Abstract
BACKGROUND AND PURPOSE
Strategies designed to enhance cerebral cAMP have been proposed as symptomatic treatments to counteract cognitive deficits. However, pharmacological therapies aimed at reducing PDE4, the main class of cAMP catabolizing enzymes in the brain, produce severe emetic side effects. We have recently synthesized a 3-cyclopentyloxy-4-methoxybenzaldehyde derivative, structurally related to rolipram, and endowed with selective PDE4D inhibitory activity. The aim of the present study was to investigate the effect of the new drug, namely GEBR-7b, on memory performance, nausea, hippocampal cAMP and amyloid-β (Aβ) levels.
EXPERIMENTAL APPROACH
To measure memory performance, we performed object recognition tests on rats and mice treated with GEBR-7b or rolipram. The emetic potential of the drug, again compared with rolipram, was evaluated in rats using the taste reactivity test and in mice using the xylazine/ketamine anaesthesia test. Extracellular hippocampal cAMP was evaluated by intracerebral microdialysis in freely moving rats. Levels of soluble Aβ peptides were measured in hippocampal tissues and cultured N2a cells by elisa.
KEY RESULTS
GEBR-7b increased hippocampal cAMP, did not influence Aβ levels and improved spatial, as well as object memory performance in the object recognition tests. The effect of GEBR-7b on memory was 3 to 10 times more potent than that of rolipram, and its effective doses had no effect on surrogate measures of emesis in rodents.
CONCLUSION AND IMPLICATIONS
Our results demonstrate that GEBR-7b enhances memory functions at doses that do not cause emesis-like behaviour in rodents, thus offering a promising pharmacological perspective for the treatment of memory impairment.
Keywords: PDE4 inhibitor, memory, phosphodiesterase, cyclic AMP, rolipram, emesis
Introduction
cAMP is a central component of signalling pathways regulating a wide range of biological functions, including the cognitive process of memory consolidation. Cerebral cAMP activates PKA and the subsequent downstream target cAMP response element-binding (CREB) protein, generating a signalling cascade that is important in the mediation of synaptic plasticity and memory (Frey et al., 1993; Bailey et al., 1996). A large body of evidence has shown that long-term potentiation (LTP), the neurochemical substrate of learning and memory processes, requires the functioning of the cAMP/PKA/CREB pathway, and its genetic or pharmacological manipulation can affect cognitive functions (Barad et al., 1998; Bourtchouladze et al., 2003; Li et al., 2011).
Cerebral levels of cAMP critically depend on the activity of PDE4, a family of cAMP-catabolizing enzymes. PDE4s are encoded by four independent genes (Pde4a to Pde4d), which can give rise to multiple spliced variants differently expressed in the CNS (Cherry and Davis, 1999; Perez-Torres et al., 2000; Houslay and Adams, 2003). Their differential distribution in brain regions has suggested distinct roles of individual PDE4 subtypes in CNS functions. Among the different classes of PDE4 presently known, PDE4D is the subtype that is likely to influence memory processes. This is consistent with its predominant expression in hippocampal CA1 region (Perez-Torres et al., 2000) and with the important role played in hippocampal LTP (Rutten et al., 2008). Moreover, different lines of evidence, showing that pharmacologically induced overexpression of PDE4D impairs memory (Giorgi et al., 2004), and that its knocking-out exerts cognitive-enhancing effects and stimulates hippocampal neurogenesis (Li et al., 2011), have strongly supported the concept that selective PDE4D inhibition may be beneficial for the treatment of memory loss characterising several CNS disorders (e.g. Alzeimer's disease; AD). Indeed, it has been consistently demonstrated that PDE4 inhibitors, such as rolipram, can facilitate LTP and improve memory functions in a variety of cognitive tasks, both under physiological and pathological conditions (Reneerkens et al., 2009). In particular, rolipram has been found effective in reducing cognitive impairments in different transgenic AD models (Gong et al., 2004; Comery et al., 2005; Costa et al., 2007), as well as in rats injected with amyloid-β (Aβ) peptides (Cheng et al., 2010). PDE4 inhibitors have been widely investigated also for their antidepressant effects in both animals and humans (Zhang, 2009), and, within this context, PDE4D has been proposed as the PDE subtype responsible for the antidepressant activity of rolipram (Zhang et al., 2002). However, despite its potential clinical relevance, therapeutic use of rolipram is limited because of the acute emesis occurring after its administration (Hebenstreit et al., 1989).
Our studies, aimed at developing novel PDE4 inhibitors, led us to synthesize a series of 3-cyclopentyloxy-4-methoxybenzaldehyde derivatives structurally related to rolipram and endowed with selective PDE4D inhibitory activity (Bruno et al., 2009).
Here, to ascertain the potential clinical relevance of the new PDE4D inhibitors, we evaluated the effect of the most active and selective one, namely GEBR-7b, on the memory performance of rodents in the object recognition test (Ennaceur and Delacour, 1988) and object location test, a spatial variant of the former (Rutten et al., 2009). Given the emetic properties of PDE4 inhibitors, we also analysed the ability of GEBR-7b to stimulate conditioned gaping in rats and to shorten α2-adrenoceptor-mediated xylazine/ketamine anaesthesia in mice, two well-established surrogate measures of emesis in rodents, which are non-vomiting species (Grill and Norgren, 1978; Robichaud et al., 2002; Parker and Limebeer, 2006). In addition, using intracerebral microdialysis, we assessed the effect of the drug on the hippocampal levels of cAMP in freely moving rats. Finally, as it has been suggested that PDE4D inhibitors might be useful in AD (Burgin et al., 2010; Li et al., 2011), and that Aβ could differentially modulate hippocampal-dependent memory processes by positively or negatively influencing LTP (Haass and Selkoe, 2007; Puzzo et al., 2008), we also evaluated the effect of GEBR-7b on Aβ levels both in vivo and in vitro.
Methods
Object location and recognition tests
Twenty-four 3 month-old male Wistar rats and twenty-four 7 week-old male C57Bl6/6NCrl mice (Charles River, Lille, France) were used. In order to test the animals in their active phase, they were kept under a reversed 12/12 h light/dark cycle. All animals were housed individually in standard Makrolon home cages and had free access to food and water. GEBR-7b was dissolved in dimethylsulphoxide (DMSO) and kept at 4°C; this stock solution was used for further dilutions in 0.5% methylcellulose. All injection solutions consisted of 0.5% methylcellulose with fixed DMSO percentages (0.01% for rats and 0.005% for mice). Based on previous findings, administration of PDE4 inhibitors was performed 3 h after the first trial, as this has an optimum effect on object memory performance (Rutten et al., 2007, 2009). Injection volumes were 1 mL·kg−1 for rats (i.p.) and 8 mL·kg−1 for mice (s.c.).
Object recognition testing (ORT) was performed as described elsewhere (Ennaceur and Delacour, 1988). The details on the apparatus and objects are identical to those described in previous studies with rats (Rutten et al., 2009) and mice (Sik et al., 2003). A testing session consisted of two trials. The duration of each trial was 3 min for rats and 4 min for mice. Only mice were placed in an empty cage for 4 min before the first trial (T1), in order to increase arousal at testing. During T1, the apparatus contained two identical objects. After the first exploration period, the animal was put back in its home cage. Exploration was defined directing the nose to the object at a distance of not more than 2 cm and/or touching the object with the nose. After a 24 h delay interval, the animal was put in the apparatus for the second trial (T2). In T2 of the object location test (OLT), two identical objects as in T1 were used; one object was placed in the previously used position, whereas the other was placed in a novel position. The novel position of the object could be either a fixed distance towards the front or a fixed distance towards the back of the arena for both objects (for details see Rutten et al., 2009; Vanmierlo et al., 2009). All mice and rats were tested in the OLT. In the ORT, one of the two familiar objects in T2 was replaced by a new object. Only rats were tested in the ORT. All objects and locations were used in a balanced manner to exclude possible object and/or location preferences. To avoid olfactory cues, the objects were thoroughly cleaned with 70% ethanol after each trial. Before the drugs were tested, animals were handled daily and adapted to the procedures in 2 days; that is, they were allowed to explore the apparatus twice for 3 min each day. All four objects used in this study were presented in these two subsequent days. Afterwards, animals were adapted to the treatment by one i.p. or s.c. saline injection for rats and mice, respectively. The testing order of conditions was determined randomly. A within-design was used, and all animals were treated once with each condition. T1 was always on Monday and Thursday in order to have a sufficient wash-out period between compound sessions. The experimenter was unaware of the testing protocol. All experimental procedures were approved by the ethical committee of the Maastricht University and met the governmental guidelines on animal experiments.
The time spent exploring the two identical objects in T1 was indicated as ‘a1’ and ‘a2’, respectively. The time spent exploring the familiar and the new object or location in T2 was indicated as ‘a’ and ‘b’ respectively. The following variables were calculated: e1 = a1 + a2; e2 = a + b and d2 = (b − a) / e2. e1 and e2 are measures of the total exploration time of both objects during T1 and T2 respectively. d2 is a relative measure of discrimination, corrected for exploration activity (e2). Animals with an e2 lower than 7.5 s were excluded. Comparisons within a treatment condition were based on a special paired t-test (i.e. the one-sample t-test), which evaluates the significance of d2 for a given session by comparing its mean value with zero. Statistical significance between treatment conditions was calculated using a one-way anova followed by Bonferroni's post hoc comparison test.
Taste reactivity test
Twenty-three male (2 to 3 month-old) Sprague–Dawley rats were housed individually at an ambient temperature of 21°C, with a reversed 12/12 h light/dark cycle and free access to food and water. Surgical procedure was as reported in detail elsewhere (Rock et al., 2009). Briefly, all rats were implanted with intra-oral cannulae inserted at the base of the neck, directed around the ear and brought out behind the first molar inside the mouth. For 3 days following surgery, rats were monitored and had their cannulae flushed daily with chlorhexidine. The taste reactivity test (Grill and Norgren, 1978) room was dark with two 50 Watt white lights on either side of the conditioning chamber, which was made of clear Plexiglass sides (22.5 × 26 × 20 cm) with a clear lid. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat. A video camera (Sony DCR-HC48, Edcom Multimedia, Waterloo, ON, Canada) with fire-wire feed to a computer was used to record the orofacial and somatic reactions of the rat during conditioning and testing. Three days after recovering from surgery, the rats received an adaptation trial in which they were placed in the chamber with their cannula attached to an infusion pump (KDS 100, KD Scientific Inc, Holliston, MA, USA) for fluid delivery. Water was infused into their intra-oral cannulae for 5 min at the rate of 1 mL·min−1, and rats were then returned to their home cage. The animals received the first conditioning trial 24 h after the adaptation trial. There were a total of three conditioning trials, with 72–96 h between each trial. On each conditioning trial, rats were individually placed in the chamber and were intra-orally infused with 17% sucrose solution for 5 min at a rate of 1 mL·min−1, while the orofacial responses were video-recorded. Immediately following the sucrose infusion, the rats were injected with: vehicle (n = 5), 0.003 mg·kg−1 GEBR-7b (n = 6), 0.3 mg·kg−1 GEBR-7b (n = 5) and 0.3 mg·kg−1 rolipram (n = 7). All drugs were dissolved in DMSO, diluted in 0.5% methylcellulose and administered i.p. The control group received the same concentration of DMSO administered to the highest GEBR-7b dose group (maximally 0.005% DMSO). The fourth trial proceeded just as a conditioning trial, except that no injection was delivered afterwards. The videotapes were later scored by an observer blind to the experimental conditions using ‘The Observer’ (Noldus Information Technology, Sterling, VA, USA) for the behaviours of gaping (large openings of the mouth and jaw, with lower incisors exposed). Statistical significance between treatment conditions was calculated using a one-way anova followed by Bonferroni's post hoc comparison test. All procedures adhered to the guidelines of the Canadian Council of Animal Care and were approved by the Animal Care Committee of the University of Guelph.
Xylazine/ketamine induced α2-adrenoceptor-mediated anaesthesia test
Seven-week-old male C57Bl6/6NCrl mice were anaesthetized with an i.p. injection consisting of 60 mg·kg−1 ketamine (Eurovet Animal Health, Bladel, the Netherlands) and 10 mg·kg−1 xylazine (CEVA Santé Animale, Naaldwijk, the Netherlands). Fifteen minutes after induction of anaesthesia, seven to eight animals per group were treated with rolipram (0.03 and 0.1 mg·kg−1 s.c.), GEBR-7b (0.003, 0.03, 0.1 and 0.3 mg·kg−1 s.c.) or vehicle (0.07% DMSO in 0.5% methylcellulose) and placed in dorsal position. The time delay to the recovery of the righting reflex was used as an endpoint to measure the duration of anaesthesia. Animals recovering from anaesthesia before the treatment injection were excluded from analysis. Statistical significance between treatment conditions was calculated using a one-way anova followed by Bonferroni's post hoc comparison test.
Microdialysis surgery and experiments
Detailed description of the surgical procedure is reported elsewhere (Fedele et al., 1997). Briefly, male Sprague–Dawley rats (2 to 3 month-old; Charles River, Italy) were anaesthetized with chloral hydrate (400 mg·kg−1), placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) and implanted with microdialysis probes, which were transversely positioned (horizontal microdialysis) into the dorsal hippocampi according to the following coordinates: AP =+3.8, H =+6.5 from the interaural line (Paxinos and Watson, 1986). A piece of dialysis fibre made of a co-polymer of acrylonitrile sodium methallyl sulphonate (AN69HF Hospal S.p.A., Italy; 0.3 mm outer diameter, 40 kDa mol. wt. cut-off) was covered with epoxy glue to confine dialysis to the area of interest (8 mm glue-free length). The skull was exposed, and two holes were drilled on the lateral surfaces; the dialysis probe, held straight by a tungsten wire inside, was inserted transversely into the brain so that the glue-free zone was exactly located into the target area. The tungsten wire was withdrawn, and stainless steel cannulae (22 gauge diameter, 15-mm long) were glued to the ends of the fibre, bent up and fixed vertically to the skull with dental cement and modified Eppendorf tips. Rats were allowed to recover in their home cages for 24 h. The day after surgery, rats were placed into observation cages, and the probes were infused at a flow rate of 5 µL·min−1 (CMA/100 microinjection pump, CMA Microdialysis, Solna, Sweden) with modified Ringer's medium containing (in mM): NaCl 145, KCl 3, MgCl2 1, CaCl2 1.26, buffered at pH 7.4 with 2 mM phosphate buffer. Following a stabilization period of 1 h, consecutive samples were collected every 20 min; GEBR-7b or rolipram were locally administered at different concentrations through the dialysis probe (retrodialysis) after three control samples had been collected to estimate cAMP basal values and were present in the infusion solution for the time reported in the figures. GEBR-7b and rolipram were dissolved in DMSO at the concentration of 3 or 10 mM and diluted down to the desired concentration in modified Ringer's medium. 1% DMSO was also present in the infusion solution for 120 min before drug administration. Under our experimental conditions, the in vitro recovery for cAMP amounted to 12.35 ± 0.11% (mean ± SD, n = 3 different probes). At the end of the experiment, rats were killed, and the correct position of the probe was verified by optical examination of the fibre tract. Animals presenting haemorrhagic lesions were rejected. cAMP content in the dialysates was assayed with a commercially available radioimmunoassay kit (IZOTOP, Institute of Isotopes Co., Budapest, Hungary) using the acetylation protocol (standard curve range 2–128 fmol/100 µL) and a modified bound/unbound separation protocol (Wang et al., 2004b). The data presented (mean ± SEM) are expressed as percentages of the mean basal value (defined as 100%), which was determined by averaging the content of the first three samples collected before drug treatments. Differences were analysed by two-way anova for repeated measures followed by Bonferroni's post hoc comparison test. Experimental procedures were carried out in accordance with the European legislation on the use and care of laboratory animals (CEE 86/609).
Cell culture and treatments
The cells used in this study (mouse Neuro-2a, N2a, stably expressing wild type human APP 695) were obtained from Peter Davies (Albert Einstein College of Medicine, Bronx, NY) and grown in 50% Dulbecco's modified Eagle medium, 50% OptiMEM, with 0.1 mM non-essential amino acids, 200 µg·mL−1 geneticin and 5% fetal bovine serum. Treatments started 16–18 h after plating the cells onto six-well plates (300 000 cells·per well). Serial dilutions of 10 mM GEBR-7b stock in DMSO were used in order to add 1 µL·mL−1 DMSO to each well. Twenty-four hours after the treatment, conditioned media were collected, centrifuged at 1000×g for 10 min to remove cell debris and stored at −80°C for later assessments.
Preparation of hippocampal homogenates
Male Sprague–Dawley rats (2 to 3 month-old) were injected with: 0.003 mg·kg−1 (n = 6) or 1 mg·kg−1 GEBR-7b (n = 6). Controls for each dosage (n = 6) received the corresponding volume of DMSO. All drugs were prepared in 0.5% methylcellulose vehicle and i.p. administered (injection volume 1 mL·kg−1). Three rats per group were killed by decapitation either 6 or 18 h after the treatment. The hippocampi were immediately extracted, flash frozen in liquid nitrogen and stored at −80°C. On assay days, frozen tissue samples were weighed and homogenized in ice-cold buffer containing 1% Complete protease inhibitor (Roche S.p.A., Monza, Italy), 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 5 mM EDTA, at a ratio of 100 mg tissue/0.5 mL buffer. The homogenates were centrifuged at 28 000×g for 30 min. (4°C), and the supernatant was stored at −80°C until analysed.
Aβelisa
Conditioned media and hippocampal homogenates were analysed for soluble Aβx-40 and Aβx-42 by sandwich elisa (Wako Chemicals GmbH, Neuss, Germany) according to manufacturer protocols. Statistical analysis was performed with a two-tailed Student's t-test.
Results
Spatial and object recognition memory
To determine whether the new rolipram-related compound GEBR-7b (Figure 1) affects the cognitive process of memory in rats and mice, we used the object location and the object recognition tests, which are based on the natural tendency of rodents to explore unfamiliar or displaced objects (Ennaceur and Delacour, 1988; Rutten et al., 2009).
Figure 1.
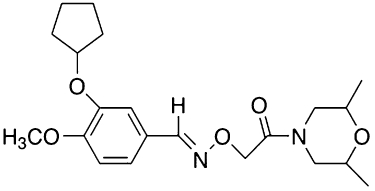
Chemical structure of GEBR-7b.
Total exploration times of the treatments with GEBR-7b or rolipram were not different from the vehicle treatments in all spatial and object memory tests (data not shown). In the spatial location test for rats, one-sample t-tests revealed that d2 values after 0.01 and 0.03 mg·kg−1 rolipram treatments significantly differed from zero, indicating that the rats discriminated between locations at these doses. d2 values after rolipram treatment were found to be different from each other [F(3,89)= 3.34, P < 0.05]. Post hoc analysis revealed that the d2 value after 0.03 mg·kg−1 rolipram treatment was significantly higher than that of the vehicle condition, indicating a full spatial memory enhancement (Figure 2). One-sample t-tests revealed that the d2 values after all GEBR-7b treatments differed from zero. The d2 values differed between GEBR-7b treatments [F(4,114)= 3.12, P < 0.05]. Post hoc analysis revealed that the d2 value after 0.003 mg·kg−1 GEBR-7b treatment was higher than after vehicle treatment (Figure 2). Thus, GEBR-7b greatly improved spatial memory at 0.003 mg·kg−1.
Figure 2.
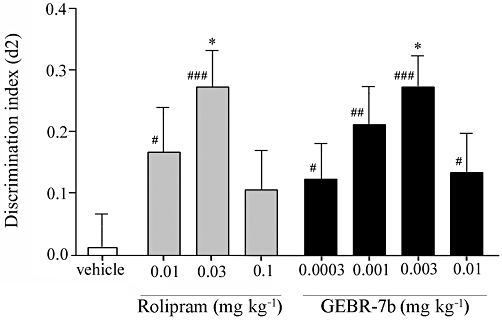
Influence of rolipram and GEBR-7b on the discrimination index (d2) in the object location test in rats (means ± SEM). A difference from the vehicle condition is depicted with asterisks (Bonferroni's comparison t-test: *P < 0.05). A difference from zero is depicted with hashes (one-sample t-tests: #P < 0.05, ##P < 0.01, ###P < 0.001). n = 22–24.
Spatial memory of mice was assessed to verify the effects of GEBR-7b on memory in a different animal species. In the object location task, paired t-tests revealed that the d2 value after 0.03 mg·kg−1 rolipram treatment differed from zero. There were differences in d2 values between rolipram treatments [F(3,64)= 7.698, P < 0.001]. Post hoc analysis indicated that the d2 value after 0.03 mg·kg−1 rolipram treatment was higher than after the vehicle administration (Figure 3). One-sample t-tests revealed that d2 values after 0.001 and 0.003 mg·kg−1 GEBR-7b treatments differed from zero. Differences between d2 values after GEBR-7b treatment were present [F(4,79)= 5.898, P < 0.001]. Post hoc analysis indicated that the d2 value after 0.003 mg·kg−1 GEBR-7b injection was higher than after vehicle treatment (Figure 3).
Figure 3.
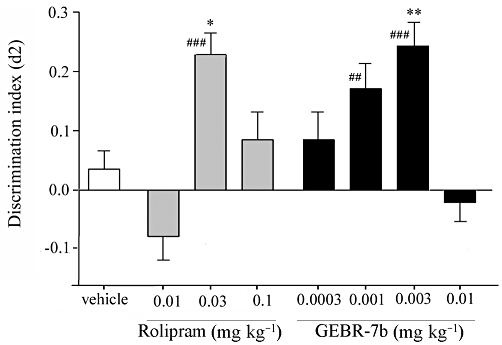
Influence of rolipram and GEBR-7b on the discrimination index (d2) in the object location in mice (means ± SEM). A difference from the vehicle condition is depicted with asterisks (Bonferroni's comparison t-test: *P < 0.05, **P < 0.01). A difference from zero is depicted with hashes (one-sample t-tests: #P < 0.05, ##P < 0.01, ###P < 0.001). n = 14–16, except for the vehicle group, where n = 23.
In the object recognition test in rats, one-sample t-tests indicated that the d2 value after 0.01 mg·kg−1 rolipram treatment differed from zero. Differences in d2 values between rolipram treatments were found [F(3,85)= 6.123, P < 0.001]. Post hoc analysis revealed that the d2 value after 0.01 mg·kg−1 rolipram treatment was higher than after vehicle treatment, thus showing complete object memory enhancement (Figure 4). One-sample t-tests showed that d2 values after 0.003 mg·kg−1 GEBR-7b treatment differed from zero. There were differences between the d2 values of the GEBR-7b treatment conditions [F(3,78)= 4.724, P < 0.01]. Post hoc analysis revealed that the d2 value in the 0.003 mg·kg−1 GEBR-7b condition was higher than in the vehicle condition (Figure 4).
Figure 4.
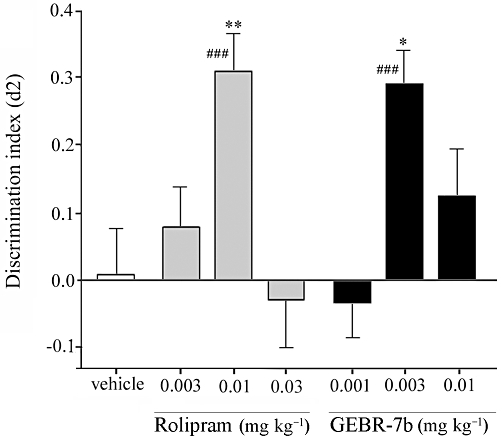
Influence of rolipram and GEBR-7b on the discrimination index (d2) in the object recognition test in rats (means ± SEM). A difference from the vehicle condition is depicted with asterisks (Bonferroni's comparison t-test: *P < 0.05, **P < 0.01). A difference from zero is depicted with hashes (one-sample t-tests: ###P < 0.001). n = 21–23.
Conditioned gaping in the taste reactivity test
Because nausea and vomiting are common side effects of PDE4 inhibitors (Zeller et al., 1984; Robichaud et al., 2001; Hirose et al., 2007), we used the taste reactivity test (Grill and Norgren, 1978) to evaluate the emetic potential of GEBR-7b in rats. Although rodents are unable to vomit, they display characteristic gaping reactions when exposed to flavoured solutions previously paired with nausea-inducing agents; therefore, conditioned gaping in rats has been introduced as a selective index of nausea (Parker and Limebeer, 2006; Rock et al., 2009). Figure 5 shows the mean number of gapes elicited during the 5 min intra-oral infusion of 17% sucrose solution paired with GEBR-7b or rolipram across trials. Statistical data analysis revealed a significant group effect only on trial 4 [F(3,19)= 8.6; P < 0.01]. Bonferroni's post hoc comparison test indicated that 0.3 mg·kg−1 rolipram stimulated a conditioned gaping response, following three conditioning trials, significantly greater than that elicited by vehicle, 0.003 or 0.3 mg·kg−1 GEBR-7b (P < 0.01), with no other significant effects.
Figure 5.
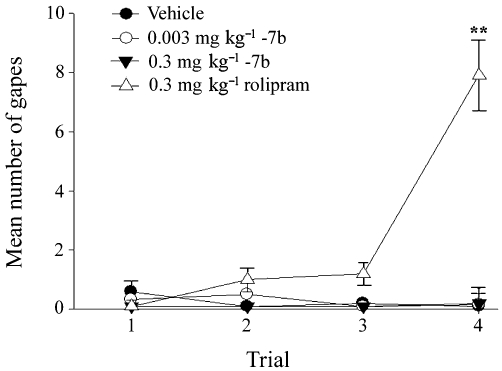
Influence of rolipram and GEBR-7b on conditioned gaping in rats. Graph presents mean number (±SEM) of gapes elicited by the 5 min intra-oral infusion of 17% sucrose solution paired with the various conditioning drugs across three conditioning trials (trials 1–3) and the test trial (trial 4). Vehicle (n = 5), 0.003 mg·kg−1 GEBR-7b (n = 6), 0.3 mg·kg−1 GEBR-7b (n = 5), 0.3 mg·kg−1 rolipram (n = 7). Bonferroni's post hoc comparison test: **P < 0.01 versus the other conditions tested in trial 4.
Recovery after xylazine/ketamine induced α2-adrenoceptor-mediated anaesthesia
PDE4 inhibitors mimic the pharmacological actions of α2-adrenoceptor antagonists. This has been postulated as the mechanism by which PDE4D inhibitors induce emesis. Since α2-adrenoceptor antagonists are also known to reverse xylazine/ketamine-induced anaesthesia, we used this latter effect as a surrogate measure of emesis in mice (Robichaud et al., 2002). As expected, rolipram treatments significantly affected the duration of xylazine/ketamine-induced anaesthesia [F(2,18)= 14.53, P < 0.001] (Figure 6). Post hoc analysis indicated that the duration of xylazine/ketamine-induced anaesthesia was reduced, compared with vehicle, upon injection of the dose of rolipram effective in the memory tasks (0.03 mg·kg−1). GEBR-7b appeared to affect the recovery time [F(4,31)= 2.84, P < 0.05], but post hoc analysis revealed that this was not the case after the injection of 0.003, 0.03 and 0.1 mg·kg−1. Only a dose (0.3 mg·kg−1) 100 times higher than that effective in the memory tasks reduced the recovery time (Figure 6).
Figure 6.
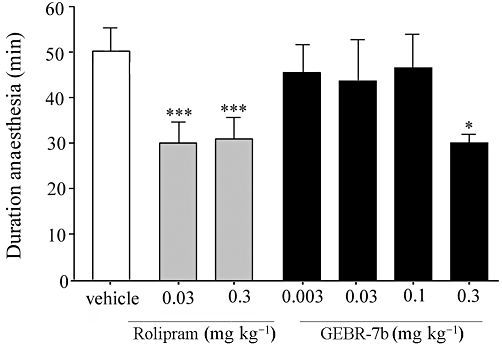
Influence of rolipram and GEBR-7b on the recovery time after xylazine/ketamine induced α2-adrenoceptor-mediated anaesthesia in mice (means ± SEM). A difference from the vehicle condition is depicted with asterisks (Bonferroni's comparison t-test: *P < 0.05, ***P < 0.001; n = 5–7 per group).
Hippocampal levels of cAMP in freely moving rats
To evaluate in vivo the effects of PDE4D inhibition on the hippocampal levels of cAMP, we performed intracerebral microdialysis experiments in freely-moving rats. Local administration of GEBR-7b by retrodialysis caused a concentration-dependent (30–100 µM) increase in extracellular cAMP. anova analysis revealed that both time [F(8,64)= 4.19, P < 0.001] and concentrations [F(1,64)= 6.77, P < 0.05] had significant effects, and that there was a significant interaction [F(8,64)= 2.13, P < 0.05]. Post hoc test indicated that, although at 30 µM the drug slightly augmented extracellular cAMP (max. increase 20%), the effect did not reach statistical relevance; on the other hand, when GEBR-7b was infused at the concentration of 100 µM, a significant and constant 40–50% increase was observed (Figure 7). Infusion of rolipram (30–100 µM) also resulted in the increase of extracellular cAMP, but the effect was more pronounced. anova analysis revealed that both time [F(8,48)= 43.33, P < 0.0001] and concentrations [F(1,48)= 287.37, P < 0.0001] had significant effects, and that there was a significant interaction [F(8,48)= 13.17, P < 0.0001]. Post hoc test indicated that both rolipram concentrations elicited significant effects. In fact, a 50–60% elevation of extracellular cAMP was already evident at the concentration of 30 µM, whereas infusion of 100 µM rolipram resulted in a 150–200% increase over basal levels (Figure 7).
Figure 7.
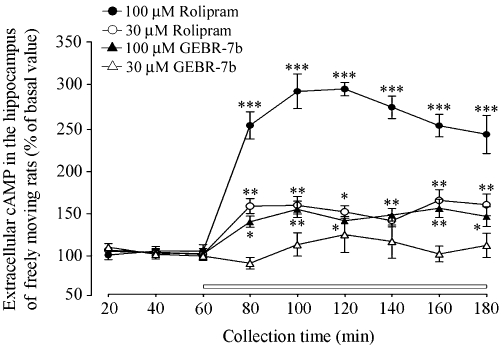
Effects of rolipram and GEBR-7b on cAMP extracellular levels in hippocampi of freely moving rats. Rolipram or GEBR-7b was infused through the dialysis probe after three consecutive basal samples had been collected and for the time indicated by the horizontal bar. The data represent means ± SEM of four (rolipram) or five (GEBR-7b) independent experiments (Bonferroni's comparison test: *P < 0.05, **P < 0.01 and ***P < 0.001 vs. basal level).
Aβ levels in hippocampal tissues and neuronal cultured cells
Since it has been reported that picomolar concentrations of Aβ peptides may enhance synaptic plasticity (Puzzo et al., 2008), whereas higher concentrations inhibit hippocampal LTP and impair memory (Haass and Selkoe, 2007; Puzzo et al., 2008), it was of interest to investigate whether the selective PDE4D inhibitor could influence the hippocampal content of Aβ peptides. To this purpose, rats were injected i.p. with vehicle, 0.003 or 0.1 mg·kg−1 GEBR-7b and killed 6 or 18 h after the treatment. Hippocampal levels of soluble Aβ40 and Aβ42 were measured by specific sandwich elisa and normalized to total protein concentration in each sample. The results shown in Figure 8A indicate that, at least under the tested conditions, neither the low nor the high dose of GEBR-7b significantly affected the hippocampal content of Aβ peptides. To further investigate this issue, we tested the ability of GEBR-7b to modulate the secretion of Aβ40 and Aβ42 in a mouse neuronal cell line (N2a) stably transfected to express the human amyloid precursor protein (APP). Assuming a 150 mL of body fluids for a rat weighing 250 g, a complete absorption of the drug, no metabolic degradation or excretion and a homogeneous distribution throughout the body, the i.p. injection of 0.003 and 0.1 mg·kg−1 GEBR-7b would lead to a concentration of ∼0.013 and 0.43 µM, respectively (Fedele et al., 1998). Thus, we exposed N2a cells to 0.01, 0.1 and 1 µM GEBR-7b for 24 h and performed Aβ-specific sandwich elisa on the conditioned culture media. Consistent with the results obtained in hippocampal tissues, statistical analysis revealed that, compared with the controls, GEBR-7b did not change the amount of Aβ40 and Aβ42 secreted by the cells in the extracellular medium (Figure 8B).
Figure 8.
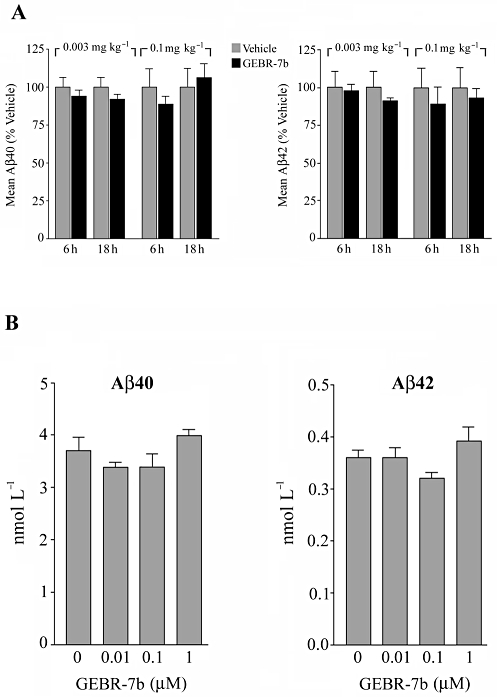
Effect of GEBR-7b on Aβ levels in vivo and in vitro. (A) Effect of GEBR-7b on the hippocampal content of Aβ40 and Aβ42. Rats were injected i.p. with the indicated doses of drug or vehicle and killed either 6 or 18 h after the treatment. Hippocampal homogenates were subjected to soluble Aβ40 and Aβ42 specific elisa. Data, calculated as pmol·g−1 protein, are expressed as a percentage (means ± SD; n = 3) of the corresponding control group. (B) Effect of GEBR-7b on the secretion of Aβ40 and Aβ42 in neuronal cultured cells. Conditioned media from N2a cells treated for 24 h with GEBR-7b or DMSO were subjected to specific Aβelisa. Results are expressed as means ± SD for four independent experiments. Statistically significant differences were not detected either in (A) or in (B) (P > 0.05 vs. the corresponding control group).
Discussion and conclusions
The data presented here show that the new selective PDE4D inhibitor GEBR-7b is able to improve late-phase consolidation processes of spatial and object recognition memory and to increase the hippocampal levels of cAMP in vivo. cAMP is known to play a key role in regulating the complex mechanisms of cognitive processes. In fact, it has been reported that hippocampal LTP can be blocked by PKA inhibitors, whereas pharmacological activation of adenylyl cyclase (AC) results in the induction of long-lasting synaptic potentiation that occludes tetanus-induced LTP (Huang and Kandel, 1994; Nguyen et al., 1994). Accordingly, AC1 and AC8 double knockout mice do not express LTP and exhibit significant memory deficits (Wong et al., 1999), whilst overexpression of AC1 results in enhanced LTP and recognition memory (Wang et al., 2004a). A large body of evidence has also convincingly demonstrated that blocking cAMP degradation improves memory functions in unimpaired rodents (i.e. attenuates natural forgetting) and ameliorates cognitive alterations in a variety of pharmacologically induced and transgenic-based memory deficit models, including murine models of AD (Zhang et al., 2000; Bourtchouladze et al., 2003; Reneerkens et al., 2009).
GEBR-7b shows selective PDE4D inhibitory properties, it being almost inactive towards PDE4A4, PDE4B2 and PDE4C2 isoforms (Bruno et al., 2009), and is effective in two different cognitive tasks and in two different animal species. Thus, our behavioural results provide further evidence that PDE4D plays a critical role in the mediation of memory processes (Zhang et al., 2005; Burgin et al., 2010; Li et al., 2011).
Collectively, the behavioural effects shown by GEBR-7b indicate that the optimum dose to improve spatial and object memory consolidation processes is approximately 0.003 mg·kg−1. While lower doses are not effective enough, higher doses show a decrease in efficacy, most likely due to the accumulation of side effects. In view of the potential use of PDE4D inhibitors in AD (Burgin et al., 2010; Li et al., 2011) and based on the evidence that Aβ inhibits hippocampal LTP at high concentrations and improves synaptic plasticity and memory when applied in lower amounts (Haass and Selkoe, 2007; Puzzo et al., 2008), it was of interest to investigate whether GEBR-7b could affect the production of Aβ peptides. In this context, however, our in vivo and in vitro results suggest that the lack of memory-enhancing effects, seen at high doses of GEBR-7b, as well as the efficacy of the lower doses, may not be related to the production of Aβ. This is also consistent with the observation that chronic rolipram treatment did not affect cerebral levels of Aβ40 and Aβ42 in a mouse model of AD (Gong et al., 2004).
Despite the beneficial effects of PDE4 inhibitors, such as rolipram, on memory functions, one major problem for their clinical application concerns the emetic side effect that occurs after acute administration. Within this context, it is worth noting that the effects on memory consolidation of GEBR-7b in rats and mice were observed at doses between 3 and 10 times lower than that of rolipram. These findings, together with the notion that GEBR-7b exerts a more isoform-specific activity, compared with rolipram, led us to hypothesize a reduced emetic effect. In fact, when GEBR-7b was used in the taste reactivity test, we did not observe emesis-like behaviour in rats, even at doses up to 100 times higher than those effective in the object recognition and location tasks. Using the xylazine/ketamine test, this lack of emetic effect was also observed in mice at doses 30 times higher than those improving behavioural performances in the object location test; GEBR-7b induced an emetic-like response only when administered at a 100 times higher dose. However, PDE4D seems the predominant PDE4 subtype in emesis-associated brain regions such as the area postrema and the nucleus of the solitary tract (Cherry and Davis, 1999; Lamontagne et al., 2001; Mori et al., 2010). In addition, both PDE4 and PDE4D selective inhibitors (i.e. rolipram, D157140, D159382) have been shown to cause emetic-like behaviour in the xylazine/ketamine test in mice already at doses that are effective in the object recognition test (Burgin et al., 2010). It is therefore likely that the greatly reduced emetic effect shown by GEBR-7b in the present study is due to the relative low doses required to improve memory.
As PDE4s are involved in the degradation of the second messenger cAMP, which seems to play a critical role in cognitive processes, we also evaluated whether GEBR-7b could influence, in vivo, the hippocampal levels of cAMP. Indeed, results obtained by intracerebral microdialysis show that GEBR-7b causes a significant 40% increase of extracellular cAMP in the hippocampus of freely moving rats. It could be argued that the concentrations of GEBR-7b used in the microdialysis experiments (30 and 100 µM) are not comparable with the doses found effective in the cognitive tests (0.001–0.003 mg·kg−1). However, since microdialysis measures cAMP in the extracellular milieu and not directly in the intracellular compartment, where it is supposed to influence physiological functions (e.g. cognition), it is difficult to establish a direct correlation between the doses effective in behavioural tests and microdialysis experiments. Cyclic nucleotides (both cAMP and cGMP) are extruded from cells into the extracellular milieu by low-affinity, active transport systems (MRP4/5) and represent only a small fraction of the intracellular counterpart (Wielinga et al., 2003). Therefore, it is likely that in order to get an intracellular cAMP elevation able to be significantly reflected in the extracellular space, we need a much stronger PDE4 inhibition than that necessary to improve cognition in the behavioural tasks. Consistent with this, rolipram elicited a significant response even at 30 µM and elevated extracellular cAMP by approx. 160% at 100 µM. Thus, although not directly correlating with the behavioural data, our microdialysis results represent the ‘proof of concept’ that GEBR-7b does increase in vivo the hippocampal levels of cAMP. Moreover, the evidence that GEBR-7b has a markedly lower influence on extracellular cAMP, compared to rolipram is consistent with its selectivity towards PDE4D.
In conclusion, our data show that GEBR-7b increases hippocampal cAMP and is able to enhance memory functions at doses that do not cause emesis-like behaviour in rodents. These findings provide further support to the idea that targeting PDE4D with selective inhibitors may represent an innovative cognitive-enhancing pharmacological strategy.
Acknowledgments
This research was supported by grants from Genoa University, Italian Minister of University Research (PRIN 2008N9N9KL_002 and 2007E8CRF3_003), Internationale Stichting Alzheimer Onderzoek (ISAO 09513) and Natural Sciences and Engineering Research Council of Canada.
Glossary
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer's disease
- CREB
cAMP response element-binding protein
- DMSO
dimethylsulphoxide
- LTP
long-term potentiation
- N2a
mouse Neuro-2a cells
- OLT
object location test
- ORT
object recognition test
Conflict of interest
The authors declare that they have no competing financial interests.
References
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno O, Romussi A, Spallarossa A, Brullo C, Schenone S, Bondavalli F, et al. New selective phosphodiesterase 4D inhibitors differently acting on long, short, and supershort isoforms. J Med Chem. 2009;52:6546–6557. doi: 10.1021/jm900977c. [DOI] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Cheng YF, Wang C, Lin HB, Li YF, Huang Y, Xu JP, et al. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25-35 or Abeta1-40 peptide in rats. Psychopharmacology (Berl) 2010;212:181–191. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, et al. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28:831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Raiteri M. In vivo microdialysis study of GABA(A) and GABA(B) receptors modulating the glutamate receptor/NO/cyclic GMP pathway in the rat hippocampus. Neuropharmacology. 1997;36:1405–1415. doi: 10.1016/s0028-3908(97)00113-5. [DOI] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Ansaldo MA, Raiteri M. Nicotine administration stimulates the in vivo N-methyl-D-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol. 1998;125:1042–1048. doi: 10.1038/sj.bjp.0702130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154:99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, et al. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–160. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- Hirose R, Manabe H, Nonaka H, Yanagawa K, Akuta K, Sato S, et al. Correlation between emetic effect of phosphodiesterase 4 inhibitors and their occupation of the high-affinity rolipram binding site in Suncus murinus brain. Eur J Pharmacol. 2007;573:93–99. doi: 10.1016/j.ejphar.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Lamontagne S, Meadows E, Luk P, Normandin D, Muise E, Boulet L, et al. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O'Donnell JM, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Pérez-Torres S, De Caro R, Porzionato A, Macchi V, Beleta J, et al. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat. 2010;40:36–42. doi: 10.1016/j.jchemneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL. Conditioned gaping in rats: a selective measure of nausea. Auton Neurosci. 2006;129:36–41. doi: 10.1016/j.autneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Benzaquen J, Limebeer CL, Parker LA. Potential of the rat model of conditioned gaping to detect nausea produced by rolipram, a phosphodiesterase-4 (PDE4) inhibitor. Pharmacol Biochem Behav. 2009;91:537–541. doi: 10.1016/j.pbb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol. 2007;558:107–112. doi: 10.1016/j.ejphar.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, et al. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28:625–632. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- Rutten K, Van Donkelaar EL, Ferrington L, Blokland A, Bollen E, Steinbusch HW, et al. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology. 2009;34:1914–1925. doi: 10.1038/npp.2009.24. [DOI] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Vanmierlo T, Rutten K, Dederen J, Bloks VW, van Vark-van der Zee LC, Kuipers F, et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol Aging. 2009;32:1262–1272. doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004a;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Wang M, Urenjak J, Fedele E, Obrenovitch TP. Effects of phosphodiesterase inhibition on cortical spreading depression and associated changes in extracellular cyclic GMP. Biochem Pharmacol. 2004b;67:1619–1627. doi: 10.1016/j.bcp.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–190. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SC, Frith SA, Suvarna N, Conti N, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Suvarna NU, Deng C, Crissman AM, Hopper AT, et al. Effects of the novel PDE4 inhibitors MEM1018 and MEM1091 on memory in the radial-arm maze and inhibitory avoidance tests in rats. Psychopharmacology (Berl) 2005;179:613–619. doi: 10.1007/s00213-004-2085-2. [DOI] [PubMed] [Google Scholar]


