Abstract
Mutations in the phosphatidylinositol 4,5-bisphosphate (PtdIns4,5P2) 5-phosphatase OCRL cause Lowe syndrome, which is characterised by congenital cataracts, central hypotonia, and renal proximal tubular dysfunction. Previous studies have shown that OCRL interacts with components of the endosomal machinery; however, its role in endocytosis, and thus the pathogenic mechanisms of Lowe syndrome, have remained elusive. Here, we show that via its 5-phosphatase activity, OCRL controls early endosome (EE) function. OCRL depletion impairs the recycling of multiple classes of receptors, including megalin (which mediates protein reabsorption in the kidney) that are retained in engorged EEs. These trafficking defects are caused by ectopic accumulation of PtdIns4,5P2 in EEs, which in turn induces an N-WASP-dependent increase in endosomal F-actin. Our data provide a molecular explanation for renal proximal tubular dysfunction in Lowe syndrome and highlight that tight control of PtdIns4,5P2 and F-actin at the EEs is essential for exporting cargoes that transit this compartment.
Keywords: actin, early endosomes, Lowe syndrome, megalin, phosphoinositides
Introduction
Mutations in OCRL, which encodes a phosphatidylinositol 4,5-bisphosphate (PtdIns4,5P2) 5-phosphatase, result in oculo-cerebro-renal syndrome of Lowe (Lowe syndrome). This is a severe disease that is characterised by congenital cataracts, central hypotonia, and renal proximal tubular dysfunction, with low molecular weight (LMW) proteinuria and acidosis (Lowe et al, 1952; Attree et al, 1992).
OCRL is localised to the plasma membrane (PM), clathrin-coated vesicles (CCVs), multiple endosomal compartments, and the trans-Golgi network (TGN; Ungewickell et al, 2004; Choudhury et al, 2005; Faucherre et al, 2005; Hyvola et al, 2006). OCRL is a multidomain protein with a split N-terminal pleckstrin-homology (PH) domain (Mao et al, 2009), a central 5-phosphatase catalytic domain (Tsujishita et al, 2001; Schmid et al, 2004), an ASPM-SPD-2-Hydin (ASH) domain (Ponting, 2006; Erdmann et al, 2007; McCrea et al, 2008), a C-terminal inactive Rho-GTPase-activating protein (GAP) domain (Faucherre et al, 2003), and multiple clathrin-binding motifs (Choudhury et al, 2005, 2009) and Rab-binding regions (Hyvola et al, 2006). Through these domains and motifs, OCRL interacts with key components of the membrane-trafficking machineries, such as clathrin, the clathrin adaptor AP2, small GTPases (like Rab5, Rab6, Rab14, and Arf6), endocytic adaptors (like APPL1), and the recently identified Ses proteins (Ungewickell et al, 2004; Choudhury et al, 2005, 2009; Hyvola et al, 2006; Lichter-Konecki et al, 2006; Erdmann et al, 2007; Fukuda et al, 2008; McCrea et al, 2008; Swan et al, 2010; Noakes et al, 2011; Pirruccello et al, 2011).
In spite of the detailed knowledge we have gained over the last few years regarding the subcellular localisation, molecular organisation, and interactors of OCRL, we still lack a real and coherent understanding of the cellular roles of OCRL and thus of pathogenetic mechanisms of Lowe syndrome. The role of OCRL in membrane trafficking, for instance, has remained debated and ill defined. In spite of reports showing that the depletion of OCRL impairs endosome-to-Golgi trafficking (Choudhury et al, 2005) in mammal cells, or that depletion of a distant homologue of OCRL induces the appearance of giant endocytic vacuoles in Drosophila (Ben El Kadhi et al, 2011), other reports conclude that OCRL does not directly modulate endocytosis or post-endocytic membrane trafficking in mammal cells (Coon et al, 2009; Cui et al, 2010).
On the other side, OCRL has been shown to have a role in actin cytoskeleton regulation, cell migration, and more recently in cytokinesis (Suchy and Nussbaum, 2002; Coon et al, 2009; Ben El Kadhi et al, 2011; Dambournet et al, 2011); however, the significance of these roles of OCRL for the pathogenesis of Lowe syndrome remains to be understood.
Here, with the aim of uncovering roles of OCRL that are relevant for the pathogenesis of Lowe syndrome, we analysed the impact of the loss of OCRL (both in cells knocked down (KD) for OCRL using small-interfering (si)RNAs and in renal proximal tubule cells (PTCs) from Lowe syndrome patients) on membrane trafficking pathways that govern protein reabsorption in PTCs, as this process is compromised in patients with Lowe syndrome. These pathways involve the multiligand receptor megalin, which mediates retrieval of the major fraction of the LMW proteins that are present in the ultrafiltrate. This is achieved by continuous cycling of megalin between the apical PM, where it binds the LMW proteins and other ligands in the ultrafiltrate, and the endosomal compartment, where it releases its bound ligands (Christensen and Birn, 2002; Saito et al, 2010).
We show here that via its 5-phosphatase activity, OCRL is essential for early endosome (EE) function. Indeed, OCRL-KD cells and OCRL mutations in PTCs from patients with Lowe syndrome result in a ‘traffic jam’ at the level of the EEs, where different classes of endocytic and signalling receptors are retained, including megalin. We demonstrate that this trafficking defect involves ectopic accumulation of the OCRL substrate PtdIns4,5P2, and PtdIns4,5P2- and N-WASP-dependent increases in F-actin on EE membranes. Our data provide a molecular explanation for PTC dysfunction in Lowe syndrome, and they also highlight how tight temporal and spatial control of PtdIns4,5P2 and F-actin on EE membranes is essential for effective sorting and export of cargoes that pass through this compartment.
Results
OCRL is required for endocytic recycling of megalin
We assessed the involvement of OCRL in endocytic trafficking pathways that control protein reabsorption in PTCs, and that involve the multiligand receptor megalin (Christensen and Birn, 2002; Saito et al, 2010). To this end, and due to the difficulties of obtaining satisfactory staining of endogenous megalin by immunofluorescence, we combined two approaches: a study of the distribution and trafficking of megalin in kidney cell lines (HK2 and MDCK cells) expressing a tagged form of megalin, and an analysis of the uptake and recycling of specific megalin ligands in PTCs from healthy subjects and from patients with Lowe syndrome.
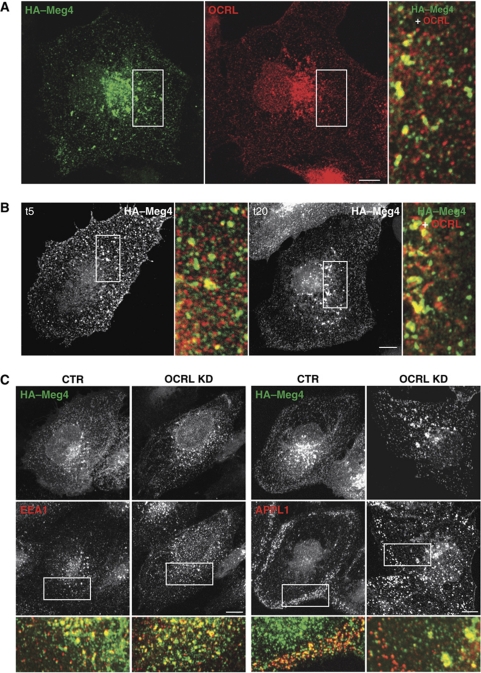
For the transfected megalin model, we exploited the megalin mini-receptor model (HA–Meg4), an accepted surrogate for full-length megalin (Li et al, 2001; Marzolo et al, 2003; Takeda et al, 2003; Yuseff et al, 2007) expressed in HK2 cells. At steady state, HA–Meg4 was distributed mainly to the PM, to both peripheral and central endosomal structures as labelled by APPL1, EEA1, and Mannose-6 Phosphate Receptor (MPR) (Figure 1A and C; Supplementary Figure S1B and C). Interestingly, about 30% of the megalin-positive structures also contained OCRL (Figure 1A). However, this percentage of colocalisation varied over time for the population of HA–Meg4 that was moving synchronously from the PM through the endosomal compartments. This population was followed using an anti-HA antibody that binds the lumenal HA epitope of HA–Meg4 (Figure 1B). When incubated at 4°C, the anti-HA antibody stained the PM, and then after 5 min at 37°C, the anti-HA antibody appeared in peripheral structures 30% of which contained OCRL. After a further 15 min at 37°C, the anti-HA antibody was in perinuclear structures 78% of which contained OCRL (Figure 1B).
Figure 1.
OCRL associates with megalin-containing endosomes. (A) HK2 cells expressing the HA–megalin (HA-Meg4) mini-receptor at steady state were stained for megalin (green) and OCRL (red), as indicated. OCRL and megalin partially colocalised in endosomal perinuclear structures (28% of megalin-containing structures were positive for OCRL). Inset: detail of boxed area. (B) HK2 cells expressing HA–Meg4 were initially kept for 2 h in serum-free medium, then incubated with a mouse anti-HA monoclonal antibody on ice for 30 min (to label the PM pool of HA–Meg4). They were then warmed to 37°C for 5 and 20 min (t5 and t20), given an acid wash, fixed, and incubated with an anti-OCRL polyclonal antibody and with secondary anti-mouse and anti-rabbit antisera. Insets: enlargement of the boxed area, with merged images of HA–Meg4 (green) and OCRL (red). After 5 min at 37°C, about 30% of the megalin-positive puncta contained OCRL. After 20 min, 78% of the perinuclear structures that contained megalin also contained OCRL. (C) HK2 cells were treated with non-targeting siRNA (CTR) or OCRL siRNAs (OCRL KD) for 96 h, transfected with HA–Meg4 for the last 18 h, and double labelled with antibodies against HA (upper panels) and against EEA1 or APPL1 (lower panels) as indicated. Insets: merged images of the boxed area, with megalin (green) and endocytic markers (red). Scale bar, 10 μm.
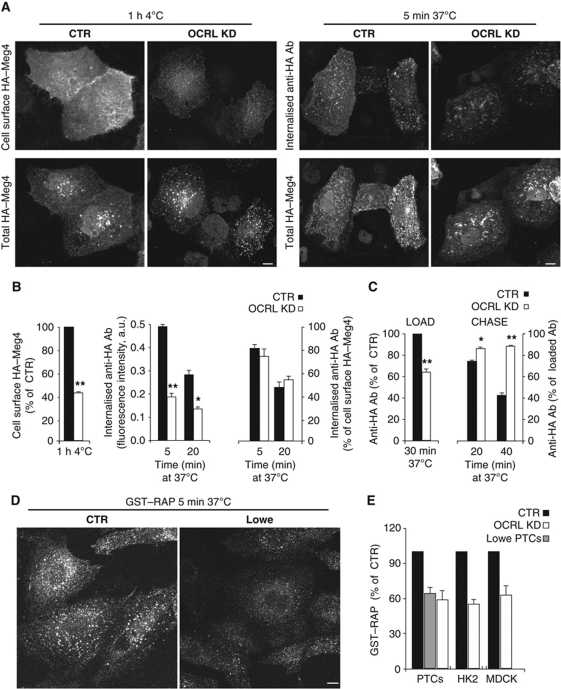
The distribution of HA–Meg4 was markedly affected by OCRL KD, as HA–Meg4 was less visible at the PM and accumulated in EEA1- and MPR-positive endosomes (Figure 1C; Supplementary Figure S1). These changes in HA–Meg4 distribution induced by OCRL KD prompted us to investigate its impact on the trafficking of megalin and of its ligands. We found that the levels of surface-exposed HA–Meg4 were markedly reduced in the OCRL-KD HK2 cells, compared with control HK2 cells, in spite of comparable total levels of HA–Meg4 (Figure 2A and B, see also Supplementary Figure S1D for immuno-electron microscopy). Furthermore, the uptake of the anti-HA antibody in HA–Meg4 HK2 cells was significantly lower with OCRL KD, compared with control cells (Figure 2A and B). This reduced uptake was mainly due to impaired exposure of HA–Meg4 at the cell surface in OCRL-KD cells, rather than to a defect in the internalisation process per se, as the fraction of internalised/bound anti-HA antibody was not different with OCRL KD, compared with control HK2 cells (Figure 2B). Furthermore, from experiments designed to directly follow re-exposure of the internalised HA–Meg4 at the cell surface, we concluded that the impaired exposure of HA–Meg4 at the cell surface was due to a defect in the recycling of HA–Meg4 to the PM (Figure 2C).
Figure 2.
OCRL is required for megalin recycling to the PM. (A) HK2 cells were treated with non-targeting siRNA (CTR) or OCRL siRNAs (OCRL KD) for 96 h and transiently transfected with HA–Meg4 for the last 18 h. The PM exposure of HA–Meg4 and its internalisation were measured through binding at 4°C (cell surface HA–Meg4) and internalisation at 37°C for 5 min (internalised anti-HA Ab) of an anti-HA monoclonal antibody. The total amount of HA–Meg4 expressed was measured using an anti-HA polyclonal antibody (total HA–Meg4) in permeabilised HA–Meg4 cells. (B) Quantitative analysis of cell surface HA-Meg4 measured in cells exposed to the anti-HA monoclonal antibody for 1 h at 4°C (left graph), internalisation of HA–Meg4 measured at 5 and 20 min as uptake of anti-HA monoclonal antibody by HA–Meg4 HK2 cells treated as described in (A) (middle graph). Right graph: ratios of internalised/bound anti-HA monoclonal antibody at 5 and 20 min. All of the fluorescence intensities of the anti-HA monoclonal antibody (either at the cell surface or internalised) are normalised for total HA–Meg4 content (measured as described in (A), and expressed as the ratio between the mean fluorescence intensity of the anti-HA monoclonal antibody and that of the anti-HA polyclonal antibody). (C) HA–Meg4 recycling: HA–Meg4 HK2 cells were loaded with the anti-HA monoclonal antibody at 37°C for 30 min (LOAD) and then chased in fresh medium for 20 and 40 min at 37°C, and acid washed (CHASE). Data are mean values±s.d. (n=100 cells; three independent experiments). *P<0.01 and **P<0.001. (D, E) Uptake of GST–RAP in control PTCs (CTR; D, E), Lowe PTCs (D, E), and OCRL-KD PTCs, HK2, and MDCK cells (E) (by siRNA treatment), as indicated. RAP internalisation was quantified in (E) as the amount of cell-associated fluorescence, and expressed as % CTR. Scale bar, 10 μm. Data are mean values±s.d. (n=100 cells; three independent experiments).
Of note, the defects in megalin trafficking were apparent only upon almost complete depletion of OCRL (below 5% of control values, reached by treating HK2 cells for 96 h with the OCRL siRNA; Supplementary Figure S1A). Less complete depletion of OCRL (20% of control values) might explain why Cui et al (2010) failed to see an effect of OCRL siRNA treatment on uptake/degradation of lactoferrin, which they took as an index of endogenous megalin trafficking in HK2 cells.
Furthermore, we observed the same defects in HA–Meg4 exposure at the cell surface in MDCK cells stably expressing HA–Meg4 either with transient (Supplementary Figure S2A–C) or with stable (Supplementary Figure S2D–F) KD for OCRL.
We also followed megalin trafficking in PTCs from healthy subjects and Lowe syndrome patients (obtained and characterised as described in Supplementary data and Supplementary Figure S2G–I); and in particular, we analysed the uptake of one of its ligands: the receptor-associated protein (RAP) that escorts neosynthesised megalin in its ER-to-Golgi transport (Bu et al, 1995; Willnow et al, 1996). RAP binds the lumenal domain of megalin with high affinity, and because of this, RAP has become a widely used tool to study megalin dynamics. When RAP is administered exogenously to cells as a recombinant protein, it is taken up with an efficiency that strictly depends on the efficiency of the trafficking and recycling of megalin (Czekay et al, 1997; Yuseff et al, 2007).
While PTCs from healthy subjects readily internalised exogenously administered RAP, the Lowe PTCs took up RAP much less efficiently (Figure 2D and E). Importantly, a similar difference in efficiency was seen with OCRL KD by RNA interference in PTCs from healthy subjects and in HA–Meg4 HK2 and MDCK cells (Figure 2E).
Altogether, the above data indicate that OCRL is required for the correct trafficking of megalin, and they provide a possible explanation for the impairment of LMW protein reabsorption by PTCs in Lowe syndrome. We then determined whether the requirement for OCRL was restricted to megalin trafficking or was extended to the trafficking of other endocytic cargoes/receptors.
OCRL is required for endosomal trafficking of different classes of receptors
We assessed the impact of OCRL KD on the trafficking of different classes of receptors that follow distinct itineraries: (i) the transferrin receptor (TfR), which follows two recycling routes to the PM, one fast and direct from the EEs, and the other slower and through the recycling endosomes (REs) (Ullrich et al, 1996; Maxfield and McGraw, 2004); (ii) the cation-independent MPR, which normally recycles between the TGN, EEs, late endosomes (LEs), and the PM (Ghosh et al, 2003; Pfeffer, 2009); and (iii) the epidermal growth factor receptor (EGFR), which after EGF-induced internalisation, is sorted towards degradation compartments (LEs/lysosomes) (Scita and Di Fiore, 2010).
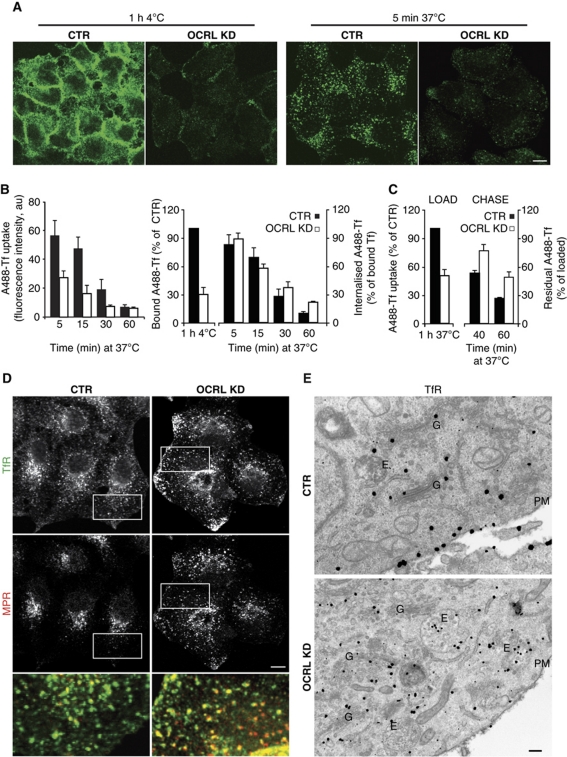
Compared with control cells, the OCRL-KD cells showed lower PM binding of Tf at 4°C (Figure 3A and B) (indicative of lower exposure of the TfR at the cell surface), along with impaired uptake of Tf (Figure 3A and B). As reported above for megalin ligands, also in the case of Tf, the ratio of internalised/bound Tf and the rate of internalisation of surface-bound Tf were not significantly different in the OCRL-KD cells, as compared with control cells (Figure 3B). This indicates that there was no defect in the internalisation process per se. Indeed, slower recycling rates of internalised Tf (Figure 3C) were measured in OCRL-KD cells, as compared with mock cells. The immunofluorescence and immuno-electron microscopy analyses indicated that the TfR accumulated in anomalously enlarged endosomal structures at the cell periphery and was less concentrated at the PM, as compared with control cells (Figure 3D and E).
Figure 3.
OCRL KD impairs recycling of the TfR. (A) Control (CTR) or OCRL-KD HeLa cells were exposed to Alexa-Fluor-488 (A488)-Tf for 1 h at 4°C and then warmed to 37°C in complete medium for 5 min. Scale bar, 10 μm. (B) Quantification of cell-associated A488-Tf, evaluated as mean fluorescence intensities at indicated times, and expressed as indicated. (C) For Tf recycling, the cells were loaded with Alexa-Fluor-488-Tf for 1 h at 37°C (LOAD) and chased in complete medium for 40 and 60 min (CHASE). The fluorescence intensities remaining in the cells after 40 and 60 min of chase were quantified and expressed as percentages of the loaded Tf. Data are mean values±s.d. (n=150 cells; three independent experiments). (D) Steady-state distributions of the TfR and MPR in CTR and OCRL-KD HeLa cells. Insets: enlargement of the boxed area, with merged images of TfR (green) and MPR (red). Scale bar, 10 μm. (E) Steady-state localisation of the TfR in CTR and OCRL siRNA-treated HeLa cells was visualised by pre-embedding immuno-gold labelling with an anti-TfR antibody. E, endosomes; G, Golgi complex; PM, plasma membrane. Scale bar, 100 nm.
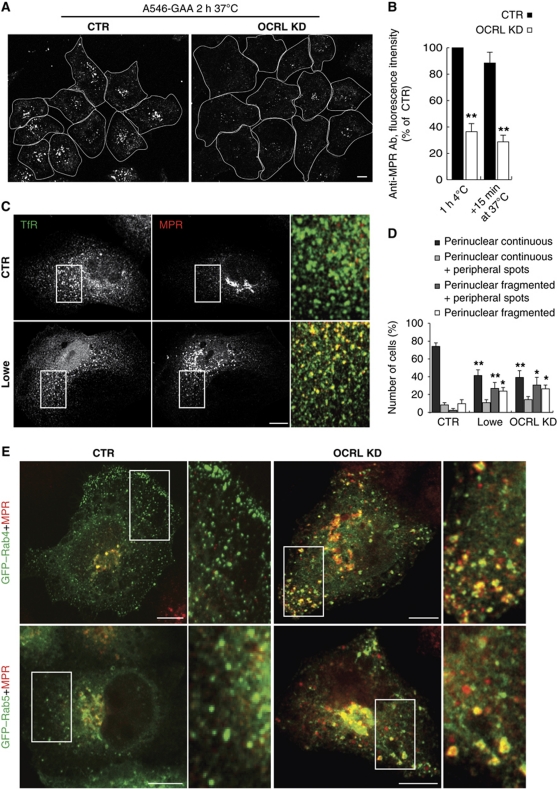
The trafficking of the MPR was also significantly altered in the OCRL-KD cells. This was indicated by impaired MPR-dependent uptake of the recombinant lysosomal enzyme α-glycosidase (which was reduced by 50%) and by reduced binding at 4°C and subsequent internalisation at 37°C of an antibody directed against a luminal epitope of MPR in the OCRL-KD cells, as compared with control cells (Figure 4A and B). Furthermore, the recycling of the MPR from the endosomes to the Golgi complex was impaired in the OCRL-KD cells and in Lowe PTCs. This was seen as an increase in the MPR associated with peripheral endosomal structures that also contained the TfR, and a decrease in the central Golgi pool of the MPR in PTCs from Lowe patients, and OCRL-KD PTCs, as compared with control PTCs (Figure 4C and D) and in HeLa cells (Supplementary Figure S3A). The impaired endosome-to-Golgi trafficking of the MPR prompted us to investigate the transport of MPR-dependent lysosomal enzymes, and we found that OCRL-KD cells release greater amounts of lysosomal enzymes in their precursor forms into the extracellular medium, consistent with their mis-routing to the PM as secondary to MPR mistrafficking (Supplementary Figure S3B and C).
Figure 4.
OCRL KD impairs recycling of the MPR to the PM and the Golgi complex. (A) Uptake of human α-glycosidase (GAA) was determined in control (CTR) and OCRL-KD HeLa cells incubated with Alexa-Fluor-546 (A546)-recombinant human GAA (A546-GAA) for 2 h at 37°C. White lines, approximate cell contours. The amount of internalised A546-hGAA (quantified by fluorescence intensity) decreased by 50% (±5%) of the CTR in the OCRL-KD cells (n=80 cells; three independent experiments). (B) PM exposure of the MPR and its internalisation were measured through binding at 4°C (1 h 4°C) and internalisation for 15 min at 37°C (+15 min 37°C) of an anti-MPR antibody directed against the luminal epitope of MPR (anti-MPR Ab) in control (CTR) and OCRL-KD HeLa cells. After fixation and treatment with the Alexa-568 secondary antibody, the cell-associated fluorescence was quantified and expressed as %CTR. (C, D) Steady-state distributions of the MPR and TfR in PTCs from healthy subjects (CTR) and Lowe syndrome patients (Lowe). In (C), the insets show enlargements of the boxed areas, with merged images of the TfR (green) and the MPR (red). (D) Images from control PTCs (CTR), Lowe PTCs, and PTCs from healthy subjects knock down for OCRL via siRNA treatment (OCRL KD) labelled for the MPR, as acquired and quantified by Scan^R automated microscopy (see Supplementary data). The percentages of cells showing the different MPR staining patterns were classified as indicated; these are mean values±s.d. (n=1000 cells; three independent experiments, each performed in quadruplicate). *P<0.01 and **P<0.001. Scale bar, 10 μm. (E) Colocalisation of the MPR with Rab proteins in control (CTR) and OCRL-KD HeLa cells transiently expressing GFP-tagged Rab4 and Rab5. Insets: enlargements of boxed areas, with merged images of Rab4 or Rab5 (green) and the MPR (red). Scale bar, 10 μm.
Furthermore, we tested the impact of OCRL KD on EGF-induced trafficking of the EGFR. After 30 and 60 min of EGF stimulation, in control cells the EGFR was efficiently degraded (by up to 70%), while at the same time the EGFR levels remained high in the OCRL-KD cells (Supplementary Figure S4B and C), and the EGFR appeared to have accumulated in TfR-positive structures (Supplementary Figure S4A). Importantly, this delayed degradation of the EGFR was accompanied by more persistent signalling, as indicated by the prolonged phosphorylation state of ERK (Supplementary Figure S4B and C).
The finding that OCRL depletion affects endosomal trafficking in HeLa cells might appear surprising considering the tissue specificity of the clinical manifestations of Lowe syndrome. Although the reasons for this tissue specificity remain to be defined, it appears likely that they involve compensatory mechanisms in non-affected tissues, such as high levels of the INPP5B 5-phosphatase. These compensatory mechanisms might be defective in the HeLa cell line. Indeed, Cui et al (2010) reported a lack of detectable levels of INPP5B mRNA in HeLa cells, and we also found very low levels of the INPP5B protein in this cell line (data not shown). Furthermore, it is likely that in addition to the cells that generate overt clinical signs, other cells might also suffer from trafficking defects in Lowe syndrome patients, as suggested by the platelet dysfunction (Lasne et al, 2010) and the higher blood levels of lysosomal enzymes reported in Lowe patients (Ungewickell and Majerus, 1999) and as supported by our data that OCRL-depleted cells release more lysosomal enzymes compared with control cells (Supplementary Figure S3B and C).
OCRL controls receptor trafficking at EEs
Altogether, the above data indicate that OCRL has a pivotal role in the trafficking and recycling of different classes of receptors. This prompted us to identify which of the different endosomal stations were these receptors pass through depends on OCRL for its correct functioning. To this end, we compared the distribution of TfR and MPR, two receptors with distinct endosomal recycling pathways: EE–LE for MPR (Ghosh et al, 2003; Pfeffer, 2009) and EE–RE for TfR (Ullrich et al, 1996; Maxfield and McGraw, 2004). In control cells, TfR and MPR show only 34% colocalisation while in OCRL-KD cells (Figure 3D) and in Lowe PTCs (Figure 4C) they show 72 and 63% colocalisation, respectively. This raises the question whether this increased colocalisation is due to the mis-routing of the TfR to LEs or to a retention/misrouting of the MPR into EEs/REs, respectively, and thus, whether the compartment affected by this OCRL KD is part of the EEs, REs, or LEs. To address this question, we took advantage of the well-defined Rab map (Stenmark, 2009) that allows identification of the nature of different endosomal compartments according to the types of their associated Rabs. We defined the EE nature of the ‘mixed’ (i.e., containing both TfR and MPR) endosomal compartment induced by the loss of OCRL by analysing a panel of Rabs (as Rabs 4, 5, 7, 9, and 11). Indeed, the peripheral structures containing the TfR and MPR in the OCRL-KD cells were marked by the EE Rabs, Rab4 and Rab5 (showing colocalisation of 87 and 58%, respectively; Figure 4E) and much less by the other Rabs analysed (with 22% colocalisation with Rab11, a marker for REs, and 18 and 13% with Rab7 and Rab9, respectively, markers for LEs). Immuno-electron microscopy analysis of these TfR/MPR-containing structures in the OCRL-KD cells confirmed their Rab4 and Rab5 positivity, and indicated that they corresponded to enlarged endosomes containing numerous internal vesicles (Supplementary Figure S3D–F).
The catalytic activity of OCRL is required for its role at EEs
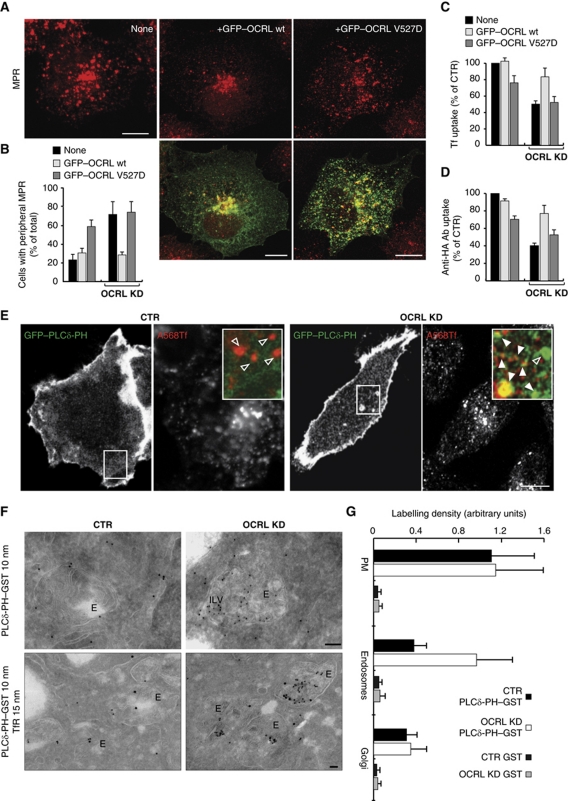
To investigate whether the catalytic activity of OCRL is required for its role at EEs, we carried out gene rescue in the OCRL-KD cells using wild-type (wt) OCRL and V527D-OCRL, a single point mutant of OCRL that results in catalytically inactive OCRL, which has also been reported in patients with Lowe syndrome (Addis et al, 2004). In both cases, we used GFP-tagged plasmids carrying silent mutations to make them resistant to the siRNA treatment, and we evaluated their ability to rescue the endocytic phenotypes induced by OCRL KD (i.e., peripheral redistribution of the MPR and reduced uptake of Tf and RAP). The OCRL-depleted cells that received the wt OCRL plasmid regained a concentrated, perinuclear MPR distribution (Figure 5A and B) and also partly recovered their Tf and megalin ligand uptake (Figure 5C and D). In contrast, the V527D-OCRL mutant protein not only failed to induce any rescue of MPR distribution (Figure 5A and B), and Tf and megalin ligand uptake in OCRL-KD cells, but also exerted a negative effect itself on the above endocytic parameters in control cells (Figure 5C and D).
Figure 5.
OCRL regulates the phosphoinositide composition of early endosomes. (A) OCRL-KD HeLa cells (left panel) and those injected with cDNA encoding for siRNA-resistant GFP-tagged wild-type (OCRL wt, middle panels) or catalytically inactive (OCRL V527D, right panels) OCRL were stained for the MPR (red). (B–D) The distribution of MPR (B), uptake of Alexa 568-Tf (C), and uptake of the anti-HA antibody (D) were evaluated and quantified in HeLa cells (B, C), and HA–Meg4 HK2 cells (D), which were knocked down for OCRL and transfected with siRNA-resistant GFP-tagged wild-type (OCRL wt) or catalytically inactive (OCRL V527D) OCRL for the last 6 h. HeLa cells were exposed to Alexa 568-Tf for 1 h at 37°C and HK2 cells were exposed to the anti-HA antibody for 30 min at 37°C. The data are expressed as % analysed cells with peripheral redistribution of the MPR in (B) or as % control cells (cells which were neither transfected with GFP–OCRL nor treated with siRNA for OCRL) in (C) and (D). The data in each graph refer to at least three independent experiments. (E–G) Subcellular localisation of PtdIns4,5P2 in CTR and OCRL-KD cells determined using the PH-PLCδ PtdIns4,5P2 probe. (E) GFP–PLCδ-PH-expressing cells were exposed to 50 μg/ml Alexa-Fluor-568 (A568)-Tf for 30 min, rinsed in complete medium, and imaged at 37°C for 40 min. Still images from a time-lapse movie are shown. PLCδ-PH-negative and PLCδ-PH-positive Tf-containing structures are indicated in the boxed areas by empty and filled arrowheads, respectively. As the OCRL-KD cells take up less Tf compared with mock cells, the setting of the A568-Tf channel was adjusted (with higher laser power and detector amplifier) to visualise an adequate number of Tf-containing structures in these cells. (F) CTR and OCRL-KD cells were processed for immuno-gold labelling with GST–PH-PLCδ (10 nm gold particles) and an anti-TfR antibody (15 nm gold particles). E, endosomes. Note that some of the endosome intralumenal vesicles (ILVs) in OCRL-KD cells are positive for GST–PH-PLCδ. (G) Morphometric analysis (performed as described in Materials and methods) of the labelling density of GST–PH-PLCδ in different cellular compartments in CTR cells and in OCRL-KD cells. The data are expressed as mean±s.d.; for PM, n=43 (CTR) and 48 (OCRL KD) cells; for endosomes, n=61 (CTR) and 82 (OCRL KD) endosomes; for Golgi, n=28 (CTR) and 24 (OCRL KD) stacks. Scale bars, 10 μm (A, E) and 100 nm (F).
Thus, the catalytic activity of OCRL is required for its role in receptor recycling from EEs.
OCRL controls PtdIns4,5P2 levels on early endosomal membranes
With the 5-phosphatase activity of OCRL required for correct functioning of EEs, we asked whether OCRL has a role in the transition from PM-derived, clathrin-coated structures rich in PtdIns4,5P2 into EE membranes rich in PtdIns3P, and thus whether OCRL can modulate the phosphoinositide composition of EEs (De Matteis and Godi, 2004). To this end, we followed the subcellular distribution of a PtdIns4,5P2 probe, the PH domain of the PLC-delta protein (PLCδ-PH; Watt et al, 2002). In agreement with previous reports (Watt et al, 2002), PLCδ-PH localised to the PM in control cells (including to PM ruffles). However, with OCRL KD, PLCδ-PH was detectable not only at the PM, but also on EEs, which were loadable with fluorescent Tf (Figure 5E; Supplementary Movies S1 and S2) and also contained the MPR and TfR (Supplementary Figure S5A). In particular, in control cells, PtdIns4,5P2 was detected in endosomes only in a small fraction of cells (15%), while 56% of the OCRL-KD cells had enlarged endosomal structures (containing the MPR and TfR) that were positive for PtdIns4,5P2.
This altered subcellular distribution of PtdIns4,5P2 was confirmed by immuno-electron microscopy performed using the GST–PLCδ-PH domain to stain cryosections (Watt et al, 2002). The morphometric analysis of the subcellular distribution of this PtdIns4,5P2 probe showed that the labelling density for PtdIns4,5P2 was significantly higher in OCRL-KD cells, as compared with control cells, only at the level of the endosomal compartments (Figure 5F and G).
We asked whether these increased levels of PtdIns4,5P2 on endosomal membranes in the OCRL-KD cells might promote the ectopic recruitment/activation of PtdIns4,5P2-dependent machineries that usually operate at the PM. AP2 is the prototype of a PtdIns4,5P2-controlled protein that acts at the PM as clathrin adaptor and that is released from endosomal membranes as a consequence of PtdIns4,5P2 removal (Cremona et al, 1999; Semerdjieva et al, 2008). When we investigated the distribution of AP2 in the OCRL-KD cells, we found that it not only associated with the PM, but also with the enlarged PtdIns4,5P2- and MPR-containing endosomes (Supplementary Figure S5B). Here, 60% of the OCRL-KD cells showed accumulation of AP2 in PtdIns4,5P2-containing endosomes, as compared with 19% in control cells.
We finally investigated whether accumulation of PtdIns4,5P2 in endosomal membranes was accompanied by impaired generation of the endosomal phosphoinositide, PtdIns3P. To this end, we assessed the distribution of a PtdIns3P probe, the FYVE domain of SARA (Hayakawa et al, 2004). This FYVE domain was still associated with endosomal membranes in the OCRL-KD cells, which indicated that the levels of PtdIns3P were not changed, at least grossly, by OCRL KD (not shown).
OCRL controls actin polymerisation on early endosomal membranes
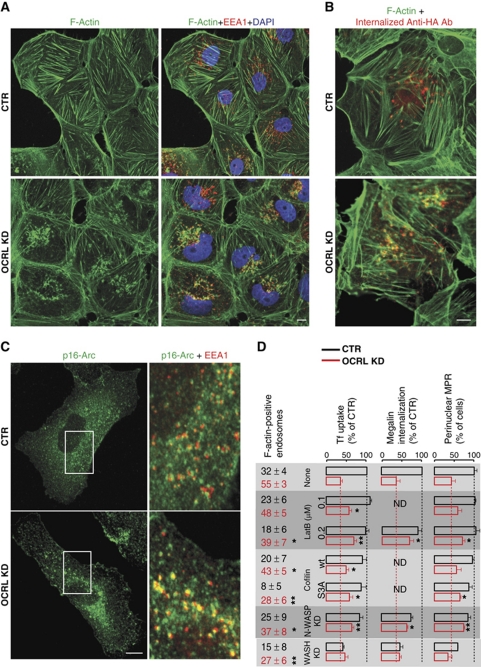
Actin dynamics is under the strict control of PtdIns4,5P2 (Hilpela et al, 2004), and OCRL has been shown to be involved in control of the actin cytoskeleton (Suchy and Nussbaum, 2002; Allen, 2003; Coon et al, 2009; Cui et al, 2010; Dambournet et al, 2011). As filamentous actin (F-actin) can control different steps in the endocytic pathway, ranging from CCV internalisation to cargo sorting and trafficking at EEs (Yarar et al, 2005; Puthenveedu et al, 2010), we asked whether OCRL has a specific role in determining the dynamics of endosomal actin. In agreement with previous reports (Suchy and Nussbaum, 2002; Allen, 2003; Cui et al, 2010; Ben El Kadhi et al, 2011), we observed that in cells depleted of OCRL, the levels of actin stress fibres decreased and foci of F-actin accumulated on internal membranes (Figure 6; Supplementary Figure S6A). We investigated the nature of these membranes, and found that a large fraction of them (56%) were EEs, as assessed by their staining with EEA1 (Figure 6A), colocalisation with internalised megalin (Figure 6B), and filling with Tf (Supplementary Figure S6A). Indeed, the percentage of endosomes positive for F-actin at steady state increased from 32% in control cells to 55% in OCRL-KD cells. This increase in endosomal actin induced by OCRL KD was also assessed in living cells transfected with GFP–Lifeact (Riedl et al, 2008) and undergoing endocytic cargo loading (Supplementary Movies S3 and S4).
Figure 6.
OCRL KD induces an increase in F-actin on early endosomes, which has a causative role in early endosome dysfunction. (A) Control (CTR) and OCRL-KD HK2 cells were stained with Alexa-Fluor-488 (A488)-phalloidin (green) to visualise F-actin and with anti-EEA1 antibody (red) to visualise EE compartments. (B) Control (CTR) and OCRL-KD HK2 cells expressing HA–mini-megalin were incubated with an anti-HA antibody at 37°C for 30 min, then fixed and stained with Alexa-Fluor-488 (A488)-phalloidin (green) to visualise F-actin, and with secondary antibody directed against the anti-HA antibody (red) to visualise internalised megalin. (C) Control (CTR) and OCRL-KD HK2 cells were stained with anti-p16Arc antibodies to visualise the Arp2/3 complex, and with anti-EEA1 antibodies to visualise EE. Insets show enlargements of the boxed areas, with merged images of p16-Arc (green) and EEA1 (red). (D) Control cells and OCRL-KD cells were either left untreated (none) or were treated with latrunculin B (LatB) at the indicated concentrations or transfected with wild-type (wt) cofilin or a constitutively active non-phosphorylatable mutant of cofilin (S3A) 1 day before the experiment, or treated with siRNAs directed against N-WASP (N-WASP-KD) or WASH (WASH KD) for 3 days before the experiments. The cells were then either stained for F-actin (with phalloidin) or for the MPR, or were exposed to Tf (1 h at 37°C, HeLa cells) or to anti-HA antibody (1 h at 37°C, HA–megalin-expressing HK2 cells). The amounts of F-actin associated with endosomes loaded with Tf were analysed as described in Materials and methods and expressed as % endosomes positive for F-actin±s.d. Uptake of Tf and of the anti-HA antibody and the number of cells with a central distribution of MPR were analysed and quantified as described in Materials and methods and are expressed as % CTR cells (i.e., untreated cells). *P<0.01 and **P<0.001.
We next determined whether this increase in endosomal F-actin had a causative role in the dysfunction of EEs in OCRL-KD cells, by assessing the impact of treatments aimed at interfering with F-actin on the endocytic function of OCRL-depleted cells. To this end, we used two approaches: treatment with low doses of the actin polymerisation inhibitor latrunculin B and expression of the actin-severing protein cofilin (which is inhibited by PtdIns4,5P2; Arber et al, 1998; Gorbatyuk et al, 2006) in its wt form and in its non-phosphorylatable constitutively active form (S3A) (von Blume et al, 2009). Interestingly, at concentrations that did not affect the general actin organisation at the level of the stress fibres or the cortical web (0.1–0.2 μM), latrunculin B was effective in decreasing the levels of F-actin associated with endosomes (Figure 6D; Supplementary Figure S6A); and importantly, it also rescued in part the endocytic trafficking defects in OCRL-KD cells (Figure 6D). In an analogous way, transfection of cofilin decreased endosomal F-actin and improved the endocytic defects in OCRL-depleted cells, with constitutively active cofilin being more effective than wt cofilin (Figure 6D).
These data indicate that the increased association of F-actin with EEs has a causative role in endosomal dysfunction induced by OCRL depletion, and prompted us to search for the molecular mechanisms underlying this increase. We found that the actin polymerisation-activating complex Arp2/3 (Rottner et al, 2010) was significantly more associated with endosomal membranes in OCRL-KD cells, as compared with control cells (Figure 6C). We, thus, investigated through which of the two main activators of Arp2/3 complex that operate in the endocytic pathway OCRL was controlling endosomal actin polymerisation; that is, through the PtdIns4,5P2-regulated N-WASP (Merrifield et al, 2004) or through WASH (Derivery et al, 2009; Gomez and Billadeau, 2009). To this end, we analysed the impact of depleting either N-WASP or WASH on increased endosomal F-actin and on endosomal dysfunction induced by OCRL KD. The KD of WASH did not rescue the endocytic defects of OCRL-depleted cells, while impaired endosomal sorting by itself, as previously reported (Derivery et al, 2009; Gomez and Billadeau, 2009; Figure 6D; Supplementary Figure S6B).
In contrast, depletion of N-WASP (which only partially decreased the efficiency of clathrin-mediated endocytosis, as also reported; Kessels and Qualmann, 2002; Benesch et al, 2005) not only prevented the accumulation of endosomal actin induced by OCRL KD, but also partly rescued the inhibitory effects of OCRL KD on Tf and megalin ligand uptake and on the MPR distribution (Figure 6D).
These data indicate that N-WASP, which is a known PtdIns4,5P2 target (Miki et al, 1996), mediates the effects of OCRL depletion on endosomal F-actin and endosomal function.
Decreasing PtdIns4,5P2 production rescues endocytic and endosomal F-actin defects in OCRL-depleted cells
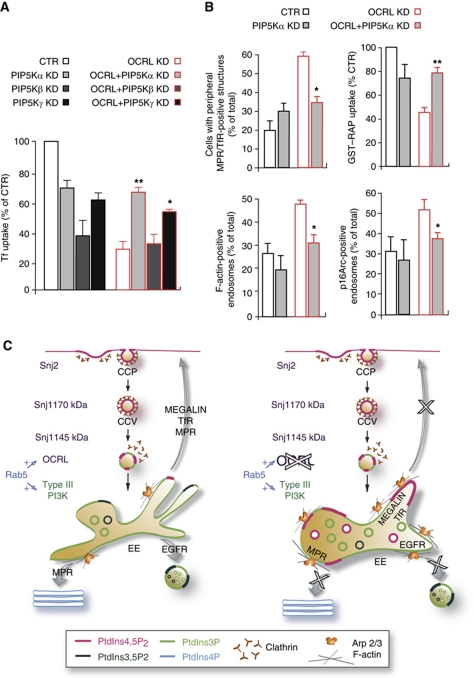
The above data indicated that loss of OCRL resulted in misfunctioning of EEs, in the anomalous presence of PtdIns4,5P2, and in increases in F-actin on their membranes. At this point, we asked whether these events induced by OCRL KD were causally linked. We reasoned that if this were the case, then rebalancing the PtdIns4,5P2 levels should ameliorate endocytic function in the OCRL-KD cells and decrease the endosome-associated F-actin. To achieve this rebalance, we inhibited the production of PtdIns4,5P2 by inhibiting the PtdIns4P 5-kinases (PIP5Ks), and we determined whether this inhibition had any effects on the endocytic and actin phenotypes induced by the loss of function of OCRL (dispersal of the MPR to the periphery, and impaired Tf or RAP uptake). We first checked the levels of expression and the subcellular distribution of the different PIP5Ks (α, β, and γ) in control and OCRL-KD cells, and found no significant differences (data not shown). We then lowered their levels of expression through siRNA treatments (Supplementary Figure S7). The nearly complete KD of each of these three PIP5Ks markedly inhibited uptake of the ligands examined (Tf and RAP) per se (not shown). Thus, we set conditions to lower the levels of the 5-kinases to 50%. While 50% KD of PIP5Kβ, the kinase isoform with a recognised role in Tf endocytosis (Padron et al, 2003), still induced a marked inhibition of Tf uptake (60% inhibition), lowering the levels of PIP5Kα and PIP5Kγ to 50% of control levels caused only moderate impairment in Tf uptake in control cells (30% inhibition; Figure 7A). However, under these conditions (with 50% lowering of PIP5Kα and PIP5Kγ) there was partial rescue of the uptake of Tf in OCRL-KD cells (from 30% control to 70% control). We also noted that KD of PIP5Kα was more effective in this rescue than that of PIP5Kγ (Figure 7A). In addition, this partial KD of PIP5Kα also rescued the uptake of RAP and counteracted the dispersal of the MPR into peripheral structures that was induced by OCRL KD (Figure 7B; Supplementary Figure S7B). Finally, establishing a causal link between accumulation of PtdIns4,5P2 in EEs and increase in F-actin in the same compartment and confirming the involvement of the PtdIns4,5P2-regulated Arp2/3 activator WASP, this partial KD of PIP5Kα also counteracted the increase in endosomal F-actin and in Arp2/3 complex in OCRL-KD cells (Figure 7B).
Figure 7.
Knockdown of PIP 5-kinases rescues the endosomal trafficking defects and the F-actin changes in OCRL-depleted cells. (A) Internalised Alexa-Fluor-488 (A488)-Tf (5 min at 37°C) was visualised and quantified in control cells (CTR), OCRL-KD cells (OCRL KD), PIP5Kα-KD cells, PIP5Kβ-KD cells, PIP5Kγ-KD, and OCRL-KD cells upon siRNA-mediated depletion of the different PIP5K isoforms (OCRL+PIP5Kα KD, OCRL+PIP5Kβ KD, and OCRL+PIP5Kγ KD). (B) MPR distribution, GST–RAP uptake (5 min at 37°C), F-actin and Arp2/3 complex associated with Tf-loaded endosomes, expressed as indicated, were quantified in CTR cells, OCRL-KD cells, PIP5Kα-KD cells, and in OCRL-KD cells upon siRNA-mediated depletion of PIP5Kα (OCRL+PIP5Kα KD). The data are mean values±s.d. (n=100 cells for Tf and GST–RAP uptake, MPR distribution; n=10 cells for F-actin and Arp2/3 endosomal localisation; from three independent experiments); *P<0.01 and **P<0.001. (C) Model of the role of OCRL in the early endocytic trafficking pathway. Left panel: Snj2 and brain-specific, 145 kDa Snj1 are recruited at the early and late steps, respectively, of clathrin-coated pit (CCP) and clathrin-coated vesicles (CCVs) formation; the ubiquitously expressed 170 kDa SJ1 is present throughout CCP and CCV formation. OCRL associates with CCV just before the release of clathrin and with early endosomes. Consumption of PtdIns4,5P2 and generation of PtdIns3P via type III PI3K are under the control of the GTPase Rab5. Right panel: impact of OCRL loss of function on endosomal PtdIns4,5P2 and F-actin levels and, as a consequence, on recycling from early endosomes (see text for detail).
Altogether, these data indicate that the loss of OCRL causes EE misfunctioning through local accumulation of PtdIns4,5P2 along with PtdIns4,5P2 and N-WASP-dependent increase in F-actin, and they identify in PIP5Kα a candidate drug target for treatment of patients with Lowe syndrome.
Discussion
The mechanistic links bridging OCRL mutations and renal proximal tubular dysfunction in Lowe syndrome have remained obscure to date. Here, we report that OCRL depletion or mutation severely affects trafficking of the multiligand receptor megalin, which is responsible for protein reabsorption at the proximal tubule, by impairing its recycling to the PM and inducing its retention in EEs.
We have provided here various lines of evidence that the PtdIns4,5P2 5-phosphatase activity of OCRL is important for its role in controlling the trafficking through EEs of megalin and other receptors, including the TfR, EGFR, and MPR. First, loss of OCRL results in ectopic accumulation of PtdIns4,5P2 at EEs. Second, the endocytic defects of the OCRL-KD cells can be rescued by catalytically active, but not by catalytically inactive OCRL. Third, a decrease in PtdIns4,5P2 production achieved through lowering the levels of PIP5Kα also alleviates the disruption of endocytic trafficking that is caused by this OCRL loss. Hence, PIP5Kα will be a promising target for pharmacological treatment of patients with Lowe syndrome, through the use of specific inhibitors (van den Bout and Divecha, 2009).
The levels of PtdIns4,5P2 along the clathrin-dependent endocytic pathway result from the activities of the PIP5Ks and the phosphoinositide 5-phosphatases, with multiple 5-phosphatases being recruited at different times and different stages in the lifespan of CCVs. While the ubiquitously expressed 170 kDa synaptojanin 1 (SJ1) is present throughout the duration of clathrin-coated pit (CCP) formation, SJ2 and 145 kDa SJ1 are recruited at the early and late stages of CCP formation, respectively (Rusk et al, 2003; Perera et al, 2006). In contrast, OCRL associates with CCVs just before the clathrin is released, and persists on the uncoated vesicles and endosomes (Erdmann et al, 2007; Figure 7C). These data indicate that there is continuous PtdIns4,5P2 turnover throughout the internalisation process, as highlighted by De Camilli and colleagues (2009) (Zoncu et al, 2009), which indeed persists after clathrin is released, and also occurs on endosomal membranes (the present study). Indeed, the observations that the 5-phosphatase activity of OCRL is required for trafficking through EEs indicate that although enriched in PtdIns3P, EEs have domains where PtdIns4,5P2 hydrolysis takes place. Our data show the functional importance of controlling this hitherto unsuspected dynamic endosomal pool of PtdIns4,5P2. What remains to be defined is whether this endosomal PtdIns4,5P2 pool (which is barely detectable under control conditions) is a residual PM-derived pool or whether it is generated in a ‘second wave’ of PtdIns4,5P2 synthesis, as recently demonstrated for phagosomes (Bohdanowicz et al, 2010).
We show here that in the absence of OCRL, this endosomal PtdIns4,5P2 pool becomes enlarged, and hence clearly detectable, thus loosening the boundaries between PtdIns4,5P2-containing (PM) and PtdIns3P-containing (endosomal) membranes (Clague et al, 2009). The GTPase Rab5 is a key factor in establishing this boundary, as it negatively regulates PtdIns4,5P2 levels and stimulates PtdIns3P production in EEs (Christoforidis et al, 1999; Semerdjieva et al, 2008; Compagnon et al, 2009). Indeed, the phenotype of Rab5 inactivation is reminiscent of that seen with OCRL KD, at least in terms of the presence of PtdIns4,5P2 and clathrin on endosomal membranes (Compagnon et al, 2009). As Rab5 binds and recruits OCRL to EEs (Hyvola et al, 2006; Erdmann et al, 2007; Fukuda et al, 2008), we envisage OCRL as the main downstream effector for Rab5 in mediating the removal of PtdIns4,5P2 from EEs (Figure 7C). Indeed, our data experimentally support the hypothesis according to which OCRL has a role in the ‘phosphoinositide switch’ that controls maturation of the early endocytic intermediates into EEs (Zoncu et al, 2009).
With regard to the mechanisms by which a lack of OCRL 5-phosphatase activity and an accumulation of PtdIns4,5P2 in EEs is detrimental to EE function, we have shown that these involve PtdIns4,5P2-dependent increases in EE-associated F-actin. Previous reports have indicated that the general organisation of actin cytoskeleton is altered in fibroblasts from patients with Lowe syndrome (Suchy and Nussbaum, 2002), that actin comets are more frequently seen in these fibroblasts (Allen, 2003), and that depletion of OCRL also induces actin comets in MDCK cells (Guerriero et al, 2006; Cui et al, 2010). These changes in actin organisation have been linked to impaired cell migration (Coon et al, 2009) and to increased release of apical reporter cargoes (Guerriero et al, 2006) leaving open the question of whether and/or how these actin cytoskeleton changes are relevant for the pathogenesis of Lowe syndrome. What we demonstrate here is that the loss of OCRL induces a PtdIns4,5P2- and N-WASP-dependent increase in actin polymerisation on EEs, which is responsible for megalin retention in this compartment. This, thus, identifies a pathogenetic mechanism for impaired reabsorption capacity of PTCs in Lowe syndrome, and at the same time, an unprecedented pathological correlate for F-actin dysregulation at EEs. Furthermore, our data indicate that this PtdIns4,5P2- and N-WASP-dependent endosomal F-actin pool is not functionally equivalent to the WASH-dependent pool (Derivery et al, 2009; Duleh and Welch, 2010). Indeed, the N-WASP-dependent actin pool (increased in OCRL-KD cells) cannot substitute for the lack of the WASH-dependent actin pool (induced by WASH KD) for endosomal function, as this function remains severely impaired in cells KD for both OCRL and WASH, in spite of levels of F-actin on EEs that are comparable to those in control cells.
We have also shown that the PtdIns4,5P2-dependent increase in actin polymerisation on EEs induced by OCRL depletion can be efficiently counteracted by expression of the constitutively active form of the actin-depolymerising factor cofilin (von Blume et al, 2009). Interestingly, with its actin-severing activity inhibited by phosphorylation and by PtdIns4,5P2 (Arber et al, 1998; Gorbatyuk et al, 2006), cofilin has recently been shown to be recruited to CCVs approximately at the same time when OCRL is recruited and when N-WASP and the Arp 2/3 complex are released (Taylor et al, 2011). Thus, the causal relationships that we have established here between the PtdIns4,5P2 5-phosphatase activity of OCRL and the mobilisation of F-actin from internalised membranes perfectly matches with the timing of the recruitment of OCRL and the shut-off of the PtdIns4,5P2-dependent actin polymerisation module on the same membranes (i.e., N-WASP with the Arp2/3 complex) and with the recruitment of the F-actin destabilising factor cofilin. Indeed, cofilin has very recently emerged as a key factor in promoting the maturation of EE into retrograde transport-competent endosomes (Harrington et al, 2011). In addition, cofilin has been shown to be involved in albumin uptake in PTCs and to interact with the ClC-5 (Hryciw et al, 2003) chloride channel, mutations of which cause Dent disease (Fisher et al, 1995), a condition that shows significant clinical overlap with Lowe syndrome in terms of proximal tubular dysfunction. Furthermore, the impairment to megalin trafficking reported for the loss of ClC-5 (i.e., slowing down of the recycling of megalin to the PM of PTCs, and retention of megalin in EEs; Christensen et al, 2003) appears to be remarkably similar to that we have highlighted here as a consequence of OCRL depletion/mutation. Our findings, thus, provide a further example of how two clinically overlapping pathological conditions share defects along the same cellular pathway, which in this case turns out to involve the early endocytic trafficking pathway.
Materials and methods
Antibodies, cDNAs, and reagents
The polyclonal and monoclonal antibodies used for immunofluorescence, immuno-electron microscopy, and western blotting are described in Supplementary data. Human recombinant RAP was expressed and isolated as described in Bu et al (1995). Full-length OCRL (isoform b) was amplified from the IMAGE clone (30528571) in the pEGFP-C3 expression vector (BD Biosciences Clontech). Mutations in the 5-phosphatase domain (V527D) and in sequences targeted by siRNAs (to generate siRNA-resistant cDNAs) were carried out by PCR. The duplex sequences were 5′-TGACATAGCTTCTAACAGT-3′ and 5′-GAAATTACCTCCCAAGTTGTT-3′. The DNA sequences in the duplex regions after mutations were (mutated bases underlined) 5′-TGACATCGCTTCCAATAGT-3′ and 5′-GAAATTATCTCCCTAGCTGTT-3′.
All of the chemical reagents were of analytical grade or higher, and were purchased from Sigma-Aldrich, unless otherwise specified.
RNA interference
The siRNA oligonucleotides targeting OCRL were purchased from Dharmacon (mix of four Upgrade siRNA duplexes for human OCRL, D-010026-01, and D-010026-05 and a single siRNA duplex for canine OCRL, D-010026-01). In addition, three different shRNAs were used to knowk down OCRL levels (not shown). Thus, a total of seven different OCRL siRNA sequences were used in human cell lines, which generated similar phenotypes, in terms of Tf uptake and the MPR redistribution. The siRNAs for the PIP5Ks were from Qiagen (mix of two siRNA duplexes) and the siRNAs for WASH and N-WASP were from Dharmacon (mix of four Upgrade siRNA duplexes).
All of the siRNA oligonucleotides and shRNA sequences are listed in Supplementary Table S1.
The rescue experiments were performed in cells treated with the D-010026-01 and D-010026-05 oligonucleotides and transfected with the siRNA-resistant OCRL cDNA (see above). HK2, MDCK, and HeLa cells were transfected with siRNAs as described in Supplementary data, and the control cells were treated with identical concentrations of non-targeting siRNAs (Dharmacon D-001810-01-20 and D-001810-02-20).
Microinjection
Plasmid DNAs encoding GFP-tagged PH-PLCδ and GFP-tagged wt and V527D (siRNA-resistant) OCRL were injected into the cell nuclei using an Eppendorf micromanipulator and transjector; the cells were fixed after 6–8 h. The injection concentration used in all of the experiments was 10 ng/μl.
Immunofluorescence and live-cell imaging
Immunofluorescence labelling was performed as previously described (Daniele et al, 2008) and live-cell imaging was performed as described in Supplementary data.
Confocal fluorescence microscopy, image processing, and colocalisation analysis
The samples were examined under a Zeiss LSM 510 confocal laser-scanning microscope equipped with × 63 oil-immersion objective. To perform quantitative image analysis, 10–15 randomly chosen fields that included 8–10 cells each were scanned, using the same setting parameters (i.e., laser power and detector amplification) below pixel saturation. The mean intensity per cell was determined using the histogram function in the Zeiss LSM 510 Software (version 3.2), and all of the pixel values above background levels were quantified. All of the experiments were repeated at least three times, and representative images are shown.
To quantify the levels of colocalisation (i.e., MPR/TfR, Rabs/MPR, clathrin/MPR, megalin/OCRL, megalin/APPL1, megalin/EEA1, megalin/MPR, and Tf/F-actin colocalisation), confocal serial sections were acquired from 8 to 10 cells per experimental condition, exported in TIFF format, and processed as previously described (Sonnichsen et al, 2000). At least two sections per cell were counted, ensuring that peripheral and perinuclear structures were taken into account equally. The percentage colocalisation represents the percentage of total megalin-containing structures positive for OCRL or other endosomal markers, of MPR-labelled endosomes positive for TfR or Rab proteins, and of Tf-containing endosomes positive for F-actin staining.
For GFP–PH-PLCδ structure detection, 20 serial images were taken in 0.25 μm steps or 10 serial images in 0.5 μm steps, beginning 1–2 μm below the focal plane of the bottom of the monolayer and proceeding upwards to 1–2 μm above the top of the cells. GFP–PH-PLCδ-positive structures continuously present in three ‘central’ (i.e., two sections below or above the PM sections labelled with an anti-MHCI antibody) consecutive serial optical sections and co-stained with endocytic markers (such as the MPR, TfR, or internalised Tf) were referred to as endosomal structures. All of the experiments were carried out at least three times.
Automated analysis of MPR distribution
The automated analysis of the MPR distribution was performed as described in Supplementary data.
Endocytosis assays
The GST–RAP, Tf, and α-glycosidase uptake assays, and the megalin trafficking assays, were all performed as described in Supplementary data.
Electron microscopy
Samples were processed for pre-embedding immuno-EM as previously described (Daniele et al, 2008), using anti-GFP, anti-TfR, and anti-MPR antibodies. The labelling of PtdIns4,5P2 using PLCδ1-PH–GST in ultrathin cryosections, and the quantification of immunolabelling were performed as described previously (Watt et al, 2002).
Statistics
Data are expressed as mean values±s.d. Student’s t-tests were used to compare differences between treated groups relative to their paired controls. P-values are indicated in the figures and figure legends.
Supplementary Material
Acknowledgments
We thank CP Berrie for editorial assistance; A Luini and F Emma for insightful discussions; S Corvera for providing the FYVE domain of SARA; V Malhotra for providing the cofilin cDNAs; A Gautreau for providing the anti-WASH antibodies; A Ballabio and D Medina for providing reagents to measure lysosomal enzyme secretion. MADM acknowledges the support of Telethon (grant numbers GSP08002 and GGP06166), AIRC (AIRC, grant IG 8623) and AISLO; ADC acknowledges the support of AIRC (AIRC, MFAG 6325); MV is a recipient of a Fellowship from FIRC; MPM is funded by Fondo Nacional de Ciencia y Tecnología, FONDECYT, grants # 1070373 and 1110382 and the Millenium Nucleus in Regenerative Biology (MINREB); and LS is supported by a CONICYT Fellowship for PhD students.
Author contributions: MV designed and performed the experiments, analysed the data, and contributed to writing the manuscript; ADC designed and performed the experiments and analysed the data; EP performed the EM analysis; MS and GDT provided technical assistance; AG performed the initial experiments; EL provided PTCs; MGDL performed some of the experiments on HK2 cells; RP performed the EM analysis; LS performed the experiments on MDCK cells treated with OCRL shRNA; MM provided HA–Meg4 and GST–RAP cDNAs and discussed the results; MADM supervised the experimental work, discussed the data, and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Addis M, Loi M, Lepiani C, Cau M, Melis MA (2004) OCRL mutation analysis in Italian patients with Lowe syndrome. Hum Mutat 23: 524–525 [DOI] [PubMed] [Google Scholar]
- Allen PG (2003) Actin filament uncapping localizes to ruffling lamellae and rocketing vesicles. Nat Cell Biol 5: 972–979 [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393: 805–809 [DOI] [PubMed] [Google Scholar]
- Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL (1992) The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242 [DOI] [PubMed] [Google Scholar]
- Ben El Kadhi K, Roubinet C, Solinet S, Emery G, Carreno S (2011) The Inositol 5-Phosphatase dOCRL Controls PI (4,5) P2 Homeostasis and Is Necessary for Cytokinesis. Curr Biol 21: 1074–1079 [DOI] [PubMed] [Google Scholar]
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K (2005) N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci 118: 3103–3115 [DOI] [PubMed] [Google Scholar]
- Bohdanowicz M, Cosio G, Backer JM, Grinstein S (2010) Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. J Cell Biol 191: 999–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Geuze HJ, Strous GJ, Schwartz AL (1995) 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J 14: 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M (2005) Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16: 3467–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, Noakes CJ, McKenzie E, Kox C, Lowe M (2009) Differential clathrin binding and subcellular localization of OCRL1 splice isoforms. J Biol Chem 284: 9965–9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Birn H (2002) Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266 [DOI] [PubMed] [Google Scholar]
- Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ (2003) Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci USA 100: 8472–8477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, de Lartigue J (2009) Phosphoinositides and the endocytic pathway. Exp Cell Res 315: 1627–1631 [DOI] [PubMed] [Google Scholar]
- Compagnon J, Gervais L, Roman MS, Chamot-Boeuf S, Guichet A (2009) Interplay between Rab5 and PtdIns (4,5)P2 controls early endocytosis in the Drosophila germline. J Cell Sci 122: 25–35 [DOI] [PubMed] [Google Scholar]
- Coon BG, Mukherjee D, Hanna CB, Riese DJ II, Lowe M, Aguilar RC (2009) Lowe syndrome patient fibroblasts display Ocrl1-specific cell migration defects that cannot be rescued by the homologous Inpp5b phosphatase. Hum Mol Genet 18: 4478–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P (1999) Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99: 179–188 [DOI] [PubMed] [Google Scholar]
- Cui S, Guerriero CJ, Szalinski CM, Kinlough CL, Hughey RP, Weisz OA (2010) OCRL1 function in renal epithelial membrane traffic. Am J Physiol Renal Physiol 298: F335–F345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekay RP, Orlando RA, Woodward L, Lundstrom M, Farquhar MG (1997) Endocytic trafficking of megalin/RAP complexes: dissociation of the complexes in late endosomes. Mol Biol Cell 8: 517–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A (2011) Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol 13: 981–988 [DOI] [PubMed] [Google Scholar]
- Daniele T, Di Tullio G, Santoro M, Turacchio G, De Matteis MA (2008) ARAP1 regulates EGF receptor trafficking and signalling. Traffic 9: 2221–2235 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Godi A (2004) PI-loting membrane traffic. Nat Cell Biol 6: 487–492 [DOI] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A (2009) The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17: 712–723 [DOI] [PubMed] [Google Scholar]
- Duleh SN, Welch MD (2010) WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 67: 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P (2007) A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13: 377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Desbois P, Nagano F, Satre V, Lunardi J, Gacon G, Dorseuil O (2005) Lowe syndrome protein Ocrl1 is translocated to membrane ruffles upon Rac GTPase activation: a new perspective on Lowe syndrome pathophysiology. Hum Mol Genet 14: 1441–1448 [DOI] [PubMed] [Google Scholar]
- Faucherre A, Desbois P, Satre V, Lunardi J, Dorseuil O, Gacon G (2003) Lowe syndrome protein OCRL1 interacts with Rac GTPase in the trans-Golgi network. Hum Mol Genet 12: 2449–2456 [DOI] [PubMed] [Google Scholar]
- Fisher SE, van Bakel I, Lloyd SE, Pearce SH, Thakker RV, Craig IW (1995) Cloning and characterization of CLCN5, the human kidney chloride channel gene implicated in Dent disease (an X-linked hereditary nephrolithiasis). Genomics 29: 598–606 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Ishibashi K, Itoh T (2008) Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics 7: 1031–1042 [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4: 202–212 [DOI] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD (2009) A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17: 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk VY, Nosworthy NJ, Robson SA, Bains NP, Maciejewski MW, Dos Remedios CG, King GF (2006) Mapping the phosphoinositide-binding site on chick cofilin explains how PIP2 regulates the cofilin-actin interaction. Mol Cell 24: 511–522 [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Weixel KM, Bruns JR, Weisz OA (2006) Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem 281: 15376–15384 [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD (2011) Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell 146: 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa A, Hayes SJ, Lawe DC, Sudharshan E, Tuft R, Fogarty K, Lambright D, Corvera S (2004) Structural basis for endosomal targeting by FYVE domains. J Biol Chem 279: 5958–5966 [DOI] [PubMed] [Google Scholar]
- Hilpela P, Vartiainen MK, Lappalainen P (2004) Regulation of the actin cytoskeleton by PI (4,5) P2 and PI (3,4,5) P3. Curr Top Microbiol Immunol 282: 117–163 [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Wang Y, Devuyst O, Pollock CA, Poronnik P, Guggino WB (2003) Cofilin interacts with ClC-5 and regulates albumin uptake in proximal tubule cell lines. J Biol Chem 278: 40169–40176 [DOI] [PubMed] [Google Scholar]
- Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M (2006) Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J 25: 3750–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels MM, Qualmann B (2002) Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J 21: 6083–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne D, Baujat G, Mirault T, Lunardi J, Grelac F, Egot M, Salomon R, Bachelot-Loza C (2010) Bleeding disorders in Lowe syndrome patients: evidence for a link between OCRL mutations and primary haemostasis disorders. Br J Haematol 150: 685–688 [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, Marzolo MP, Bu G (2001) Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J Biol Chem 276: 18000–18006 [DOI] [PubMed] [Google Scholar]
- Lichter-Konecki U, Farber LW, Cronin JS, Suchy SF, Nussbaum RL (2006) The effect of missense mutations in the RhoGAP-homology domain on ocrl1 function. Mol Genet Metab 89: 121–128 [DOI] [PubMed] [Google Scholar]
- Lowe CU, Terrey M, Mac LE (1952) Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 83: 164–184 [DOI] [PubMed] [Google Scholar]
- Mao Y, Balkin DM, Zoncu R, Erdmann KS, Tomasini L, Hu F, Jin MM, Hodsdon ME, De Camilli P (2009) A PH domain within OCRL bridges clathrin-mediated membrane trafficking to phosphoinositide metabolism. EMBO J 28: 1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo MP, Yuseff MI, Retamal C, Donoso M, Ezquer F, Farfan P, Li Y, Bu G (2003) Differential distribution of low-density lipoprotein-receptor-related protein (LRP) and megalin in polarized epithelial cells is determined by their cytoplasmic domains. Traffic 4: 273–288 [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132 [DOI] [PubMed] [Google Scholar]
- McCrea HJ, Paradise S, Tomasini L, Addis M, Melis MA, De Matteis MA, De Camilli P (2008) All known patient mutations in the ASH-RhoGAP domains of OCRL affect targeting and APPL1 binding. Biochem Biophys Res Commun 369: 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Qualmann B, Kessels MM, Almers W (2004) Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol 83: 13–18 [DOI] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T (1996) N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J 15: 5326–5335 [PMC free article] [PubMed] [Google Scholar]
- Noakes CJ, Lee G, Lowe M (2011) The PH domain proteins IPIP27A and B link OCRL1 to receptor recycling in the endocytic pathway. Mol Biol Cell 22: 606–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron D, Wang YJ, Yamamoto M, Yin H, Roth MG (2003) Phosphatidylinositol phosphate 5-kinase Ibeta recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol 162: 693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D (2006) Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci USA 103: 19332–19337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (2009) Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett 583: 3811–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirruccello M, Swan LE, Folta-Stogniew E, De Camilli P (2011) Recognition of the F&H motif by the Lowe syndrome protein OCRL. Nat Struct Mol Biol 18: 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP (2006) A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics 22: 1031–1035 [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M (2010) Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5: 605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Hanisch J, Campellone KG (2010) WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol 20: 650–661 [DOI] [PubMed] [Google Scholar]
- Rusk N, Le PU, Mariggio S, Guay G, Lurisci C, Nabi IR, Corda D, Symons M (2003) Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis. Curr Biol 13: 659–663 [DOI] [PubMed] [Google Scholar]
- Saito A, Sato H, Iino N, Takeda T (2010) Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium. J Biomed Biotechnol 2010: 403272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AC, Wise HM, Mitchell CA, Nussbaum R, Woscholski R (2004) Type II phosphoinositide 5-phosphatases have unique sensitivities towards fatty acid composition and head group phosphorylation. FEBS Lett 576: 9–13 [DOI] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463: 464–473 [DOI] [PubMed] [Google Scholar]
- Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E (2008) Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol 183: 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525 [DOI] [PubMed] [Google Scholar]
- Suchy SF, Nussbaum RL (2002) The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am J Hum Genet 71: 1420–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan LE, Tomasini L, Pirruccello M, Lunardi J, De Camilli P (2010) Two closely related endocytic proteins that share a common OCRL-binding motif with APPL1. Proc Natl Acad Sci USA 107: 3511–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Yamazaki H, Farquhar MG (2003) Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol 284: C1105–C1113 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Perrais D, Merrifield CJ (2011) A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 9: e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujishita Y, Guo S, Stolz LE, York JD, Hurley JH (2001) Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell 105: 379–389 [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell A, Ward ME, Ungewickell E, Majerus PW (2004) The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci USA 101: 13501–13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell AJ, Majerus PW (1999) Increased levels of plasma lysosomal enzymes in patients with Lowe syndrome. Proc Natl Acad Sci USA 96: 13342–13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bout I, Divecha N (2009) PIP5K-driven PtdIns (4,5) P2 synthesis: regulation and cellular functions. J Cell Sci 122: 3837–3850 [DOI] [PubMed] [Google Scholar]
- von Blume J, Duran JM, Forlanelli E, Alleaume AM, Egorov M, Polishchuk R, Molina H, Malhotra V (2009) Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J Cell Biol 187: 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM (2002) Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363: 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J (1996) RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J 15: 2632–2639 [PMC free article] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL (2005) A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell 16: 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuseff MI, Farfan P, Bu G, Marzolo MP (2007) A cytoplasmic PPPSP motif determines megalin′s phosphorylation and regulates receptor′s recycling and surface expression. Traffic 8: 1215–1230 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136: 1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.