137H (21–28), which causes complete NDI; R137L (29), which causes the neonatal syndrome of inappropriate antidiuresis (NSIAD), and S329R (7), which is located near to Ser-333del and causes partial NDI. The characteristics of these V2R mutants and the effects of V2R antagonists OPC41061 (OPC4) and OPC31260 (OPC3) (30, 31) enabled us to elucidate the molecular defects underlying partial NDI and reveal a unique example of protean agonism.
EXPERIMENTAL PROCEDURES
Patient Profiles
Patient 1
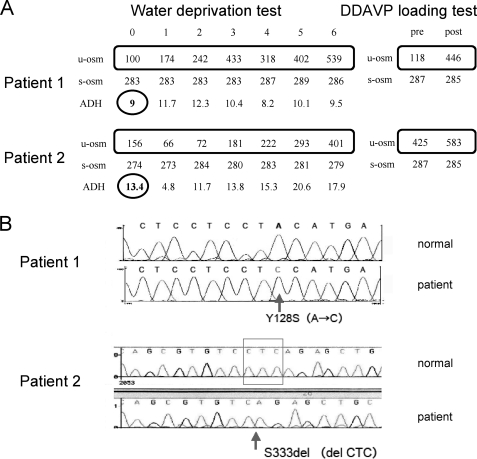
A 7-year-old boy who had been diagnosed with psychogenic polydipsia was admitted to the department of pediatrics in our hospital to evaluate the underlying cause of his polydipsia and polyuria using a water deprivation test followed by a DDAVP loading test (Fig. 1A), which showed an apparently normal response. On the basis of the effectiveness of the DDAVP treatment and the high levels of ADH, his V2 receptor was genotyped, and a Y128S mutation was identified (Fig. 1B).
FIGURE 1.
Clinical testing and V2 receptor gene mutation profiles of the patients examined in this study. A, water deprivation and DDAVP loading test results for the two patients. B, V2R gene mutations in the patients.
Patient 2
A 3-year-old boy who had been diagnosed with psychogenic polydipsia was admitted to our hospital for an evaluation of the polydipsia and polyuria using water deprivation and DDAVP loading tests (Fig. 1A). Although laboratory testing data appeared to be compatible with psychogenic polydipsia, his basal ADH was high (Fig. 1A). His V2R was therefore sequenced and revealed a Ser-333 deletion (Ser-333del, Fig. 1B).
Pharmacological Chaperones
The V2R antagonists OPC4 and OPC3 (30, 31), were kindly provided by Otsuka Pharmaceutical Company.
Expression Constructs, Cell Culture, and Transfection
An expression construct encoding a myc-tagged wild-type V2R was kindly provided by M. Bouvier (Université de Montreal, Canada), and each mutant causative for diabetes insipidus was generated using a manual procedure (Stratagene, La Jolla, CA). COS-7 cells, maintained in DMEM containing 10% (v/v) fetal bovine serum, were transfected with constructs encoding myc-tagged WT-V2R or myc-tagged mutant V2R using Lipofectamine 2000 (Invitrogen).
cAMP Assay
cAMP accumulation in COS-7 cells transiently expressing WT-V2R and each V2R mutant was assayed as described previously (32–35). Briefly, 1 day after transfection, cells were reseeded into 24-well plates to which [3H]adenine (2 μCi/ml) was added. 16 h before performing the assay, the cells were pretreated with OPC3 or OPC4 at the indicated concentrations. 24 h later, the cells were washed and incubated in 0.5 ml of assay medium containing 1 mm isobutylmethylxanthine with or without the indicated concentrations of vasopressin, OPC3, or OPC4. Reactions were terminated after 30 min by removing the medium and lysing the cells in 5% trichloroacetic acid containing ATP and cAMP (1 mm each). [3H]cAMP and [3H]ATP were separated on AG 50W-X4 Dowex and alumina columns, and the data are presented as the ratio of [3H]cAMP to [3H]cAMP plus [3H]ATP, as described previously (32–35).
Cell Surface ELISA
The cell surface expression of exogenous receptors in COS-7 cells transiently transfected with myc-tagged WT-V2R or each V2R mutant was quantified by ELISA (36, 37). Briefly, 1 day after transfection, cells were reseeded into 12-well plates. 16 h before performing the assay, cells were pretreated with OPC3 or OPC4 at the indicated concentrations. After 24 h, the cells were then washed twice with ice-cold 1% BSA/PBS twice, placed on ice for 5 min, and incubated in an anti-myc antibody solution (1:4000) diluted in 1% BSA/PBS for 1 h at 4 °C. This was followed by two further washes in 1% BSA/PBS. The cells were next fixed at 4 °C for 15 min in 4% paraformaldehyde/PBS and again washed twice with 1% BSA/PBS and incubated in anti-mouse HRP-conjugated secondary antibody solution (1:12000) diluted in 1% BSA/PBS at room temperature for 1 h. This was followed by two washes in 1% BSA/PBS for 20 min and a final wash in PBS. Finally, the cells were treated with substrate (o-phenylenediamine dihydrochloride, Sigma) for 5 min at room temperature. This reaction was stopped by the addition of an equivalent volume of 2.5 n HCl. The absorption levels were then read at 492 nm using a plate reader (Bio-Rad).
Immunofluorescence Microscopy
Immunofluorescence staining was performed as described previously (18, 37). Briefly, transiently transfected COS-7 cells were placed onto glass coverslips. 16 h before performing the assay, cells were pretreated with OPC3 or OPC4 at the indicated concentrations. Cells were then washed with PBS twice, fixed with ice-cold 100% methanol, and incubated at −20 °C for 2 min to evaluate whole cell expression (with permeabilization). To evaluate cell surface expression (without permeabilization), the cells were fixed at 4 °C for 15 min in 4% paraformaldehyde/PBS. After two further washes in PBS, the cells were blocked using Block Ace at room temperature for 20 min and sequentially incubated with anti-myc antibody (1:4000) or anti PDI (protein disulfide isomerase, an ER marker) antibody (1:4000) diluted in 20% Block Ace/PBS at 4 °C overnight. The cells were then further washed twice in 20% Block Ace/PBS and incubated with anti-mouse antibody 488 (for anti-myc antibody) or anti-rabbit antibody 594 (for anti-PDI antibody) diluted in 20% Block Ace at room temperature for 1 h. After two additional washes in 0.1% Tween 20/PBS and once in PBS alone, the cells were mounted on glass slides for analysis.
Genetic Analysis of the V2 Receptor Gene (AVPR2) in Two Patients
Genetic analysis of the V2 receptor gene was performed under approval of the Institutional Review Board of the University of Tokyo. After written informed consent was obtained from the parents of the patients, genomic DNA was prepared from the peripheral blood leukocytes with the use of a DNA isolation kit, and all exons and exon-intron boundaries of AVPR2 were analyzed using a PCR direct sequencing method.
Water Deprivation Test and DDAVP Administration Test
Water deprivation tests were performed as follows. Patients were allowed to drink water freely prior to commencing the test. After the final urination, the test was started. Urine samples were collected at hourly intervals until 6 h, accompanied by the measurement of body weight, serum osmolarity, plasma concentration of ADH, and urine volume. One day after the fluid deprivation test, DDAVP (5 units/m2) was administered subcutaneously. Samples were collected before the test and at 30, 60, and 120 min after DDAVP administration. The urine volume, gradation, osmolarity, and concentration of cAMP with serum osmolarity were then measured.
Statistical Method and Analysis
The statistical method used was the Dunnett's multiple comparison procedure. To evaluate the dose response, Williams multiple comparison was used. Averaged data of three independent experiments are shown, and error bars represent mean ± S.D. These analyses were performed using Microsoft Excel software. *, p < 0.05; **, p < 0.01.
RESULTS
Identification of V2 Receptor Mutants in Partial NDI Patients
Two patients showing polydipsia were suspected of having partial NDI (see “Experimental Procedures” for a full description of these patients). In both cases, the original diagnosis had been psychogenic polydipsia because of an apparently normal response to two standard diagnostic tests, the water deprivation and DDAVP loading tests (Fig. 1A). However, an instructive clue was the increase in their basal ADH levels (Fig. 1A), which was suggestive of a possible partial ADH resistance. Subsequently, in each patient, we identified a different V2R mutation, Y128S and Ser-333del (Fig. 1B) (see “Introduction”).
cAMP Accumulation Associated with WT, Partial NDI, and Control Mutant V2R
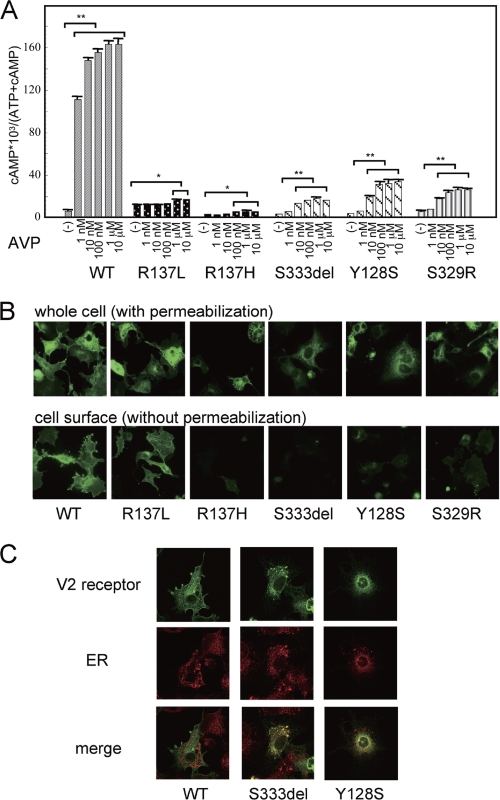
To further characterize the properties of a range of V2R mutations, including those identified in our current patients, we first examined AVP-dependent cAMP accumulation in COS-7 cells transiently expressing the wild-type V2R (COS-WT) or a different V2R mutant (COS-R137L, COS-R137H, COS-Ser-333del, COS-Y128S, and COS-S329R; Fig. 2A). COS-WT cells showed a robust AVP-dependent cAMP accumulation response in a dose-dependent manner. COS-R137L cells constitutively accumulated cAMP at basal levels and showed a moderate further accumulation in the presence of AVP compatible with the NSIAD clinical phenotype (29). COS-R137H cells showed a weak cAMP accumulation response where even the maximum levels were lower than the normal basal levels seen in the COS-WT cells. This phenotype is compatible with the clinical manifestation of complete NDI. In contrast, the COS-Ser-333del, COS-Y128S, and COS-S329R cells showed only a partial AVP-dependent cAMP accumulation response in a dose-dependent manner, with the maximum levels far below those of the COS-WT cells. This is compatible with a clinical phenotype of partial NDI.
FIGURE 2.
cAMP accumulation and immunofluorescence microscopy analysis of V2R-WT and V2R mutant-expressing cells. A, cAMP accumulation associated with the partial NDI mutant, the control mutant, and WT V2R. COS-7 cells (1 × 106 cells) were transiently transfected with V2R-WT, control V2R mutants (V2R-R137L, V2R-R137H), and partial NDI mutants (V2R-Ser-333del, V2R-Y128S, and V2R-S329R) (0.2 μg of pcDNA3 containing each myc-tagged V2R was used). 24 h later, the cells were reseeded into 24-well plates at 0.75 × 105 cells/well to which [3H]adenine (2 μCi/ml, Amersham Biosciences) was added. 48 h later, after washing in assay medium with 1 mm isobutylmethylxanthine, the cells were incubated for 30 min at 37 °C in serum-free medium with or without increasing concentrations of AVP (from 1 nm to 10 μm). cAMP accumulation was measured as described under “Experimental Procedures.” Data are mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01. B, immunofluorescence microscopy analysis of COS-7 cells transiently expressing myc-tagged V2R-WT or the indicated V2R mutant was performed as described under “Experimental Procedures.” C, colocalization of myc-tagged V2R-Ser-333del or V2R-Y128S with ER marker. Immunofluorescence microscopy analysis of COS-7 cells transiently expressing myc-tagged V2R-WT or the indicated V2R mutant was performed using confocal microscopy, and fluorescence images were collected either with anti-myc antibody or with anti-protein disulfide isomerase (ER marker) antibody as described under “Experimental Procedures.” Each set of results is representative of at least two additional experiments.
Cell Surface Expression of WT and Mutant V2 Receptors
We next examined the receptor localization patterns in cells expressing a myc-tagged wild-type V2 receptor (WT) and myc-tagged mutant V2R (R137L, R137H, Ser-333del, Y128S, and S329R) by immunofluorescence microscopy (Fig. 2B). The WT and each mutant receptor were all found to be expressed (by whole cell examination performed with permeabilization), but loss of function mutants did not localize at the cell surface (by cell surface examination performed without permeabilization) but in intracellular lesions. Immunofluorescence confocal microscopy data (Fig. 2C) showed that the Ser-333del and Tyr-128 mutants appeared to be localized in ER compartments assessed by their colocalization with an ER marker, protein disulfide isomerase.
The Effects of Inhibiting Internalization upon Cell Surface Expression of the V2 Receptor
We next examined why loss of function V2R mutants failed to localize at the cell surface. It has been reported previously that many V2R mutants are trapped in the ER so that they cannot reach the cell surface and interact with AVP (3, 4). Consistently, several non-peptide V2R antagonists, by operating as pharmacological chaperones, have been found to rescue the cell surface expression of some of these intracellularly trapped V2R mutants (3, 4). We thus speculated that many more such V2R mutants would be blocked from localizing at the cell surface because of a trafficking disorder. However, the constitutive internalization of these mutants was an alternative possibility. In this regard, it was recently reported that the R137H and R137L V2R mutants cannot localize at the cell surface because they are constitutively internalized because of their increased affinity for β-arrestin, in addition to a trafficking disorder arising from impaired maturation (38).
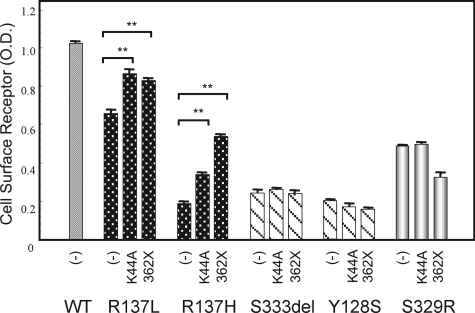
We first investigated whether constitutive internalization was the primary cause of the reduced cell surface expression of the partial NDI mutants. First, we assessed the effects of a dominant negative dynamin mutant, DynK44A, that has been shown to inhibit both constitutive and agonist-induced internalization by preventing the pinching off of the endocytic vesicles without preventing the assembly of the internalization machinery (39, 40). Cotransfection of DynK44A did not increase the cell surface expression of the partial NDI mutants but did so significantly for R137H and R137L, as reported previously (Fig. 3). We next assessed the effects of a C-tail truncation of the V2 receptor, which has been reported to decrease its ability to bind β-arrestin (41). In the experiments, the cell surface expression of the C-tail truncation mutants of R137H, R137L, R137H-362X, and R137L-362X was found to be significantly enhanced above that of the receptor harboring the R137H and R137L single mutations (Fig. 3). In contrast, cell surface expression was not augmented for the C-tail truncation mutants of Ser-333del, Y128S, and S329R, Ser-333del-362X, Y128S-362X, and S329R-362X (Fig. 3). These results suggest that constitutive internalization is not likely to be the cause of the defective cell surface expression of partial NDI mutants of V2R.
FIGURE 3.
Cell surface expression of WT and mutant V2R proteins measured by ELISA and the effects of a dominant-negative dynamin mutant, DynK44A, and C-tail truncations of the V2R mutants. COS-7 cells (1 × 106 cells) were cotransfected with myc-tagged V2R-WT, R137L, R137H, Ser-333del, Y128S, R329R (0.2 μg of pcDNA3 containing each myc-tagged V2R), and either DynK44A or pcDNA3.1 (1.0 μg) or with C-tail truncation mutants in addition to myc-tagged V2R-WT or each mutant (V2R-WT-362X, R137L-362X, R137H-362X, Ser-333del-362X, Y128S-362X, and S329R-362X; 0.2 μg of pcDNA3 containing each myc-tagged V2R). ELISA measurements with an anti-myc antibody were then performed. Data are mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01. Each set of results is representative of at least two additional experiments.
The Effects of Pharmacological Chaperones on V2R Mutants
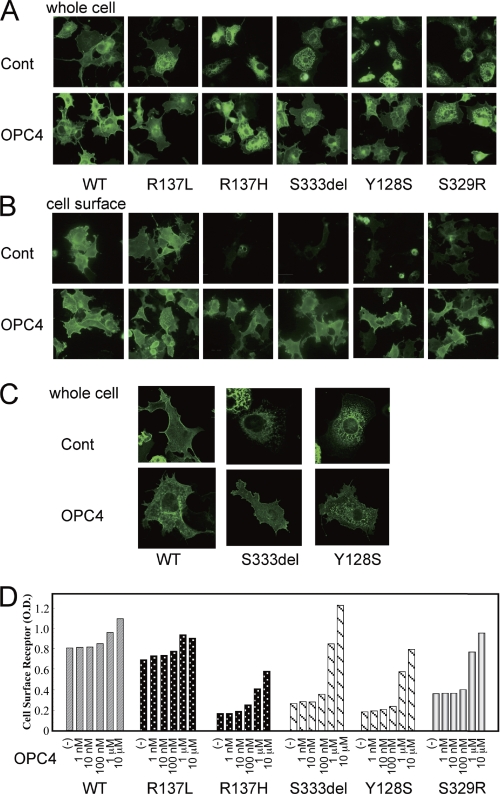
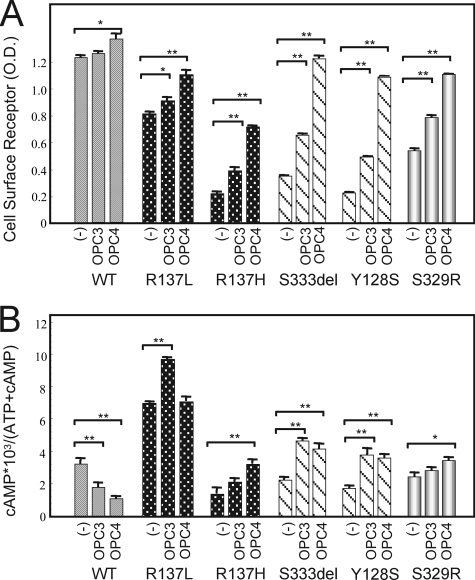
It has been reported that non-peptide V2R antagonists can stabilize ER-retained immature V2R mutants and rescue their cell surface expression by functioning as pharmacological chaperones (3, 4). We investigated the effects of the V2R antagonists OPC4 and OPC3 upon our series of mutant V2 receptors. In COS-7 cells expressing the partial NDI mutants as well as R137H, the cell surface expression of the receptors became obvious after OPC4 treatment for 16 h (Fig. 4, A and B). The cell surface expression of the Ser-333del and Tyr-128 mutants after OPC4 treatment was confirmed by confocal microscopy experiments (Fig. 4C). Using a cell surface ELISA assay, we further confirmed that OPC4 had these same effects on all of the loss of function mutants in our series, including R137H, in a dose-dependent manner (Fig. 4D). We further found that OPC3 also increased the expression of those mutants at the cell surface but was less potent in this regard than OPC4 (Fig. 5A). Accordingly, OPC4 and OPC3 augmented the basal cAMP accumulation levels in the NDI mutants (Fig. 5B). In contrast, OPC4 and OPC3 were found to operate as inverse agonists against wild-type V2R by suppressing the basal cAMP accumulation caused by wild-type V2R (Fig. 5B). Interestingly, in our experiments, OPC3 increased the constitutive cAMP accumulation caused by the R137L mutant, whereas OPC4 did not appear to do so. These data are consistent with the prediction that OPC4 and OPC3 function as inverse agonists against wild-type V2R and yet also operate as pharmacological chaperones of inactivating V2R mutants. In both cases, OPC4 appears to be more potent than OPC3. (The mechanism underlying the apparently paradoxical effects of OPC4 and OPC3 against the R137L mutant is expanded upon under “Discussion.”) Taken together, our current data suggest that OPC4 and OPC3 exert different bidirectional actions against different V2R mutants, i.e. protean agonism.
FIGURE 4.
The effects of the OPC4 chaperone on the immunofluorescence microscopy results and cell surface expression of V2R-WT or V2R mutants. A–C, after COS-7 cells had been transfected as described in Fig. 2 and treated for 16 h at 37 °C with OPC4 at 10 μm or with vehicle (Cont.), they were fixed and incubated with the antibodies as described under “Experimental Procedures.” D, after the cells had been treated for 16 h at 37 °C with increasing concentrations of OPC4 (from 1 nm to 10 μm) or vehicle, the cell surface expression of the exogenous receptors was measured by ELISA as described under “Experimental Procedures.” Each set of results is representative of at least two additional experiments.
FIGURE 5.
The effects of the OPC3 and OPC4 chaperones against COS-7 cells transiently expressing the myc-tagged V2R-WT or V2R mutants. A, cell surface expression of V2R-WT or V2R mutants. After the cells had been transfected as described in Fig. 2 and treated for 16 h at 37 °C with OPC3 (10 μm), OPC4 (10 μm), or with vehicle, they were fixed and incubated with the antibodies as described under “Experimental Procedures.” The cell surface expression of the exogenous receptors was measured by ELISA as described under “Experimental Procedures.” B, cAMP accumulation by WT or each mutant with or without pharmacological chaperones. Transiently transfected COS-7 cells with V2R-WT or each mutant were reseeded as described in Fig. 2 and incubated with 10 μm of OPC3 or OPC4 for 16 h at 37 °C. After washing by assay medium (1 mm isobutylmethylxanthine), cells were incubated again for 30 min at 37 °C in serum-free medium with or without 10 μm of OPC3 or OPC4. cAMP accumulation was measured as described under “Experimental Procedures.” The data are mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01. Each set of results is representative of at least two additional experiments.
DISCUSSION
In this study, we have identified two mutations in the V2R gene in patients who had been initially diagnosed with psychogenic polydipsia but were finally diagnosed with partial NDI. On the basis of the clinical phenotypes associated with the partial NDI in these cases, we suspected that the receptor mutant proteins must be expressed and that an analysis of their functional properties may provide further clues to the mechanisms underlying normal V2R functions. Our intact cell experiments showed that both mutant proteins were indeed expressed but did not localize at the cell surface. Previous functional analysis has shown that 70% of the missense V2R mutants thus far investigated show loss of function because of misfolding and retention in the ER (42). In additional studies, constitutive internalization was also found to affect the cell surface expression of some V2R mutants, such as R137H and R137L, at least in part (38, 41, 43). Examination of the partial NDI V2R mutants in our current cases revealed that the decreased cell surface expression underlying their loss of function was due to defective trafficking rather than constitutive internalization.
Protean Agonism Properties of Non-peptide V2 Receptor Antagonists
We further found in our current analysis that the V2 receptor antagonists OPC4 and OPC3 produce different pharmacological actions against different V2R mutants (Fig. 5B), which is known as protean agonism (13, 15, 16, 44) (see “Introduction”). OPC4 and OPC3 function as pharmacological chaperons against inactivating V2R mutants, thereby rescuing their cell surface expression and basal functions. In contrast, these compounds work as pure inverse agonists against wild-type V2R by suppressing its basal cAMP accumulation function without showing large effects against its cell surface expression levels. Although OPC4 enhances the cell surface expression of the Ser-333del and Y128S mutants more prominently than OPC3, the cAMP signal in NDI mutants is increased by both agents to similar levels (Fig. 5, A and B). These data may be explained by the prediction that OPC4 operates more strongly as an inverse agonist than OPC3, even against these V2R mutants.
Tolvaptan (OPC4) has been shown to be beneficial for the treatment of hyponatremia with heart failure (45, 46) and is now being studied as a primary therapy to delay progression of autosomal dominant polycystic kidney disease in a randomized trial (47, 48). Interestingly, in the studies on hyponatremia with heart failure, tolvaptan appeared to be effective even when the ADH levels of the patient were not increased, which could be explained by the speculation that basal activity of V2 receptor (shown in Fig. 5B) might play a pathophysiological role, at least in part. If so, we also speculate that OPC4 might be more effective than OPC3 because OPC4 is a more potent inverse agonist than OPC3 (Fig. 5B).
We surmised that if OPC4 and OPC3 can efficiently suppress the constitutive activity of the R137L mutant V2R by working as inverse agonists, they may have potential utility as therapeutics against nephrogenic syndrome of inappropriate antidiuresis NSIAD. However, this was subsequently found not to be the case. OPC3 augments the constitutive activity of the R137L mutant, whereas OPC4 had no such effect. This finding may be at least partly explained by the fact that OPC4 and OPC3 enhance the cell surface expression of the R137L mutant. It is therefore likely that although OPC4 in particular works as inverse agonist even in the case of the R137L mutant V2R, this inhibitory effect is neutralized by its pharmacological chaperone activity that up-regulates the membrane expression of the receptor protein. In addition, the inverse agonist activity itself may also increase the membrane expression of the R137L mutant by suppressing its constitutive internalization. A paradoxical increase in the constitutive activity of the R137L mutant has been reported previously and discussed for another V2R inverse agonist, satavaptan (SR121463) (38). Another report has shown that SR121463 has little effect upon the constitutive activity of the R137L mutant (49). Nevertheless, a more potent inverse agonist may still be capable of suppressing the constitutive accumulation of cAMP by the R137L mutant V2R and aid in the future treatment of neonatal syndrome of inappropriate antidiuresis patients.
V2 Receptor Ligands as Potential Therapeutics against NDI
We demonstrate also from our current analysis that the V2R antagonists, OPC4 and OPC3, can function as pharmacological chaperones for three partial NDI V2R mutants, as reported for several other V2R mutants (31). From a clinical point of view, however, these agents would not be suitable as therapeutics, as they also inhibit AVP action. The identification of a pharmacological chaperone that does not inhibit AVP action because it is easily replaced by AVP or functions allosterically or acts as an agonist would be of interest, as it could potentially be used to treat NDI patients. As reported recently, some pharmacochaperones may operate as a biased agonist against a limited repertoire of NDI mutants, thus revealing that they behave as full agonists for AC activation but that they are unable to phosphorylate ERK1/2 (50). Some NDI cases could be treated with such agents.
Insights Gained from Analyzing of Partial NDI V2R Mutants
An additional clinical implication of our current finding is that partial NDI may often be missed or misdiagnosed. Indeed, both of our partial NDI cases were originally diagnosed with psychogenic polydipsia. Following a water deprivation test and DDAVP loading test, we found that our patients could concentrate urine in response to AVP. However, the principal indication of possible NDI was the high basal AVP levels in both cases. Only nine mutants, including those identified herein, have been reported previously as causative for partial NDI, whereas more than 200 mutants have been identified as causal for complete NDI (11). It is thus possible that partial NDI will be revealed in further patients with polydipsia and that additional V2R mutations will be found. Careful attention to the basal AVP levels is warranted in such cases.
Acknowledgments
We thank M. Bouvier for generously donating the myc-tagged V2R construct. We also thank H. R. Bourne for suggestions and Otsuka Pharmaceutical Company for providing the V2 receptor antagonists.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture, Japan.
- GPCR
- G-protein-coupled receptor
- NDI
- nephrogenic diabetes insipidus
- AVP
- arginine vasopressin
- V2R
- V2 vasopressin receptor
- OPC4
- OPC3
- ER
- endoplasmic reticulum
- DDAVP
- 1-desamino-8-arginine vasopressin
- ADH
- antidiuretic hormone
- NSIAD
- neonatal syndrome of inappropriate antidiuresis.
REFERENCES
- 1. Tao Y. X. (2006) Inactivating mutations of G protein-coupled receptors and diseases. Structure-function insights and therapeutic implications. Pharmacol. Ther. 111, 949–973 [DOI] [PubMed] [Google Scholar]
- 2. Spiegel A. M., Weinstein L. S. (2004) Inherited diseases involving G proteins and G protein-coupled receptors. Annu .Rev. Med. 55, 27–39 [DOI] [PubMed] [Google Scholar]
- 3. Robben J. H., Knoers N. V., Deen P. M. (2006) Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Renal Physiol. 291, F257–270 [DOI] [PubMed] [Google Scholar]
- 4. Bichet D. G. (2006) Nephrogenic diabetes insipidus. Adv. Chronic Kidney Dis. 13, 96–104 [DOI] [PubMed] [Google Scholar]
- 5. Ala Y., Morin D., Mouillac B., Sabatier N., Vargas R., Cotte N., Déchaux M., Antignac C., Arthus M. F., Lonergan M., Turner M. S., Balestre M. N., Alonso G., Hibert M., Barberis C., Hendy G. N., Bichet D. G., Jard S. (1998) Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus. Molecular basis of a mild clinical phenotype. J. Am. Soc. Nephrol. 9, 1861–1872 [DOI] [PubMed] [Google Scholar]
- 6. Chen C. H., Chen W. Y., Liu H. L., Liu T. T., Tsou A. P., Lin C. Y., Chao T., Qi Y., Hsiao K. J. (2002) Identification of mutations in the arginine vasopressin receptor 2 gene causing nephrogenic diabetes insipidus in Chinese patients. J. Hum. Genet. 47, 66–73 [DOI] [PubMed] [Google Scholar]
- 7. Faerch M., Christensen J. H., Corydon T. J., Kamperis K., de Zegher F., Gregersen N., Robertson G. L., Rittig S. (2008) Clin. Endocrinol. 68, 395–403 [DOI] [PubMed] [Google Scholar]
- 8. Inaba S., Hatakeyama H., Taniguchi N., Miyamori I. (2001) The property of a novel v2 receptor mutant in a patient with nephrogenic diabetes insipidus. J. Clin. Endocrinol. Metab. 86, 381–385 [DOI] [PubMed] [Google Scholar]
- 9. Pasel K., Schulz A., Timmermann K., Linnemann K., Hoeltzenbein M., Jääskeläinen J., Grüters A., Filler G., Schöneberg T. (2000) Functional characterization of the molecular defects causing nephrogenic diabetes insipidus in eight families. J. Clin. Endocrinol. Metab. 85, 1703–1710 [DOI] [PubMed] [Google Scholar]
- 10. Sadeghi H., Robertson G. L., Bichet D. G., Innamorati G., Birnbaumer M. (1997) Biochemical basis of partial nephrogenic diabetes insipidus phenotypes. Mol. Endocrinol. 11, 1806–1813 [DOI] [PubMed] [Google Scholar]
- 11. Faerch M., Christensen J. H., Rittig S., Johansson J. O., Gregersen N., de Zegher F., Corydon T. J. (2009) Diverse vasopressin V2 receptor functionality underlying partial congenital nephrogenic diabetes insipidus. Am. J. Physiol. Renal Physiol. 297, F1518–1525 [DOI] [PubMed] [Google Scholar]
- 12. Gether U. (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 [DOI] [PubMed] [Google Scholar]
- 13. Kenakin T. (2007) Functional selectivity through protean and biased agonism. Who steers the ship? Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 14. Leach K., Sexton P. M., Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 28, 382–389 [DOI] [PubMed] [Google Scholar]
- 15. Hill S. J. (2006) G-protein-coupled receptors. Past, present and future. Br. J. Pharmacol. 147, S27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 17. Azzi M., Charest P. G., Angers S., Rousseau G., Kohout T., Bouvier M., Piñeyro G. (2003) β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 100, 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makita N., Sato J., Manaka K., Shoji Y., Oishi A., Hashimoto M., Fujita T., Iiri T. (2007) An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proc. Natl. Acad. Sci. U.S.A. 104, 5443–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makita N., Iiri T. (2008) AfCS Nature Molecule Pages doi: 10.1038/mp.a004000.01 [DOI] [Google Scholar]
- 20. Kenakin T. (2001) Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 15, 598–611 [DOI] [PubMed] [Google Scholar]
- 21. Bichet D. G., Arthus M. F., Lonergan M., Hendy G. N., Paradis A. J., Fujiwara T. M., Morgan K., Gregory M. C., Rosenthal W., Didwania A. (1993) X-linked nephrogenic diabetes insipidus mutations in North America and the Hopewell hypothesis. J. Clin. Invest. 92, 1262–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bichet D. G., Birnbaumer M., Lonergan M., Arthus M. F., Rosenthal W., Goodyer P., Nivet H., Benoit S., Giampietro P., Simonetti S. (1994) Nature and recurrence of AVPR2 mutations in X-linked nephrogenic diabetes insipidus. Am. J. Hum. Genet. 55, 278–286 [PMC free article] [PubMed] [Google Scholar]
- 23. Arthus M. F., Lonergan M., Crumley M. J., Naumova A. K., Morin D., De Marco L. A., Kaplan B. S., Robertson G. L., Sasaki S., Morgan K., Bichet D. G., Fujiwara T. M. (2000) Report of 33 novel AVPR2 mutations and analysis of 117 families with X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 11, 1044–1054 [DOI] [PubMed] [Google Scholar]
- 24. Boson W. L., Della Manna T., Damiani D., Miranda D. M., Gadelha M. R., Liberman B., Correa H., Romano-Silva M. A., Friedman E., Silva F. F., Ribeiro P. A., De Marco L. (2006) Novel vasopressin type 2 (AVPR2) gene mutations in Brazilian nephrogenic diabetes insipidus patients. Genet. Test. 10, 157–162 [DOI] [PubMed] [Google Scholar]
- 25. Carroll P., Al-Mojalli H., Al-Abbad A., Al-Hassoun I., Al-Hamed M., Al-Amr R., Butt A. I., Meyer B. F. (2006) Novel mutations underlying nephrogenic diabetes insipidus in Arab families. Genet. Med. 8, 443–447 [DOI] [PubMed] [Google Scholar]
- 26. Kalenga K., Persu A., Goffin E., Lavenne-Pardonge E., van Cangh P. J., Bichet D. G., Devuyst O. (2002) Intrafamilial phenotype variability in nephrogenic diabetes insipidus. Am. J. Kidney Dis. 39, 737–743 [DOI] [PubMed] [Google Scholar]
- 27. Schoneberg T., Schulz A., Biebermann H., Gruters A., Grimm T., Hubschmann K., Filler G., Gudermann T., Schultz G. (1998) V2 vasopressin receptor dysfunction in nephrogenic diabetes insipidus caused by different molecular mechanisms. Hum. Mutat. 12, 196–205 [DOI] [PubMed] [Google Scholar]
- 28. Shoji Y., Takahashi T., Suzuki Y., Suzuki T., Komatsu K., Hirono H., Yokoyama T., Kito H., Takada G. (1998) Hum. Mutat. 1, S278–283 [DOI] [PubMed] [Google Scholar]
- 29. Feldman B. J., Rosenthal S. M., Vargas G. A., Fenwick R. G., Huang E. A., Matsuda-Abedini M., Lustig R. H., Mathias R. S., Portale A. A., Miller W. L., Gitelman S. E. (2005) Nephrogenic syndrome of inappropriate antidiuresis. N. Engl. J. Med. 352, 1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macion-Dazard R., Callahan N., Xu Z., Wu N., Thibonnier M., Shoham M. (2006) Mapping the binding site of six nonpeptide antagonists to the human V2-renal vasopressin receptor. J. Pharmacol. Exp. Ther. 316, 564–571 [DOI] [PubMed] [Google Scholar]
- 31. Robben J. H., Sze M., Knoers N. V., Deen P. M. (2007) Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones. Relevance to therapy of nephrogenic diabetes insipidus. Am. J. Physiol. Renal Physiol. 292, F253–260 [DOI] [PubMed] [Google Scholar]
- 32. Iiri T., Herzmark P., Nakamoto J. M., van Dop C., Bourne H. R. (1994) Rapid GDP release from Gs α in patients with gain and loss of endocrine function. Nature 371, 164–168 [DOI] [PubMed] [Google Scholar]
- 33. Iiri T., Farfel Z., Bourne H. R. (1997) Conditional activation defect of a human Gs α mutant. Proc. Natl. Acad. Sci. U.S.A. 94, 5656–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iiri T., Bell S. M., Baranski T. J., Fujita T., Bourne H. R. (1999) A Gs α mutant designed to inhibit receptor signaling through Gs. Proc. Natl. Acad. Sci. U.S.A. 96, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makita N., Sato J., Rondard P., Fukamachi H., Yuasa Y., Aldred M. A., Hashimoto M., Fujita T., Iiri T. (2007) Human G(sα) mutant causes pseudohypoparathyroidism type Ia/neonatal diarrhea, a potential cell-specific role of the palmitoylation cycle. Proc. Natl. Acad. Sci. U.S.A. 104, 17424–17429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernier V., Lagacé M., Lonergan M., Arthus M. F., Bichet D. G., Bouvier M. (2004) Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol. Endocrinol. 18, 2074–2084 [DOI] [PubMed] [Google Scholar]
- 37. Bernier V., Morello J. P., Zarruk A., Debrand N., Salahpour A., Lonergan M., Arthus M. F., Laperrière A., Brouard R., Bouvier M., Bichet D. G. (2006) Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 17, 232–243 [DOI] [PubMed] [Google Scholar]
- 38. Rochdi M. D., Vargas G. A., Carpentier E., Oligny-Longpré G., Chen S., Kovoor A., Gitelman S. E., Rosenthal S. M., von Zastrow M., Bouvier M. (2010) Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis. Implications for treatments. Mol. Pharmacol. 77, 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki J., Ohnsihi H., Shibata H., Wada A., Hirayama T., Iiri T., Ueda N., Kanamaru C., Tsuchida T., Mashima H., Yasuda H., Fujita T. (2001) Dynamin is involved in human epithelial cell vacuolation caused by the Helicobacter pylori-produced cytotoxin VacA. J. Clin. Invest. 107, 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baldassarre M., Pompeo A., Beznoussenko G., Castaldi C., Cortellino S., McNiven M. A., Luini A., Buccione R. (2003) Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell 14, 1074–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barak L. S., Oakley R. H., Laporte S. A., Caron M. G. (2001) Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. U.S.A. 98, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morello J. P., Bichet D. G. (2001) Nephrogenic diabetes insipidus. Annu. Rev. Physiol. 63, 607–630 [DOI] [PubMed] [Google Scholar]
- 43. Kocan M., See H. B., Sampaio N. G., Eidne K. A., Feldman B. J., Pfleger K. D. (2009) Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis. Mol. Endocrinol. 23, 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neubig R. R. (2007) Missing links. Mechanisms of protean agonism. Mol. Pharmacol. 71, 1200–1202 [DOI] [PubMed] [Google Scholar]
- 45. Farmakis D., Filippatos G., Parissis J., Kremastinos D. T., Gheorghiade M. (2009) Hyponatremia in heart failure. Heart Fail. Rev. 14, 59–63 [DOI] [PubMed] [Google Scholar]
- 46. Zmily H. D., Daifallah S., Ghali J. K. (2011) Tolvaptan, hyponatremia, and heart failure. Int. J. Nephrol. Renovasc. Dis. 4, 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Irazabal M. V., Torres V. E., Hogan M. C., Glockner J., King B. F., Ofstie T. G., Krasa H. B., Ouyang J., Czerwiec F. S. (2011) Kidney Int. 80, 295–301 [DOI] [PubMed] [Google Scholar]
- 48. Torres V. E., Meijer E., Bae K. T., Chapman A. B., Devuyst O., Gansevoort R. T., Grantham J. J., Higashihara E., Perrone R. D., Krasa H. B., Ouyang J. J., Czerwiec F. S. (2011) Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 Study. Am. J. Kidney Dis. 57, 692–699 [DOI] [PubMed] [Google Scholar]
- 49. Tenenbaum J., Ayoub M. A., Perkovska S., Adra-Delenne A. L., Mendre C., Ranchin B., Bricca G., Geelen G., Mouillac B., Durroux T., Morin D. (2009) The constitutively active V2 receptor mutants conferring NSIAD are weakly sensitive to agonist and antagonist regulation. PLoS ONE 4, e8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jean-Alphonse F., Perkovska S., Frantz M. C., Durroux T., Méjean C., Morin D., Loison S., Bonnet D., Hibert M., Mouillac B., Mendre C. (2009) Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 20, 2190–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]