Abstract
Neutrophil migration to inflamed sites is crucial for both the initiation of inflammation and resolution of infection, yet these cells are involved in perpetuation of different chronic inflammatory diseases. Gastrin-releasing peptide (GRP) is a neuropeptide that acts through G protein coupled receptors (GPCRs) involved in signal transmission in both central and peripheral nervous systems. Its receptor, gastrin-releasing peptide receptor (GRPR), is expressed by various cell types, and it is overexpressed in cancer cells. RC-3095 is a selective GRPR antagonist, recently found to have antiinflammatory properties in arthritis and sepsis models. Here we demonstrate that i.p. injection of GRP attracts neutrophils in 4 h, and attraction is blocked by RC-3095. Macrophage depletion or neutralization of TNF abrogates GRP-induced neutrophil recruitment to the peritoneum. In vitro, GRP-induced neutrophil migration was dependent on PLC-β2, PI3K, ERK, p38 and independent of Gαi protein, and neutrophil migration toward synovial fluid of arthritis patients was inhibited by treatment with RC-3095. We propose that GRPR is an alternative chemotactic receptor that may play a role in the pathogenesis of inflammatory disorders.
Neuropeptides are used by neurons as signaling molecules to regulate synaptic transmission and plasticity (1). Nonetheless, these molecules can be versatile, also acting as chemical messengers outside the nervous system. Recent reports showed that neuropeptides are produced as a result of immune pathologies (2), whereas others appear to induce cytokine production by immune cells (3).
Gastrin-releasing peptide (GRP) is a neuropeptide that induces gastrin secretion in the gastric tract (4). It acts by binding to the gastrin-releasing peptide receptor (GRPR or BB2), a member of the G protein coupled receptor (GPCR) superfamily expressed in the gastric, respiratory, and nervous systems, as well as endocrine glands and muscle (5). GRPR mediates gastrointestinal motility and hormone and neurotransmitter release in the gut, intestine, colon, and other organs (6). It has roles in the nervous system, controlling the circadian cycle, anxiety, fear, stress, and modulation of memory (7). It is overexpressed in cancer cells, and the production of GRP together with GRPR overexpression results in autocrine growth stimulation (6). Selective GRPR antagonists were produced as candidate anticancer drugs, including RC-3095 (8).
More recently, RC-3095 has been demonstrated to have antiinflammatory effects in arthritis (9) and sepsis (10, 11) models, down-regulating the production of proinflammatory cytokines IL-1β, IL-6, and TNF-α. Interestingly, GRPR has been found to be expressed in immune cells (12). Inflammation is a protective immune response initiated by exposure of innate immune cells to molecular patterns that signal infection or injury (13), and the migration of neutrophils to sites of inflammation can promote tissue damage (14), although it is also critical for healing of the affected areas (15).
The mechanisms underlying the actions of GRPR-binding drugs in inflammatory scenarios have not been elucidated. In this study, we report that GRP can be an endogenous inflammatory mediator, acting as a chemoattractant through GRPR. In addition, it activates specific signaling pathways that promote neutrophil migration. We propose that GRP triggers neutrophil recruitment both indirectly, through macrophages, as well as directly, binding to GRPR in these cells.
Results
GRP Induces Neutrophil Migration in Vivo.
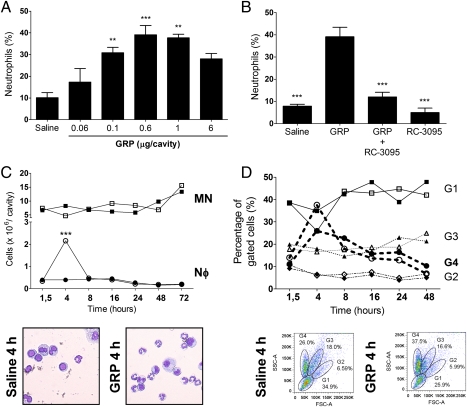
It has been previously shown that GRPR antagonist RC-3095 has antiinflammatory activity in animal models of inflammation (9, 10, 16). We hypothesized that GRP could have proinflammatory potential, so we tested whether GRP would have a dose-dependent effect on neutrophil recruitment in vivo. We performed a kinetic analysis, looking at different time points after GRP injection. I.p. injection of human GRP induced neutrophil recruitment after 4 h in a dose-dependent fashion, the highest numbers being obtained with 0.6 μg per cavity (Fig. 1A). Pretreatment of mice with RC-3095 immediately before injection of GRP abolished neutrophil accumulation in the peritoneum (Fig. 1B), indicating that GRP-induced neutrophil migration occurs specifically through activation of GRPR. The chemoattractant effect of GRP in vivo was restricted to neutrophils, because no recruitment of macrophages or B or T cells was observed in any of the time points, from 1.5 to 72 h (Fig. 1 C and D), as assessed by both Diff-Quick staining and flow cytometry. In our hands, a peritoneal lavage of an unmanipulated mouse yielded between 5 and 10 million cells, and injection of GRP did not significantly alter total numbers of cells. The absolute numbers of neutrophils migrating to the peritoneum after GRP i.p. were thus comparable between both methods. Collectively, these results indicate that GRP has a selective effect over neutrophils in vivo, inducing their migration through activation of GRPR.
Fig. 1.
GRP induces neutrophil recruitment to the peritoneal cavity of mice. Mice were injected i.p. with (A) different doses of GRP (0.06–6 μg/cavity) and recovered cells were counted after 4 h, **P < 0.01 compared with saline-treated group; (B) GRP (0.6 μg/cavity), RC-3095 (6 μg/cavity), or RC-3095 (6 μg/cavity) + GRP (0.6 μg/cavity) and control group, saline, ***P < 0.001 compared with GRP-injected group; and (C) GRP (0.6 μg/cavity). After 1.5, 4, 8, 16, 24, 48, and 72 h, animals were killed, cells were cytocentrifuged, stained with Diff-Quick, and counted. Filled circles, neutrophils (Nφ) in saline groups; open circles, GRP-treated groups. Filled squares, mononuclear cells (MN) in controls; open squares, mononuclear cells counted in GRP-treated groups. ***P < 0.001 compared with saline-injected group; (D) GRP (0.6 μg/cavity). After 1.5, 4, 8, 16, 24, 48, and 72 h, cells in the peritoneal fluid were analyzed by FACS. Gates 1 to 4 were determined on the basis of FSC × SSC distribution and staining with anti-CD14, CD11c, CD4, and B220. G1 = lymphocytes; G2 = larger lymphocytes and DCs; G3 = macrophages; G4 = neutrophils. Filled forms, saline groups; open forms, GRP-treated groups. Data representative of four independent experiments (n = 4 for each group of treatment) and expressed as the mean ± SE of the percentage or number of cells.
GRP-Induced Neutrophil Recruitment in Vivo Depends on Macrophages and TNF-α Production.
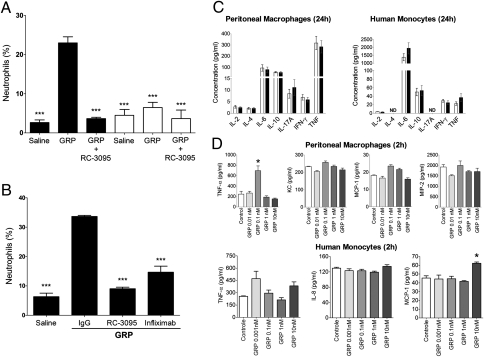
Neutrophil migration to sites of inflammation in vivo is mediated by the release of cytokines and chemokines by resident cells. We decided to investigate the role of macrophages on neutrophil migration induced by GRP in vivo. We performed macrophage depletion by i.p. injection of chlodronate liposomes in mice, later injecting GRP or saline i.p. Depletion of macrophages almost completely inhibited GRP-induced neutrophil migration (Fig. 2A), suggesting that macrophages play a role in neutrophil recruitment to the peritoneum in response to GRP injection.
Fig. 2.
GRP-induced neutrophil recruitment in vivo depends on macrophages and TNF-α production. (A) Mice were injected i.p. with chlodronate liposomes (500 μg per cavity) (open bars) or saline (filled bars), 24 h before GRP (0.6 μg per cavity) or RC-3095 (6 μg per cavity) + GRP (0.6 μg per cavity) injection. After 4 h of i.p. GRP with/without RC-3095 injection, differential counts in the peritoneal fluid were determined. ***P < 0.001 compared with GRP-injected group. (B) Mice were pretreated with infliximab or IgG control before i.p. injection of GRP (0.6 μg per cavity). After 4 h, differential counts in the peritoneal fluid were determined. (C) Murine peritoneal macrophages or human monocytes were isolated, stimulated with GRP (1 nM) or medium, and 24 h later the supernatant was analyzed for cytokines by cytometric bead array. Open bars, control; filled bars, GRP-treated cells; ND, not detectable. (D) Peritoneal macrophages or human monocytes were stimulated with GRP (1 nM) or medium alone, and 2 h later the supernatant was harvested and analyzed for TNF or chemokine production by ELISA. Data are representative of three independent experiments, performed in triplicate for each sample, and expressed as mean ± SE.
TNF-α is one of the major cytokines released by macrophages in response to inflammatory stimuli, enhancing neutrophil migration by increasing the expression of adhesion molecules on endothelial cells and neutrophils, facilitating cell arrival to inflamed sites (17). To investigate the role of TNF-α in our system, we pretreated mice with a therapeutic TNF-α–specific neutralizing antibody (infliximab), a chimeric (mouse/human) antibody that cross-reacts with murine TNF-α (18). Mice were treated with infliximab or human IgG, immediately before GRP injection, and blocking of TNF-α significantly inhibited GRP-induced neutrophil recruitment to the peritoneum (Fig. 2B). To determine whether GRP could induce the production of proinflammatory cytokines, we performed cytokine analysis (IL-4, IL-6, IL-10, IL-17A, INF-γ, and TNF-α) in response to GRP stimulation of murine peritoneal macrophages and human monocytes. Twenty-four hours after stimulation with 1 nM GRP (19, 20), none of these mediators were induced (Fig. 2C). An alternative explanation for our observations was that GRP induced chemokine production by macrophages, which in turn could up-regulate TNF-α production in vivo, enhancing GRP-induced neutrophil recruitment. Chemokines peak earlier than some cytokines (17) and it was possible that a dose other than 1 nM was necessary to induce these mediators in macrophages/monocytes, so we used a range of concentrations of GRP for stimulation (10–0.001 nM), for 2 h. Supernatants were analyzed for TNF-α, KC/IL-8, MIP-2, and MCP-1 production. Results shown in Fig. 2D reveal that in 2 h, GRP induces TNF-α in murine macrophages, at 0.1 nM, and MCP1 in human monocytes, at 10 nM. Together, these results suggest that in vivo neutrophil recruitment through GRPR depends on macrophage presence and TNF-α production, and that TNF/chemokine production by macrophages/monocytes can be triggered by GRP.
GRP Has a Direct Chemoattractant Effect on Neutrophils.
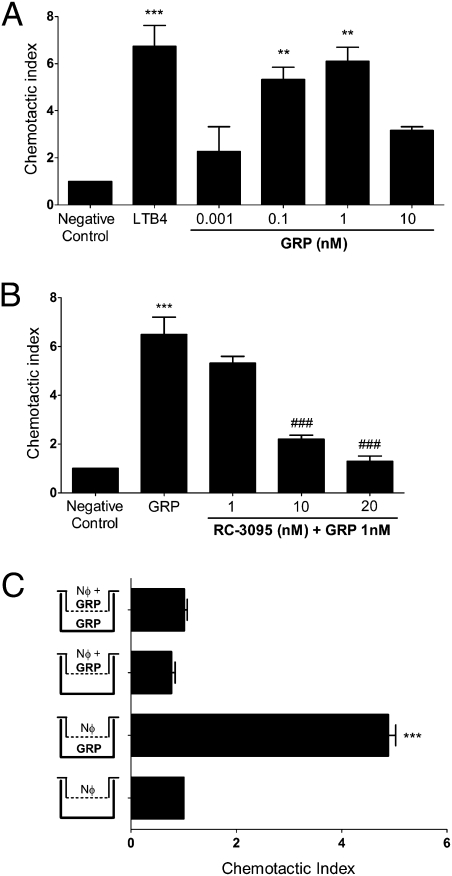
It has recently been demonstrated that neutrophils express GRPR (12). Chemokines (17) and leukotrienes (21) and molecules released by damaged tissues (22, 23) act as chemoattractants, acting directly on neutrophils to induce migration. We investigated whether GRP, a neuropeptide, would induce neutrophils to migrate up a gradient of GRP in vitro, in a Transwell system. Nanomolar amounts of GRP induce neutrophil migration in a dose-dependent manner (Fig. 3A) in a bell-shaped curve. These results reflect previous studies on macrophages (19), lymphocytes (20), and mast cells (24), showing that GRP modulates immune cell function in a nanomolar range. To investigate the effects exerted by RC-3095 over migration, we performed a dose–response curve of this inhibitor using the optimal dose of GRP determined previously. We observed that RC-3095 does not inhibit GRP-induced migration while in equivalent nanomolar amounts (Fig. 3B). However, a 10-fold increase of the inhibitor blocks the effect of GRP on neutrophil migration, and a 20-fold increase completely abolishes it.
Fig. 3.
GRP has a direct chemoattractant effect on neutrophils. (A) Human neutrophils were allowed to migrate toward GRP (0.001–10 nM) with 2% FCS for 2 h. **P < 0.01 and ***P < 0.001 compared with negative control. (B) Neutrophils were preincubated with RC-3095 (1–20 nM) for 1 h at 37 °C under 5% CO2 and stimulated to migrate toward GRP (1 nM) for 2 h at 37 °C under 5% CO2. (C) Neutrophils were placed in the upper chamber alone, or together with GRP (1 nM), and exposed in the lower chamber to medium alone or GRP (1 nM) with 2% FCS for 2 h. ***P < 0.001 compared with negative control and ###P < 0.001 compared with GRP-treated group. Data are representative of four independent experiments, performed in triplicate for each sample, and expressed as mean ± SE.
To exclude the possibility that GRP was simply inducing chemokinesis, and not chemotaxis, we performed a checkerboard analysis. For this, the optimal concentration of GRP (1 nM) was placed in the upper wells, lower wells, or both upper and lower wells of the Transwell chamber (25). GRP was only able to induce influx of neutrophils when it was added to the lower wells, confirming chemotaxis rather than chemokinesis (Fig. 3C).
Neutrophil Migration Induced by GRP Is Independent of Gαi Proteins, but Dependent on PLC-β, PI3K, and MAPK.
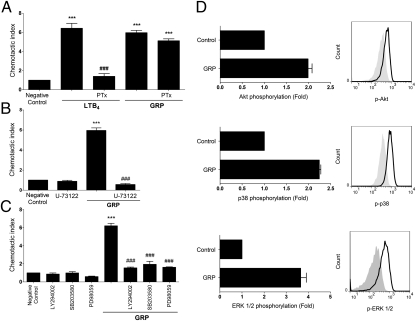
The classical pathway of neutrophil migration involves triggering GPCR receptors, which release Gβγ subunits from Gαi proteins, activating specific signaling pathways (26). However, GRPR preferentially couples to Gαq proteins (27), which have not been identified with neutrophil migration. Given the complexity of GPCR biology, we hypothesized that GRPR could associate with Gαi protein to induce migration. To investigate this, we pretreated neutrophils with pertussis toxin (PTx), which irreversibly inhibits Gαi proteins (28) and allowed cells to migrate toward GRP or leukotriene B4 (LTB4). Pretreating neutrophils with PTx abrogated their migration to LTB4 but did not affect migration toward GRP (Fig. 4A), suggesting that Gαi proteins are not involved in neutrophil chemotaxis induced by GRP.
Fig. 4.
GRP induces neutrophil migration independently of Gαi protein and dependently on PLC-β, PI3K, and MAPK. Neutrophils were preincubated with: (A) Pertussis toxin 100 ng/mL for 2 h; (B) U-73122 1 μM (PLC-β inhibitor); (C) LY294002 50 μM (PI3K inhibitor), SB203580 10 μM (p38 inhibitor), or PD98059 30 μM (ERK inhibitor) for 1 h, and stimulated to migrate toward GRP (1 nM) or LTB4 (1 nM) for 2 h. (D) Neutrophils were stimulated with GRP (1 nM) for 5 min and stained for phosphorylated proteins (AKT, p38, and ERK 1/2). Filled histograms are control neutrophils and black solid lines are GRP-stimulated neutrophils. Phosphorylation of protein pathways are presented as fold increase relative to unstimulated neutrophils (Left column). Data are representative of three independent experiments, performed in triplicate for each sample, and expressed as mean ± SE. ***P < 0.001 compared with negative control and ###P < 0.001 compared with LTB4- or GRP-treated group.
Because GRPR is coupled to Gαq protein, and triggering Gαq-coupled receptors leads to activation of phospholipase C-β (PLC-β) (29), we investigated its involvement on GRP-induced migration. The pretreatment of cells with a selective inhibitor of PLC-β, U-73122, abolished neutrophil migration toward GRP (Fig. 4B), indicating that GRP activates PLC-β to induce chemotaxis.
Neutrophil migration induced by GPCR ligation is known to be dependent on PI3K and MAPK signaling routes (30). To determine downstream signaling pathways activated in GRP-induced neutrophil migration, we pretreated the cells with selective inhibitors of PI3K (LY29004), as well as of ERK 1/2 (PD98059) and p38 MAPK (SB203580). Blocking these pathways completely inhibited recruitment toward GRP (Fig. 4C), indicating that GRP activates PI3K and MAPK, similarly to other chemoattractant molecules (31). We also investigated whether treatment of neutrophils with GRP would activate these signaling pathways, analyzing phosphorylation of MAPKs and AKT (as a reading of PI3K activation) (32). Results showed in Fig. 4D support the findings that GRP rapidly signals through GRPR, activating these pathways, which promote neutrophil chemotaxis.
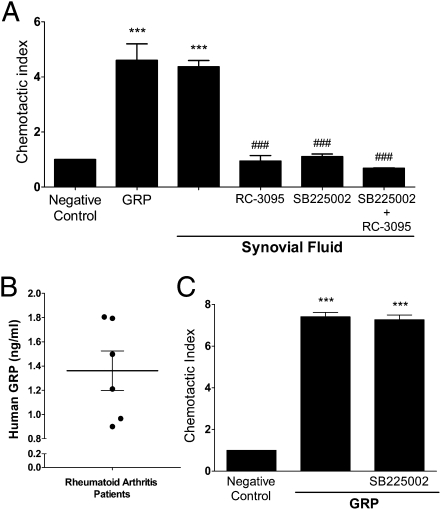
Neutrophil Migration Toward Synovial Fluid (SF) of Rheumatoid Arthritis (RA) Patients Can Be Blocked by RC-3095.
Previous studies revealed an antiinflammatory effect of RC-3095 in murine arthritis (9) and sepsis models (10). This effect was attributed to the inhibition of IL-1β and TNF-α production. Other studies reported an increase of GRP concentrations in SF of arthritis patients, correlating with severity of disease (33, 34).
We hypothesized that at least part of the effect of RC-3095 in arthritis could be explained by an inhibition of neutrophil recruitment to the arthritic joint. We analyzed neutrophil migration toward SF from RA patients. The concentrations of GRP in samples of SF from patients varied between 0.8 and 1.8 ng/mL in our samples (n = 6) (Fig. 5B). Analysis in the Transwell system indicated that SF induced neutrophil migration, comparably to GRP alone (Fig. 5A). This migration was abrogated by RC-3095, indicating that GRP in the SF can activate GRPR and trigger neutrophil chemotaxis. Interestingly, the inhibition of neutrophil migration by RC-3095 was comparable to that observed with pretreatment of neutrophils with a CXCR2 antagonist (SB225002). The concentration of IL-8 in SF samples of RA was greater than 300 pg/mL (311.12 ± 3.45 pg/mL). To exclude the possibility that GRP in SF could be binding directly to CXCR2, we allowed neutrophils pretreated with SB225002 to migrate toward GRP. This pretreatment did not inhibit GRP-induced neutrophil migration (Fig. 5C), suggesting the effect of GRP was restricted to GRPR binding.
Fig. 5.
Synovial fluid-induced migration of neutrophils is partially inhibited by RC-3095. (A) Human neutrophils were preincubated with RC-3095 (10 nM) or SB225002, a CXCR2 antagonist (300 nM), for 1 h and allowed to migrate toward GRP (1 nM) or synovial fluid (SF) of rheumatoid arthritis (RA) patients. (B) Samples of RA patients’ SF were assayed for the presence of GRP by ELISA (n = 6). (C) Neutrophils were preincubated with SB225002 (300 nM) for 1 h at 37 °C under 5% CO2 and stimulated to migrate toward GRP (1 nM) for 2 h. Data are representative of three independent experiments, performed in triplicate for each sample, and expressed as mean ± SE. ***P < 0.001 compared with negative control; ###P < 0.001 compared with SF-treated group.
Discussion
Neutrophil migration is governed by gradients of chemoattractants, which include chemokines, complement-derived peptides (26), bacterial peptides (35), and lipid mediators (21). Despite the different nature of the ligands, all of them bind to and activate GPCR (26). In this study, we describe a unique function for the neuropeptide GRP and its receptor, GRPR, in the chemotaxis of neutrophils, showing that GRP can directly induce the GRPR-mediated neutrophil migration.
In vivo, macrophages play a role in the recruitment of neutrophils to the peritoneum by GRP. When we stimulated murine peritoneal macrophages or human monocytes with GRP in vitro and analyzed TNF-α and chemokine secretion by these cells, we observed induction of TNF-α in murine macrophages and MCP-1 in human monocytes 2 h after GRP stimulation. These results suggest that an early induction of such mediators in macrophages by GRP may occur in vivo, contributing to neutrophil recruitment, and this is consistent with neutrophil peaking at 4 h after GRP injection. Our results show that at 24 h, these mediators are no longer induced, and consistently, no neutrophils migrate to the peritoneum at this time point. Different stimuli induce inflammatory responses with prominent neutrophil influx, even though they are not chemotactic agents (for example, LPS) (36). However, in our system, we believe that in vivo GRP acts both directly and indirectly as a chemoattractant for neutrophils. The direct effect is demonstrated by the in vitro experiments, and the checkerboard analysis indicates that it has a chemotactic effect, and not simply a chemokinetic one. In vivo, besides acting directly on neutrophils, GRP would act on macrophages, inducing TNF-α production, leading to up-regulation of adhesion molecules that are necessary for neutrophil extravasation (17).
It is possible that GRP also acts on cells other than macrophages, contributing to the production of neutrophil-recruiting chemokines. A previous study reported that bombesin treatment induced production of IL-8 and IL-1β in human keratinocytes, contributing to wound healing (37). It is also possible that in addition to being a chemotactic factor for neutrophils, GRP can prime cells to migrate toward other stimuli—a property that would be consistent with the growth factor function described for this neuropeptide (6) and that needs to be further investigated. Other studies showed that peritoneal macrophages (19) or total lymph node cells (20) incubated with GRP migrated more efficiently toward casein and formyl-Met-Leu-Phe (fMLP), respectively.
We report here that GRPR mediates chemotaxis, acting independently of Gαi proteins (Fig. 4A). Gαi-coupled GPCR are known as “classical” chemokine receptors, inducing cell migration through release of the βγ subunits from the G protein, which can activate selective signaling pathways, such as PI3K, PKC, and MAPKs, culminating in cell chemotaxis (29). However, chemokine receptors can couple to other classes of G proteins, including Gαq proteins (38). An alternative signaling pathway has recently been demonstrated to be dependent on Gαq-containing G proteins (39). Gαq-deficient neutrophils were not able to migrate toward fMLP in vitro, although still able to migrate toward IL-8, indicating that IL-8 is a ligand of the classical pathway and fMLP is a ligand of the alternative pathway (39). The same study also evidenced that this was a pathway used by neutrophils, but not other cells, such as bone marrow-derived dendritic cells (BMDCs) and T cells. We believe our results are in agreement with these findings. Also, the activation of Gαq-coupled receptors leads to dissociation of Gαq and βγ subunits. The signature activity of Gαq protein is to activate one or more PLC-β isoforms, which catalyze the production of the intracellular messengers, promoting intracellular calcium release (29). PLC-β2 and PLC-β3 were shown to be crucial for neutrophil activation induced by chemokines (40). Our results indicate that GRP activates the PLC-β2 isoform, because the U-73122 inhibitor is specific for this isoenzyme (41). We propose that GRP is a ligand of the alternative signaling pathway.
We showed that activation of PI3K is essential for GRP-induced neutrophil migration. We extended our observations by showing that besides the requirement of PLC-β2 and PI3K, GRP also activates MAPK, such as ERK and p38, to induce neutrophil chemotaxis. These are pathways known to be downstream of GPCR, and different neutrophil chemoattractants, namely fMLP, IL-8, LTB4, and C5a, and ERK and p38 to stimulate neutrophil migration (31, 42), after triggering either classical or alternative chemokine receptors.
Several neuropeptide receptors have seven-spanned structure and are associated with G proteins (2). It is possible that the phenomenon characterized in this study is actually a general mechanism mediating the interaction of endogenous neuromodulators and immune cells. Some neuropeptides produced by immune cells have potent antiinflammatory actions and were found to have important roles in the maintenance of tolerance in different immunological disorders (2). However, here we demonstrate the proinflammatory activity of the neuropeptide GRP. Our results also provide a unique candidate mechanism for the antiinflammatory effect of the GRPR antagonist RC-3095 in models of arthritis (9) and sepsis (10). We can also extend our model to explain the proinflammatory effect of GRP, because a GRP blocking agent effectively prevented asthma exacerbation in animal models (12). The effective role of GRP in inflammation has not been characterized. The present study demonstrates that injection of GRP can induce an inflammatory response.
An unexpected finding was the suggestion of an interaction between GRPR and CXCR2, implicating some level of hierarchy or cooperation between the two receptors. Neutrophils prioritize between different chemoattractant cues, favoring p38-controlled migration toward bacterial end targets over PI3K-controlled migration toward endogenous chemoattractants (42). Priorization is regulated by the phosphatase and tensin homolog (PTEN) (43), which inhibits chemoattractant-derived PI3K signaling, allowing unidirectional migration to an end-target chemoattractant. It is possible that GRP and IL-8 are viewed by neutrophils as equivalent chemoattractants, and the blockage of either receptor in the presence of the other's agonist inhibits the PI3K pathway, inhibiting migration toward the other molecule. Alternatively, GRPR could be interacting with CXCR2 directly. A recent study demonstrated that GRPR can heteromerize with an isoform of the μ-opioid receptor MOR1D (44). This interaction explains the usual itch that accompanies morphine treatments, which could now be inhibited by preventing the interaction between the two GPCRs. In that study, heteromerization was followed by internalization of the complexed receptors. It is possible that in our system, GRPR and CXCR2 form a complex and this complex is internalized upon treatment with each of the antagonists. These two intriguing possibilities deserve further investigation and will probably reveal new properties of chemoattractant receptors, including GRPR.
Finally, the migration of neutrophils to arthritic joints has been linked to disease severity (45). The presence of GRP in inflammatory scenarios, independently of infection, could directly recruit neutrophils via a nonclassical pathway, as well as induce macrophages to produce other neutrophil-recruiting molecules (Fig. S1), and both are blocked by RC-3095. The fact that GRP is a neuropeptide poses the intriguing possibility that neutrophil recruitment, and consequent disease exacerbation, could ensue in situations of psychological stress, when GRP can be released by neurons (46). Disease relapse in response to stress is a common aspect of autoimmune diseases (47), as well as asthma (48). Future studies on the in vivo mechanisms mediating cell migration induced by GRP will test this hypothesis.
Methods
Further information can be found in SI Methods.
Mice and Patients.
C57BL/6 females, 6–8 wk old, were purchased from FEPPS and housed in accordance with National Institutes of Health Guidelines. Synovial fluid from RA patients were provided by the Rheumatology Service of São Lucas Hospital (PUCRS). All patients signed an informed consent term approved under Ethics protocol no. 858/05-CEP. This study was approved by the Ethics committee at PUCRS under protocol no. CEUA 10/00186.
Human Neutrophil Isolation and Chemotaxis Assay.
Neutrophils were isolated from heparin-treated venous blood of healthy human volunteers using Histopaque-1077 (Sigma-Aldrich). Neutrophil chemotaxis was assayed in a Transwell system (Corning). Stimuli was added to the bottom wells, and neutrophils were added to top wells and incubated for 2 h at 37 °C. For checkerboard analysis, neutrophils were placed in the upper chamber alone, or with GRP (1 nM), and exposed in the lower chamber with medium alone or GRP (1 nM). Involvement of PLC-β, PI3K, MAPK (p38 and ERK), GαI, and CXCR2 was tested by pretreating neutrophils with selective inhibitors at 37 °C for 1 h.
In Vivo Neutrophil Migration Assay.
For neutrophil migration, GRP (0.06–6 μg per cavity) and/or RC-3095 (6 μg per cavity) or endotoxin-free saline were injected i.p., in 200 μL. After 4 h, peritoneal cavities were rinsed with 3 mL PBS. Differential counting of leukocytes was carried out on Diff-Quick-stained slides.
Murine Macrophage and Human Monocyte Purification.
Peritoneal macrophages were obtained by peritoneal and adherent cells stimulated with GRP (0.001–10 nM) for 2 or 24 h. Human monocytes were isolated from peripheral blood mononuclear cells obtained by density gradient centrifugation with Histopaque-1077, and adherent cells were stimulated with GRP for 2 or 24 h. Supernatants were collected and frozen until cytokine/chemokine analysis.
Flow Cytometry.
Peritoneal cells were stained with anti-CD14 PE, CD11c PE-Cy7, CD4 FITC, and B220 PE-Cy5.5. Murine macrophages and human monocytes were stimulated with GRP for 24 h and culture supernatants analyzed for cytokines by Th1/Th2/Th17 Cytometric Bead Array (BD Biosciences), according to the manufacturer's instructions.
p-Akt, p-p38, and p-ERK 1/2 in human neutrophils were measured by flow cytometry using BD Phosflow protocol for human whole blood sample. Data were obtained using FACSCantoII (Beckton Dickinson) and BD FACSDiva software, and analyzed using Flowjo v 7.5.
ELISA.
GRP (Phoenix Pharmaceuticals), TNF, and chemokines were determined according to the supplier's instructions.
Statistical Analysis.
Data are presented as mean ± SE. Results were analyzed using GraphPad Prism 5. Statistical differences among the experimental groups were evaluated by ANOVA with Tukey or Bonferroni correction or with Student's t test. The level of significance was set at P < 0.05.
Supplementary Material
Acknowledgments
The authors thank Dr. José C. Alves-Filho for the PTx gift, Drs. Maria M. Campos and Rafael Zanin for Phosflow reagents, and Dr. Marcelo Bozza for the U-73122 gift. The authors acknowledge grant support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grant 474697/2008-8. R.S.C. is the recipient of a CNPq fellowship and B.N.P., a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Programa Nacional de Pós-Doutorado (CAPES-PNPD) fellowship. R.R. and G.S. are supported by CNPq Grant 303703/2009-1 (to R.R.), the National Institute for Translational Medicine, and the South American Office for Anticancer Drug Development.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110996109/-/DCSupplemental.
References
- 1.Burbach JP. Neuropeptides from concept to online database www.neuropeptides.nl. Eur J Pharmacol. 2010;626(1):27–48. doi: 10.1016/j.ejphar.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 3.Hernanz A, Tato E, De la Fuente M, de Miguel E, Arnalich F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha production by whole blood cells from healthy young and old subjects. J Neuroimmunol. 1996;71:25–30. doi: 10.1016/s0165-5728(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 4.McDonald TJ, et al. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979;90:227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- 5.Xiao D, Wang J, Hampton LL, Weber HC. The human gastrin-releasing peptide receptor gene structure, its tissue expression and promoter. Gene. 2001;264:95–103. doi: 10.1016/s0378-1119(00)00596-5. [DOI] [PubMed] [Google Scholar]
- 6.Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18:1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- 7.Roesler R, Henriques JA, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS Neurol Disord Drug Targets. 2006;5:197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- 8.Schwartsmann G, et al. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest New Drugs. 2006;24:403–412. doi: 10.1007/s10637-006-6886-5. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira PG, et al. Effects of an antagonist of the bombesin/gastrin-releasing peptide receptor on complete Freund's adjuvant-induced arthritis in rats. Peptides. 2008;29:1726–1731. doi: 10.1016/j.peptides.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Dal-Pizzol F, et al. Gastrin-releasing peptide receptor antagonist effects on an animal model of sepsis. Am J Respir Crit Care Med. 2006;173:84–90. doi: 10.1164/rccm.200507-1118OC. [DOI] [PubMed] [Google Scholar]
- 11.Petronilho F, Roesler R, Schwartsmann G, Dal Pizzol F. Gastrin-releasing peptide receptor as a molecular target for inflammatory diseases. Inflamm Allergy Drug Targets. 2007;6:197–200. doi: 10.2174/187152807783334319. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S, Potts EN, Cuttitta F, Foster WM, Sunday ME. Gastrin-releasing peptide blockade as a broad-spectrum anti-inflammatory therapy for asthma. Proc Natl Acad Sci USA. 2011;108:2100–2105. doi: 10.1073/pnas.1014792108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 15.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petronilho F, et al. Protective effect of gastrin-releasing peptide receptor antagonist in carrageenan-induced pleural inflammation in rats. Inflamm Res. 2010;59:783–789. doi: 10.1007/s00011-010-0190-8. [DOI] [PubMed] [Google Scholar]
- 17.Vieira SM, et al. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009;158:779–789. doi: 10.1111/j.1476-5381.2009.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seadi Pereira PJ, et al. Nociceptive and inflammatory responses induced by formalin in the orofacial region of rats: Effect of anti-TNFalpha strategies. Int Immunopharmacol. 2009;9:80–85. doi: 10.1016/j.intimp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.De la Fuente M, Del Rio M, Ferrandez MD, Hernanz A. Modulation of phagocytic function in murine peritoneal macrophages by bombesin, gastrin-releasing peptide and neuromedin C. Immunology. 1991;73:205–211. [PMC free article] [PubMed] [Google Scholar]
- 20.Medina S, Del Rio M, Hernanz A, Guaza C, De la Fuente M. Nitric oxide released by accessory cells mediates the gastrin-releasing peptide effect on murine lymphocyte chemotaxis. Regul Pept. 2005;131:46–53. doi: 10.1016/j.regpep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Noiri E, et al. An in vivo approach showing the chemotactic activity of leukotriene B(4) in acute renal ischemic-reperfusion injury. Proc Natl Acad Sci USA. 2000;97:823–828. doi: 10.1073/pnas.97.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porto BN, et al. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem. 2007;282:24430–24436. doi: 10.1074/jbc.M703570200. [DOI] [PubMed] [Google Scholar]
- 23.Chen GY, Nuñez G. Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramaniam M, et al. Bombesin-like peptides and mast cell responses: Relevance to bronchopulmonary dysplasia? Am J Respir Crit Care Med. 2003;168:601–611. doi: 10.1164/rccm.200212-1434OC. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield PJ, Suchard SJ. Thrombospondin promotes both chemotaxis and haptotaxis in neutrophil-like HL-60 cells. J Immunol. 1993;150:1959–1970. [PubMed] [Google Scholar]
- 26.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 27.Hellmich MR, Battey JF, Northup JK. Selective reconstitution of gastrin-releasing peptide receptor with G alpha q. Proc Natl Acad Sci USA. 1997;94:751–756. doi: 10.1073/pnas.94.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 29.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 30.Niggli V. Signaling to migration in neutrophils: Importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619–1638. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 31.Coxon PY, et al. MAPK-activated protein kinase-2 participates in p38 MAPK-dependent and ERK-dependent functions in human neutrophils. Cell Signal. 2003;15:993–1001. doi: 10.1016/s0898-6568(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 32.Hong CW, et al. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 2010;184:4401–4413. doi: 10.4049/jimmunol.0902814. [DOI] [PubMed] [Google Scholar]
- 33.Grimsholm O, Rantapää-Dahlqvist S, Forsgren S. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R416–R426. doi: 10.1186/ar1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Origuchi T, et al. Reduction in serum levels of substance P in patients with rheumatoid arthritis by etanercept, a tumor necrosis factor inhibitor. Mod Rheumatol. 2010;21:244–250. doi: 10.1007/s10165-010-0384-5. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira C, et al. Effect of plant neutrophil elastase inhibitor on leucocyte migration, adhesion and cytokine release in inflammatory conditions. Br J Pharmacol. 2010;161:899–910. doi: 10.1111/j.1476-5381.2010.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baroni A, et al. Bombesin: A possible role in wound repair. Peptides. 2008;29:1157–1166. doi: 10.1016/j.peptides.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Gerard C, Gerard NP. The pro-inflammatory seven-transmembrane segment receptors of the leukocyte. Curr Opin Immunol. 1994;6:140–145. doi: 10.1016/0952-7915(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 39.Shi G, et al. Identification of an alternative Galphaq-dependent chemokine receptor signal transduction pathway in dendritic cells and granulocytes. J Exp Med. 2007;204:2705–2718. doi: 10.1084/jem.20071267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 41.Hou C, et al. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- 42.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heit B, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 44.Liu XY, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos LL, et al. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum. 2011;63:960–970. doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez V, Taché Y. Bombesin and the brain-gut axis. Peptides. 2000;21:1617–1625. doi: 10.1016/s0196-9781(00)00293-x. [DOI] [PubMed] [Google Scholar]
- 47.Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9:A271–A276. doi: 10.1016/j.autrev.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allergy Asthma Immunol. 2003;90(6) Suppl 3:34–40. doi: 10.1016/s1081-1206(10)61658-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.