Abstract
Information on the long-term prognosis of nonalcoholic fatty liver disease (NAFLD) is limited. We sought to describe the long-term morbidity and mortality of patients with NAFLD with advanced fibrosis or cirrhosis.
We conducted this prospective cohort study including 247 patients with NAFLD and 264 patients with HCV infection that were either naïve or non-responders to treatment. Both cohorts were Child-Pugh class A and had advanced (stage 3) fibrosis or cirrhosis (stage 4) confirmed by liver biopsy at enrolment.
In the NAFLD cohort, followed-up for 85.6 months mean (range 6-297), there were 48 (19.4%) liver-related complications and 33 (13.4%) deaths or liver transplants. In the HCV cohort, followed-up for 74.9 months mean (range 6-238), there were 47 (16.7%) liver-related complications and 25 (9.4%) deaths or liver transplants. When adjusting for baseline differences in age and gender, the cumulative incidence of liver-related complications was lower in the NAFLD than the HCV cohort (p=0.03), including incident hepatocellular cancer (6 vs 18; p=0.03), but that of cardiovascular events (p=0.17) and overall mortality (p=0.6) was similar in both groups. In the NAFLD cohort, platelet count, stage 4 fibrosis, and serum levels of cholesterol and ALT were associated with liver-related complications; an AST/ALT ratio >1 and older age were associated with overall mortality; and higher serum bilirubin levels and stage 4 fibrosis were associated with liver-related mortality.
Conclusions
Patients with NAFLD with advanced fibrosis or cirrhosis have lower rates of liver-related complications and hepatocellular cancer than corresponding patients with HCV infection, but similar overall mortality. Some clinical and laboratory features predict outcomes in patients with NAFLD.
Keywords: Natural history, Non-alcoholic fatty liver disease, hepatitis C virus, liver cancer
Non-alcoholic fatty liver disease (NAFLD) has become the most prevalent cause of chronic liver disease worldwide.(1-3) Regarded as the hepatic manifestation of the metabolic syndrome, NAFLD represents a histological spectrum of disease that extends from simple steatosis to steatohepatitis (NASH).(1-5) NAFLD may be associated with advanced fibrosis or cirrhosis which is a concern as many of the liver-related complications and mortality(e.g., liver failure, varices, etc.), occurs in these patients.(6) In addition to an increasing need for transplantation,(7) patients with NAFLD with and without cirrhosis may develop hepatocellular carcinoma (HCC).(8, 9)
Despite its prevalence, the prognosis of NAFLD with advanced fibrosis or cirrhosis remains poorly studied. Previous studies have been hampered by problems with case ascertainment, definition and have generally had limited numbers and/or follow-up, which could potentially lead to inaccurate estimates of disease burden.(10-12) It is well established that cirrhotic patients presenting with overt synthetic liver dysfunction are more likely to develop liver-related complications, and have a high overall mortality. However, some important aspects of the prognosis of patients with NAFLD still remain unclear. First, it is unclear how the long term prognosis of patients with NAFLD compares with patients with liver disease of other aetiologies, such as chronic hepatitis C virus (HCV) infection. Secondly, what are the risks of liver-related complications, including HCC, in patients with NAFLD with advanced fibrosis or cirrhosis and no overt synthetic dysfunction (i.e., Child-Pugh class A)? Thirdly, the impact of NAFLD on non-liver-related sequelae such as vascular outcomes (eg, myocardial infarction, strokes and vascular deaths) remains poorly described.(13) Finally, it is unclear which, if any, risk factors can independently predict liver-, vascular-, and overall-morbidity and mortality.
In order to answer these questions, we carried out an international, multi-center prospective study to assess the natural history and outcomes of liver biopsy-confirmed NAFLD with advanced fibrosis or cirrhosis from four medical centers. We sought to assess complications that occurred in these patients, and identify the predictors of such events; we also compared their long-term morbidity and mortality to a group of patients with histologically-confirmed chronic hepatitis C virus (HCV) infection and advanced fibrosis or cirrhosis.
PATIENTS AND METHODS
Participants & setting
Two-hundred and forty seven Child-Pugh class A patients with biopsy-confirmed NAFLD and advanced fibrosis or cirrhosis comprised the NAFLD cohort. This cohort was recruited from 1984 to 2006. Patients were previously untreated and consecutively biopsied at 4 centers: Mayo Clinic Rochester, USA (n = 105); Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK (n = 57); Westmead Hospital, Sydney, Australia (n = 51) and University of Turin, Italy (n = 34). The comparator cohort consisted of 264 patients diagnosed with HCV infection and advanced fibrosis or cirrhosis who were also Child-Pugh class A enrolled from 1987 to 2005. HCV infection was confirmed by a positive polymerase chain reaction at baseline in all patients. HCV subjects were seen and consecutively biopsied at Westmead Hospital, Sydney (n = 209) and University of Turin, Italy (n = 55). They were either non-responders to antiviral treatment (n= 224; 85%) or had never had any treatment for HCV infection (n=40; 15%). These criteria were chosen because sustained virological response to anti-HCV therapy improves the outcomes of these patients. The study was approved by appropriate regulatory bodies at all centers and written informed consent was obtained from all patients for participation in medical research.
NAFLD was diagnosed based on: (i) elevated aminotransferases for at least six months; (ii) liver biopsy showing changes consistent with advanced fibrotic NAFLD (detailed below), and (iii) exclusion of other etiologies including viral, autoimmune, cholestatic, genetic, metabolic, alcoholic or drug-induced liver diseases. These other etiologies were excluded using specific biochemical, clinical, radiological, and/or histological criteria. All patients had current and past consumption of ethanol less than 20 grams per day on direct questioning of both the patients and a close relative.
A complete medical history and physical examination was undertaken. Body mass index (BMI) was calculated using the formula: weight (in kilograms)/height2 (in metres). Waist circumference (to the nearest half centimetre) was measured at the midpoint between the lower border of the ribcage and the iliac crest. Serum measurements included routine liver biochemistry (ALT and AST levels, total bilirubin, albumin, alkaline phosphatase, and gamma glutamyl transpeptidase); complete blood count; fasting glucose; fasting insulin; total cholesterol, HDL cholesterol, and total trigycerides; serology for hepatitis B and C viruses; iron studies; autoantibodies; alpha 1 antitrypsin levels and phenotype; and ceruloplasmin levels. Components of the metabolic syndrome including central obesity, hyperglycemia, hypertrigylceridemia, hypertension and low HDL cholesterol were recorded.
Liver histology
Liver biopsies were stained with hematoxylin and eosin, Masson’s trichrome, and special stains for iron and copper. Liver biopsies were read by a single liver pathologist in each participating center. Histological features of NAFLD such as steatosis, inflammation, hepatocyte ballooning, and fibrosis were scored as previously described.(14, 15) Only those patients that had steatosis of at least 5% plus stage 3 (septal/bridging) or stage 4 (cirrhosis) fibrosis were included in this analysis. Other histological changes of steatohepatitis such as inflammation and ballooning were not required as inclusion criteria.
For HCV, the degree of fibrosis was scored according to the METAVIR scale (16): briefly, stage 0: no fibrosis; stage 1: enlarged portal tract without septa; stage 2: enlarged portal tract with rare septa; stage 3: numerous septa without cirrhosis; stage 4: cirrhosis.
Only those patients with fibrosis stage 3 or 4 disease were included. There was no clinical, serological or radiological evidence of HCC at recruitment in any patient with NAFLD or HCV infection.
Data Collection
The start date for analyses was the date of liver biopsy, with any events occurring in the first 6 months excluded. Patients were monitored every 3-6 months and followed up until death or liver transplantation, whichever occurred first, or until data analysis. For those lost to follow-up, up-to date clinical information was sought by: (i) contact with the primary care physician, (ii) telephone interview, and (iii) checking the respective death and transplant registries. Patients were censored at the time of death or transplantation or last clinic visit, and those lost to follow-up were censored at the time last seen. All clinical outcomes were confirmed by a physician at each center utilising the patient records and physician diagnoses.
The following outcomes were assessed: (i) liver complications including liver failure, gastro-esophageal varices (+/− hemorrhage), ascites, encephalopathy, hepatopulmonary syndrome and HCC; (ii) liver-related death or liver transplantation (for calculation of survival probability, transplantation was considered as an equivalent end-point); (iii) all-cause mortality; and (iv) total vascular events (including myocardial infarction, stroke, and vascular deaths).(13)
HCC was diagnosed if the following were present: (1) pathological changes consistent with HCC identified by histological examination of liver tissue obtained by fine-needle aspiration, liver biopsy, or liver explant at transplantation or autopsy, or (2) one or more hepatic space-occupying lesions that had vascular patterns typical of HCC by angiography, triple-phase computed tomography or magnetic resonance imaging.
All patients were followed according to standards of care and guidelines without experimental or therapeutic interventions for NAFLD or HCV. Weight management was performed with lifestyle intervention such as dietary modification and exercise recommended at the outpatient clinic in patients with overweight/obesity. Other treatments, such as oral hypoglycemics, cholesterol-lowering medications, and anti-hypertensive medications were only given in the context of management of concomitant diabetes mellitus, hypercholesterolemia or hypertension, respectively. Neither drug use nor lifestyle interventions were recorded systematically after baseline.
Statistical Analysis
Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago IL). Results are reported as means ± standard deviation or frequency (percentage) as appropriate. Continuous variables were compared using two-tailed students’ t-test. Categorical data were compared using the Chi square test. Variables with a p-value of ≤ 0.1 on univariate analysis were further analyzed by multiple logistic regression in order to determine the independent determinants of outcome variables. The cumulative probabilities of morbidity and mortality (including 95% confidence intervals [CI]) were analysed using the Kaplan-Meier method and using the date of the liver biopsy as time zero (baseline). The effect of individual variables upon morbidity and mortality were compared using log-rank tests. When assessing the effects of multiple variables on survival, Cox-regression was used.
RESULTS
Baseline characteristics
Table 1 summarizes the baseline characteristics of the patient population. Among cases with NAFLD, the mean age was 54.7 years, 60.5% were female, 91.5% were of white ethnicity, and the mean BMI was 32.8 kg/m2. More than half had concomitant metabolic morbidity including 50% with diabetes, 44.1% with hypertension and 12.1% with previous vascular disease. At baseline, metformin was used in a third (34.4%) of patients, and statins in one fifth (21.5%) of patients. Among cases with HCV, the mean age was 48.3 years, 35.2% were female, 72.3% were white, and the mean BMI was 27.3 kg/m2. The average BMI was significantly lower in patients with HCV infection and they had lower levels of glucose, cholesterol, and triglycerides, and higher levels of ALT and AST than patients with NAFLD. Although the NAFLD cohort had lower levels of albumin and higher platelet count, other parameters indicative of liver function such as bilirubin, INR and MELD score were similar between the two groups as was the proportion of patients with fibrosis stage 3 and 4 (Table 1).
TABLE 1.
Baseline characteristics of the patient population
| Parameters | NAFLD (n=247) | HCV (n=264) | P-value |
|---|---|---|---|
| Age (years) | 54.7 (11.6) | 48.3 (11.3) | <0.001 |
| Female gender (%) | 149 (60.3) | 93 (35.2) | <0.001 |
| Caucasian race (%) | 226 (91.5) | 191 (72.3) | 0.09 |
| Body mass index (kg/m2) | 32.8 (5.6) | 27.3 (4.5) | <0.001 |
| Diabetes (%) | 125 (50.6) | 45 (17.1) | <0.001 |
| Triglyceride (mmol/L) | 2.2 (1.4) | 1.3 (0.8) | <0.001 |
| Cholesterol (mmol/L) | 5.2 (1.3) | 4.2 (1.1) | <0.001 |
| HDL Cholesterol (mmol/L) | 1.2 (0.4) | 1.2 (0.4) | 0.9 |
| Random glucose (mg/dl) | 7.6 (3.5) | 5.5 (1.3) | <0.001 |
| Bilirubin (μmol/L) | 14.1 (7.0) | 14.7 (8.4) | 0.4 |
| Albumin (g/dl) | 41.1 (5.7) | 42.5 (3.6) | 0.002 |
| Platelet (109/L) | 194.3 (79.8) | 177.2 (64.7) | 0.04 |
| International Normalised Ratio | 1.06 (0.1) | 1.05 (0.1) | 0.4 |
| ALT (IU/L) | 78.9 (65.6) | 179.4 (147.0) | <0.001 |
| AST (IU/L) | 68.2 (46.9) | 100.4 (72.7) | <0.001 |
| MELD score | 8.0 (2.7) | 7.5 (2.9) | 0.2 |
| Center (%) | |||
| Mayo Clinic, USA | 105 (42.5) | ||
| Newcastle upon Tyne, UK | 57 (23.1) | ||
| Sydney, Australia | 51 (20.6) | 209 (79.2) | |
| Turin, Italy | 34 (13.8) | 55 (20.8) | |
| Fibrosis stage (%) | 0.6 | ||
| Stage 3 | 118 (47.7) | 133 (50.4) | |
| Stage 4 | 129 (52.2) | 131 (49.6) |
FOOTNOTE: Results expressed as either mean (standard deviation) or frequency (percentage). Abbreviations: HDL, high-density lipoprotein; ALT, alanine aminotransferase; AST aspartate aminotransferase; MELD, model for end-stage liver disease
Liver-related complications and cardiovascular events
The 247 NAFLD participants had a mean (±SD) follow-up of 85.6 (±54.5) months (range 6-297 months). Complete follow-up was achieved in 235 (95.1%) patients and 12 (4.9%) patients were lost to follow-up. During follow-up, 48 (19.4%) patients developed liver-related complications, with some developing more than one complication. Twenty six (10.5%) cases developed gastroesophageal varices, 19 (7.7%) developed ascites, liver failure, hepatopulmonary syndrome and/or encephalopathy, and 6 (2.4%) developed HCC (4 of which were initially in stage 4 fibrosis). Thirteen (5.3%) had subsequent myocardial infarctions (one being fatal), and 4 (1.6%) developed strokes (all ischemic in etiology).
The 264 HCV participants had a mean (±SD) follow-up of 74.9 (±47.1) months (range 6-238 months). Complete follow-up was achieved in 254 (96.2%) patients, and 10 (3.8%) patients were lost to follow-up. During follow-up, 47 (17.8%) patients developed liver-related complications. Nine (3.4%) developed gastroesophageal varices, 20 (7.6%) developed ascites, liver failure and/or encephalopathy, and 18 (6.8%) patients developed HCC (10 of which were initially in stage 4 fibrosis). Nine (3.4%) had subsequent myocardial infarctions and one (0.3%) had a stroke (diagnosed as ischemic in etiology).
In the NAFLD cohort, the probability of liver-related complication-free survival was 98.0%, 93.4% and 81.5% at 12, 36 and 120 months respectively, whereas in the HCV cohort was 98.4%, 93.5% and 76.5% at 12, 36 and 120 months respectively (Figure 1A). In unadjusted comparison, there was a trend for more liver-related complications in the cohort with HCV infection than in the cohort with NAFLD (p=0.09; Figure 1A); there appeared to be stronger evidence of a difference between groups when adjusted in age, sex, body mass index and diabetes (p=0.03; Figures 1B & C). HCC occurred more commonly in HCV than in NAFLD (18 [6.8%] vs. 6 [2.4%] respectively, p=0.03), with time-to-event illustrated in Supplementary Figure 1. There was no significant difference in total vascular events between NAFLD and HCV groups (17 (6.9%), vs. 10 (3.8%); p=0.17).
FIGURE 1. NAFLD versus HCV.
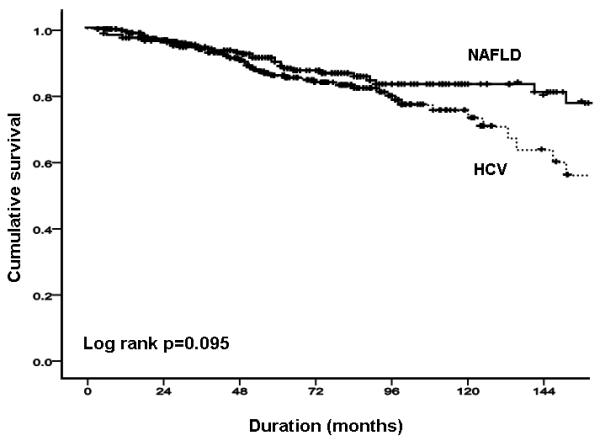
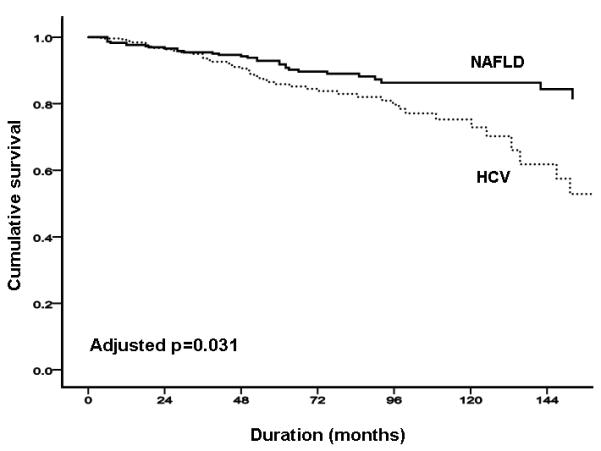
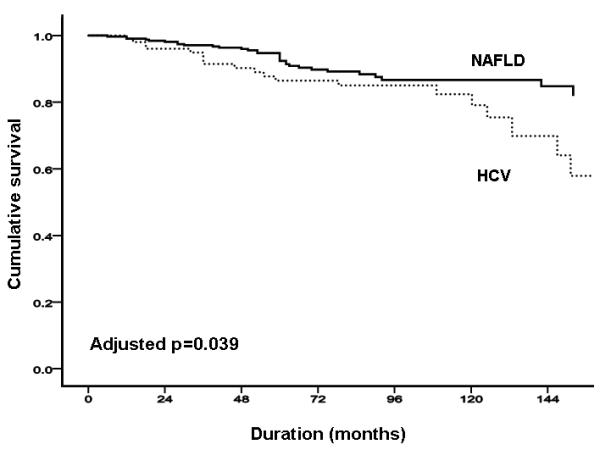
(A) total liver-related complications; (B) total liver-related complications adjusted by age and sex; (C) total liver-related complications adjusted by age, sex, body mass index and presence of diabetes.
Mortality (liver-related and all-cause) and liver transplantation
In the NAFLD cohort, there were a total of 33 deaths or liver transplants (13.4%). Of the 14 liver-related deaths and transplantations, three were related to HCC; there were four deaths related to other cancers, and one definite vascular death. The probability of overall survival was 99.6%, 96.7% and 81.6% at 12, 36 and 120 months respectively (Figure 2A). In the HCV cohort, there were a total of 25 deaths or liver transplants (9.5%). Of the 21 liver-related deaths and transplantations, 12 were related to HCC; there was one definite vascular death, and three deaths due to unknown causes. The probability of overall free survival was 99.2%, 98.3% and 82.0% at 12, 36 and 120 months respectively (Figure 2A). The overall mortality was similar in both cohorts (p=0.38; Figure 2A), with no evidence of differences after adjustment by differences between groups in age, sex, body mass index, diabetes and dislipidemia (p=0.6; Figures 2B & C).
FIGURE 2. NAFLD versus HCV.
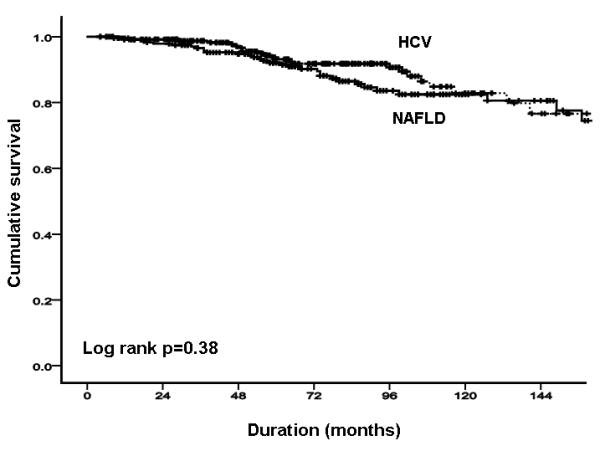
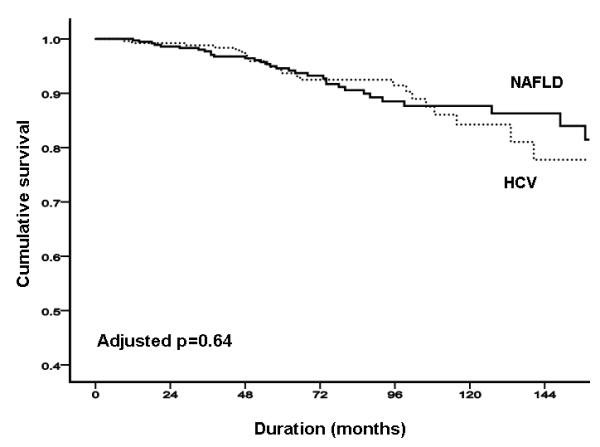
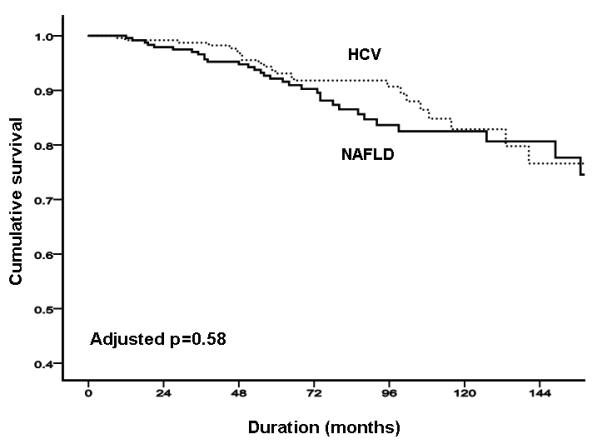
(A) overall mortality; (B) overall mortality adjusted by age and sex. (C) total liver-related complications adjusted by age, sex, body mass index, and presence of diabetes and dislipidemia.
Liver-related complications and overall mortality by fibrosis stage
In the NAFLD group there was strong evidence of differences between fibrosis stage 3 and 4 for total liver-related complications (p<0.001) and some evidence for overall mortality (p=0.05) as illustrated in Supplementary Figures 2A and 2B. In the HCV group there was little evidence of differences between fibrosis stage 3 and 4 for total liver-related complications (p=0.18) and some evidence for overall mortality (p=0.04) as illustrated in Supplementary Figures 3A and 3B.
Independent predictors for liver-related complications in the NAFLD cohort
Univariate models to characterise differences in the NAFLD group are shown in Supplementary Table 1 and a summary of the multivariate predictive factors for all categories of outcome are shown in Table 2. Stage 4 fibrosis, past history of coronary heart disease, lower serum levels of cholesterol, lower levels of ALT, and lower platelet count were all independently associated with total liver-related complications. Independent predictors were also identified for the development of ascites (lower platelet count), encephalopathy (older age), gastroesophageal varices (stage 4 fibrosis; lower levels of ALT; lower platelet count; lower levels of cholesterol), and myocardial infarction (past medical history of hypercholesterolemia, lower HDL-cholesterol). No factors were identified as predictors for HCC or stroke. All these differences remained unaffected when the center variable was included in the models.
TABLE 2.
Multivariate analyses for overall mortality, liver related mortality, overall vascular events, myocardial infarction, total liver events, varices, ascites and encephalopathy in the NAFLD cohort (n=247)
| Parameters | Adjusted Odds Ratio |
95% Confidence Intervals |
p-value | ||
|---|---|---|---|---|---|
|
OVERALL
MORTALITY |
AST/ALT >1 | 3.74 | (1.10 – 12.68) | 0.03 | |
| Age | (increase in 10 years) | 1.55 | (1.02 – 2.35) | 0.04 | |
|
LIVER-RELATED
MORTALITY |
Bilirubin | (increase in 10 μmol /L) | 2.14 | (1.09 – 4.22) | 0.03 |
| Fibrosis stage | 10.43 | (1.32 – 82.89) | 0.03 | ||
|
VASCULAR EVENT
AND DEATH (APT) |
Diabetes | 10.43 | (1.26 – 82.53) | 0.03 | |
| Hyperchol | 10.33 | (2.59 – 41.30) | 0.001 | ||
|
MYOCARDIAL
INFARCTION |
Hyperchol | 9.36 | (1.73 – 50.71) | 0.01 | |
| HDL | (decrease in 0.1 mmol/L) | 1.35 | (1.02 - 1.79) | 0.04 | |
|
TOTAL LIVER
EVENTS |
CAD | 2.99 | (1.04 – 8.58) | 0.04 | |
| Platelets | (decrease in 20 × 109/L) | 1.22 | (1.08 - 1.38) | 0.001 | |
| Fibrosis stage | 4.78 | (1.68 – 13.63) | 0.003 | ||
| ALT | (decrease in 10 IU/L) | 1.14 | (1.01 - 1.29) | 0.04 | |
| Cholesterol | (decrease in 1 mmol/L) | 1.50 | (1.05 - 2.13) | 0.03 | |
| ASCITES | |||||
| Platelets | (decrease in 20 × 109/L) | 1.27 | (1.02 - 1.63) | 0.006 | |
| ENCEPHALOPATHY | |||||
| Age | (increase in 10 years) | 3.42 | (1.09 – 10.58) | 0.04 | |
| VARICES | |||||
| ALT | (decrease in 10 IU/L) | 1.23 | (1.01 - 1.50) | 0.05 | |
| Fibrosis stage | 12.86 | (1.62 – 101.85) | 0.02 | ||
| Cholesterol | (decrease in 1 mmol/L) | 1.91 | (1.19 – 3.07) | 0.008 | |
| Platelet | (decrease in 20 × 109/L) | 1.17 | (1.02 - 1.38) | 0.04 |
Independent predictors for mortality in the NAFLD cohort
Univariate models to characterise differences in the NAFLD cohort are shown in Supplementary Table 2 and a summary of the multivariate predictive factors are shown in Table 2. By multivariate analysis and adjusting for center, older age and higher AST/ALT ratio were independently associated with overall mortality. Stage 4 fibrosis and higher serum bilirubin levels were independently associated with liver-related mortality. History of diabetes mellitus and hypercholesterolemia were associated with vascular events (ie, non-fatal myocardial infarction, non-fatal stroke, and vascular death) and vascular-related death.
DISCUSSION
In this large multi-center study from four countries, we report the natural history of the largest cohort of biopsy-proven NAFLD with advanced fibrosis or cirrhosis. The NAFLD patients had well compensated liver disease and no overt hepatic synthetic dysfunction at presentation, and they were compared with patients with HCV infection with advanced fibrosis or cirrhosis of the same functional status. There are important long-term differences, notably less liver-related complications and less HCC risk in patients with NAFLD as compared to patients with HCV infection, but also remarkable long-term similarities for vascular disease and overall mortality. In addition, we were able to identify independent risk factors for liver- and vascular-related complications and mortality in NAFLD.
This study has a number of strengths, including its relatively large sample size and the recruiting of incident cases who were extensively assessed and biopsied to ascertain the diagnosis. In particular, biopsy-confirmation avoids many of the pitfalls of studies that have described cryptogenic cirrhosis associated with risk factors for NAFLD without formal histological classification. Patients were seen in three different continents, and, hence, the results should be generalisable to at least these populations, although evidence in non-Caucasian patients is lacking. About 95% of the total cohort had complete follow-up allowing an accurate quantification of outcomes. All the centers specialise in the management of NAFLD and HCV, meaning that patients were treated according to guidelines, were regularly followed-up, and causes of events, especially those related to the liver, were verified.
Prospective observational studies do have inherent limitations and biases including those of referral (ie, all being specialist hepatology centers), lead time (ie, timing of diagnosis altering outcomes) and selection (eg, HCV nonresponders being more likely to progress). Since histology was interpreted by independent pathologists at each center, there could be some inter-rater variability – however this was likely to be low as experienced liver pathologists reviewed samples, and fibrosis stages 3/4 have the best kappa scores as compared to other histological features. (15) In particular, biopsy-confirmation avoids many of the pitfalls of studies that have described cryptogenic cirrhosis associated with risk factors for NAFLD without formal histological classification. Our histological criteria were strict to ensure that burned out autoimmune hepatitis was not mistaken as a NAFLD cases, for example. We cannot rule out NASH cases being excluded (for example, those with NASH and steatosis <5%), but, as a result of the workup to exclude other aetiologies, all included cases were managed clinically as NAFLD. Logistically, it was not possible to match every patient with NAFLD by age and gender with patients with HCV infection; however, only age was shown to have an independent effect on outcomes. We also adjusted the comparisons by age, sex, body mass index, and the presence of diabetes and dislipidemia without discernible differences.
There may be residual confounding by some parameters: for example, diabetes status differed significantly between NALFD and HCV, and even though this was adjusted for, this cannot account for severity of disglycaemia. Moreover, follow-up for medical problems that may have an effect, such as de novo diabetes mellitus, were not assessed systematically (although insulin resistance may play a role in HCV as well as in NAFLD).(17) Nor can we rule out effects of later medications for the treatment of comorbidities, although no pharmacological treatments have been shown reliably to have a substantive effect on liver fibrosis in NAFLD.(18) This also applies to any effects of non-pharmacological treatments such as exercise or diet.(19) Practice and follow-up obviously varied between the centers, although this does not affect the systematic prospective methodology used, nor should it significantly affect the event predictors.
Compared to the general population, NAFLD has been associated with an increased risk of overall death (standardised mortality ratio 1.34, 95% confidence intervals 1.003-1.76) in a community-based study of 420 patients from the United States.(20) In a similar Swedish study, just over 5% of the 129 NAFLD patients enrolled went on to develop end-stage liver disease.(21) In both studies, there was a higher vascular- and liver-related mortality in patients with NAFLD (as compared to the general population of the same age and sex).
In contrast to patients with other liver diseases, the short-term prognosis of NAFLD is largely excellent, but longer term prognosis depends crucially on the histological stage at presentation. (6, 22) In patients with bland steatosis, two studies have reported either nil (23) or minimal progression (24) to advanced disease over a median of 11.5 and 16.7 years respectively. For those with NASH on baseline liver biopsy, 11% went on to develop cirrhosis and ~40% of patients die from any cause within 15 years (of which 7.3% are from liver-related complications, especially in those with advanced fibrosis or cirrhosis).(6) Studies with subsequent liver biopsy have also prospectively evaluated the risk of fibrosis progression over time. 103 patients underwent two liver biopsies 1 to 21 years apart: baseline low fibrosis stage, diabetes, and greater BMI were independently associated with fibrosis progression.(25) In a similar study from Sweden, 70 patients underwent two liver biopsies 10 to 16 years apart; progression of fibrosis stage occurred in 41% and was associated with diabetes, weight gain and increased insulin resistance.(21)
A case control study of 23 patients from Sydney with cirrhotic stage NASH compared to those with HCV showed no difference between liver related deaths or all cause mortality between the groups after adjustment for baseline differences, despite a trend towards improved survival in NASH.(12) A larger case comparison from Virginia compared 152 patients with cirrhosis due to NASH with 150 subjects with HCV non-responders.(26) The 10-year survival in the NASH group was 80.9%, significantly better than in the HCV controls of similar age, sex, and Child-Pugh score, principally due to a lower risk of hepatic decompensation in the NASH cohort. However, the Virginia study examined less Child-Pugh Class A patients (n=74) than in our study (n= 247). More recently, a Cleveland Clinic prospective study found lower rates of HCC in 195 NASH compared to 315 HCV cirrhotics (annual risk 2.6% versus 4%; p=0.09), although their NASH group also contained those with cryptogenic cirrhosis and former heavy drinkers.(27)
This study adds important new information to our knowledge on the natural history of patients with well-compensated NAFLD (Child-Pugh class A at enrolment): a more advanced stage of liver fibrosis (F3 vs F4 cirrhosis) is associated with an increased risk of liver complications and, potentially, overall mortality. NAFLD appears to have lower rates of liver-related complications, but similar overall mortality as compared to HCV patients, even when adjusting for age (and other potential confounders). One of the key and controversial complications was the risk of HCC in NAFLD. In this large cohort, HCC was significantly more common in HCV than NAFLD (6.8% vs. 2.4%, respectively). The HCV cohort had an approximate 0.15% risk per annum of HCC development versus 0.05% risk per annum in NAFLD. The figures found in our study are much lower than those reported in the NASH studies from Virginia (17% vs. 6.7%) and the Cleveland Clinic (20.3% vs. 12.8%).(26, 27) This may be due to differences in risk factors for HCC among the patient populations (e.g., alcohol consumption and comorbidities), the inclusion of more advanced liver disease (eg, Child-Pugh class B and C,(26) higher MELD score (27) and NASH histology in both) or reduced random error with our larger sample sizes. Cirrhosis per se increases the risk of HCC,(28) but there is wide variation in carcinogenic risk depending on disease etiology: large case-control studies indicate that diabetes increases the risk of HCC by 1.3- to 2.4-fold, whereas viral hepatitis increases this risk 13- to 19-fold.(29) Taken together, we interpret the present data as indicating that the incidence of HCC is lower in NAFLD than in chronic HCV infection. However, given the high prevalence of NAFLD in the community, the population attributable risk of HCC related to NAFLD is still considerable (~13-20%).(12)
As hypercholesterolemia and diabetes are strongly associated with major vascular events, a holistic approach to NAFLD treatment is needed, including adequate treatment of metabolic conditions eg, diabetes and dyslipidemia.(18, 19) As well as the emerging relevance of NAFLD for cardiovascular diseases,(30) this collaborative study highlights the risk of liver-related events and mortality in NAFLD with advanced fibrosis. The risk factors we identified for liver-related complications are of relevance to the practising clinician, including progressive rises in serum bilirubin and fibrosis stage for liver-related mortality, and a low platelet count for both ascites and varices (consistent with a portal hypertensive etiology).
The MELD score did not predict outcomes in our NAFLD cohort, which can be explained by patients being Child-Pugh class A at enrolment rather than assessment for liver transplantation. Many of the factors that play a role in the MELD equation, such as age, were independent predictors (in this case, of overall mortality and encephalopathy). Interestingly, the AST/ALT ratio (commonly used to differentiate fatty liver clinically from other etiologies) also served as a predictor of overall mortality, having previously been shown to independently distinguish between patients with and without advanced liver fibrosis.(31)
In summary, in this multi-center collaborative study, there were independent risk factors for vascular-, liver- and all-cause outcomes in patients with NAFLD with advanced fibrosis or cirrhosis who had no overt evidence of hepatic decompensation at enrolment. At these histological stages, NAFLD appears to lead to lower rates of liver-related complications and lower rates of HCC than patients with HCV infection of a similar disease stage, albeit the overall mortality in both conditions seems to be similar. However, larger prospective studies are necessary to shed further insights on the impact of NAFLD on liver- and vascular-related morbidity and mortality.
Supplementary Material
SUPPLEMENTARY FIGURE 1. NAFLD versus HCV. hepatocellular cancer incident events
SUPPLEMENTARY FIGURE 2. NAFLD by fibrosis stage. (A) total liver-related complications
SUPPLEMENTARY FIGURE 2. NAFLD by fibrosis stage. (B) overall mortality.
SUPPLEMENTARY FIGURE 3. HCV by fibrosis stage. (A) total liver-related complications
SUPPLEMENTARY FIGURE 3. HCV by fibrosis stage. (B) overall mortality.
SUPPLEMENTARY TABLE 1 Univariate analysis for all liver-related complications in the NAFLD cohort (n=247)
SUPPLEMENTARY TABLE 2 Univariate analysis for overall mortality in the NAFLD cohort (n=247)
Acknowledgments
Grant support: Dr. Neeraj Bhala is funded by the UK Medical Research Council, and by Dame Sheila Sherlock (Royal College of Physicians) and Berkeley Trust (University College London and Gonville and Caius College, Cambridge) Travelling Fellowships for his period of study in Sydney. Dr. Leon A. Adams was supported by medical research scholarships from the National Health and Medical Research Council (no. 353710) and the University of Western Australia (Athelstan and Amy Saw Scholarship). This study was supported by an R01 DK82426 grant to Dr. Paul Angulo. These sponsors played no role in the study design, or the collection, analysis, and interpretation of data.
Footnotes
Conflict of Interest/Disclosures – all authors have nothing to disclose in relation to this study.
NB, PA, EB, CPD and JG conceived and designed the study;
NB, PA, DVP, EL, JMH, GS, LA, PC, JHT, EB, CPD, JG generated, collected, and assembled the data;
NB, PA, DVP, EB, CPD, JG analysed and/or interpretated the data;
NB, PA, DVP, EB, CPD, JG drafted and/or revised the manuscript;
NB, PA, DVP, EB, CPD, JG approved the final version of the manuscript.
Reference List
- 1.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009 Nov;13(4):511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007 Apr 15;25(8):883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Bhala N, Usherwood T, George J. Non-alcoholic fatty liver disease. BMJ. 2009;339:b2474. doi: 10.1136/bmj.b2474. [DOI] [PubMed] [Google Scholar]
- 5.Day CP. Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med. 2006 Jan;6(1):19–25. doi: 10.7861/clinmedicine.6-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010 Feb;51(2):373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009 Nov;58(11):1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002 Jul;123(1):134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 9.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010 May;51(5):1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 10.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990 Jan;11(1):74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 11.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989 Jun;20(6):594–598. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 12.Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003 Aug;38(2):420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 13.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009 May 30;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21(1):3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994 Jul;20(1 Pt 1):15–20. [PubMed] [Google Scholar]
- 17.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009 Apr;104(4):861–867. doi: 10.1038/ajg.2009.67. [DOI] [PubMed] [Google Scholar]
- 18.van der Poorten D, George J. Current and novel therapies for the treatment of nonalcoholic steatohepatitis. Hepatol Int. 2007 Sep;1(3):343–354. doi: 10.1007/s12072-007-9011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007 Dec;56(12):1760–1769. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams LA, Lymp JF, St SJ, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005 Jul;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006 Oct;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 22.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005 Jul;129(1):375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 23.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995 Dec;22(6):1714–1719. [PubMed] [Google Scholar]
- 24.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 25.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005 Jan;42(1):132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006 Apr;43(4):682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 27.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010 Jun;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 28.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999 Apr 10;353(9160):1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 29.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005 Apr;54(4):533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep 30;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 31.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007 Apr;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1. NAFLD versus HCV. hepatocellular cancer incident events
SUPPLEMENTARY FIGURE 2. NAFLD by fibrosis stage. (A) total liver-related complications
SUPPLEMENTARY FIGURE 2. NAFLD by fibrosis stage. (B) overall mortality.
SUPPLEMENTARY FIGURE 3. HCV by fibrosis stage. (A) total liver-related complications
SUPPLEMENTARY FIGURE 3. HCV by fibrosis stage. (B) overall mortality.
SUPPLEMENTARY TABLE 1 Univariate analysis for all liver-related complications in the NAFLD cohort (n=247)
SUPPLEMENTARY TABLE 2 Univariate analysis for overall mortality in the NAFLD cohort (n=247)


