Abstract
RNA processing is important for generating protein diversity and modulating levels of protein expression. The CUG-BP, Elav-like family (CELF) of RNA binding proteins regulate several steps of RNA processing in the nucleus and cytoplasm, including pre-mRNA alternative splicing, C to U RNA editing, deadenylation, mRNA decay, and translation. In vivo, CELF proteins have been shown to play roles in gametogenesis and early embryonic development, heart and skeletal muscle function, and neurosynaptic transmission. Dysregulation of CELF-mediated programs has been implicated in the pathogenesis of human diseases affecting the heart, skeletal muscles, and nervous system.
Keywords: CELF, CUG-BP, ETR-3, Bruno-like, alternative splicing, RNA editing, deadenylation, mRNA decay, translation, myotonic dystrophy
Introduction
The completion of the Human Genome Project revealed that there are far fewer genes than predicted. Combined with the development of technologies for high throughput transcriptome and proteome analyses, it has become increasingly clear that one gene can produce several proteins. Both post-transcriptional regulation of RNAs and post-translational modification of proteins contribute to proteome diversity. The amount of gene expression can also be regulated at the RNA or protein level, by modulating production or stability.
Each step in the life of a transcript, including its production, modification, transport, translation, and decay, is tightly controlled. Many RNA binding protein families differentially interact with specific subsets of transcripts and regulate one or more of these steps. An example is the CUG-BP, Elav-like family (CELF). CELF proteins are found in the nucleus and cytoplasm. They regulate multiple facets of gene expression, including pre-mRNA alternative splicing, RNA editing, deadenylation, mRNA stability, and translation. We review both the molecular and biological roles of CELF proteins, and recap evidence that implicates members of this family in human diseases. Because different family members were identified at different times in different tissues and different species, they are found in the literature under a host of names. In this review, we summarize what is known about CELF proteins using a consistent nomenclature.
THE CELF FAMILY
Before being recognized as a family, individual CELF clones were isolated. CELF1 was first isolated from human cells as a nuclear protein that binds to a (CUG) RNA.18 CELF2 was first cloned in a screen of genes expressed in fetal heart.2 It arose again in screens for genes up-regulated during apoptosis in neuroblastoma cells3 and for genes related to CELF1 in mouse liver.4 A celf3 clone was identified in Xenopus neural tissue.5
Two groups described the CELF proteins as a family. Good and colleagues called them the Bruno-like (Brunol) proteins after the homologous Drosophila protein Bruno.6 Ladd et al. dubbed them the CUG-BP and ETR-3-like factor (CELF) proteins after the first two family members identified in humans, CUG binding protein (CUG-BP, now called CELF1) and elav-type RNA binding protein 3 (ETR-3, now called CELF2).7 At that time, cDNA or EST sequences for five human CELF proteins, CELF1-5, were found in the databases, and a sixth was predicted from genomic sequence. Expression of CELF6 was validated a few years later.8 Family members have been called by a plethora of names (Table 1), until a recent consensus in the field agreed on a common nomenclature: CUGBP, Elav-like family member 1 (CELF1), CELF2, CELF3, CELF4, CELF5, and CELF6. It should be noted that in early reports, CUG-BP1 and CUG-BP2 referred to phosphoisoforms of CELF11, 9, whereas in later studies CUG-BP1 was used for all isoforms of CELF1 and CUG-BP2 referred to CELF2.
Table 1. Nomenclature and phenotypes of CELF family orthologs.
| Species | Family Member(s) |
Gene | Alternative Protein Names |
Loss of Function Phenotype |
|---|---|---|---|---|
| Vertebrates | ||||
|
Homo sapiens (human) |
CELF1 | CELF1 | CUG-BP, CUG-BP1, BRUNOL2, NAB50 |
|
| CELF2 | CELF2 | ETR-3, CUG-BP2, NAPOR, BRUNOL3 |
||
| CELF3 | CELF3 | TNRC4, BRUNOL1, CAGH4 |
||
| CELF4 | CELF4 | BRUNOL4 | ||
| CELF5 | CELF5 | BRUNOL5 | ||
| CELF6 | CELF6 | BRUNOL6 | ||
|
Mus musculus (mouse) |
CELF1 | Celf1 | CUG-BP, CUG-BP1, BRUNOL2 |
Spermatogenesis defects |
| CELF2 | Celf2 | ETR-3, CUG-BP2, NAPOR, BRUNOL3 |
||
| CELF3 | Celf3 | TNRC4, BRUNOL1, CAGH4 |
Reduced sperm count | |
| CELF4 | Celf4 | BRUNOL4 | Seizure Disorder | |
| CELF5 | Celf5 | BRUNOL5 | ||
| CELF6 | Celf6 | BRUNOL6 | ||
|
Gallus gallus (chicken) |
CELF1 | CELF1 | CUG-BP, CUG-BP1 | |
| CELF2 | CELF2 | ETR-3, CUG-BP2 | ||
| CELF3 | Deleted | |||
| CELF4 | CELF4 | BRUNOL4 | ||
| CELF5 | CELF5 | BRUNOL5 | ||
| CELF6 | CELF6 | BRUNOL6 | ||
|
Xenopus laevis (frog) |
CELF1 | celf1a | brunol1a, EDEN-BP | Somite segmentation defects |
| celf1b | brunol1b | |||
| CELF2 | celf2a | brunol2a | ||
| celf2b | brunol2b | |||
| CELF3 | celf3a | brunol3a, etr1 | Endoderm proliferation and differentiation defects |
|
| celf3b | brunol3b | |||
| CELF4 | celf4 | brunol4 | ||
| CELF5 | celf5 | brunol5 | ||
| CELF6 | Missing | |||
|
Xenopus tropicalis (frog) |
CELF1 | celf1 | cugbp1 | |
| CELF2 | celf2 | etr3 | ||
| CELF3 | celf3 | tnrc4 | ||
| CELF4 | celf4 | brunol4 | ||
| CELF5 | celf5 | brunol5 | ||
| CELF6 | Missing | |||
|
Danio rerio (Zebrafish) |
CELF1 | celf1 | Cugbp1, Brul | |
| CELF2 | celf2 | Etr-3, Cugbp2 | ||
| CELF3 | celf3 | Etr-1 | ||
| CELF4 | celf4 | |||
| CELF5 | celf5 | |||
| CELF6 | Missing | |||
| Invertebrates | ||||
|
Caenorhabditis elegans (worm) |
CELF1-2 | etr-1 | Elongation and muscle attachment defects |
|
| CELF3-6 | unc-75 | Synaptic transmission defects | ||
|
Drosophila melanogaster (fruit fly) |
CELF1-2 | bru | Gametogenesis defects | |
| bru-2 | ||||
| CELF3-6 | bru-3 | |||
CELF protein subfamilies
CELF homologs are found in deuterostomes, protostomes, and plants, but not in bacteria, Archaea, or yeast.6, 10 Phylogenetic analysis of human CELF proteins divides them into two distinct subfamilies: CELF1-2, and CELF3-6.6-8 Each subfamily is represented by at least one member in plants and animals, suggesting the ancestral CELF gene underwent a duplication soon after it arose.10 Most invertebrates, including primitive chordates, have only two CELF proteins. Drosophila, however, has three paralogs, Bruno (Bru), Bru-2, and Bru-3.6 Bru and Bru-2 likely result from a lineage-specific tandem duplication and belong to the CELF1-2 subfamily. While phylogenetic analysis suggests that Bru-3 belongs to the CELF3-6 subfamily6, it has been suggested that Bru-3 is functionally more similar to CELF1 than Bru or Bru-2.11 The number of CELF proteins expanded in vertebrates. Mice have six CELF proteins as in humans. Chicken has five due to partial deletion of the CELF3 gene.10 Paralogs of celf1-5, but not celf6, have been identified in Xenopus tropicalis and laevis.12 As a pseudotetraploid X. laevis has two of each gene, and cDNAs have been cloned for second paralogs of celf1, celf2, and celf3.12 Only celf1-5 are listed in the NCBI database for zebrafish, although ESTs and predicted protein sequences suggest there could eight Celf proteins due to expansion of the CELF3-6 subfamily.10
The CELF1-2 subfamily is broadly expressed, while the CELF3-6 subfamily exhibits more restricted expression. CELF1 and CELF2 have overlapping yet distinct expression profiles, both being highest in heart, skeletal muscle, and brain. 3, 6-8 CELF3 and CELF5 are detected primarily in the nervous system, while CELF6 is found in nervous system, kidney, and testis.6-8, 13 There are conflicting reports of CELF4 expression. In one study, CELF4 transcripts were broadly detected in most human tissues8, but in several others CELF4 mRNA was detected only in the nervous system.13-15 This discrepancy may result from using probes against different regions of the CELF4 transcript. CELF1 and CELF2 are also broadly expressed in the developing embryo with high expression in myogenic tissues, while CELF3-6 are primarily found in embryonic neural tissues.5, 10, 12, 16-18 Differential expression of the subfamilies is conserved. In C. elegans ETR-1, the CELF1-2 homolog, is expressed in developing muscle19 while UNC-75, the CELF3-6 homolog, is expressed exclusively in the nervous system.13
CELF protein structure and function
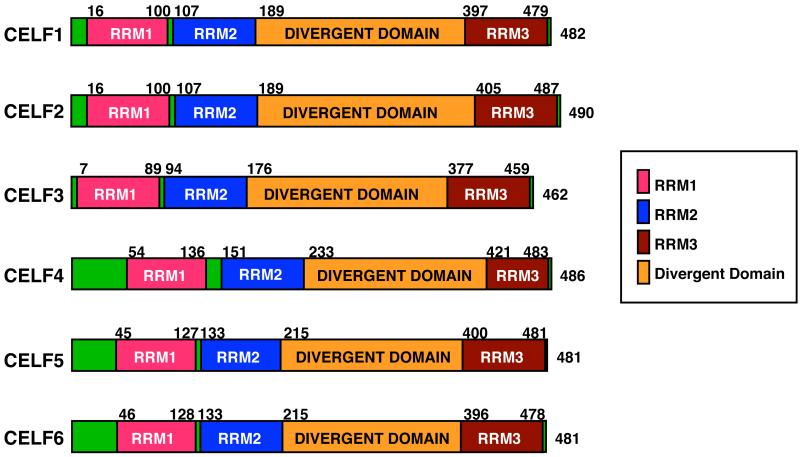
CELF proteins share a common domain structure with the Elav family (which includes the Hu antigen proteins), but are evolutionarily distinct.6 Each CELF protein contains three RNA binding domains of the RNA recognition motif (RRM) variety, two at the N terminus and one at the C terminus (Figure 1). A unique linker region called the “divergent domain” separates RRM2 and RRM3. The divergent domain was so named because sequence similarities in this domain divide CELF proteins into the two distinct subfamilies.7
FIGURE 1.
All six human CELF proteins share a common domain structure, with three RRM RNA binding domains and a divergent domain between the second and third RRM.8 Each family member exists in multiple isoforms due to alternative splicing and/or the generation of alternative 5′ ends by differential promoter usage (not shown).
Interactions with RNA
Like many RNA binding proteins, CELF protein binding sites lack a tight consensus sequence. Rather, CELF proteins have been shown to interact with a number of sequence elements in different contexts. In early studies, CELF1 and CELF2 were both identified as proteins that bind to (CUG)8 repeats1, 4, but yeast three-hybrid analysis indicated CELF1 prefers UG dinucleotide repeats over CUG repeats.20 U/G-rich sequences were also identified as preferred binding sites of recombinant CELF1 and CELF2 by systematic evolution of ligands by exponential enrichment (SELEX). CELF1 binds with high affinity to elements enriched with UGU trinucleotides21, whereas CELF2 preferentially binds to UG repeats and UGUU motifs.22 From these studies U/G-rich motifs emerge as a common theme, but CELF1 was also shown to bind to CCG repeats in the 5′ region of C/EBPβ transcripts in vitro23, to a G/C-rich region in the 5′ UTR of cyclin D124, and to U(A/G) repeats in the 3′ UTR of Eg and c-mos transcripts.25 Surface plasmon resonance analysis suggested that recombinant CELF1 prefers “pure” UG dinucleotide repeats to other U/G-rich sequences such as (UAUG)7 or (UUG)10, but cannot rule out their use in vivo.26 NMR chemical shift perturbation indicated that CELF1 RRM3 binds better to UG repeats than CUG and UA repeats in solution, although it will bind all three.27 Binding preferences for CELF3-6 are less well characterized, but are theorized to be similar based on the high degree of conservation within CELF protein RRMs.
Several studies suggest that RRM1 and RRM2 are important for CELF protein:RNA interactions. Deletional analyses indicate that CELF1 binds to CUG sequences1 and A/U-rich elements28 through the first two RRMs. Likewise, CELF2 binds to a Bruno response element through RRM1 and RRM26, and CELF4 needs either RRM1 or RRM2 for binding a muscle-specific splicing enhancer (MSE).29 In yeast three-hybrid experiments, however, CELF1 binding to UG repeats was not abrogated by loss of any single RRM, and RRM3 was sufficient to bind RNA when combined with the divergent domain.26 Furthermore, a truncated recombinant CELF1 containing RRM3 bound to a G/C-rich riboprobe, whereas truncated proteins containing either RRM1 or RRM2 did not.24 Together these studies suggest that different RRMs may bind distinct substrates. Interestingly, non-overlapping segments of CELF2 containing either RRM1 and RRM2 with the first half of the divergent domain or RRM3 with the second half of the divergent domain bind specifically to MSE RNA in vitro, suggesting the RRMs may be redundant.29 Crystal and solution structures of CELF1 domains indicate that the manner in which RRM1 and RRM2 interact with RNA is similar, but differs from that of RRM3.27, 30
Although not an RNA binding domain itself, the divergent domain likely plays a role in mediating the interaction with RNA. The largest effect on CELF1 binding in yeast three-hybrid experiments came from deletions of the divergent domain, not changes in the RRMs.20, 26 Strikingly, experiments with chimeric proteins demonstrated that binding via the first two RRMs of CELF4 is strongly influenced by the divergent domain sequence associated with them.31 It is not clear whether the divergent domain affects RNA binding by mediating protein:protein interactions, or by conveying an important conformational state.
Beyond RNA binding
The divergent domain has also been shown to contain sequences that are important for function separable from RNA binding. Deletion of a portion of the human CELF4 divergent domain immediately downstream of RRM2 resulted in loss of splicing activity, despite retaining RNA binding.29 Additionally, deletion of RRM1 and part of RRM2 in a naturally occurring splice variant of CELF4 lacking part of RRM3 results in a protein that does not bind to RNA, but can inhibit the splicing activity of full length CELF proteins.29, 32 Deletion of the CELF2 divergent domain also ablates splicing activity but not RNA binding.33 Distinct but overlapping regions within the divergent domains of human CELF2 and CELF4 activate splicing of different alternative exons.31 Regions required for repression of splicing overlap with those required for activation. Deletion and domain-swapping experiments demonstrated that its divergent domain bestows the ability to induce CFTR exon skipping on CELF2, but not CELF1.33 Similarly, replacement of the Xenopus celf1 divergent domain with that of celf3 conferred a celf3-specific translational activity to the chimeric protein.34 Together, these data suggest that different regulatory events have different requirements, perhaps due to the formation of target-specific complexes.
Several regions of CELF2 were identified that control its subcellular localization.35 Two regions confer nuclear localization, the C-terminal 20 amino acids and the last quadrant of the divergent domain. The C-terminus contains arginine and lysine residues typical of conventional nuclear localization signals, whereas the divergent domain does not. The divergent domain also contains a region that confers cytoplasmic localization via the CRM1-dependent nuclear export pathway. Deletional analyses of CELF1 indicated the divergent domain is required for exclusion from the nucleolus, localization in the perinuclear compartment, and translocation to cytoplasmic stress granules.36
Yeast two-hybrid assays suggested that CELF1 can interact with itself.28 Ablation of CELF1 oligomerization by mutating or directing a peptide against a 27-amino acid region within the divergent domain abolished these homotypic interactions and CELF1-dependent deadenylation.37 RNA binding was also lost, however, so it is not clear if CELF1:CELF1 interactions are required for deadenylation activity per se.
Regulatory effects of phosphorylation
Shortly after its discovery, CELF1 was shown to be a phosphoprotein.9 CELF1 is phosphorylated by multiple kinases, with varying effects. Hyper-phosphorylation by protein kinase C (PKC) increases its half-life.38 Although the phosphorylated residue(s) has not been identified, CELF1 has multiple PKC consensus sites. Phosphorylation by cyclinD3/cdk4 at Ser302 in the divergent domain promotes association with the initiation factor eIF2, affecting translation of CELF1 targets.39 Phosphorylation of Ser302 also increases RNA binding in vitro, as does phosphorylation of Ser28 in RRM1.24 Thus differential phosphorylation of CELF1 may control specific protein:protein and protein:RNA interactions. This is important, as many of the studies investigating in vitro RNA binding used un-phosphorylated recombinant proteins. CELF2-6 also have several predicted phosphorylation sites.7, 8 It remains to be seen whether phosphorylation is a common mechanism for regulating CELF function.
NUCLEAR ROLES OF THE CELF PROTEINS
Celf1 has been shown to co-localize with nascent transcripts on lampbrush chromosomes in amphibian oocytes.40 The loading of CELF1 onto specific transcripts as they are transcribed supports a regulatory role for CELF proteins in co-transcriptional or post-transcriptional RNA processing. Indeed, one of the earliest roles ascribed to CELF proteins is regulation of pre-mRNA alternative splicing.7, 41 Although alternative splicing regulation is generally considered the primary function of CELF proteins in the nucleus, CELF2 has also been implicated in nuclear C to U RNA editing in mammalian cells.42, 43
Alternative splicing
CELF proteins have been shown to activate inclusion of some alternatively spliced regions, but silence others. They typically bind to intronic sequences in their pre-mRNA targets. While early studies relied on the use of in vitro assays and minigene reporters in cultured cells, later studies have expanded to include regulation of endogenous transcripts in vivo.
Exogenous minigenes
Studies performed in cultured cells with transiently transfected minigenes have identified a number of alternative regions regulated by CELF proteins (Table 2). The first targets of CELF-mediated splicing activity were identified based on the presence of CUG sequences in the introns adjacent to regulated exons (e.g.41). SELEX for CELF2 suggested several others.22 Interestingly, CELF proteins can have positive or negative effects on splicing of different pre-mRNAs.
Table 2. CELF-mediated alternative splicing targets identified using minigenes.
| Target | Splicing event | Minigene species | References |
|---|---|---|---|
| PTB | Exon 11 Inclusion | Human | 31, 119 |
| cTNT | Exon 5 Inclusion | Human, Chicken | 7, 8, 22, 32, 120 |
| MTMR1 | Exon 2.1 Inclusion | Human | 22 |
| Nf1 | Exon 23a Skipping | Human | 52 |
| ClC1 | Intron 2 Retention | Human | 102 |
| APP | Exon 8 Skipping | Human | 121 |
| GABT4 | Exon 7 Inclusion | Human | 50 |
| NMDAR1 | Exon 5 Skipping | Human, Rat | 31, 122-124 |
| Exon 21 Inclusion | |||
| Insulin Receptor | Exon 11 Skipping | Human | 8, 31, 99 |
| Exon SM Inclusion | Rat | 125, 126 | |
| α-Actinin | Exon NM Skipping | ||
| β-Tropomyosin | Exon 6B Inclusion | Chicken | 127 |
| CFTR | Exon 9 Inclusion | Human | 22, 33 |
| Exon 2 Skipping | Human | 110-112, 114, 122, 128, 129 | |
| Tau | Exon 3 Skipping | Human | |
| Exon 6 Skipping | Human | ||
| Exon 10 Inclusion* | Human | ||
Minigenes can be easily modified to identify transcript features that control splicing, cis-acting regulatory elements, and trans-acting factors that bind these elements.44 Minigene data must be interpreted cautiously, however, as splicing elements may not behave the same in the context of a full-length pre-mRNA expressed under its own promoter. Likewise, regulatory factors identified in vitro or in over-expression studies may not be determinative for regulation in vivo. One example is cardiac troponin T (cTNT) exon 5. Although cTNT minigenes indicated that CELF proteins are important for activation of exon 5 inclusion7, 8, 32, 41, 45, recent transgenic mice studies suggest that cTNT exon 5 alternative splicing is unresponsive in vivo to over-expression of CELF146 or repression of CELF activity (A. Ladd, unpublished data).
Endogenous targets
The investigation of alternative splicing regulation in vivo has been aided by the development of transgenic mouse models and the advent of splicing microarrays. Splicing microarrays have identified alternative splicing events that undergo fetal-to-adult transitions in the heart.46 These changes correlate with a decline in CELF1 and CELF2 expression, and nearly half responded to over-expression of CELF1 in transgenic mice. These data support an earlier model in which it was proposed that down-regulation of CELF proteins in the heart drives developmental stage-specific alternative splicing transitions.45 Additional bioinformatically-predicted CELF targets have been shown to respond to changes in CELF activity in cells and mice.47, 48 Endogenous transcripts that respond to changes in CELF activity are summarized in Table 3.
Table 3. Endogenous CELF-mediated alternative splicing targets.
| Target | Splicing event | Tissues or cell types used | Reference |
|---|---|---|---|
| Sorbs1 | Exon 6 Skipping | cardiac tissues | 46 |
| C10orf97 | Exon 5 Inclusion | cardiac and skeletal muscle tissues |
46, 91 |
| Atp2b1 | Exon 21 Skipping | cardiac tissues | 46 |
| Mfn2 | Exon 3 Skipping | cardiac tissues | 46 |
| Exon 14-3′Spice site Skipping |
|||
| Ank2 | Exon 21 Skipping | cardiac tissues | 46 |
| Capzb | Exon 8 Skipping | cardiac and skeletal muscle tissues |
46, 91 |
| Tmem 134 | Exon 5 Inclusion | cardiac tissues | 46 |
| Mtmr3 | Exon 16 Skipping | cardiac tissues | 46 |
| Ppfibp | Exon 4 Skipping | cardiac tissues | 46 |
| Fox-2 | Exon 13 Skipping | cardiac tissues | 46 |
| 2900002H16Rik | Exon 4 Skipping | cardiac tissues | 46 |
| Ablim1 | Exon 9 Skipping | cardiac tissues | 46 |
| Mbnl2 | Exon 8 Inclusion | cardiac tissues | 46 |
| ME Exon 6 Inclusion | cardiac and skeletal muscle tissues |
46, 91 | |
| H2afy | ME Exon 7 Skipping | ||
| Actn4 | ME exon 5 Skipping | cardiac tissues | 46 |
| ME exon 6 Inclusion | |||
| Xpo7 | Exon 4-5′ Splice Site Skipping |
cardiac tissues | 46 |
| FXR1h | Exon 15 Skipping | cardiac tissues | 46 |
| Mtmr1 | Exon 2.1 Inclusion Exon 2.2* |
cardiac tissues | 48, 130 |
| Bin1 | Exon10 Skipping | cardiac tissues | 48, 130 |
| Itgb1 | Exon D Inclusion | cardiac tissues | 48, 130 |
| Mef2A | Exon 16 Inclusion | cardiac tissues | 48, 130 |
| Nrap | Exon 12 Skipping | skeletal muscle tissues | 91 |
| MAP4 | Exon 15 Skipping | HEK293T cells | 47 |
| SORBS1 | Exon 5 Skipping | HEK293T cells | 47 |
| PPF1BP1 | Exon 19 Skipping | HEK293T cells | 47 |
| SMARCE1 | Exon 4 Skipping | HEK293T cells | 47 |
| Fox2 | Exon 11 Skipping | HEK293T cells | 47 |
| CELF2 | Exon 16 Skipping | HEK293T cells | 47 |
| NFAT | Exon 2 Skipping | HEK293T cells | 47 |
| CTBP1 | Exon 2 Skipping | HEK293T cells | 47 |
| PTER | Exon 3 Skipping | HEK293T cells | 47 |
| MLLT10 | Exon 13 Skipping | HEK293T cells | 47 |
Same splicing pattern in response to over-expression of CELF1 and repression of CELF activity in the heart
Biochemical identification of direct targets
An important caveat of in vivo studies is that responding to changes in CELF activity does not necessarily indicate that a transcript is a direct target, as it could like downstream in a pathway regulated by a direct target. Crosslinking immunoprecipitation (CLIP) allows co-purification of a protein from cells with fragments of its target RNAs, called “CLIP tags”49. This can be combined with high throughput sequencing (HITS-CLIP) to generate a comprehensive putative target set.53 To date, CLIP has only been performed for one CELF protein, CELF1, from mouse hindbrain.50 Tags representing 206 genes were identified, 64% of which were intronic, consistent with a role in alternative splicing regulation. The alternative splicing of only one, Gabt4, has been shown to respond to changes in CELF1 activity thus far.50 Future studies are needed to integrate sets of targets identified by different methods, and to compare these between different tissues and family members.
Mechanisms of CELF-mediated alternative splicing
The mechanisms of CELF-mediated alternative splicing are not fully understood, but studies by several groups point to the U2 snRNP. CELF2 enhanced complex A formation on a cTNT exon 5 substrate, which was previously shown to be activated by binding of CELF2 to sites in the downstream intron.51 CELF2 bound components of the U2 snRNP and promoted binding of U2 snRNA to the upstream intron. These data suggest a model in which CELF2 binds downstream and acts across the regulated exon to promote U2 assembly on the branch site.
The U2 snRNP is also implicated in CELF-mediated silencing. In vitro binding and splicing experiments suggested that CELF proteins inhibit inclusion of NF1 exon 23a through interference with binding of U2AF65, which interacts with the polypyrimidine tract of the 3′ splice site and promotes assembly of the U2 snRNP at the branch site.52 Likewise, U2AF65 was displaced from CFTR transcripts by CELF2, inhibiting exon inclusion.33 Interestingly, CELF1 failed to displace U2AF65 and had no effect on CFTR alternative splicing. Using the NMDA R1 N1 cassette exon, Dembowski and colleagues developed a “branch site-perimeter binding” model, in which silencing depends on multiple CELF binding sites near the branch site.47 They identified a set of exons that had a similar arrangement of U/G motifs closely positioned at the boundaries of predicted branch sites, which were inhibited by CELF2.
It remains to be determined whether interactions with U2 are an essential for CELF function on all splicing targets. The generation of genome-wide data sets allows the construction of RNA binding maps, in which known regulatory outcomes (e.g. from microarray studies) are integrated with positional information from HITS-CLIP data. In addition to being a powerful tool for predicting the functional consequences of binding, RNA binding maps can offer insight into mechanism.53 The development of CELF protein RNA binding maps is a valuable objective for future investigations.
RNA editing
In mammals, the apolipoprotein B (APOB) mRNA undergoes C to U editing in the nucleus.54 APOBEC-1, an RNA cytidine deaminase, and APOBEC-1 complementation factor (ACF) form the core of the C to U editing holoenzyme. Function of this holoenzyme is regulated in part by CELF2.43 CELF2 was identified as an apobec-1 interacting protein in a yeast two-hybrid screen.42 Co-immunoprecipitation and co-localization studies in liver cells indicated that CELF2 forms a complex with APOBEC-1, ACF, and the APOB RNA.42 Addition of CELF2 to a cell-free system inhibits C to U editing, while antisense inhibition of CELF2 in hepatoma cells promoted editing.42 Likewise, knockdown of CELF2 in an intestinal cell line stimulated APOB editing.43 CELF2 levels inversely correlate with levels of APOB editing in mouse tissues and cultured human cells.43 Other transcripts undergo nuclear C to U editing in mammalian cells55, but a role for CELF proteins in these events has not yet been reported.
CYTOPLASMIC ROLES OF THE CELF PROTEINS
Just as CELF proteins bind to introns in pre-mRNAs to mediate alternative splicing in the nucleus, they have also been shown to bind to 5′ and 3′ UTRs in mature mRNAs to regulate deadenylation, mRNA stability, and translation in the cytoplasm.
Deadenylation
In Xenopus, celf1 was first identified by its ability to bind a U(A/G)-containing embryo deadenylation element (EDEN) and promote rapid EDEN-mediated deadenylation and silencing of maternal transcripts.25 When RNAs were co-immunoprecipitated with celf1 complexes from egg extracts and hybridized on microarrays over 150 putative targets were identified.56 More than 90% of these contained EDEN motifs in the 3′ UTR, and included transcripts encoding proteins involved in cell cycle control and oocyte maturation. Human CELF1 also binds EDEN motifs, and can functionally replace the deadenylation activity of Xenopus celf1 in immunodepleted egg extracts.57, 58
Deadenylation is not only important for silencing of maternal transcripts, but is also a rate-limiting step in the turnover of most eukaryotic mRNAs. CELF1 was shown to bind to C-FOS and TNFα transcripts and promote deadenylation in HeLa cell extracts.59 CELF1 also interacts with poly(A)-specific ribonuclease (PARN), suggesting CELF1 may facilitate deadenylation by recruiting or stabilizing the deadenylase on its substrates.59
While it is clear that CELF1 can promote mRNA deadenylation, it might also induce polyadenylation. Some putative targets of celf1 (e.g. frl1) are polyadenylated in the zygote.60 PARN has been shown to be part of the cytoplasmic polyadenylation element binding protein (cpeb) complex in Xenopus oocytes, where it deadenylates transcripts as the poly(A) tail is added.60 Dissociation of PARN occurs following phosphorylation of cpeb, allowing the poly(A) tail to lengthen. Recruitment of transcripts into this complex by celf1 could theoretically promote deadenylation or polyadenylation, depending on whether PARN is present. It remains to be determined whether celf1 stimulates the polyadenylation of any targets.
mRNA stability
CELF1 binds to a conserved GU-rich element (GRE) that is enriched in the 3′ UTRs of short-lived transcripts in human primary T cells, and promotes GRE-dependent mRNA decay.61 Interestingly, a CELF2 antibody failed to supershift GRE-containing complexes, suggesting this activity is specific to CELF1. Transcripts that co-purify with CELF1 in HeLa and C2C12 cytoplasmic extracts were significantly enriched for GRE and GU-repeats in the 3′ UTRs.62, 63 Many of these encode proteins involved in cell cycle, growth, and apoptosis. CELF1-associated transcripts in myoblasts have shorter half lives, and are stabilized by siRNA-mediated knockdown of CELF1.62, 64 As mRNA decay often follows poly(A) tail shortening, it remains to be determined whether CELF1-mediated degradation is a consequence of its deadenylation activity.
While CELF1 promotes mRNA decay, CELF2 binds to A/U-rich sequences in the 3′ UTR of cycloxygenase-2 (COX2) transcripts and stabilizes them, while simultaneously inhibiting their translation.65 COX-2 is an enzyme that synthesizes prostaglandins, which provide a protective effect against ionizing radiation. CELF2 is induced following irradiation, whereas knockdown of CELF2 up-regulates COX-2 expression and decreases radiation-induced apoptosis.65, 66 Prostaglandins inhibit CELF2 expression in intestinal cells, indicating COX-2 may regulate CELF2 in a negative feedback loop.67 CELF2 similarly stabilizes and inhibits translation of MCL1, an anti-apoptotic factor in the BCL2 family, via binding to its 3′ UTR.68
Translation
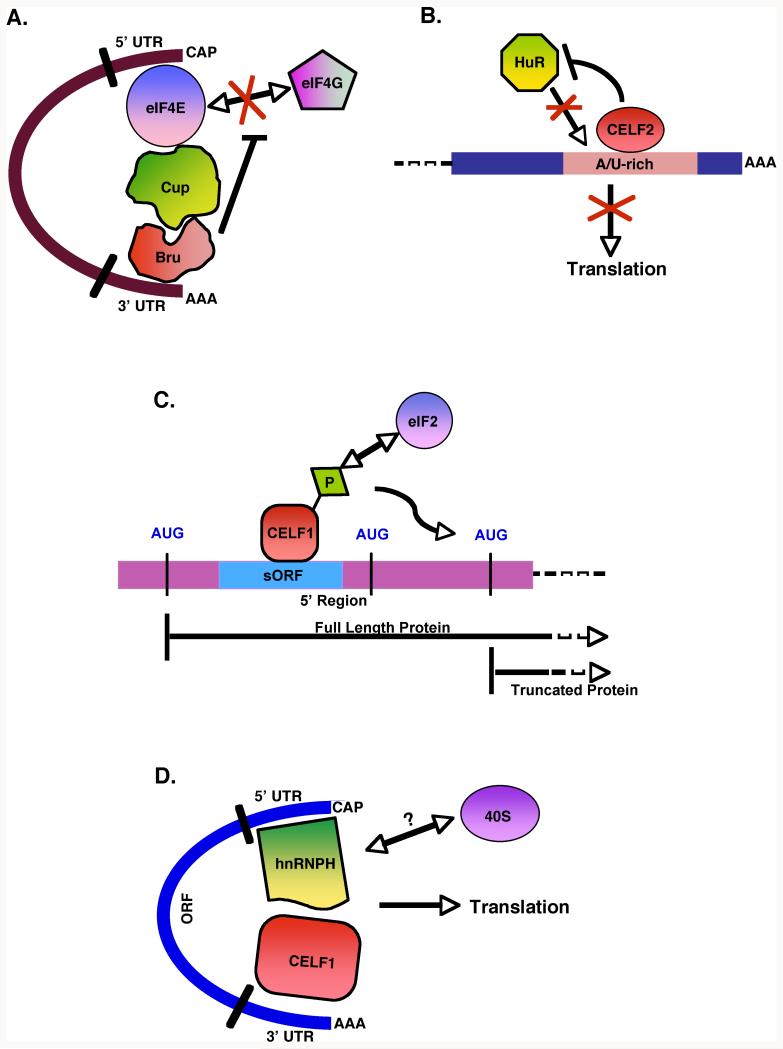
CELF proteins have been shown to regulate translation through multiple mechanisms (Figure 2). In Drosophila, Bru was first described as a repressor of oskar (osk) translation via binding to its 3′ UTR.69 Bru interacts with Cup, which associates with the 5′-cap binding initiation factor eIF4E, suggesting translational repression occurs via a 5′/3′ interaction mediated by an eIF4E-Cup-Bru complex.70 Bru also binds to gurken and Cyclin A 3′ UTRs and inhibits translation.71, 72 As described above, CELF2 has likewise been shown to inhibit the translation of COX2 and MCL1 mRNAs.65, 68 The mechanism is not well understood, but CELF2 has been shown to compete with HuR, an activator of translation, for binding to A/U-rich elements in the COX2 3′ UTR.73 Under conditions of environmental stress, CELF1 is routed to stress granules in HeLa cells, suggesting it may also play a role in translational silencing.36
FIGURE 2.
CELF proteins regulate translation through multiple mechanisms. A. The Drosophila CELF protein, Bru, forms a complex with Cup and the 5′ cap binding initiation factor, eIF4E, leading to circularization and translational repression of the mRNA. B. CELF2 inhibits translation by binding to A/U-rich elements in the 3′ UTR and competing with HuR, an activator of translation. C. Binding of CELF1 to sequences in the short open reading frame (sORF) in the 5′ region of C/EPBβ promotes usage of a downstream start codon, giving rise to the truncated LIP protein. Phosphorylated CELF1 interacts with the initiation factor, eIF2, which is thought to promote use of the downstream AUG by increased ribosome scanning. D. Interaction of CELF1 and hnRNP H at the two ends of the transcript aids circularization. This has been proposed to facilitate translation from an internal ribosome entry site by increasing ribosome recycling.
Like Bru, Xenopus celf3 was shown to bind to the 3′ UTR of cyclin A transcripts, but unlike Bru, celf3 binding stimulated translation.34 Mammalian CELF1 induces translation of p21, a cell cycle inhibitor, by binding to a G/C-rich sequence in its 5′ UTR.74, 75 CELF1 also binds to sequences in the 5′ region of C/EBPβ, where it promotes the use of a downstream AUG translation initiation codon. This gives rise to a truncated form of the C/EBPβ protein, liver inhibitory protein (LIP), in a variety of contexts.23, 39, 76-78 Phosphorylation of CELF1 activates an interaction with subunits of the initiation factor eIF2, leading to recruitment of ribosomes to the downstream AUG start site.39, 79 A CELF1-eIF2 complex also binds to the 5′ UTR of HDAC1 and increases HDAC1 protein levels in aging liver.80
Binding of CELF1 to the 3′ UTR of SHMT, a folate-dependent enzyme, has been implicated in internal ribosome entry site (IRES)-mediated translation.81, 82 A mechanism whereby CELF1-hnRNP H complexes promote circularization of the transcript by mediating 5′/3′ interactions has been proposed.81 Whether CELF1 recruits the translation machinery to the SHMT 5′ UTR via its interaction with eIF2 or another initiation factor remains to be determined.
Integration of cytoplasmic functions
Polyadenylation status, transcript stability, and translation are intimately related. For example, deadenylated transcripts are often degraded or translationally silent. How cytoplasmic CELF functions are mechanistically coupled is unclear. In addition, opposing effects may be observed in different contexts. Tethering CELF1 to the 3′ UTR of a reporter reduced RNA levels while maintaining protein production.83 This suggests a decrease in mRNA stability and increase in translation, the opposite of the effects of CELF2 on COX2 RNA. As with alternative splicing, the construction of RNA binding maps combining 5′ and 3′ UTR binding sites with regulatory information may help elucidate the mechanisms by which the functions of CELF proteins are integrated in the cytoplasm.
CELF PROTEIN PHENOTYPES
Much of what is known about the molecular roles of CELF proteins has been investigated in vitro or in cultured cells. Animal models shed light on the biological roles of CELF proteins in vivo. CELF proteins have been knocked down, knocked out, or inhibited in a variety of species. Consistent with their expression patterns, members of the CELF1-2 subfamily are important for striated muscle function, while the entire family is implicated in nervous system function. Both subfamilies likely participate in gametogenesis and zygotic development.
Mutations in invertebrate CELF proteins
Three mutant alleles in the arrest (aret) locus have been shown to disrupt the coding region of the Drosophila Bru protein.84 The aret mutants have gametogenesis defects in both males and females.85, 86 Bru translationally represses osk, which is important for germ cell formation and posterior body patterning.69 Bru also represses gurken, which is essential for axis formation, and Cyclin A, which drives the release of meiotic arrest and mitotic reentry.71, 72 Celf1 is maternally expressed in zygotes and egg extracts in zebrafish and frogs12, 87, and a cluster of mRNAs involved in the meiotic process of oocyte maturation were identified as potential targets of celf1 deadenylation.56 These data suggest that CELF1 may play a conserved role in the switch from meiosis to mitosis and oocyte to zygote.
In C. elegans, inactivation of ETR-1 by RNAi caused elongation defects and embryonic lethality.19 Worms that survived to hatching had paralysis and defects in muscle attachment. Mutations in C. elegans unc-75 caused defects in motor neuron axon sprouting and synaptic function, but not neuron differentiation.13 Mosaic analysis indicated that UNC-75 affects cholinergic and GABAergic synaptic transmission between motor neurons and target muscles. Unc-75 mutants also had behavioral defects in egg laying and feeding that are characteristic of cholinergic transmission defects.
CELF proteins in the frog embryo
While initially uniform in early Xenopus embryos, celf1 becomes enriched in dorsal mesoderm at neurula and tailbud stages, particularly presomitic mesoderm.88 Somites are transient structures that give rise to multiple tissues including body muscles. Inhibiting celf1 function by injecting antisense oligonucleotides or neutralizing antibodies leads to severe defects in somite segmentation, but not differentiation.88 This phenotype has been attributed to de-repression of Su(H), a component of the notch signaling pathway.89 CELF1 is also expressed in the somites in chick and mouse embryos, perhaps indicating a conserved role in segmentation.10, 18
Although initially isolated as a neural-enriched gene5, knockdown of Xenopus celf3 in embryos inhibited proliferation and subsequent differentiation in the endoderm.34 Conversely, over-expression of celf3 stimulated proliferation.
Knockout mice
Similar to Bru mutants in flies, Celf1 and Celf3 knockout mice both indicate a role in gametogenesis. Mice lacking CELF3 are fertile with normal testicular morphology.90 A significant reduction in the total sperm count was observed, however, suggesting that CELF3 deficiency affects the progression of spermatogenesis. Since fertility was not adversely affected, CELF3 is not essential for spermatogenesis, but compensation by another CELF protein is possible. In contrast to Celf3-null mice, disruption of Celf1 resulted in severely impaired fertility in both males and females.18 Homozygous males are hypofertile, with a blockage in spermiogenesis prior to spermatid elongation, increased apoptosis, and down-regulation of germ cell markers. Histological analysis of the ovaries revealed no obvious changes in the oocytes or follicles, so the basis for reduced fertility in females remains unknown. Celf1 knockout mice also exhibit a high rate of perinatal mortality and growth retardation, perhaps due to impaired placental function. Their failure to thrive and fertility deficits may make future characterization of the effects of loss of CELF1 on other organ systems such as heart, muscle, and brain more difficult in this model.
Consistent with neurotransmission defects in unc-75 mutants, CELF4 deficiency is associated with a seizure disorder in the Frequent flier (Ff) mouse model of epilepsy, so named for its phenotype of convulsions followed by a running-bouncing phase.15 The Ff mutation arises from a transgenic insertion in the Celf4 gene, which results in loss of function. A significantly reduced seizure threshold was observed in heterozygotes with progression of recurrent limbic and tonic clonic seizures. Homozygotes did not usually survive in the original C57BL/6J background, but those that survived in F2 hybrid backgrounds displayed spike-wave discharges, the hallmark of absence epilepsy. This suggests that although all six CELF proteins are expressed in brain, CELF4 plays a non-redundant role in neural function.
Transgenic dominant negative CELF protein models
Two mouse models have been generated that use tissue-specific expression of a nuclear dominant negative CELF protein, NLSCELFΔ, to investigate CELF-mediated alternative splicing programs in vivo. The dominant negative approach offers several advantages. First, all CELF proteins present in a tissue (e.g. CELF1 and CELF2 in striated muscle) are simultaneously inhibited. NLSCELFΔ has been shown to inhibit the splicing activity of all six family members.32 Second, because NLSCELFΔ is restricted to the nucleus, it presumably blocks only the nuclear function of CELF proteins, leaving cytoplasmic functions intact. Third, tissue-specific repression is easily achieved through use of tissue-specific promoters. Disadvantages are that individual functions of family members cannot be separated, and repression may not be complete.
MHC-CELFΔ mice express NLSCELFΔ under a cardiac muscle-specific promoter.48 MHC-CELFΔ mice develop defects in CELF-mediated alternative splicing, dilated cardiomyopathy, and severe cardiac dysfunction. These changes were attributed to loss of CELF activity because over-expression of human CELF1 in bitransgenic mice partially rescued both splicing defects and overt heart disease. Myo-CELFΔ mice express NLSCELFΔ under a skeletal muscle-specific promoter.91 Unlike the MHC-CELFΔ mice, these mice display small changes in alternative splicing and have a mild phenotype. Myo-CELFΔ mice exhibit reduced muscle interstitia, increased variability in fiber size, and a slight increase in slow twitch fibers. These modest changes may indicate that CELF proteins are more determinative for alternative splicing in heart than in skeletal muscle. Alternatively, NLSCELFΔ may more effectively inhibit CELF activity in MHC-CELFΔ hearts than in Myo-CELFΔ muscles.
CELF PROTEINS IN DISEASE
Consistent with their expression patterns and animal model phenotypes, CELF proteins have been implicated in pathogenesis in the heart, skeletal muscle, and nervous system.
CELF proteins in cardiac pathogenesis
The human CELF2 gene is located at chromosome 10p13-14. CELF2 has been proposed as a candidate gene for two genetic disorders affecting the heart that arise from mutations in this vicinity. Arrythmogenic right ventricular dysplasia (ARVD) is characterized by progressive myocardial cell loss and fibrosis.92 A familial form of ARVD, designated ARVD6, maps to the locus 10p12-14, which contains CELF2. ARVD6 has high penetrance, early onset, and a high incidence of sudden death. Although no mutation was found in ARVD6 family members that would be predicted to change the CELF2 protein sequence, variations were detected that cosegregate with the disease.92 Partial monosomy 10p is a rare chromosomal aberration associated with symptoms of the DiGeorge syndrome spectrum. Haploinsufficiency within the proximal region of 10p, DGCR2, has been linked to heart and thymus defects. CELF2 was the only known gene found within a critical 300 kb region of DGCR2 identified by deletion mapping of partial monosomy 10p patients.93 Although loss of CELF2 was not proven to cause heart defects in either ARVD6 or partial monosomy 10p, it is a promising candidate for future clinical and functional studies.
CELF proteins in muscle diseases
The best-studied example of involvement of a CELF protein in disease is that of CELF1 in myotonic dystrophy (DM). DM is a form of muscular dystrophy that affects several systems, including skeletal muscle and heart. DM results from expression of RNAs containing expanded CUG or CCUG repeats that form double-stranded hairpins, accumulate in nuclear foci, and disrupt the processing of other RNAs.94 CELF1 was first identified as a CUG-repeat binding protein1, leading to the proposal that the mutant RNAs sequester CELF1. Later studies indicated that another splicing factor, muscleblind-like 1 (MBNL1), is sequestered.95, 96 CELF1, however, does not interact with long CUG repeats or co-localize with mutant RNAs in cells.97, 98 Rather, CELF1 is up-regulated in DM heart and skeletal muscle.99, 100 PKC-mediated phosphorylation of CELF1 stabilizes the protein without a change in CELF1 transcript levels.38, 101 Increased CELF1 activity disrupts alternative splicing and translation events that are directly linked to disease symptoms.75, 99, 102 DM pathogenesis is now believed to result from a combination of effects including CELF1 over-expression and MBNL1 sequestration.
Transgenic mouse models that over-express CELF1 in striated muscle tissues support its contribution to DM pathogenesis. CELF1 is constitutively over-expressed in skeletal muscle in two models.103, 104 Both exhibited defects in muscle histology and physiology that recapitulate features of DM. Timchenko et al. showed that CELF1 over-expression enhanced translation of p21 and MEF2A104, while Ho et al. demonstrated changes in alternative splicing of muscle transcripts.103 Because embryonic muscle development is impaired in these mice, the effects of CELF1 over-expression in adult muscle are difficult to assess. Bitransgenic mice that inducibly over-express CELF1 demonstrated that elevated CELF1 in adult skeletal muscle is sufficient to induce impaired muscle performance, muscle wasting, and alternative splicing changes seen in DM.105 Inducible over-expression of CELF1 in heart likewise exhibits echocardiographic, electrocardiographic, and histological abnormalities seen in DM patients.106
While the dysregulation of CELF1 in DM is thought to be a primary pathogenic event, a recent study found that changes in alternative splicing and increases in CELF1 and CELF2 are also seen in other types of muscular dystrophy and muscle injury.107 Elevated levels of CELF2 have also been reported in Duchenne and Becker muscular dystrophies.108, 109 These changes may reflect the reiteration of early developmental programs during muscle regeneration.
CELF proteins in nervous system disorders
Although DM is primarily considered a striated muscle disease, patients also have symptoms affecting the nervous system, including cognitive impairment.94 Several exons of tau, which is involved in forming protein aggregates in Alzheimer’s disease and other dementias, are regulated by CELF proteins and are dysregulated in DM.110-114 Interestingly, skipping of tau exon 10, which encodes a microtubule binding domain, is induced by over-expression of CELF2, but not over-expression of CELF1 or MBNL1 knockdown.115 CELF1 and CELF2 are both up-regulated in DM brains, and increased exon 10 skipping correlates with elevated CELF protein levels.115 Polymorphisms in CELF2 were also identified in a genome-wide association study of late-onset Alzheimer’s disease that are significantly associated with high risk alleles of APOE.116
Expression of an expanded CUG repeat-containing RNA in the neurodegenerative disorder spinocerebellar ataxia type 8 (SCA8) may follow a similar mechanism to DM, as MBNL1 is sequestered and CELF/MBNL alternative splicing is disrupted in neurons.50 Fragile X syndrome, a form of hereditary mental retardation, is caused by a CGG repeat expansion in the 5′ UTR of the FMR1 gene that leads to silencing and loss of the FMRP protein.117 In a transgenic fly that expresses 90 CGG repeats over-expression of CELF1 suppresses the neurodegenerative phenotype through interaction with the CGG-binding protein hnRNP A2/B1.118
The mechanistic similarities between CELF-mediated pathogenesis in the nervous system and striated muscle suggest that although CELF family members are differentially expressed throughout the body, they fulfill similar functional niches in different cell types. As additional CELF targets are identified, it will be interesting to determine whether common pathways are involved in pathogenesis in different cell types.
Conclusion
CELF proteins have been shown to regulate RNA processing at several important steps in the nucleus and cytoplasm, but little is known about whether or how these disparate activities are coordinated. Dysregulation of CELF1-mediated alternative splicing and translation have been shown to contribute to pathogenesis in DM.103, 104 It is easy to imagine that shuttling of CELF1 between subcellular compartments serves to coordinate these processes in healthy tissues.
Many tissues express multiple CELF proteins, but the extent of functional redundancy and individual contributions of specific family members have not been determined in most cases. Many studies have focused on only a single CELF protein, and CELF1 and CELF2 have received greater attention than CELF3-6. The distinct phenotypes of CELF knockout mice despite overlapping expression patterns suggest that different family members do indeed play different roles in vivo. The elucidation and comparison of targets regulated by each family member will help to delineate specific CELF regulons.
Acknowledgements
We apologize to anyone whose work was not cited due to lack of space. Research in the Ladd laboratory is supported by the National Institutes of Health (1R01HL089376).
Contributor Information
Twishasri Dasgupta, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic.
Andrea N. Ladd, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic
References
- 1.Timchenko L, Miller J, Timchenko N, Devore D, Datar K, Lin L, Roberts R, Caskey C, Swanson M. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nuc Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang D, Hwang W, Liew C. Single pass sequencing of a unidirectional human fetal heart cDNA library to discover novel genes of the cardiovascular system. J Molec Cell Cardiol. 1994;26:1329–1333. doi: 10.1006/jmcc.1994.1151. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Ito T, Mitsui Y, Sakaki Y. Fluorescent differential display analysis of gene expression in apoptotic neuroblastoma cells. Gene. 1998;223:21–31. doi: 10.1016/s0378-1119(98)00364-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu X, Timchenko N, Timchenko L. Cardiac elav-type RNA-binding protein (ETR-3) binds to RNA CUG repeats expanded in myotonic dystrophy. Hum Mol Gen. 1999;8:53–60. doi: 10.1093/hmg/8.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Knecht A, Good P, Dawid I, Harland R. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–1936. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- 6.Good P, Chen Q, Warner S, Herring D. A family of human RNA-binding proteins related to the Drosophila Bruno translational regulator. J Biol Chem. 2000;275:28583–28592. doi: 10.1074/jbc.M003083200. [DOI] [PubMed] [Google Scholar]
- 7.Ladd A, Charlet-B N, Cooper T. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol. 2001;21:1285–1296. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladd A, Nguyen N, Malhotra K, Cooper T. CELF6, a member of the CELF family of RNA binding proteins, regulates muscle-specific splicing enhancer-dependent alternative splicing. J Biol Chem. 2004;279:17756–17764. doi: 10.1074/jbc.M310687200. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R, Timchenko N, Miller J, Reddy S, Caskey C, Swanson M, Timchenko L. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc Natl Acad Sci. 1997;94:13221–13226. doi: 10.1073/pnas.94.24.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brimacombe KR, Ladd AN. Cloning and embryonic expression patterns of the chicken CELF family. Dev Dyn. 2007;236:2216–2224. doi: 10.1002/dvdy.21209. [DOI] [PubMed] [Google Scholar]
- 11.Delaunay J, Le Mee G, Ezzeddine N, Labesse G, Terzian C, Capri M, Ait-Ahmed O. The Drosophila Bruno paralogue Bru-3 specifically binds the EDEN translational repression element. Nucleic Acids Res. 2004;32:3070–3082. doi: 10.1093/nar/gkh627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Li C, Zhao S, Mao B. Differential expression of the Brunol/CELF family genes during Xenopus laevis early development. Int J Dev Biol. 2010;54:209–214. doi: 10.1387/ijdb.082685jw. [DOI] [PubMed] [Google Scholar]
- 13.Loria P, Duke A, Rand J, Hobert O. Two neuronal, nuclear-localized RNA binding proteins involved in synaptic transmission. Curr Biol. 2003;13:1317–1323. doi: 10.1016/s0960-9822(03)00532-3. [DOI] [PubMed] [Google Scholar]
- 14.Meins M, Schlickum S, Wilhelm C, Missbach J, Yadav S, Glaser B, Grzmil M, Burfeind P, Laccone F. Identification and characterization of murine Brunol4, a new member of the elav/bruno family. Cytogenet Genome Res. 2002;97:254–260. doi: 10.1159/000066619. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Mahaffey CL, Berube N, Maddatu TP, Cox GA, Frankel WN. Complex seizure disorder caused by Brunol4 deficiency in mice. PLoS Genet. 2007;3:e124. doi: 10.1371/journal.pgen.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi D, Ito T, Tsukahara F, Hirai M, Sakaki Y. Developmentally regulated expression of mNapor encoding an apoptosis-induced ELAV-type RNA binding protein. Gene. 1999;237:135–142. doi: 10.1016/s0378-1119(99)00312-1. [DOI] [PubMed] [Google Scholar]
- 17.Choi DK, Yoo KW, Hong SK, Rhee M, Sakaki Y, Kim CH. Isolation and expression of Napor/CUG-BP2 in embryo development. Biochem Biophys Res Commun. 2003;305:448–454. doi: 10.1016/s0006-291x(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 18.Kress C, Gautier-Courteille C, Osborne HB, Babinet C, Paillard L. Inactivation of CUG-BP1/CELF1 causes growth, viability, and spermatogenesis defects in mice. Mol Cell Biol. 2007;27:1146–1157. doi: 10.1128/MCB.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne C, Hodgkin J. ETR-1, a homologue of a protein linked to myotonic dystropy, is essential for muscle development in Caenorhabditis elegans. Curr Biol. 1999;9:1243–1246. doi: 10.1016/s0960-9822(99)80504-1. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, Sasagawa N, Suzuki K, Ishiura S. The CUG-binding protein binds specifically to UG dinucleotide repeats in a yeast three-hybird system. Biochem Biophys Res Comm. 2000;277:518–523. doi: 10.1006/bbrc.2000.3694. [DOI] [PubMed] [Google Scholar]
- 21.Marquis J, Paillard L, Audic Y, Cosson B, Danos O, Le Bec C, Osborne HB. CUG-BP1/CELF1 requires UGU-rich sequences for high-affinity binding. Biochem J. 2006;400:291–301. doi: 10.1042/BJ20060490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faustino N, Cooper T. Identification of putative new splicing targets for ETR-3 using its SELEX sequences. Mol Cell Biol. 2005;25:879–887. doi: 10.1128/MCB.25.3.879-887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nuc Acids Res. 1999;27:4517–4525. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salisbury E, Sakai K, Schoser B, Huichalaf C, Schneider-Gold C, Nguyen H, Wang GL, Albrecht JH, Timchenko LT. Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp Cell Res. 2008;314:2266–2278. doi: 10.1016/j.yexcr.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paillard L, Omilli F, Legagneux V, Bassez T, Maniey D, Osborne H. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. EMBO J. 1998;17:278–287. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori D, Sasagawa N, Kino Y, Ishiura S. Quantitative analysis of CUG-BP1 binding to RNA repeats. J Biochem. 2008;143:377–383. doi: 10.1093/jb/mvm230. [DOI] [PubMed] [Google Scholar]
- 27.Tsuda K, Kuwasako K, Takahashi M, Someya T, Inoue M, Terada T, Kobayashi N, Shirouzu M, Kigawa T, Tanaka A, et al. Structural basis for the sequence-specific RNA-recognition mechanism of human CUG-BP1 RRM3. Nuc Acids Res. 2009;37:5151–5166. doi: 10.1093/nar/gkp546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet-Corven S, Audic Y, Omilli F, Osborne HB. An analysis of the sequence requirements of EDEN-BP for specific RNA binding. Nuc Acids Res. 2002;30:4667–4674. doi: 10.1093/nar/gkf586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh G, Charlet BN, Han J, Cooper TA. ETR-3 and CELF4 protein domains required for RNA binding and splicing activity in vivo. Nuc Acids Res. 2004;32:1232–1241. doi: 10.1093/nar/gkh275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teplova M, Song J, Gaw HY, Teplov A, Patel DJ. Structural insights into RNA recognition by the alternate-splicing regulator CUG-binding protein 1. Structure. 2010;18:1364–1377. doi: 10.1016/j.str.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Cooper TA. Identification of CELF splicing activation and repression domains in vivo. Nuc Acids Res. 2005;33:2769–2780. doi: 10.1093/nar/gki561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlet-B N, Logan P, Singh G, Cooper T. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol Cell. 2002;9:649–658. doi: 10.1016/s1097-2765(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 33.Dujardin G, Buratti E, Charlet-Berguerand N, Martins de Araujo M, Mbopda A, Le Jossic-Corcos C, Pagani F, Ferec C, Corcos L. CELF proteins regulate CFTR pre-mRNA splicing: essential role of the divergent domain of ETR-3. Nuc Acids Res. 2010;38:7273–7285. doi: 10.1093/nar/gkq573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horb LD, Horb ME. BrunoL1 regulates endoderm proliferation through translational enhancement of cyclin A2 mRNA. Dev Biol. 2010;345:156–169. doi: 10.1016/j.ydbio.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladd A, Cooper T. Multiple domains control the subcellular localization and activity of ETR-3, a regulator of nuclear and cytoplasmic RNA processing events. J Cell Sci. 2004;117:3519–3529. doi: 10.1242/jcs.01194. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura K, Kano F, Murata M. Dual localization of the RNA binding protein CUGBP-1 to stress granule and perinucleolar compartment. Exp Cell Res. 2008;314:543–553. doi: 10.1016/j.yexcr.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Cosson B, Gautier-Courteille C, Maniey D, Ait-Ahmed O, Lesimple M, Osborne HB, Paillard L. Oligomerization of EDEN-BP is required for specific mRNA deadenylation and binding. Biol Cell. 2006;98:653–665. doi: 10.1042/BC20060054. [DOI] [PubMed] [Google Scholar]
- 38.Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timchenko LT, Salisbury E, Wang GL, Nguyen H, Albrecht JH, Hershey JW, Timchenko NA. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. J Biol Chem. 2006;281:32806–32819. doi: 10.1074/jbc.M605701200. [DOI] [PubMed] [Google Scholar]
- 40.Morgan GT. Localized co-transcriptional recruitment of the multifunctional RNA-binding protein CELF1 by lampbrush chromosome transcription units. Chromosome Res. 2007;15:985–1000. doi: 10.1007/s10577-007-1179-1. [DOI] [PubMed] [Google Scholar]
- 41.Philips A, Timchenko L, Cooper T. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 42.Anant S, Henderson J, Mukhopadhyay D, Navaratnam N, Kennedy S, Min J, Davidson N. Novel role for RNA-binding protein CUGBP2 in mammalian RNA editing. J Biol Chem. 2001;276:47338–47351. doi: 10.1074/jbc.M104911200. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z, Eggerman TL, Patterson AP. ApoB mRNA editing is mediated by a coordinated modulation of multiple apoB mRNA editing enzyme components. Am J Physiol Gastrointest Liver Physiol. 2007;292:G53–65. doi: 10.1152/ajpgi.00118.2006. [DOI] [PubMed] [Google Scholar]
- 44.Cooper TA. Use of minigene systems to dissect alternative splicing elements. Methods. 2005;37:331–340. doi: 10.1016/j.ymeth.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Ladd A, Stenberg M, Swanson M, Cooper T. A dynamic balance between activation and repression regulates pre-mRNA alternative splicing during heart development. Dev. Dyn. 2005;233:783–793. doi: 10.1002/dvdy.20382. [DOI] [PubMed] [Google Scholar]
- 46.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB, Cooper TA. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci U S A. 2008;105:20333–20338. doi: 10.1073/pnas.0809045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dembowski JA, Grabowski PJ. The CUGBP2 splicing factor regulates an ensemble of branchpoints from perimeter binding sites with implications for autoregulation. PLoS Genet. 2009;5:e1000595. doi: 10.1371/journal.pgen.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladd A, Taffet G, Hartley C, Kearney D, Cooper T. Cardiac-specific repression of CELF activity disrupts alternative splicing and causes cardiomyopathy. Mol Cell Biol. 2005;25:6267–6278. doi: 10.1128/MCB.25.14.6267-6278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ule J, Jensen K, Mele A, Darnell RB. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37:376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LP. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goo YH, Cooper TA. CUGBP2 directly interacts with U2 17S snRNP components and promotes U2 snRNA binding to cardiac troponin T pre-mRNA. Nuc Acids Res. 2009;37:4275–4286. doi: 10.1093/nar/gkp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barron VA, Zhu H, Hinman MN, Ladd AN, Lou H. The neurofibromatosis type I pre-mRNA is a novel target of CELF protein-mediated splicing regulation. Nuc Acids Res. 2010;38:253–264. doi: 10.1093/nar/gkp766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darnell RB. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. WIREs RNA. 2010;1:266–286. doi: 10.1002/wrna.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanc V, Davidson NO. APOBEC-1-mediated RNA editing. Wiley Interdiscip Rev Syst Biol Med. 2010;2:594–602. doi: 10.1002/wsbm.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blanc V, Davidson NO. C-to-U RNA editing: mechanisms leading to genetic diversity. J Biol Chem. 2003;278:1395–1398. doi: 10.1074/jbc.R200024200. [DOI] [PubMed] [Google Scholar]
- 56.Graindorge A, Le Tonqueze O, Thuret R, Pollet N, Osborne HB, Audic Y. Identification of CUG-BP1/EDEN-BP target mRNAs in Xenopus tropicalis. Nuc Acids Res. 2008;36:1861–1870. doi: 10.1093/nar/gkn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paillard L, Legagneux V, Maniey D, Osborne H. c-Jun ARE targets mRNA deadenlyation by an EDEN-BP (embryo deadenylation element-binding protein)-dependent pathway. J Biol Chem. 2002;277:3232–3235. doi: 10.1074/jbc.M109362200. [DOI] [PubMed] [Google Scholar]
- 58.Paillard L, Legagneux V, Osborne H. A functional deadenylation assay identifies human CUG-BP as a deadenylation factor. Biol of the Cell. 2003;95:107–113. doi: 10.1016/s0248-4900(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 59.Moraes KC, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–1091. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JH, Richter JD. Opposing polymerase-deadenylase activities regulate cytoplasmic polyadenylation. Mol Cell. 2006;24:173–183. doi: 10.1016/j.molcel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Vlasova IA, Bohjanen PR. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol. 2008;5:201–207. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JE, Lee JY, Wilusz J, Tian B, Wilusz CJ. Systematic analysis of cis-elements in unstable mRNAs demonstrates that CUGBP1 is a key regulator of mRNA decay in muscle cells. PLoS One. 2010;5:e11201. doi: 10.1371/journal.pone.0011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, St Louis-Vlasova IA, Bohjanen PR. Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol. 2010;30:3970–3980. doi: 10.1128/MCB.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Lee JE, Wilusz J, Wilusz CJ. The RNA-binding protein CUGBP1 regulates stability of tumor necrosis factor mRNA in muscle cells: implications for myotonic dystrophy. J Biol Chem. 2008;283:22457–22463. doi: 10.1074/jbc.M802803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukhopadhyay D, Houchen C, Kennedy S, Dieckgraefe B, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell. 2003;11:113–126. doi: 10.1016/s1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 66.Mukhopadhyay D, Jung J, Murmu N, Houchen CW, Dieckgraefe BK, Anant S. CUGBP2 plays a critical role in apoptosis of breast cancer cells in response to genotoxic injury. Ann N Y Acad Sci. 2003;1010:504–509. doi: 10.1196/annals.1299.093. [DOI] [PubMed] [Google Scholar]
- 67.Murmu N, Jung J, Mukhopadhyay D, Houchen CW, Riehl TE, Stenson WF, Morrison AR, Arumugam T, Dieckgraefe BK, Anant S. Dynamic antagonism between RNA-binding protein CUGBP2 and cyclooxygenase-2-mediated prostaglandin E2 in radiation damage. Proc Natl Acad Sci U S A. 2004;101:13873–13878. doi: 10.1073/pnas.0406066101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subramaniam D, Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S. Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1025–1032. doi: 10.1152/ajpgi.00602.2007. [DOI] [PubMed] [Google Scholar]
- 69.Kim-Ha J, Kerr K, Macdonald P. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura A, Sato K, Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 71.Filardo P, Ephrussi A. Bruno regulates gurken during Drosophila oogenesis. Mech Dev. 2003;120:289–297. doi: 10.1016/s0925-4773(02)00454-9. [DOI] [PubMed] [Google Scholar]
- 72.Sugimura I, Lilly MA. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev Cell. 2006;10:127–135. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Sureban SM, Murmu N, Rodriguez P, May R, Maheshwari R, Dieckgraefe BK, Houchen CW, Anant S. Functional antagonism between RNA binding proteins HuR and CUGBP2 determines the fate of COX-2 mRNA translation. Gastroenterology. 2007;132:1055–1065. doi: 10.1053/j.gastro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 74.Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. Embo J. 2004;23:406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timchenko N, Iakova P, Cai Z-J, Smith J, Timchenko L. Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol Cell Biol. 2001;21:6927–6938. doi: 10.1128/MCB.21.20.6927-6938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPbeta-LIP in mammary epithelial cells. Mol Cell Biol. 2004;24:3682–3691. doi: 10.1128/MCB.24.9.3682-3691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 promotes liver proliferation in young mice via interactions with C/EBPbeta. J Biol Chem. 2008;283:26179–26187. doi: 10.1074/jbc.M803545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welm AL, Mackey SL, Timchenko LT, Darlington GJ, Timchenko NA. Translational induction of liver-enriched transcriptional inhibitory protein during acute phase response leads to repression of CCAAT/enhancer binding protein alpha mRNA. J Biol Chem. 2000;275:27406–27413. doi: 10.1074/jbc.M002343200. [DOI] [PubMed] [Google Scholar]
- 79.Timchenko NA, Wang GL, Timchenko LT. RNA CUG-binding protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein beta by interacting with the alpha and beta subunits of eukaryotic initiation translation factor 2. J Biol Chem. 2005;280:20549–20557. doi: 10.1074/jbc.M409563200. [DOI] [PubMed] [Google Scholar]
- 80.Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. J Biol Chem. 2008;283:26169–26178. doi: 10.1074/jbc.M803544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fox JT, Stover PJ. Mechanism of the internal ribosome entry site-mediated translation of serine hydroxymethyltransferase 1. J Biol Chem. 2009;284:31085–31096. doi: 10.1074/jbc.M109.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woeller CF, Fox JT, Perry C, Stover PJ. A ferritin-responsive internal ribosome entry site regulates folate metabolism. J Biol Chem. 2007;282:29927–29935. doi: 10.1074/jbc.M706264200. [DOI] [PubMed] [Google Scholar]
- 83.Barreau C, Watrin T, Beverley Osborne H, Paillard L. Protein expression is increased by a class III AU-rich element and tethered CUG-BP1. Biochem Biophys Rese Comm. 2006;347:723–730. doi: 10.1016/j.bbrc.2006.06.177. [DOI] [PubMed] [Google Scholar]
- 84.Webster PJ, Liang L, Berg CA, Lasko P, Macdonald PM. Translational repressor bruno plays multiple roles in development and is widely conserved. Genes Dev. 1997;11:2510–2521. doi: 10.1101/gad.11.19.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki H, Maegawa S, Nishibu T, Sugiyama T, Yasuda K, Inoue K. Vegetal localization of the maternal mRNA encoding an EDEN-BP/Bruno-like protein in zebrafish. Mech Dev. 2000;93:205–209. doi: 10.1016/s0925-4773(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 88.Gautier-Courteille C, Le Clainche C, Barreau C, Audic Y, Graindorge A, Maniey D, Osborne HB, Paillard L. EDEN-BP-dependent post-transcriptional regulation of gene expression in Xenopus somitic segmentation. Development. 2004;131:6107–6117. doi: 10.1242/dev.01528. [DOI] [PubMed] [Google Scholar]
- 89.Cibois M, Gautier-Courteille C, Vallee A, Paillard L. A strategy to analyze the phenotypic consequences of inhibiting the association of an RNA-binding protein with a specific RNA. RNA. 2010;16:10–15. doi: 10.1261/rna.1742610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dev A, Nayernia K, Meins M, Adham I, Lacone F, Engel W. Mice deficient for RNA-binding protein brunol1 show reduction of spermatogenesis but are fertile. Mol Reprod Dev. 2007 doi: 10.1002/mrd.20742. [DOI] [PubMed] [Google Scholar]
- 91.Berger DS, Moyer M, Kliment GM, van Lunteren E, Ladd AN. Expression of a dominant negative CELF protein in vivo leads to altered muscle organization, fiber size, and subtype. PLoS One. 2011;6(4):e19274. doi: 10.1371/journal.pone.0019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li D, Bachinski L, Roberts R. Genomic organization and isoform-specific tissue expression of human NAPOR (CUGBP2) as a candidate gene for familial arrhythmogenic right ventricular dysplasia. Genomics. 2001;74:396–401. doi: 10.1006/geno.2001.6558. [DOI] [PubMed] [Google Scholar]
- 93.Lichtner P, Attié-Bitach T, Schuffenhauer S, Henwood J, Bouvagnet P, Scambler P, Meitinger T, Vekemans M. Expression and mutation analysis of Brunol3, a candidate gene for heart and thymus developmental defects associated with partial monosomy 10p. J Mol Med. 2002;80:431–442. doi: 10.1007/s00109-002-0331-9. [DOI] [PubMed] [Google Scholar]
- 94.Schoser B, Timchenko L. Myotonic dystrophies 1 and 2: complex diseases with complex mechanisms. Curr Genomics. 2010;11:77–90. doi: 10.2174/138920210790886844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fardaei M, Rogers M, Thorpe H, Larkin K, Hamshere M, Harper P, Brook J. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Gen. 2002;11:805–814. doi: 10.1093/hmg/11.7.805. [DOI] [PubMed] [Google Scholar]
- 96.Mankodi A, Urbinati C, Yuan Q-P, Moxley R, Sansone V, Krym M, Henderson D, Schalling M, Swanson M, Thornton C. Muscleblind localizes to nucelar foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum Mol Gen. 2001;10:2165–2170. doi: 10.1093/hmg/10.19.2165. [DOI] [PubMed] [Google Scholar]
- 97.Fardaei M, Larkin K, Brook J, Hamshere M. In vivo co-localization of MBNL protein with DMPK expanded-repeat transcripts. Nuc Acids Res. 2001;29:2766–2771. doi: 10.1093/nar/29.13.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michalowski S, Miller J, Urbinati C, Paliouras M, Swanson M, Griffith J. Visualization of double-stranded RNAs from the myotonic dystrophy protein kinase gene and interactions with CUG-binding protein. Nuc Acids Res. 1999;27:3534–3542. doi: 10.1093/nar/27.17.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Savkur R, Phillips A, Cooper T. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29:40–47. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 100.Timchenko N, Cai Z-J, Welm A, Reddy S, Ashizawa T, Timchenko L. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem. 2001;276:7820–7826. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 101.Nezu Y, Kino Y, Sasagawa N, Nishino I, Ishiura S. Expression of MBNL and CELF mRNA transcripts in muscles with myotonic dystrophy. Neuromuscul Disord. 2007;17:306–312. doi: 10.1016/j.nmd.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Charlet-B N, Savkur R, Singh G, Philips A, Grice E, Cooper T. Loss of the muscle-specific chloride channel in type I myotonic dystrophy lead to misregulated alternative splicing. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 103.Ho TH, Bundman D, Armstrong DL, Cooper TA. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum Mol Genet. 2005;14:1539–1547. doi: 10.1093/hmg/ddi162. [DOI] [PubMed] [Google Scholar]
- 104.Timchenko NA, Patel R, Iakova P, Cai ZJ, Quan L, Timchenko LT. Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J Biol Chem. 2004;279:13129–13139. doi: 10.1074/jbc.M312923200. [DOI] [PubMed] [Google Scholar]
- 105.Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet. 2010;19:3614–3622. doi: 10.1093/hmg/ddq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koshelev M, Sarma S, Price RE, Wehrens XH, Cooper TA. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum Mol Genet. 2010;19:1066–1075. doi: 10.1093/hmg/ddp570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Orengo JP, Ward AJ, Cooper TA. Alternative splicing dysregulation secondary to skeletal muscle regeneration. Ann Neurol. 2010 doi: 10.1002/ana.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bakay M, Zhao P, Chen J, Hoffman E. A web-accessible complete transcriptome of normal human and DMD muscle. Neuromusc Disorders. 2002;12:S125–S141. doi: 10.1016/s0960-8966(02)00093-7. [DOI] [PubMed] [Google Scholar]
- 109.Sironi M, Cagliani R, Pozzoli U, Bardoni A, Comi G, Giorda R, Bresolin N. The dystrophin gene is alternative spliced throughout its coding sequence. FEBS Lett. 2002;517:163–166. doi: 10.1016/s0014-5793(02)02613-3. [DOI] [PubMed] [Google Scholar]
- 110.Leroy O, Dhaenens CM, Schraen-Maschke S, Belarbi K, Delacourte A, Andreadis A, Sablonniere B, Buee L, Sergeant N, Caillet-Boudin ML. ETR-3 represses Tau exons 2/3 inclusion, a splicing event abnormally enhanced in myotonic dystrophy type I. J Neurosci Res. 2006;84:852–859. doi: 10.1002/jnr.20980. [DOI] [PubMed] [Google Scholar]
- 111.Leroy O, Wang J, Maurage CA, Parent M, Cooper T, Buee L, Sergeant N, Andreadis A, Caillet-Boudin ML. Brain-specific change in alternative splicing of Tau exon 6 in myotonic dystrophy type 1. Biochim Biophys Acta. 2006;1762:460–467. doi: 10.1016/j.bbadis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Sergeant N, Sablonniere B, Schraen-Maschke S, Ghestem A, Maurage CA, Wattez A, Vermersch P, Delacourte A. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet. 2001;10:2143–2155. doi: 10.1093/hmg/10.19.2143. [DOI] [PubMed] [Google Scholar]
- 113.Vermesch P, Sergeant N, Ruchoux M, Hofmann-Radvanyi H, Wattez A, Petit H, Dwailly P, Delacourte A. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology. 1996;47:711–717. doi: 10.1212/wnl.47.3.711. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, Gao QS, Wang Y, Lafyatis R, Stamm S, Andreadis A. Tau exon 10, whose missplicing causes frontotemporal dementia, is regulated by an intricate interplay of cis elements and trans factors. J Neurochem. 2004;88:1078–1090. doi: 10.1046/j.1471-4159.2003.02232.x. [DOI] [PubMed] [Google Scholar]
- 115.Dhaenens CM, Tran H, Frandemiche ML, Carpentier C, Schraen-Maschke S, Sistiaga A, Goicoechea M, Eddarkaoui S, Van Brussels E, Obriot H, et al. Mis-splicing of Tau exon 10 in myotonic dystrophy type 1 is reproduced by overexpression of CELF2 but not by MBNL1 silencing. Biochim Biophys Acta. 2011;1812:732–742. doi: 10.1016/j.bbadis.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 116.Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, Lee JH, Bird TD, Bennett DA, Diaz-Arrastia R, et al. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259–277. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- 118.Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wollerton MC, Gooding C, Wagner EJ, Garcia-Blanco MA, Smith CW. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol Cell. 2004;13:91–100. doi: 10.1016/s1097-2765(03)00502-1. [DOI] [PubMed] [Google Scholar]
- 120.Philips A, Cooper T. RNA processing and human disease. Cell Molec Life Sci. 1999 doi: 10.1007/PL00000687. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poleev A, Hartmann A, Stamm S. A trans-acting factor, isolated by the three-hybrid system, that influences alternative splicing of the amyloid precursor protein minigene. Eur J Biochem. 2000;267:4002–4010. doi: 10.1046/j.1432-1327.2000.01431.x. [DOI] [PubMed] [Google Scholar]
- 122.Jiang H, Mankodi A, Swanson MS, Moxley RT, Thornton CA. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet. 2004;13:3079–3088. doi: 10.1093/hmg/ddh327. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Liu W, Grabowski P. Coordinate repression of a trio of neuron-specific splicing events by the splicing regulator PTB. RNA. 1999;5:117–130. doi: 10.1017/s1355838299981530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang W, Haiying L, Kyoungha H, Grabowski P. Region-specific alternative splicing in the nervous system: Implications for regulation by the RNA-binding protein NAPOR. RNA. 2002;8:671–685. doi: 10.1017/s1355838202027036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gromak N, Matlin A, Cooper T, Smith C. Antagonistic regulation of a-actinin alternative splicing by CELF proteins and polypyrimidine tract binding protein. RNA. 2003;9:443–456. doi: 10.1261/rna.2191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suzuki H, Jin Y, Otani H, Yasuda K, Inoue K. Regulation of alternative splicing of a-actinin transcript by Bruno-like proteins. Genes to Cells. 2002;7:133–141. doi: 10.1046/j.1356-9597.2001.00506.x. [DOI] [PubMed] [Google Scholar]
- 127.Sureau A, Sauliere J, Expert-Bezancon A, Marie J. CELF and PTB proteins modulate the inclusion of the beta-tropomyosin exon 6B during myogenic differentiation. Exp Cell Res. 2011;317:94–106. doi: 10.1016/j.yexcr.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Chapple JP, Anthony K, Martin TR, Dev A, Cooper TA, Gallo JM. Expression, localization and tau exon 10 splicing activity of the brain RNA-binding protein TNRC4. Hum Mol Genet. 2007;16:2760–2769. doi: 10.1093/hmg/ddm233. [DOI] [PubMed] [Google Scholar]
- 129.Li K, Arikan MC, Andreadis A. Modulation of the membrane-binding domain of tau protein: splicing regulation of exon 2. Brain Res Mol Brain Res. 2003;116:94–105. doi: 10.1016/s0169-328x(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 130.Terenzi F, Brimacombe KR, Penn MS, Ladd AN. CELF-mediated alternative splicing is required for cardiac function during early, but not later, postnatal life. J Mol Cell Cardiol. 2009;46:395–404. doi: 10.1016/j.yjmcc.2008.10.030. [DOI] [PubMed] [Google Scholar]