Abstract
Liver disease and liver cancer associated with childhood-acquired chronic hepatitis B are leading causes of death among adults in China. Despite expanded newborn hepatitis B vaccination programs, approximately 20% of children under age 5 years and 40% of children aged 5-19 years remain unprotected from hepatitis B. Although immunizing them will be beneficial, no studies have examined the cost-effectiveness of hepatitis B catch-up vaccination in an endemic country like China. We examined the cost-effectiveness of a hypothetical nationwide free hepatitis B catch-up vaccination program in China for unvaccinated children and adolescents aged 1 to 19 years. We used a Markov model for disease progression and infections. Cost variables were based on data published by the Chinese Ministry of Health, peer-reviewed Chinese and English publications, and the GAVI Alliance. We measured costs (2008 U.S. dollars and Chinese renminbi), quality-adjusted life years (QALYs), and incremental cost-effectiveness from a societal perspective. Our results show that hepatitis B catch-up vaccination for children and adolescents in China is cost-saving across a range of parameters, even for adolescents aged 15-19 years old. We estimate that if all 150 million susceptible children under 19 were vaccinated, more than 8 million infections and 65,000 deaths due to hepatitis B would be prevented.
Conclusion
The adoption of a nationwide free catch-up hepatitis B vaccination program for unvaccinated children and adolescents in China, in addition to ongoing efforts to improve birth dose and newborn vaccination coverage, will be cost-saving and can generate significant population-wide health benefits. The success of such a program in China could serve as a model for other endemic countries.
Keywords: Hepatitis B, Vaccination, Cost-Effectiveness Analysis
INTRODUCTION
China has the greatest burden of chronic hepatitis B virus (HBV) infection and liver cancer in the world, with an estimated 95 million people (7.3% of China’s population) living with chronic HBV infection and more than 40% of the worldwide deaths associated with HBV infection. Based on 2006 World Health Organization (WHO) estimates, liver cancer and liver disease caused by acute and chronic HBV infection account for 263,000-300,000 deaths in China each year—a burden that exceeds the combined mortality from tuberculosis (200,000), HIV/AIDS (38,000), and malaria (37) (1-7).
In 1992, the Chinese government recommended HBV vaccination of all newborns; however, because of high vaccine prices and vaccination fees, many newborns were not vaccinated (1). In 2002, the Chinese Ministry of Health partnered with the Global Alliance for Vaccines and Immunization (now known as the GAVI Alliance) to provide nationwide free HBV vaccines for all newborns and officially added infant HBV vaccination to China’s National Immunization Program. These recent initiatives successfully increased newborn immunization nationwide, even in the poorer western provinces that traditionally had low vaccination rates (1). The estimated three-dose vaccination coverage among infants increased from 70.7% among those born in 1997 to 89.8% among those born in 2003 (1).
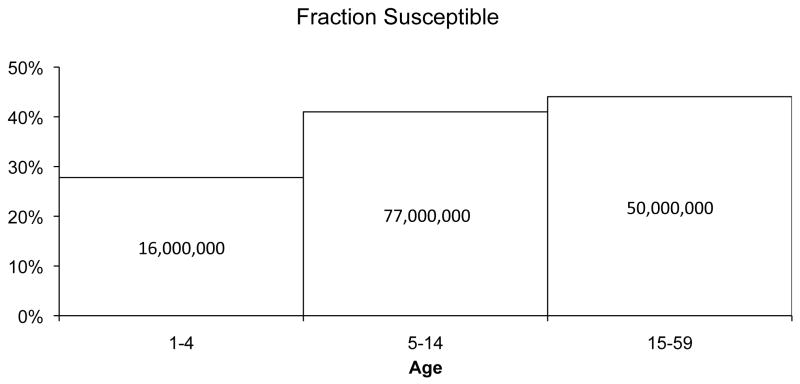
However, a substantial number of children and adolescents, particularly those born before 2002 in poorer rural provinces, are not protected from the virus. Data released by the Chinese Ministry of Health from its 2006 national seroepidemiologic study of more than 81,000 people showed that more than 20% of 1- to 4-year-olds and 40% of 5- to 19-year-olds remain unprotected from HBV (Figure 1) (8). This equates to almost 150 million children and adolescents across all of China (9). Although China encourages catch-up vaccination for unvaccinated children based on the observation of prior recommendations for newborn vaccination, compliance was poor until the vaccinations were made totally free (1).
Figure 1.
Fraction and number of children and adolescents in China susceptible to HBV infection, by age group. The height of the bars represents the fraction of the population in that age group susceptible to HBV infection. Numbers inside the bars represent total numbers of people susceptible. Based on data from the 2006 China National Hepatitis Seroprevalence Survey (8).
* HBV = Hepatitis B virus.
Traditionally, catch-up vaccination for unprotected children has not been included in the global strategy to control hepatitis B, primarily based on the assumption that without vaccination at birth, most children or adolescents will have been infected (10, 11). Very few studies have evaluated HBV vaccination in areas of high endemicity, and none have examined the cost-effectiveness of catch-up vaccination (10). However, in light of the reduced vaccine costs and large numbers of unprotected individuals, it is important to reconsider the infant-only HBV vaccination paradigm and examine new programs to expand vaccination to children and adolescents in China who did not receive the HBV vaccine at birth.
We assessed the cost-effectiveness of a nationwide catch-up HBV vaccination program for children aged 1 to 19 years in China, taking a societal perspective and measuring all costs and health benefits (measured in quality-adjusted life years (QALYs)) of the vaccination programs.
METHODS
Overview
We considered a strategy of catch-up vaccination for children and compared it to the status quo with current levels of vaccination coverage. The catch-up vaccination strategy provides hepatitis B vaccination immediately for children and adolescents who missed newborn vaccination. For children not participating in the intervention (and for all individuals under the status quo), we assumed that 0.5% would voluntarily seek the vaccine themselves each year in the future.
We developed a model to project costs and health impacts of both acute and chronic HBV infection. We developed a probability tree to evaluate the outcomes of acute HBV infection (new infection that is either asymptomatic or symptomatic) and a Markov model of disease progression to evaluate the health and economic impacts accruing from treated and untreated chronic HBV infection. The Markov model of disease progression captures the long-term effects of chronic HBV infection, which represent a much larger part of the overall disease burden of HBV than the effects of acute infection (12). We modeled costs and health states for a hypothetical cohort of 10,000 children and adolescents age 1-19 throughout their lifetimes. We calculated costs and benefits for the overall cohort, and we examined individual cohorts of age 1-4 years, 5-10 years, 11-14 years, and 15-19 years. We measured costs and health outcomes in discrete time increments of one year, similar to other analyses (13, 14).
According to WHO criteria (15), an intervention can be considered “very cost-effective” if the cost per disability-adjusted life year (DALY) gained is less than the annual per capita GDP, which in China is approximately $2,500 (16-18) [17,000 RMB]. An intervention is considered to be good value for money if it costs less than three times the per capita GDP. Because QALYs are roughly similar to DALYs (19), we use a threshold of 17,000 RMB [$2,500] per QALY gained for the intervention being very cost-effective.
Data and Sources
To gather data needed for our model (Table 1, Appendix Tables 1 and 2), we performed a Medline search to review previous cost-effectiveness analyses of HBV vaccination in both China and the United States (1, 20-22) and, more importantly, published Chinese government reports, national seroepidemiologic surveys, and peer reviewed articles published in Chinese. Values for model parameters were drawn from the existing literature when available. In most instances, Chinese data was used, although parameters that were less specific to China (e.g., those relating to the natural history of disease) were taken from international literature (Appendix Table 2). When no data were available, informed expert judgment was used (personal communication with China CDC, China Foundation for Hepatitis Prevention and Control’s national experts and the WHO’s technical experts in China and the Western Pacific Region). In sensitivity analysis we considered low and high values for all variables, using ranges to reflect values in the literature, where available.
Table 1.
Parameter values: base value and range considered in sensitivity analysis*
| Parameter | Base Value | Range
|
Source | |
|---|---|---|---|---|
| Minimum Value | Maximum Value | |||
| Population | ||||
| Discount rate | 3% | 0% | 5% | (35) |
| Probabilities | ||||
| Starting Population | ||||
| Compliance with vaccine intervention | 70% | 10% | 95% | Assumed |
| Percent chronically infected who are aware of infection† | 50% | 33% | 95% | (45, 46) |
| Percent aware who receive medical management | 50% | 0% | 100% | Assumed |
| Chronic infections that have elevated ALT | 2.0% | 0.5% | 5.0% | Assumed |
| Already immune (given no chronic infection) | 50% | 33% | 75% | Assumed |
| Aware of immunity (previous vaccination) | 75% | 50% | 100% | Assumed |
| Protected by three doses of HBV vaccine | 95% | 92% | 99% | (23-25) |
| Annual voluntary vaccination | 0.5% | 0.0% | 2.0% | Assumed |
| Acute Infection | ||||
| Annual acute HBV infection incidence | 100/100,000 | 20/100,000 | 500/100,000 | (6, 8, 20, 21, 29, 30) |
| Asymptomatic acute infections | 90% | 40% | 99% | (31, 32, 37, 47, 48) |
| Symptomatic acute infections that require hospitalization | 12% | 2% | 50% | (31, 32, 37) |
| Hospitalized cases that are fulminant | 4% | 1% | 51% | (31, 32, 37) |
| Fulminant cases that result in death | 70% | 10% | 100% | (31, 32, 37) |
| Disease Progression parameters (annual probabilities) | ||||
| Normal ALT to elevated ALT‡ | 0.15% | 0.10% | 0.20% | (49) |
| Normal ALT to HCC | 0.34% | 0.15% | 0.50% | (51) |
| Durable virologic response while on treatment | 15% | 5% | 30% | (13, 38, 52-54) |
| Chronic HBV infection with elevated ALT to compensated cirrhosis | 3.8% | 0.5% | 12.3% | (13, 36) |
| Chronic HBV infection with elevated ALT to HCC | 1.5% | 0.5% | 9.5% | (13, 36) |
| Durable response relapse to elevated ALT | 7% | 2% | 15% | (13, 55, 56) |
| Durable response to HCC | 0.34% | 0.15% | 0.50% | (51) |
| Compensated cirrhosis to decompensated cirrhosis | 7% | 3% | 10% | (13, 36) |
| Mortality from compensated cirrhosis | 4.8% | 2.0% | 13.1% | (13, 36) |
| Mortality from decompensated cirrhosis | 17.3% | 5.8% | 22.1% | (13, 36) |
| Cirrhosis to HCC | 3.3% | 1.0% | 11.3% | (13, 36, 57) |
| Cirrhosis to cirrhosis with ascites | 68% | 50% | 90% | (13) |
| Cirrhosis to cirrhosis with variceal bleeding | 14.6% | 7.0% | 30.0% | (13) |
| Cirrhosis to cirrhosis with encephalopathy | 10% | 5% | 30% | (13) |
| Receiving a liver transplant while in decompensated cirrhosis | 1.5 % | 0% | 40% | (13, 36, 58-60) |
| Mortality from HCC | 40.0% | 32.0% | 47.3% | (13, 36, 61, 62) |
| Mortality from HCC while on medical management (due to early detection) | 20% | 10% | 40% | (62) |
| Receiving a liver transplant while in HCC | 0.1% | 0% | 40% | (13, 36, 60, 63-67) |
| Mortality first year after liver transplantation | 15% | 7.5% | 30% | (13, 36) |
| Mortality second and subsequent years after liver transplantation | 1.5% | 0.75% | 3.0% | (13, 36) |
| Costs ($) | ||||
| Vaccine costs | ||||
| Vaccine (per dose) | 0.34 | 0.26 | 0.43 | (68-70)§ |
| Vaccine administration (per dose) | 0.60 | 0.35 | 7.66 | (69)§ |
| Liver transplantation cost | 30000 | 7500 | 120000 | (36, 71) |
| Annual treatment costs | ||||
| Fraction of patients on drug therapy while in durable response** | 50% | 0% | 100% | Assumed |
| Drugs | 2000 | 500 | 8000 | (36, 72) |
| Regular health monitoring | 250 | 62.50 | 1000 | (22, 71) |
| Cirrhosis | 2000 | 500 | 8000 | (22, 36, 71) |
| Ascites | 2500 | 625 | 10000 | (22, 36, 71) |
| Encephalopathy | 2500 | 625 | 10000 | (22, 36, 71) |
| Variceal hemorrhage | 2500 | 625 | 10000 | (22, 36, 71) |
| HCC | 5000 | 1250 | 20000 | (22, 36, 71) |
| Transplantation followup | 3000 | 750 | 12000 | (36) |
| Annual normal healthcare costs | (17) | |||
| Quality Multipliers | ||||
| Acute HBV infection | 0.94 | 0.90 | 1.00 | (37) |
| Chronic HBV infection, normal ALT | 1.00 | 0.95 | 1.00 | (37, 38) |
| Chronic HBV infection, elevated ALT | 0.99 | 0.90 | 1.00 | (36-38) |
| Durable response | 1.00 | 0.90 | 1.00 | (13) |
| Compensated cirrhosis | 0.80 | 0.70 | 0.93 | (13, 36, 38) |
| Decompensated cirrhosis | 0.60 | 0.50 | 0.70 | (13, 36, 38) |
| HCC | 0.73 | 0.50 | 0.80 | (13, 36, 38) |
| Liver transplant | 0.86 | 0.70 | 0.90 | (13, 36, 38) |
ALT = alanine aminotransferase; anti-HBs = hepatitis B surface antibody; HBsAg = hepatitis B surface antigen; HBsAg+ = hepatitis B surface antigen positive; HBV = hepatitis B virus; HCC = hepatocellular carcinoma.
Percentage of all chronically infected individuals who are aware of their infection
This value was estimated from (49), and then calibrated to yield approximately 25% mortality from untreated liver disease (23, 50).
Personal communication by Dr. So with physicians in China
Some therapies are discontinued if the therapy suppresses the virus. This parameter is the fraction of patients who continue on drug therapy after the therapy has suppressed the virus into a “durable response”.
Some parameters varied by age (Appendix Table 1). The initial prevalence of chronic HBV infection and of HBV immunity are different for different age groups. In addition, background mortality and the chance that an acute infection develops into a chronic infection vary by age. For example, an acute infection in a 1-year old has an 86% chance of becoming chronic, whereas an acute infection in a 5-year-old has only a 30% chance of becoming chronic. As the model steps forward in time, these parameters update according to the aging of the children. We assumed that all other parameters relating to disease progression did not vary by age.
Compliance and Vaccine Effectiveness
We assumed that parents who knew of their children’s infection or prior hepatitis B vaccination history would not have their children vaccinated. Of those unaware of their vaccination history or infection, we assumed that 70% complied with the intervention, regardless of the age of the children.
We assumed that the vaccination would consist of a three-dose series, and that the protection rate from three doses of the vaccine was 95% (23-25). If the children complied with the intervention, we assumed that they all received all three doses, based on known high completion rates in the target population (1, 26). We assumed that costs associated with program implementation would be similar to that of a recently completed two-year, province-wide, school-based, pilot catch-up vaccination program that one of the authors (S So) helped to initiate in partnership with the China CDC and Ministry of Health, the China Foundation for Hepatitis Prevention and Control, and the Qinghai provincial CDC and government (27). The program provided free HBV catch-up vaccination and education for more than 500,000 children in kindergarten and primary school in the entire remote province of Qinghai between 2006-08 (26). Vaccination at each school clinic was administered through the existing provincial CDC immunization offices that have branches to serve the immunization needs of the various districts in the province. We did not include program setup costs in the model as such costs could be highly variable depending on the size and scope of a vaccination program. Setup cost for the recent Qinghai province catch-up vaccination program was approximately $0.40 [2.7 RMB] per child; we included this setup cost in sensitivity analysis.
Epidemiologic Parameters
Epidemiological studies of HBV in China over the past 15 years have shown a reduction in the national estimates of HBV prevalence and incidence due to the expanded newborn immunization efforts. Estimates of the prevalence of chronic HBV infection in the general Chinese population dropped from 9.75% in a national survey conducted between 1992-95, to 7.18% in a more recent 2006 national survey (8, 28). The prevalence of chronic HBV infection among young children is lower because children have had higher vaccination coverage than older members of the population. The latest national serological survey of more than 80,000 people conducted in 2006 in China estimated chronic HBV infection prevalence to be 0.96% among the almost 60 million children aged 1-4 years and 2.42% among the almost 190 million children aged 5-14 years (8). Using prevalence data from this serological survey (8), we estimated base case incidence of acute HBV infection to be 100/100,000 per year per unvaccinated person, regardless of age (calculations in Appendix). Because estimates of incidence vary, we varied this parameter between 20/100,000 and 500/100,000 in sensitivity analysis (6, 8, 20, 21, 29, 30). We discuss incidence in terms of acute HBV infection per unvaccinated person since the policy affects unvaccinated persons and we wished to provide a comparable risk of infection across age groups. This incidence number is higher than published incidence numbers that are based on entire populations (for the latter, the denominator is larger and thus the reported incidence statistic is lower).
Acute HBV infections were modeled in a similar manner to those of other published cost-effectiveness studies (31, 32). The likelihood of an acute HBV infection becoming chronic varies by age. We used a data from a meta-analysis (33) to relate this likelihood with age; estimated values are shown in Appendix Table 1. There are differences of opinion about this likelihood, especially at older ages (34), so we performed sensitivity analysis on this probability.
Health Outcomes and Costs
We measured health and economic outcomes for all children and adolescents in the cohort over their lifetimes. Since newborn vaccination coverage is rapidly increasing in China (1), we excluded the effects of infection on future children born to girls currently in the cohort. We took a societal perspective and included all costs and savings, regardless of source or beneficiary, and we discounted all costs and health outcomes to the present at 3% (35).
We included the costs of vaccination, treatment, and all other healthcare costs for HBV-infected and uninfected individuals in the cohort over their lifetimes. We did not count time from work or school due to disease since that morbidity is accounted for in quality-of-life adjustments (35). We gathered treatment cost data from several recent studies of the cost of chronic HBV infection in China (22, 36) and then converted them to US dollars. Since costs vary significantly for different regions of China, we conducted sensitivity analysis on all cost parameters.
We measured several health outcomes including deaths, HBV infections averted, and QALYs gained. Quality adjustments for health states were taken from published literature (13, 36-38).
RESULTS
Base Case
Base case results for the entire cohort of children and adolescents ages 1-19, and for individual age cohorts, are shown in Table 2. In all cases, catch-up vaccination is cost-saving: it increases QALYs and saves costs compared to the status quo.
Table 2.
Results – Costs and health outcomes, by age groups*
| Ages 1-4 | Ages 5-10 | Ages 11-14 | Ages 15-19 | Ages 1-19 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Status Quo | Catch-up Vaccination | Status Quo | Catch-up Vaccination | Status Quo | Catch-up Vaccination | Status Quo | Catch-up Vaccination | Status Quo | Catch-up Vaccination | |
|
| ||||||||||
| Costs ($1000’s) | ||||||||||
| Program cost | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 |
| Health care costs | 35,706 | 35,650 | 35,777 | 35,738 | 34,815 | 34,786 | 36,849 | 36,828 | 35,819 | 35,786 |
| Total costs | 35,706 | 35,662 | 35,777 | 35,751 | 34,815 | 34,799 | 36,849 | 36,840 | 35,819 | 35,798 |
| Outcomes | ||||||||||
| Vaccinations | 0 | 4367 | 0 | 4354 | 0 | 4354 | 0 | 4300 | 0 | 4339 |
| Acute infections | 339 | 113 | 314 | 105 | 292 | 98 | 252 | 85 | 292 | 98 |
| New chronic infections | 23.1 | 7.7 | 16.3 | 5.4 | 12.0 | 4.0 | 8.6 | 2.9 | 13.7 | 4.6 |
| HBV-related deaths† | 29 | 26 | 61 | 59 | 56 | 55 | 178 | 177 | 91 | 90 |
| QALYs experienced | 297,126 | 297,147 | 291,048 | 291,061 | 283,391 | 283,400 | 273,524 | 273,530 | 284,228 | 284,239 |
| Incremental cost-effectiveness ratio | Cost-Saving | Cost-Saving | Cost-Saving | Cost-Saving | Cost-Saving | |||||
Each case assumes a cohort of 10,000 children. HBV = hepatitis B virus; QALY = quality-adjusted life year. All costs and QALYs were discounted to the present at 3%. Costs are expressed in 2008 US dollars.
Deaths are HBV-related deaths for the entire cohort, including deaths of persons who were chronically infected prior to the intervention.
Sensitivity Analysis
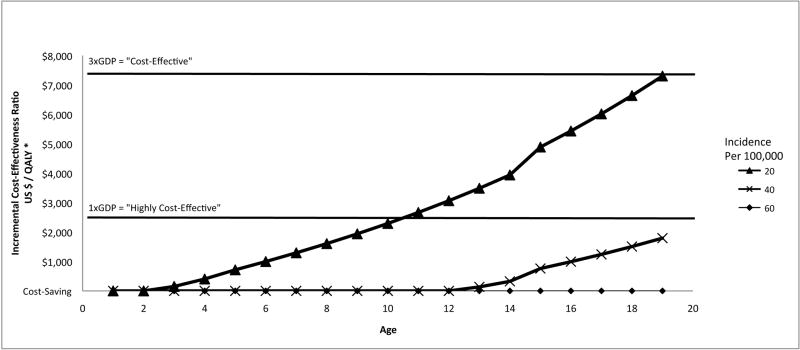
We performed two-way sensitivity analysis on the incidence of acute HBV infection and the age of the cohort (Figure 2). In areas where annual incidence of acute HBV infection is 100/100,000 or higher among susceptible children (base case), it is cost-saving to vaccinate all children aged 1 to 19 years. If annual incidence is 20/100,000 (one-fifth as high as estimated in the base case), catch-up vaccination is still cost-saving for children aged 2 years and younger, and would be highly cost-effective for children aged 3-10 years, costing less than $2,500 [17,000 RMB] per QALY gained. The catch-up vaccination program becomes less cost-effective as acute HBV infection incidence decreases and as the age of the children vaccinated increases.
Figure 2.
Cost-effectiveness of catch-up HBV vaccination in China (incremental cost in US dollars per quality-adjusted life year gained) as a function of cohort age and annual incidence of acute HBV infection (base case incidence 100/100,000 per year)*
* Incremental cost-effectiveness ratio of vaccination versus no vaccination. HBV = hepatitis B virus. QALY = quality-adjusted life year. When the line intersects the horizontal axis, the intervention is cost-saving.
We performed one-way sensitivity analysis across the ranges of all parameters (Appendix Table 3). In almost all cases, catch-up vaccination was cost-saving. In addition to cohort age and incidence of acute HBV infection (discussed above), the cost-effectiveness ratio was most sensitive to the costs of administering the vaccine. If the total cost of vaccine and administration is at the high end of our range ($8 [55 RMB] per child vaccinated), then the intervention costs $5,800 [40,000 RMB] per QALY gained for a cohort of 10-year-olds.
In our base-case analysis, the chance an acute infection becomes chronic is based on a meta-analysis (33). One previous study, not included in that meta-analysis, found a lower likelihood (34). Reflecting that study, we lowered the chance of an acute infection becoming chronic (calculations in Appendix) and found that vaccination is still cost-saving for children up to age 10 and costs less than $2,500 per QALY gained up until age 15 (Appendix Figure 3).
In the base case, we did not include setup costs since they can be highly variable depending on the size and scope of the program. When we included setup costs similar to those in a recent catch-up vaccination program in Qinghai province (about $0.40 [2.7 RMB] per child), the catch-up vaccination program was still cost-saving.
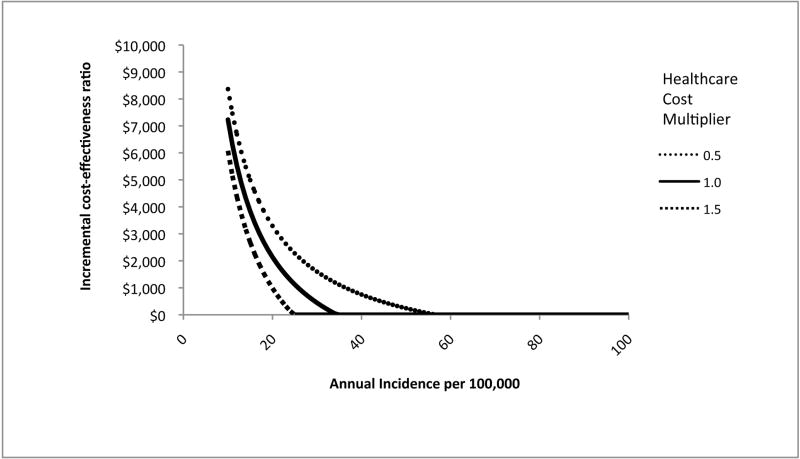
China has recently experienced rapid economic growth and increased prosperity. This economic growth may lead to higher costs for HBV treatment, and for health care in general. In addition, rural areas in China may have lower healthcare costs and higher HBV incidence (due to lower vaccination coverage) than urban areas. Thus, we conducted two-way sensitivity analysis on healthcare costs and incidence (Figure 3). We varied annual incidence of acute HBV infection between 10/100,000, a rate similar to that among vaccinated individuals (20, 21), and 100/100,000 (the base case incidence). We varied all costs of health care and HBV treatment simultaneously from 50% to 150% of the base case values. If incidence is lower than in the base case, there is less benefit from the vaccination program. Similarly, if the costs of treating HBV and its complications are lower, then the program has less benefit. However, treatment costs must be significantly lower and incidence significantly lower than in the base case before catch-up vaccination is not cost-effective.
Figure 3.
Cost-effectiveness of catch-up HBV vaccination in China (incremental cost in US dollars per quality-adjusted life year gained) as a function of acute HBV incidence and healthcare cost*
* HBV = hepatitis B virus. Incremental cost-effectiveness ratio of vaccination versus no vaccination. Assumes a cohort age of 10 years old. Incidence represents acute (symptomatic and asymptomatic) HBV infections per 100,000 persons per year. All healthcare costs except for those related to vaccination were varied proportionally: thus, for example, 1.0 represents the base case, and 1.5 represents all costs (except for vaccination cost) being 150% of the value assumed in the base case. The base case incidence was 100/100,000 persons per year. All scenarios with incidence higher than 60/100,000 persons per year were cost-saving.
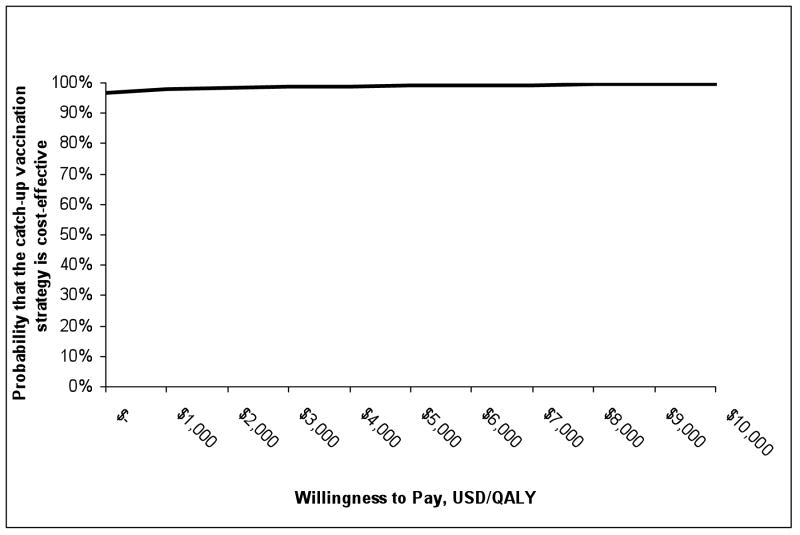
We performed probabilistic sensitivity analysis in which we varied all parameters simultaneously in a Monte Carlo simulation (Figure 4). Parameters were drawn from a uniform probability distribution between the low and high values (Table 1) and were assumed to be uncorrelated. With these assumptions, the catch-up vaccination program has a 97% chance of being cost-saving and a 98% chance of having an incremental cost-effectiveness ratio of less than $2,500 [17,000 RMB] per QALY gained.
Figure 4.
Results of Monte-Carlo sensitivity analysis: cost-effectiveness acceptability curve*
* Results are based on 10,000 Monte Carlo simulations in which all parameters were varied simultaneously. Each parameter was uniformly distributed between the high and low estimates shown in Table 1, and parameter values were chosen independently. QALY = quality-adjusted life year.
DISCUSSION
In recent years, China has made great strides in protecting newborns from HBV infection, but many children and adolescents still remain unprotected, particularly those born before 2003. Incomplete vaccination coverage is particularly acute in rural areas (8), so catch-up vaccination efforts in such areas could be especially cost-effective, and could help reduce regional disparities in the burden of disease caused by HBV.
Our study is the first cost-effectiveness analysis of catch-up HBV vaccination for children in an endemic country. We found that free nationwide catch-up vaccination for children and adolescents in China is likely to be cost-saving, or to cost less than $2,500 [17,000 RMB] per QALY gained for many combinations of parameter values.
Previous studies of childhood vaccination for other diseases in Asia have reported a wide range of cost-effectiveness estimates. Some vaccinations, such as those for Japanese encephalitis, are estimated to be cost-saving (39). Vaccination of infants and children for rotavirus is estimated to be cost-saving or to cost less than about $10,000 [68,000 RMB] per DALY gained (40-42). Catch-up vaccination for HBV compares very favorably with these programs.
Our analysis has several limitations. Because of limited quality-of-life studies in China, we used health-related quality-of-life data from several international studies. However, in sensitivity analysis we showed that catch-up vaccination is cost-saving even with significantly different quality-of-life estimates. We did not include the costs of time spent by vaccine recipients or their parents. We did not include the effects of catch-up vaccination on future hepatitis B incidence (as more people are vaccinated, there will be fewer infectious people to transmit the disease). Because our model ignores this effect, our analysis underestimates the value of the catch-up program. Our analysis is based on current treatment options and current costs. However, chronic HBV infection is a disease that individuals may live with for many years. As treatments for chronic HBV infection evolve, the costs and health benefits of HBV treatment may change. We showed in sensitivity analysis that higher HBV treatment costs make catch-up vaccination even more economically attractive because vaccination helps avert those costs.
A free, nationwide catch-up vaccination program for children and adolescents in China is likely to be cost-saving – and feasible. Recent pilot programs for HBV catch-up vaccination in China, including an aggressive vaccination program in Beijing (20, 21, 43), have been successful in increasing vaccination coverage. In addition, a demonstration program in the remote, rural province of Qinghai provided HBV education and free vaccination to 54,680 grade school students at 331 schools between September 2006 and April 2007 (26)(S So, manuscript submitted). The program led to an unprecedented 99.4% three-dose HBV vaccine coverage. The program was expanded in 2007-2008 to educate and provide free vaccination to the remaining 500,000 kindergarteners and grade school children in the province (26). Replication of such school-based catch-up vaccination efforts implemented by existing provincial health department staff nationwide is likely to be both feasible and highly cost-effective, and can disseminate vaccination programs even to isolated regions of China, where the need for catch-up vaccination is particularly urgent.
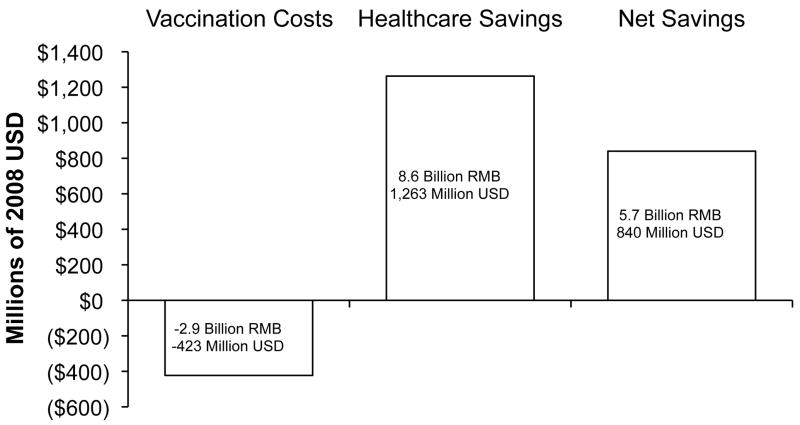
The overall health and economic impacts of catch-up vaccination could be immense. Approximately 150 million children and adolescents in China ages 19 and younger are unprotected from HBV (8, 9, 44). We estimate that the cost to vaccinate these children against HBV would be on the order of $423 million [2.9 billion RMB] ($2.82 [19 RMB] per three-dose vaccination series × 150 million vaccinations), and would lead to net present cost savings of almost $840 million [5.7 billion RMB] (calculated using our model) (Figure 5). Even if some HBV-infected or immune children were vaccinated in such a program (thus leading to cost for extra vaccination), and even if such a one-time vaccination program incurred significant setup costs, it would still be highly cost-saving. Such catch-up vaccination could prevent as many as 8.2 million people from becoming HBV-infected, 390,000 from developing chronic HBV infection, and 65,000 from dying of HBV-related liver disease and liver cancer. This study provides strong evidential support for the new national program introduced by China in 2009 to immunize all children under 15 years old who have not received hepatitis B vaccination.
Figure 5.
Estimated vaccination program costs, healthcare savings, and net savings for a hypothetical free catch-up vaccination program for children and adolescents age 1-19 in China. Negative numbers represent costs; positive numbers are net savings. All numbers are expressed in net present 2008 currency.
* USD = US Dollar, RMB = Chinese Yuan Renminbi
Supplementary Material
Acknowledgments
The authors thank the China Foundation for Hepatitis Prevention and Control, Professor Ji-Dong Jia from the Capital University of Medical Science in Beijing and president of the Asian Pacific Association in the Study of the Liver, Dr. Stephen Hadler, Medical Officer in the World Health Organization and China-GAVI program officer, Beijing, China office, and Dr. Ellen Chang of the Northern California Cancer Center and the Asian Liver Center at Stanford University for their valuable input and suggestions. The authors thank Amanda Wong at Cornell University for her assistance with researching sources. David Hutton is supported by a Stanford Graduate Fellowship and Grant Number R18PS000830 from the US Centers for Disease Control and Prevention. Samuel So is supported by Grant Number R18PS000830 from the US Centers for Disease Control and Prevention, R21CA122317 from the National Institute of Health, and CP1MP071051 from the Office of Minority Health. Margaret Brandeau is supported by Grant Number R01-DA15612 from the National Institute on Drug Abuse and Grant Number R18PS000830 from the US Centers for Disease Control and Prevention.
Grant Support/Role of the Funding Source: No grant support was provided for this study.
Footnotes
Potential Financial Conflicts of Interest: None disclosed.
Model and Statistical Code: Available to interested readers by contacting David Hutton at david.hutton@stanfordalumni.org.
References
- 1.Progress in hepatitis B prevention through universal infant vaccination--China, 1997-2006. MMWR Morb Mortal Wkly Rep. 2007;56:441–445. [PubMed] [Google Scholar]
- 2.World Health Organization. [November 18, 2008];WHO report 2008, Global tuberculosis control - surveillance, planning, financing. Available at: http://www.who.int/tb/publications/global_report/2008/pdf/annex_3.pdf.
- 3.World Health Organization. [November 18, 2008];Epidemiological Fact Sheet on HIV and AIDS: China. Available at: http://www.who.int/globalatlas/predefinedreports/EFS2008/full/EFS2008_CN.pdf.
- 4.World Health Organization. [October 30, 2008];WHO Disease and injury country estimates. Available at: http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html.
- 5.World Health Organization. [November 18, 2008];Malaria annual data. Available at: http://www.wpro.who.int/sites/mvp/data/malaria/2006.htm.
- 6.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol. 2002;67:447–450. doi: 10.1002/jmv.10094. [DOI] [PubMed] [Google Scholar]
- 8. [August 1, 2008];The Ministry of Health conference on planning and hepatitis B immunization, malaria prevention and control work [Chinese] Available at: http://www.gov.cn/xwfb/2008-04/21/content_950425.htm.
- 9.United States Census Bureau. [November 18, 2008];China International Database Country Summary. Available at: http://www.census.gov/ipc/www/idb/country/chportal.html#TAB.
- 10.Beutels P. Economic evaluations of hepatitis B immunization: a global review of recent studies (1994-2000) Health Econ. 2001;10:751–774. doi: 10.1002/hec.625. [DOI] [PubMed] [Google Scholar]
- 11.Wittet S. Hepatitis B Vaccine Introduction - Lessons Learned in Advocacy, Communication, and Training. Seattle, Washington: Program for Appropriate Technology in Health [PATH], Bill and Melinda Gates Children’s Vaccine Program; 2001. Report No.: (Bill and Melinda Gates Children’s Vaccine Program Occasional Paper No. 4) [Google Scholar]
- 12.World Health Organization. Western Pacific regional plan for hepatitis B control through immunization. Manila, Philippines: World Health Organization; 2007. Report No.: (WP)/ICP/EPI/5.2/001-E. [Google Scholar]
- 13.Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Intern Med. 2005;142:821–831. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460–469. doi: 10.7326/0003-4819-147-7-200710020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Murray CJ, Lopez A. World Health Report 2002: Reducing risks, promoting healthy life. Geneva: World Health Organization; 2002. [Google Scholar]
- 16.International Monetary Fund. World Economic Outlook Database for October 2007. 2007 October 17; [Google Scholar]
- 17.World Bank. [August 1, 2008];World Development Indicators database. Available at: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK:20394822~isCURL:Y~menuPK:1192694~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html.
- 18. [August 1, 2008];CIA - The World Factbook -- China. Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/ch.html#Econ.
- 19.Murray CJL, Salomon JA, Mathers CD, Lopez AD, editors. Concepts, Ethics, Measurement and Applications. Geneva: World Health Organization; 2002. Summary Measures of Population Health. [Google Scholar]
- 20.Gong XH, Li YH, Liu LR, Jia L, Xing YL, Wang YQ. Study on the afficacy [sic] of hepatitis B immunization among youngsters in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:388–390. [PubMed] [Google Scholar]
- 21.Gong XH, Liu LR, Jia L, Li YH, Xing YL, Wang QY. Epidemiological effect of hepatitis B immunization among newborn babies in Beijing. Zhonghua Gan Zang Bing Za Zhi. 2003;11:201–202. [PubMed] [Google Scholar]
- 22.Zhiqiang G, Zhaohui D, Qinhuan W, Dexian C, Yunyun F, Hongtao L, Iloeje UH. Cost of chronic hepatitis B infection in China. J Clin Gastroenterol. 2004;38:S175–178. doi: 10.1097/00004836-200411003-00010. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) Hepatitis B Fact sheet no 204. Geneva: World Health Organization; 2008. [Google Scholar]
- 24.Shivananda, Somani V, Srikanth BS, Mohan M, Kulkarni PS. Comparison of two hepatitis B vaccines (GeneVac-B and Engerix-B) in healthy infants in India. Clin Vaccine Immunol. 2006;13:661–664. doi: 10.1128/CVI.00087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alimonos KK, Nafziger AAN, Murray JJ, Bertino JJS. Prediction of response to hepatitis B vaccine in health care workers: whose titers of antibody to hepatitis B surface antigen should be determined after a three-dose series, and what are the implications in terms of cost-effectiveness? Clin Infect Dis. 1998;26:566–571. doi: 10.1086/514575. [DOI] [PubMed] [Google Scholar]
- 26.Chen JJ. A model HBV catch-up immunization and education project in Qinghai, China. National Immunization Conference 2008. 2008 March 17; [Google Scholar]
- 27.People’s Daily Online. [November 18, 2008];Chinese, U S agencies help hepatitis B prevention in northwest China. Available at: http://english.peopledaily.com.cn/200609/11/eng20060911_301654.html.
- 28.Liang XF, Chen YS, Wang XJ, He X, Chen LJ, Wang J, Lin CY, et al. A study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:655–658. [PubMed] [Google Scholar]
- 29.Li RC, Yang JY, Gong J, Li YP, Huang ZN, Fang KX, Xu ZY, et al. Efficacy of hepatitis B vaccination on hepatitis B prevention and on hepatocellular carcinoma. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:385–387. [PubMed] [Google Scholar]
- 30.Gong J, Li RC, Yang JY, Li YP, Chen XR, Xu ZY, Liu CB, et al. Long-term efficacy of infant hepatitis B immunization program. Zhonghua Gan Zang Bing Za Zhi. 2003;11:203–205. [PubMed] [Google Scholar]
- 31.Margolis H, Coleman P, Brown R, Mast E, Sheingold S, Arevalo J. Prevention of hepatitis B virus transmission by immunization: an economic analysis of current recommendations. JAMA. 1995;274:1201–1208. [PubMed] [Google Scholar]
- 32.Pisu M, Meltzer M, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine. 2002;21:312–321. doi: 10.1016/s0264-410x(02)00457-7. [DOI] [PubMed] [Google Scholar]
- 33.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 34.Seeff L, Beebe G, Hoofnagle J, Norman J, Buskell-Bales Z, Waggoner J, Kaplowitz N. A serologic follow-up of the 1942 epidemic of post-vaccination hepatitis in the United States Army. New Engl J Med. 1987;316:965–970. doi: 10.1056/NEJM198704163161601. [DOI] [PubMed] [Google Scholar]
- 35.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. NY: Oxford University Press; 1996. [Google Scholar]
- 36.Chen D, Yao G-b, Chen W. Economic evaluation of peginterferon Alfa-2a and lamivudine in the treatment of HBeAg-negative chronic hepatitis B [Chinese] Chinese Hepatology. 2007;12:164–167. [Google Scholar]
- 37.Jacobs R, Saab S, Meyerhoff A. The cost effectiveness of hepatitis immunization for US college students. J Am Coll Health. 2003;51:227–236. doi: 10.1080/07448480309596355. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation. Health Technol Assess. 2006;10:1–200. doi: 10.3310/hta10280. [DOI] [PubMed] [Google Scholar]
- 39.Ding D, Kilgore PE, Clemens JD, Wei L, Zhi-Yi X. Cost-effectiveness of routine immunization to control Japanese encephalitis in Shanghai, China. Bull World Health Organ. 2003;81:334–342. [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer TK, Anh DD, Antil L, Cat ND, Kilgore PE, Thiem VD, Rheingans R, et al. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192:1720–1726. doi: 10.1086/497339. [DOI] [PubMed] [Google Scholar]
- 41.Podewils LJ, Antil L, Hummelman E, Bresee J, Parashar UD, Rheingans R. Projected cost-effectiveness of rotavirus vaccination for children in Asia. J Infect Dis. 2005;192(Suppl 1):S133–145. doi: 10.1086/431513. [DOI] [PubMed] [Google Scholar]
- 42.Ho AM, Nelson EA, Walker DG. Rotavirus vaccination for Hong Kong children: an economic evaluation from the Hong Kong Government perspective. Arch Dis Child. 2008;93:52–58. doi: 10.1136/adc.2007.117879. [DOI] [PubMed] [Google Scholar]
- 43.Viral Hepatitis in China: Seroepidemiological Survey in Chinese Population (part one) 1992~1995. Beijing, China: Scientific and Technical Documents Publishing House; 1997. [Google Scholar]
- 44.State Statistical Bureau of the People’s Republic of China. China statistical yearbook. English. Hong Kong: International Centre for the Advancement of Science & Technology; 2006. [Google Scholar]
- 45.Bristol-Myers Squibb Foundation and China Foundation for Hepatitis Prevention and Control collaborate to fight one of China’s most pressing health problems. [November 1, 2008]; Available at: http://investor.bms.com/phoenix.zhtml?c=106664&p=;irol-newsArticle&ID=638504&highlight=
- 46.Ho L. [November 1, 2008];Public ’lacks awareness’ of hepatitis B. Available at: http://www.chinadaily.com.cn/cndy/2007-12/11/content_6311290.htm.
- 47.Hoofnagle JH, Shafritz DA, Popper H. Chronic type B hepatitis and the “healthy” HBsAg carrier state. Hepatology. 1987;7:758–763. doi: 10.1002/hep.1840070424. [DOI] [PubMed] [Google Scholar]
- 48.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 49.Lin X, Robinson NJ, Thursz M, Rosenberg DM, Weild A, Pimenta JM, Hall AJ. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: review of disease progression. J Gastroenterol Hepatol. 2005;20:833–843. doi: 10.1111/j.1440-1746.2005.03813.x. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Hepatitis B fact sheet. Atlanta, GA: [April 8, 2008]. http://www.cdc.gov/ncidod/diseases/hepatitis/b/fact.htm. [Google Scholar]
- 51.Chen CJ, Yang HI, Su JUN, Jen CL, You SL, Lu SN, Huang GT, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 52.Perrillo R, Hann H, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 53.Peters MG, Hann HW, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 54.Chan HL-Y, Leung NW-Y, Hui AY, Wong VW-S, Liew C-T, Chim AM-L, Chan FK-L, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated Interferon-{alpha}2b and Lamivudine with Lamivudine alone. Ann Intern Med. 2005;142:240–250. doi: 10.7326/0003-4819-142-4-200502150-00006. [DOI] [PubMed] [Google Scholar]
- 55.Cooksley WG. Treatment with interferons (including pegylated interferons) in patients with hepatitis B. Semin Liver Dis. 2004;24:45–53. doi: 10.1055/s-2004-828678. [DOI] [PubMed] [Google Scholar]
- 56.Krogsgaard K. The long-term effect of treatment with interferon-alpha 2a in chronic hepatitis B. J Viral Hepat. 1998;5:389–397. doi: 10.1046/j.1365-2893.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 57.Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132–2137. [PubMed] [Google Scholar]
- 58.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-{alpha}2b in patients with histologically mild chronic Hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 59.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. [November 1, 2008];OPTN: Organ Procurement and Transplantation Network: Data. Available at: http://www.optn.org/latestData/viewDataReports.asp.
- 61.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 62.Yuen M-F, Cheng C-C, Lauder IJ, Lam S-K, Ooi CG, Lai C-L. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 63.Terrault NA, Carter JT, Carlson L, Roland ME, Stock PG. Outcome of patients with hepatitis B virus and human immunodeficiency virus infections referred for liver transplantation. Liver Transpl. 2005;2006:801–807. doi: 10.1002/lt.20776. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 10. Washington DC: Public Health Foundation; 2007. [Google Scholar]
- 65.Hu RH, Ho MC, Wu YM, Yu SC, Lee PH. Feasibility of salvage liver transplantation for patients with recurrent hepatocellular carcinoma. Clin Transplant. 2005;19:175–180. doi: 10.1111/j.1399-0012.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 66.Pierie JP, Muzikansky A, Tanabe KK, Ott MJ. The outcome of surgical resection versus assignment to the liver transplant waiting list for hepatocellular carcinoma. Ann Surg Oncol. 2005 doi: 10.1245/ASO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Maluf DG, Stravitz RT, Williams B, Cotterell AH, Mas VR, Heuman D, Luketic V, et al. Multimodality therapy and liver transplantation in patients with cirrhosis and hepatocellular carcinoma: 6 years, single-center experience. Transplant Proc. 2007;39:153–159. doi: 10.1016/j.transproceed.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 68.Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, Treleaven S, et al. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006;24:1975–1982. doi: 10.1016/j.vaccine.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 69.Juan S. [November 1, 2008];China ‘has 120m hepatitis B carriers’. Available at: http://www.chinadaily.com.cn/china/2007-09/01/content_6073020.htm#.
- 70.UNICEF. 2003 Vaccine Projection: Quantities and Pricing. [November 18, 2008]; Available at: http://www.unicef.org/supply/files/2003_Vaccine_Projection.pdf.
- 71.Li SC, Ong SC, Lim SG, Yeoh KG, Kwong KS, Lee V, Lee W, et al. A cost comparison of management of chronic hepatitis B and its associated complications in Hong Kong and Singapore. J Clin Gastroenterol. 2004;38:S136–143. doi: 10.1097/00004836-200411003-00004. [DOI] [PubMed] [Google Scholar]
- 72.Lacey L, Tan A. Economic evaluation of different treatments of HBeAg-negative chronic hepatitis B in China [Chinese] Chin J Infect Dis. 2007;25:473–479. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.