Abstract
Syntenin-1 is a PDZ domain-containing adaptor that controls trafficking of transmembrane proteins including those associated with tetraspanin-enriched microdomains. We describe the interaction of syntenin-1 with ubiquitin through a novel binding site spanning the C terminus of ubiquitin, centered on Arg72, Leu73, and Arg74. A conserved LYPSL sequence in the N terminus, as well as the C-terminal region of syntenin-1, are essential for binding to ubiquitin. We present evidence for the regulation of this interaction through syntenin-1 dimerization. We have also established that syntenin-1 is phosphorylated downstream of Ulk1, a serine/threonine kinase that plays a critical role in autophagy and regulates endocytic trafficking. Importantly, Ulk1-dependent phosphorylation of Ser6 in the LYPSL prevents the interaction of syntenin-1 with ubiquitin. These results define an unprecedented ubiquitin-dependent pathway involving syntenin-1 that is regulated by Ulk1.
Keywords: Adaptor Proteins, NMR, Ubiquitin, Ubiquitination, Ubiquitylation, Ulk1, Phosphorylation, Syntenin, Tetraspanin
Introduction
Tetraspanin-enriched microdomains (TERMs)4 are molecular platforms within the plasma membrane which control the migration, proliferation, and fusion of cells (1). Tetraspanins, the main structural component of TERMs, are also master regulators of lateral clustering, dynamics, and endocytic trafficking of associated transmembrane receptors, including integrins and growth factor receptors (2). The molecular pathways controlling the composition and dynamics of proteins within the TERMs remain poorly understood.
Syntenin-1/melanoma differentiation-associated gene 9 is a recently identified component and regulator of TERMs (3). Originally described as a partner of syndecans (4, 5), syntenin-1 was subsequently found to associate with a number of cytoplasmic and transmembrane proteins, including the tetraspanin CD63 (for review, see Ref. 6). Most of these interactions involve tandem PDZ domains, which were also shown to play a critical role in the activities ascribed to syntenin-1. Recent reports have shown that syntenin-1 regulates motility, invasiveness, and proliferation of tumor cells through signaling pathways involving focal adhesion kinase (7), c-Src (8), p38 (9), Akt kinases (10), and activation of NF-κB (7). Syntenin-1 is also involved in regulation of axonal outgrowth (11), and it has been proposed that it may play a role in determining the formation and maturation of synapses (12). As an adaptor for several transmembrane cargos, syntenin-1 was reported to control various aspects of intracellular trafficking, including biosynthetic trafficking of TGFα (13), endocytosis of CD63 (3), and recycling of syndecans (14). Although it has been shown that syntenin-1 regulates syndecan recycling via mechanisms involving phosphoinositides and the small GTPase Arf6 (14), molecular pathways underlying the role of syntenin-1 in other trafficking events remain unknown.
Protein ubiquitylation and ubiquitin (Ub)-based protein interactions are central to a number of endocytic pathways (15). Although the structural basis for Ub recognition by many Ub-binding protein domains is well established (16), the molecular pathways that control Ub-protein interactions remain virtually unknown. It was recently reported that syntenin-1 binds Ub in yeast two-hybrid assays (17). This interaction was suggested to be weak and transient, involving the C-terminal glycine residues of Ub. It was also suggested that this interaction requires the intact syntenin-1 molecule and is important for the ability of syntenin-1 to induce formation of filopodia.
Here, we establish that syntenin-1 facilitates recruitment of ubiquitylated proteins to its transmembrane partners. Importantly, we show that syntenin-1 binds Ub directly through a unique pocket not employed by previously established Ub ligands. Moreover, the recognition involves both N and C termini of syntenin-1, rather than its PDZ domains, which instead mediate prerequisite dimerization. Importantly, the binding is regulated by phosphorylation downstream of Ulk1, a Ser/Thr kinase that is known to control various trafficking pathways and plays a critical role in autophagy. Together, these results provide a novel conceptual framework for the Ub-dependent trafficking of transmembrane proteins via syntenin-1.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
HEK293T and HeLa cells were transfected with plasmids using polyethyleneimine (Sigma) and cultured in DMEM (Invitrogen) supplemented with 10% FCS (Invitrogen). The cells were then harvested and the lysates used for Western blotting or immunoprecipitation. Plasmids used to express syntenin-1 included pcDNA3-syntenin-1(WT)-HA, pcDNA3-syntenin-1ΔN, and syntenin-1ΔC as described previously (3). The FLAGTM-tagged syntenin-1WT and point mutants were generated using PCR or QuikChange site-directed mutagenesis (Stratagene). The Myc-tagged Ulk1WT and kinase-dead Ulk1-KD (K46R) mutant DNA constructs were provided by Dr. S. Tooze (London Research Institute, London, UK). The plasmid encoding the glutathione S-transferase (GST)-tagged form of human syntenin-1 was used as reported previously (3), and point mutants were generated. Expression plasmids pET30a containing chemically synthesized His6-tagged SmpA (control protein), wild-type Ub, or Ub mutant (L71A/L73A/R74Q and I44A) cDNAs inserted between the Nde1-Xho1 site were purchased from GeneScript (Piscataway, NJ). Pure His-Ub mutant proteins (G76A, ΔGG, G75A/G76A, K33R) and His-tagged poly-Ub (Lys63 and Lys48) were purchased from Boston Biochem and Enzo Life Sciences, respectively.
Antibodies used included anti-Ub mouse mAb, clone FK2 (Enzo Life Sciences); mouse mAb against CASK (Transduction Laboratories); anti-FLAG epitope mouse mAb (clone M2), and rabbit pAb (Sigma). Rabbit anti-Hrs pAbs were generously provided by Dr. S. Urbe (Liverpool). Rabbit anti-HA Ab (Santa Cruz Biotechnology); rabbit anti-Myc pAb (Cell Signaling Technology); mouse anti-actin mAb (Sigma); and mouse anti-syntenin-1 mAb (Abnova) were also used. Electroporation of HeLa cells was carried out using siRNA 5′-GCUAUAGCAUAGCUGCUUAtt-3′ and SilencerTM negative control #1 siRNA (Ambion catalogue no. 4611) and assessed for knockdown by Western blot analysis. Mouse mAb against CD63, 6H1, has been previously described (3).
Recombinant Protein Purification
GST-tagged human syntenin-1 and His-SmpA/His-Ub were expressed in Escherichia coli BL21 (DE3), lysed, and affinity-purified using GSTrap or HisTrap FF column (GE Healthcare), followed by size exclusion chromatography using HiLoad Superdex 75 26/60 (GE Healthcare) in 50 mm NaPO4, pH 7.5, 0.15 m NaCl, 0.5 mm tris(2-carboxyethyl)phosphine buffer. Tobacco etch virus protease was used to remove the GST tag from proteins, prior to size exclusion chromatography.
Immunoprecipitation, Pulldown, and Western Blotting
Immunoprecipitation and pulldown experiments from cellular lysates were performed as described previously (3). In brief, cells were lysed in 1% Triton X-100/PBS containing PMSF (2 mm), leupeptine (10 μg/ml), aprotinin (10 μg/ml), and the lysates were incubated with 15 μl of mono-ubiquitin (mUb) covalently bound to agarose beads (Sigma). Proteins retained by mUb-agarose were eluted from the beads in Laemmli buffer, and the eluate was divided into the appropriate number of equal aliquots, which were resolved by SDS-PAGE. The proteins were transferred to the nitrocellulose membrane and visualized with specific antibodies. In some of the experiments involving cellular lysates the functionality of mAb-agarose beads was additionally tested by adding 3 μg of hHR23A tandem UBA domain, TUBE1 (Boston Biochem), as a “Ub-binding tracer,” to all aliquots of cellular lysates used (supplemental Figs. S4 and S5). For pulldown experiments with recombinant proteins, syntenin-1 was incubated with His-tagged proteins immobilized on nickel-Sepharose beads, and bound proteins were eluted with 50 mm EDTA. The protein samples were resolved using SDS-PAGE and visualized by Coomassie staining or used for Western blotting.
NMR Spectroscopy
Lyophilized untagged 15N-labeled Ub (ASLA Biotech, Riga, Latvia) was reconstituted to 1 mm in NMR buffer (50 mm NaPO4 pH 7.5, 150 mm NaCl). NMR spectra were recorded on 100 μm Ub at 30 °C in a Varian Inova 800-MHz spectrometer equipped with a 5 mm 1H/13C/15N z-PFG cold probe and analyzed using CcpNmr Analysis 2.2. Chemical shift assignments for 15N-Ub at pH 7.5 were obtained from BMRB entry 4769 and as published (18). The concentration of syntenin-1 was increased stepwise as 1H,15N-resolved two-dimensional NMR spectra of 15N-labeled Ub were collected. Titration was discontinued beyond 1.4-fold excess of syntenin-1 due to extensive line broadening of Ub resonances. The intensities of individual cross-peaks in free Ub versus the Ub-syntenin-1 complex were normalized, and decreases in cross-peak intensities were mapped onto the Ub structure. Molecular graphics images were produced using the Chimera package (Computer Graphics Laboratory, University of California, San Francisco).
Immunofluorescence Staining
HeLa cells grown on glass coverslips were transfected with appropriate DNA plasmids using GeneJammer (Stratech). Staining was performed 40–48 h after transfection. Cells were fixed with 2% paraformaldehyde/PBS for 10–15 min. The staining with primary and fluorochrome-conjugated secondary Abs was carried out as described previously (19). The images were captured using Zeiss LSM510 META confocal system with 63× oil immersion objective (NA 1.4). The images were captured using Zeiss LSM510 META confocal system with 63× oil immersion objective (NA 1.4). Co-localization was quantified using the “Profile” function of Zeiss software.
RESULTS
Syntenin-1 Links Transmembrane Partners to Ubiquitylated Proteins
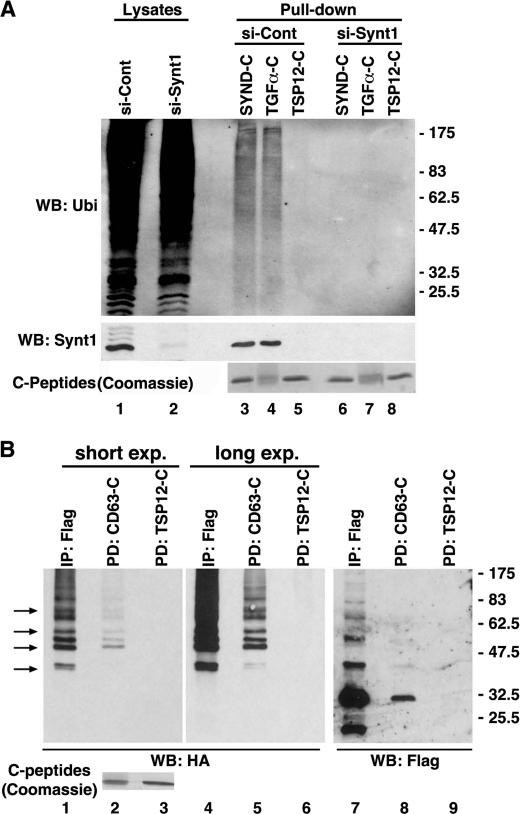
We investigated whether syntenin-1 may control trafficking of its transmembrane partners via ubiquitylation-dependent pathways. Pulldown assays with immobilized syndecan-2, TGFα, and CD63 C-terminal peptides (“C-tails”) on HeLa cell lysates specifically retained ubiquitylated protein species (Fig. 1A and supplemental Fig. S1). Importantly, the signals for all three peptides were diminished in lysates from syntenin-1 knockdown cells. In the control experiments we found that the peptide corresponding to the C-terminal cytoplasmic region of the tetraspanin Tspan-12, which does not bind syntenin-1, did not retain ubiquitylated proteins (Fig. 1A, lane 5). This indicates that syntenin-1 can link a diverse range of transmembrane partners with ubiquitylated proteins.
FIGURE 1.
Syntenin-1 links its transmembrane partners to ubiquitylated proteins. A, lysates prepared from HeLa cells transfected with either the siRNA targeting syntenin-1 or control siRNA were incubated with biotinylated peptides corresponding to the cytoplasmic tails of syndecan-2 (SYND-C), TGFα (TGFα-C), and Tspan-12-C (TSP12-C). Complexes were collected on NeutrAvidin-agarose and resolved on SDS-PAGE. Pulled-down ubiquitylated proteins interacting with the peptides were visualized with anti-Ub mAb (FK2). Shown are results of the pulldown experiments. Lower panel shows efficiency of syntenin-1 knockdown. Note, knockdown of syntenin-1 markedly disrupted the interaction of the peptides with ubiquitylated proteins. WB, Western blotting. B, lysates prepared from HEK293T cells expressing FLAG-syntenin-1 and HA-Ub were incubated with either biotinylated CD63-C and Tspan12-C (control) peptides or anti-FLAG mAb. Protein complexes were collected on NeutrAvidin-agarose, resolved on SDS-PAGE, and subsequently probed with anti-HA and anti-FLAG polyclonal Abs in Western blotting. Arrows point to ubiquitylated proteins from the syntenin-1 immunoprecipitate (IP; lane 1) that are distinct from the ubiquitylated species of syntenin-1 (characteristic ladder, lane 7). Note that CD63-C interacts with both ubiquitylated syntenin-1 and the associated proteins. Two exposures are shown to compare the patterns of proteins precipitated with anti-FLAG mAb and CD63-C.
We then tested whether any of the C-tail-bound proteins correspond to ubiquitylated forms of syntenin. Pulldown and immunoprecipitation experiments revealed a characteristic ladder of ubiquitylated species of syntenin-1, suggesting its poly- or multiubiquitylation (Fig. 1B). Notably, the most abundant of the ubiquitylated syntenin-1 molecules were also detected in the CD63 pulldown lane. Moreover, the syntenin-1 immunoprecipitate contained ubiquitylated proteins that are clearly distinct from the ubiquitylated syntenin-1 species (Fig. 1B). Thus, syntenin-1 interacts with a set of ubiquitylated proteins which it links to transmembrane partners, forming Ub-based molecular hubs.
Syntenin Binds Directly to Ubiquitin
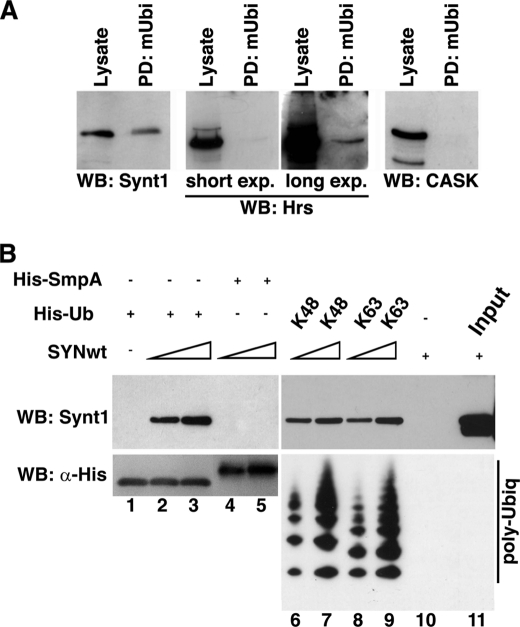
Binding assays showed that agarose-conjugated mUb specifically retained endogenous syntenin from the lysates of HeLa cells, whereas CASK, an unrelated PDZ domain-containing protein, did not bind Ub (Fig. 2A). Interestingly, the interaction of Ub with Hrs, a protein with a well recognized double-sided Ub-interacting motif (20), was somewhat weaker (Fig. 2A). These results indicate that syntenin-1 (or one of its partners) interacts with Ub. To analyze whether interaction between syntenin-1 and Ub is direct we tested the binding of Ub to purified recombinant syntenin-1 protein. As illustrated in Fig. 2B, syntenin-1 binds to immobilized mUb in pulldown assays (lanes 2 and 3). Importantly, syntenin-1 interacted equally well with Lys48- or Lys63-linked poly-Ub chains (Fig. 2B, lanes 6–9). Together, this shows that full-length syntenin-1 binds directly and tightly to Ub, including its polyubiquitylated forms.
FIGURE 2.
Syntenin-1 interacts with Ub. A, syntenin-1 is retained by immobilized mono-Ub from cellular lysates. Cellular lysates prepared from HeLa cells were incubated with mono-Ub covalently attached to agarose beads (mUbi); interacting proteins were eluted from the beads and equal volumes of the eluate (controlled by Western blotting with mAb to actin) were resolved on SDS-PAGE. Syntenin-1, Hrs, and CASK were detected by Western blotting (WB). B, upper, recombinant syntenin-1 (0.1 and 0.5 μg) was incubated with immobilized His-Ub or His-SmpA (negative control) and His-poly-Ub (2–7) chains (Lys48-linked) or His-poly-Ub (2–7) chains (Lys63-linked). Interactions between the proteins were visualized by Western blotting using anti-syntenin-1 mAb. Lower, loading of His-Ub species was controlled using anti-His HRP mAb antibody.
Syntenin-1 Contacts the C Terminus of Ubiquitin
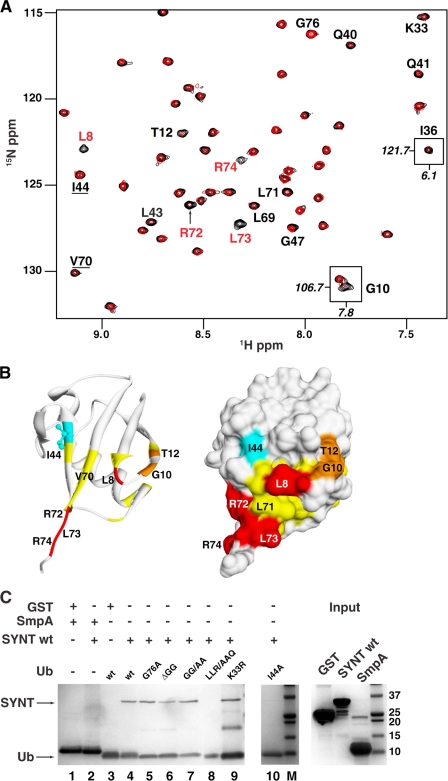
The Ub binding pocket for full-length syntenin-1 was mapped by NMR spectroscopy. Significant and progressive line broadening of a set of cross-peaks in 1H,15N-resolved two-dimensional spectra of Ub was seen with the addition of increasing amounts of unlabeled syntenin-1 (Fig. 3A). This indicated the intermediate exchange of Ub residues between free and complexed states with syntenin-1. The interface was mapped by monitoring Ub resonances which exhibited decreased intensities upon syntenin-1 binding. As seen in Fig. 3B, syntenin-1 binds to a well defined region spanning the C terminus and centered on Arg72, Leu73, and Arg74, resulting in severe decrease in the cross-peak intensities. In addition, Leu8, Gly10, and Thr12 also exhibited significant intensity reduction. Other regions exhibiting decreased peak intensities involved residues Lys33, Ile36, Gln40, Gly47, and Leu71, all nearby and circumscribing a novel C-terminal binding site. Apart from Leu8 there was very minimal perturbation of the conservative hydrophobic pocket spanning Ile44 and Val70. The extreme C terminus of Ub was not involved in syntenin-1 binding because no significant shift or broadening of the resolved Gly76 resonance was induced during NMR titration. Although Gly75 could not be observed by NMR under the pH conditions employed in this study, it was not required based on the mutational analysis as shown below. The entire HSQC spectral comparison of syntenin-bound and free Ub is shown in supplemental Fig. S2. The affinity (KD) of the Ub-syntenin-1 interaction was 27.3 μm, as measured by isothermal calorimetry (supplemental Fig. S3). This value is consistent with the intermediate exchange kinetics apparent by NMR, and is relatively tight compared with most ubiqutin binding domains (21).
FIGURE 3.
Syntenin-1 binds to the C terminus of Ub. A, overlays of a region from 1H-15N heteronuclear single quantum correlation spectra of 100 μm Ub without (black) and with 140 μm syntenin (red). Residues that exhibit significant intensity reduction are labeled, as well as Ile44 and Val70 that lie outside the binding interface. Shown as insets are Gly10 and Ile36 that lie outside the extracted region and undergo significant intensity reduction. B, syntenin-1 binding interface mapped onto crystal structure of Ub are shown as ribbon (left) and molecular surface (right) representations, in similar orientations. Residues with missing cross-peaks are colored red whereas those with >50% or between 40 and 50% reduction in peak intensities are colored orange and yellow, respectively. The exposed Ile44 side chain is shown in cyan. C, recombinant syntenin-1 or GST (control) was incubated with immobilized His-Ub and a panel of His-Ub mutants (G76A, ΔGG, GG/AA, K33R, and I44A) as indicated, or His-SmpA (negative control). Bound proteins were then visualized by Coomassie Brilliant Blue staining.
The Ub binding site for syntenin-1 was validated by mutagenesis. Although the wild-type syntenin-1 exhibited compromised binding to the UbLLR/AAQ Ub mutant (Leu71 to Ala, Leu73 to Ala, and Arg74 to Gln substitutions) (Fig. 3C, lane 8), binding of syntenin-1 to three different C-terminal glycine mutants including UbG76A, UbG75A,G76A (GG/AA mutant), and UbΔG75,G76 (ΔGG deletion mutant) was not affected in the pulldown assays (Fig. 3C, lanes 4–7). Although the resonances of the Ub Ile44 residue showed no perturbation, the Ile44 to Ala mutant exhibited reduced binding to syntenin-1 (Fig. 3C, lane 9). We speculate that this could be due to an indirect effect caused by a local disruption of the hydrophobic pocket around Ile44 in the mutant protein. UbK33R still bound syntenin-1 (Fig. 3C, lane 7), suggesting that any basic residue will suffice at position 33. Thus, we concluded that residues 71–74 represent the core of the syntenin recognition site, with residues 8–12 (but not the C-terminal glycine residues) providing additional stabilizing interactions.
Syntenin Dimerization Is Essential for Ubiquitin Interaction
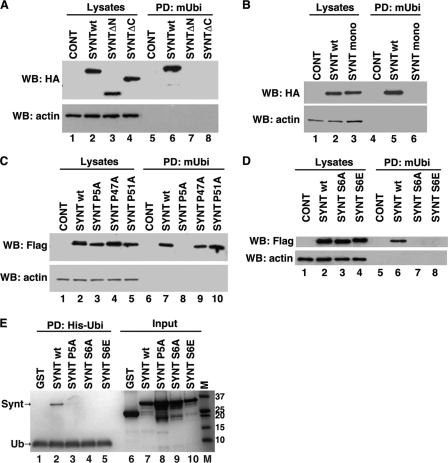
To identify the region of syntenin-1 recognized by Ub we initially tested the N- and C-terminal deletion mutants of syntenin-1 (synt-ΔN and synt-ΔC). Pulldown assays using cellular lysates containing these truncated syntenin-1 proteins revealed that the presence of the first 101 and last 25 residues were critical for Ub interaction (Fig. 4A, lanes 6–8). The requirement for both N and C termini for the syntenin-1 Ub interaction suggests their spatial juxtaposition during Ub binding. Head-to-tail dimerization could yield such proximity of the termini. Indeed, the crystal structure of the PDZ tandem of syntenin-1 reveals head-to-tail dimers (22), and the native protein dimerizes in cells (23). To investigate whether syntenin-1 protein dimerization is important for Ub binding, we performed Ub pulldown experiments using a SyntF195A/L233A mutant (Synt-mono), which is impaired in dimer formation (24). Fig. 4B illustrates that the Synt-mono was not retained by mUb-agarose (lane 6). Together, this indicates that dimerization of syntenin-1 is critical for Ub binding.
FIGURE 4.
Both the N- and C-terminal regions are important for the interaction of syntenin-1 with Ub: role of dimerization. A, protein lysates prepared from HEK293T cells expressing syntenin-1WT-HA, syntenin-ΔN-HA (SYNT-ΔN), and syntenin-ΔC-HA (SYNT-ΔC) were incubated with mUb covalently attached to agarose beads, and interacting proteins were resolved on SDS-PAGE. Syntenin-1 proteins were detected by Western blotting (WB) with anti-HA Ab. In the control experiments cells were transfected with pcDNA (lanes 1 and 5). B, Protein lysates prepared from HEK293T cells expressing HA-tagged syntenin-1WT or dimerization mutant (F195A/L233A, referred to as SYNTmono), were incubated with mUb covalently attached to agarose beads, and interacting proteins were resolved on SDS-PAGE. Syntenin-1 was detected by Western blotting with anti-HA Ab. C and D, protein lysates prepared from HEK293T cells expressing syntenin-1WT-FLAG, and various FLAG-tagged mutants of syntenin-1 were incubated with mUb covalently attached to agarose beads. Interacting proteins were resolved on SDS-PAGE. Syntenin-1 was detected by Western blotting with anti-FLAG Ab. In the control experiments cells were transfected with pcDNA (lanes 1 and 5 for B and D; lanes 1 and 4 for C). E, recombinant wild-type syntenin-1 (SYNTwt) and syntenin-1 point mutants (P5A, SYNT P5A; S6A, SYNT S6A; and S6E, SYNT S6E) (0.5 μg each) were incubated with 30 μl of His-Ub-immobilized nickel-Sepharose beads in 50 mm NaPO4, pH 7.4, 500 mm NaCl, 0.5 mm tris(2-carboxyethyl)phosphine, 20 mm imidazole, 0.2% Triton X-100, 0.02% BSA. Bound proteins were eluted with 50 mm EDTA. The protein samples were resolved using SDS-PAGE and visualized with Coomassie Brilliant Blue staining. Note, additional low molecular mass protein bands correspond to proteolytic products of full-length syntenin. PD, pulldown.
Discovery of the Syntenin Motif Recognized by Ub
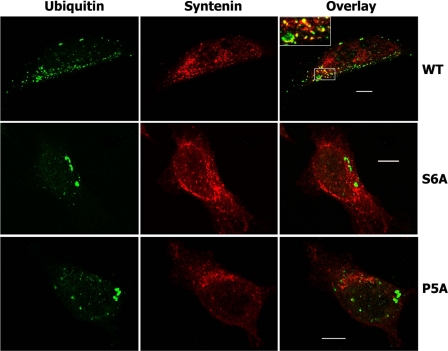
We observed that the syntenin-1 N terminus contains three conserved LYPXL sequences centered at positions 5, 47, and 51, respectively, where X is any residue. To investigate their role in Ub interaction, we examined Ub binding by syntenin-1 mutants in which each central proline was mutated to Ala. Mutation of Pro5 to Ala (Synt P5A) completely abolished binding of syntenin-1 to Ub in pulldown experiments with cellular lysates (Fig. 4C, lane 8) or with recombinant proteins (Fig. 4E, lane 3). By contrast, P47A and P51A substitutions had no detectable effects on the interaction (Fig. 4C, lanes 9 and 10). Accordingly, immunofluorescence experiments reveal that the co-localization of Ub puncta with the Synt P5A mutant was severely compromised (Fig. 5).
FIGURE 5.
Co-localization of syntenin-1 and ubiquitylated proteins. HeLa cells expressing FLAG-tagged syntenin-1 WT or syntenin-1 S6A (S6A) and syntenin-1 P5A (P5A) mutants were fixed with 2% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were co-stained with anti-FLAG rabbit pAb and anti-Ub mouse mAb (clone FK2). Staining was visualized with Alexa Fluor 488-conjugated goat anti-mouse antibody and labeled with Alexa Fluor 594-conjugated anti-rabbit antibody. Overlay panels and insets show partial co-localization of wild-type syntenin-1 (red) with ubiquitylated cellular proteins (green) and lack of co-localization in cells expressing mutants of syntenin-1 (red). Scale bars, 10 μm.
The first LYPXL motif of syntenin-1 is unique in containing a conserved Ser residue at the X position. To investigate the role of Ser6, we generated Synt S6A and Synt S6E mutants. Either mutation completely abolished the interaction of syntenin-1 with Ub (Fig. 4D, lanes 7 and 8). Results from the pulldown assays using recombinant syntenin-1 mutant proteins further corroborated this observation (Fig. 4E, lanes 4 and 5). Accordingly, Ser6 was important for the recruitment syntenin-1 to ubiquitylated proteins in cells (Fig. 5). Together, this establishes the L4YPSL8 sequence of syntenin-1 as a novel Ub binding motif and a determinant in the formation of Ub-based regulatory complexes.
Ulk1 Controls the Syntenin-Ubiquitin Interaction
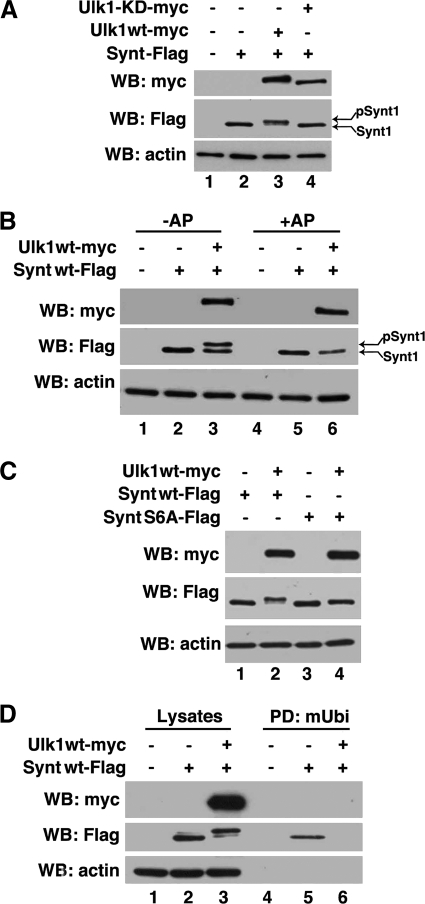
The importance of Ser6 prompted us to define the role of phosphorylation in the regulation of Ub-syntenin-1 interaction. Co-expression of syntenin-1 and Ulk1, a partner for syntenin-1 (25), in HEK293T cells yielded a slower migrating additional protein band (Fig. 6A, lane 3). This band disappeared when cellular lysates were treated with alkaline phosphatase (Fig. 6B, lane 6), indicating that syntenin-1 phosphorylation was responsible for this slower migrating species. Accordingly, no extra band was detectable when syntenin-1 was co-expressed with the kinase-dead mutant of Ulk1 (Fig. 6A, lane 4). Next, we examined the extent and effect of syntenin-1 phosphorylation by Ulk1. Although the S6A mutant migrates faster than the phosphorylated variant of the wild-type syntenin-1 in SDS-PAGE, its position was still above the nonphosphorylated form (Fig. 6C, middle). Not only did these results show that Ulk1 phosphorylates syntenin-1 on Ser6, but they also indicate additional phosphorylation sites in syntenin-1. Pulldown experiments were carried out to examine whether Ulk1-dependent phosphorylation of syntenin-1 regulates its binding to Ub. mUb-agarose did not retain the phosphorylated form of syntenin-1 from Ulk1-overexpressing cellular lysates (Fig. 6D, middle, lanes 5 and 6). Hence, Ulk1-dependent phosphorylation of syntenin-1 on Ser6 can represent a physiological switch which controls the interaction of Ub with syntenin-1. Together, this establishes the substrate specificity of Ulk1 and implicates syntenin-1 as its critical downstream target.
FIGURE 6.
Ulk1 modifies syntenin via phosphorylation. A, protein lysates prepared from HEK293T cells expressing syntenin-1WT-FLAG, Ulk1-Myc, and the Ulk1-KD-Myc mutant were resolved by SDS-PAGE. B, samples prepared as above were subjected to treatment with alkaline phosphatase (AP) for 40 min at 37 °C (lanes 4–6) and subsequently resolved on 10% SDS-PAGE. Note, alkalkine phosphatase treatment resulted in the loss of modification indicating that syntenin-1 is phosphorylated in the presence of Ulk1. C, protein lysates prepared from 293T cells expressing syntenin-1WT-FLAG, syntenin-1 S6A-FLAG mutant, and Ulk1-Myc were resolved on SDS-PAGE. D, protein lysates prepared from 293T cells expressing syntenin-1WT-FLAG and Ulk1-Myc were incubated with immobilized monoubiquitin (mUbi), and proteins were resolved on SDS-PAGE. In the control experiments, cells were transfected with pcDNA (lanes 1 and 4). Syntenin-1 and Ulk1 were detected by Western blotting (WB) with anti-FLAG and anti-Myc Abs, respectively (A–D). Actin was used as a loading control in all blots.
Ubiquitylation Influences Recruitment of Synteinin-1 to Endosomes
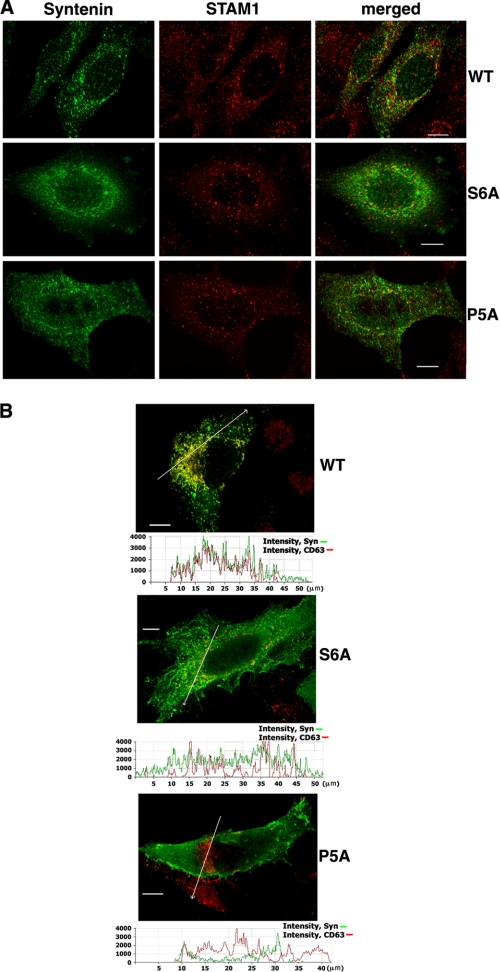
Ubiquitylation of transmembrane proteins serves as an important signal for their endocytic sorting (26). Accordingly, ubiquitylated proteins could be found on both early and late endosomes (27). To examine whether binding to Ub has a role in distribution of syntenin-1 to various endocytic compartments, we carried out immunofluorescence experiments using HeLa cells expressing the wild-type syntenin-1 and its mutants which lost their Ub binding property (i.e. Synt S6A and Synt P5A). There was only limited co-localization of the wild-type syntenin-1 or the mutants with markers of early endosomes (i.e. EEA-1 and STAM) (Fig. 7A and data not shown). Similarly, we found no evidence for co-localization of syntenin-1 with transferrin receptors on recycling endosomes (data not shown). By contrast, a significant proportion of syntenin-1-positive vesicles also contained CD63, a well established marker for late endosomes and lysosomes (Fig. 7B). Importantly, recruitment of both syntenin-1 mutants to these compartments was markedly suppressed. These results demonstrated that binding to ubiquitylated proteins may be an important factor that controls the distribution of syntenin-1 to late endocytic compartments.
FIGURE 7.
Role of Ub binding in cellular localization of syntenin-1. HeLa cells expressing FLAG-tagged syntenin-1 (WT or syntenin-1 S6A (S6A) and syntenin-1 P5A (P5A) mutants) were fixed with 2% paraformaldehyde and permeabilized with 0.1% Triton X-100. A, cells were co-stained with anti-FLAG mouse mAb (M2) and anti-STAM rabbit pAb. Staining was visualized with Alexa Fluor 488-conjugated goat anti-mouse antibody and labeled with Alexa Fluor 594-conjugated anti-rabbit antibody. Overlay panels show minimal co-localization syntenin-1 proteins (green) with STAM (red). B, cells were co-stained with anti-FLAG rabbit pAb and anti-CD63 mouse mAb (6H1). Staining was visualized with Alexa Fluor 594-conjugated goat anti-mouse antibody and labeled with Alexa Fluor 488-conjugated anti-rabbit antibody. Shown are only overlay panels. Co-localization profiles (along the lines on the images directed toward the arrows) are presented as the fluorescence intensity distribution for each channel (shown underneath each of the panels). Note the differences in degree of syntenin-1/CD63 co-localization in cells expressing the wild-type syntenin-1 (top) and syntenin-1 mutants (middle and bottom). Scale bars, 10 μm.
DISCUSSION
A structural mechanism is presented here for Ub-mediated interactions. Phosphorylation of the novel Ub binding motif discovered here in syntenin-1 serves as a negative switch that causes disassembly of the Ub-syntenin-1 complex. Furthermore, our results represent the first validated example of a Ub-based interaction regulated by dimerization and phosphorylation of a Ub partner.
The mode of interaction between syntenin-1 and Ub is unique as syntenin-1 does not contain a clearly identifiable Ub binding module. Instead, our results indicate that the Ub interaction is necessarily stabilized by the concerted action of two termini of syntenin-1. This mode of binding to Ub is likely to be facilitated by the head-to-tail dimerization of syntenin-1 which is controlled by its tandem PDZ domains (22). Such dimerization permits simultaneous binding of syntenin-1 to two Ub molecules (or ubiquitylated proteins) in a 2:2 stoichiometry. Thus, the tight affinity of Ub-syntenin-1 interaction is probably manifested by the syntenin-1 dimer. Not only would this offer enhanced avidity within the Ub-syntenin-1 complex but could also result in amplification of cellular pathways controlled by this interaction.
Secondary structure prediction of the unfolded N-terminal region of syntenin-1 indicates α-helical propensities for residues Ser6-Ser23, Glu52-Gly58, and Glu63-Ser74, and also at the C-terminal region between residues Phe275 and Asp292 (supplemental Fig. S6). By analogy, Ub-binding proteins that interact through CUE domains do so through two discontinuous α-helices (28, 29). Thus, although the syntenin N and C termini lack sequence homology with CUE domains, its binding to Ub may also involve α-helical ligand conformations.
Our results with purified recombinant proteins using NMR and pulldown assays show that syntenin-1 binds both mUb and poly-Ub molecules. Notably, binding of syntenin-1 to Ub differs from that of most Ub-binding proteins: it engages a novel pocket near the Ub C-terminal LRLR74 element. In addition to this region, syntenin-1 binding to Ub encompasses Leu8, which is a part of the conserved hydrophobic pocket that includes Ile44 and Val70 (16). Despite this, our NMR data strongly suggest that neither Ile44 nor Val70 is involved in direct contact with syntenin-1. Curiously, binding of recombinant syntenin-1 to the I44A mutant of Ub was severely compromised. Although further experiments are required to establish the reason for this discrepancy, one possibility is that the decreased syntenin-1 binding exhibited by the I44A mutant might be due to a more general disruption of the hydrophobic pocket (the side chains of Leu8, Ile44, and Val70 are tightly packed against each other in this pocket). Interestingly, similar results (i.e. minimal perturbation in the NMR experiments and disruption in binding to the I44A mutant) have been reported for the Ub binding GAT domains of clathrin-associated GGA proteins (30). Nonengagement of the region around Ile44 may explain syntenin's lack of preference for Lys48- or Lys63-linked poly-Ub and allows broader specificity for ubiquitylated ligands as well as multiprotein docking. Significantly, this would also permit recruitment of other Ub-binding proteins (that bind to Ile44 interface) into the complex and thereby further diversify the adaptor function of syntenin-1. Finally, we found that syntenin-1 is ubiquitylated in cells and does not affect interactions of the protein with its transmembrane partners. Thus, syntenin-1 has been shown to play a critical role in nucleating various Ub-dependent interactions that would explain the multiplicity of its cellular functions.
Deubiquitinating enzymes remove the C-terminal peptide extensions from Ub by recognizing the C terminus of Ub and a wider patch encompassing 20–40% of the Ub surface (31). Specifically, the residues Arg74 and Gly75 are shown to be crucial for specific recognition of Ub by deubiquitinating enzymes (32). Thus, the primary deubiquitinating enzyme binding site in Ub (18, 33) partially overlaps with that for syntenin-1. Given the fact that Ub-syntenin-1 interaction is relatively tight (KD of 27.3 μm), the complex of syntenin-1 with monoubiquitylated partners may be shielded from the activity of deubiquitinating enzymes, thus ensuring prolongation of syntenin-1-dependent pathways.
Recently, Okumura et al. have suggested that the C-terminal glycines of Ub are the primary syntenin-1 binding determinants (17). However, these authors failed to detect binding of syntenin-1 to Ub in pulldown experiments with recombinant proteins. By contrast, direct binding of syntenin-1 to Ub using recombinant proteins was consistently observed in our NMR, pulldown, and isothermal calorimetry experiments. Furthermore, our results clearly ruled out the requirement of Gly75 and Gly76 for syntenin-1 binding, based on chemical shift mapping analysis and in vitro binding assays involving Ub mutants. A possible explanation for the discrepancies is the differences in the assays and variations in syntenin-1 constructs used in these studies. Indeed, we found that GST-tagged syntenin-1 as used by Okumura et al. fails to bind recombinant Ub (data not shown), and hence we have used untagged protein in all our experiments with recombinant proteins.
We found that binding to Ub controls recruitment of syntenin-1 to CD63-positive late endosomes. These results strongly suggest that syntenin-1 may play an important role in Ub-dependent sorting of its transmembrane cargos. In this regard, endocytic trafficking of several transmembrane partners of syntenin-1 is regulated by ubiquitylation, including syndecan-4 (34), GlyT2 (35), and IL5R (36). Thus, ubiquitylation of the cargo on one side and the ability of syntenin-1 to bind Ub on the other may play an important role in either stabilization of the complex or nucleating an Ub-dependent protein network required for efficient sorting of its transmembrane partners.
Phosphorylation-regulated interactions of syntenin-1 with ubiquitylated proteins implicate Ulk1 kinase as a key regulator of syntenin-1-dependent trafficking. Ulk1 is a component of the autophagy-initiating complex which controls membrane deformation and elongation (25). It also appears to regulate both clathrin-dependent and clathrin-independent endocytosis (37, 38) and cycling of transmembrane proteins from the Golgi to endosomes (39). However, the molecular mechanisms underlying these nonautophagic activities of Ulk1 remain unknown. With no published reports on the involvement of previously reported Ulk1 substrates FIP200 and mAtg13 in endocytic trafficking (40), syntenin-1 represents not only a new substrate but also a pivotal candidate for linking Ulk1 with the trafficking of a diverse range of transmembrane proteins. With several missing linkages now revealed, the biological ramifications of the Ub-syntenin-1 interaction and its impact on context of phosphorylation-dependent receptor trafficking are now open for investigation and exploitation.
Supplementary Material
Acknowledgments
We thank Dr. D. Powner for generating a FLAG-tagged syntenin-1 construct, Prof. J. Baz Jackson for isothermal calorimetry access, and Drs. T. Cierpicki and Z. Derewenda (University of Virginia) for their GST-syntenin-1 expression plasmid. NMR data were collected at the Wellcome Trust-funded Henry Wellcome Building for Nuclear Magnetic Resonance.
This work was supported by the Breast Cancer Campaign (to F. B.), Cancer Research UK (to F. B. and M. O.), and the Medical Research Council and the Biotechnology and Biological Sciences Research Council (to M. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- TERM
- tetraspanin-enriched microdomain
- KD
- kinase-dead
- mUb
- monoubiquitin
- Ub
- ubiquitin.
REFERENCES
- 1. Hemler M. E. (2003) Annu. Rev. Cell Dev. Biol. 19, 397–422 [DOI] [PubMed] [Google Scholar]
- 2. Berditchevski F., Odintsova E. (2007) Traffic 8, 89–96 [DOI] [PubMed] [Google Scholar]
- 3. Latysheva N., Muratov G., Rajesh S., Padgett M., Hotchin N. A., Overduin M., Berditchevski F. (2006) Mol. Cell. Biol. 26, 7707–7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grootjans J. J., Zimmermann P., Reekmans G., Smets A., Degeest G., Dürr J., David G. (1997) Proc. Nat. Acad. Sci. U.S.A. 94, 13683–13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmermann P., Tomatis D., Rosas M., Grootjans J., Leenaerts I., Degeest G., Reekmans G., Coomans C., David G. (2001) Mol. Biol. Cell 12, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beekman J. M., Coffer P. J. (2008) J. Cell Sci. 121, 1349–1355 [DOI] [PubMed] [Google Scholar]
- 7. Boukerche H., Su Z. Z., Emdad L., Sarkar D., Fisher P. B. (2007) Cancer Res. 67, 1812–1822 [DOI] [PubMed] [Google Scholar]
- 8. Boukerche H., Su Z. Z., Prévot C., Sarkar D., Fisher P. B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15914–15919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boukerche H., Su Z. Z., Emdad L., Baril P., Balme B., Thomas L., Randolph A., Valerie K., Sarkar D., Fisher P. B. (2005) Cancer Res. 65, 10901–10911 [DOI] [PubMed] [Google Scholar]
- 10. Hwangbo C., Park J., Lee J. H. (2011) J. Biol. Chem. 286, 33601–33612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomoda T., Kim J. H., Zhan C., Hatten M. E. (2004) Genes Dev. 18, 541–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McClelland A. C., Sheffler-Collins S. I., Kayser M. S., Dalva M. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20487–20492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ureña J. M., Merlos-Suárez A., Baselga J., Arribas J. (1999) J. Cell Sci. 112, 773–784 [DOI] [PubMed] [Google Scholar]
- 14. Zimmermann P., Zhang Z., Degeest G., Mortier E., Leenaerts I., Coomans C., Schulz J., N'Kuli F., Courtoy P. J., David G. (2005) Dev. Cell 9, 377–388 [DOI] [PubMed] [Google Scholar]
- 15. Hurley J. H., Emr S. D. (2006) Annu. Rev. Biophys. Biomol. Struct. 35, 277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dikic I., Wakatsuki S., Walters K. J. (2009) Nat. Rev. Mol. Cell Biol. 10, 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okumura F., Yoshida K., Liang F., Hatakeyama S. (2011) Mol. Cell. Biochem. 352, 163–172 [DOI] [PubMed] [Google Scholar]
- 18. Sakamoto T., Tanaka T., Ito Y., Rajesh S., Iwamoto-Sugai M., Kodera Y., Tsuchida N., Shibata T., Kohno T. (1999) Biochemistry 38, 11634–11642 [DOI] [PubMed] [Google Scholar]
- 19. Berditchevski F., Odintsova E. (1999) J. Cell Biol. 146, 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirano S., Kawasaki M., Ura H., Kato R., Raiborg C., Stenmark H., Wakatsuki S. (2006) Nat. Struct. Mol. Biol. 13, 272–277 [DOI] [PubMed] [Google Scholar]
- 21. Hurley J. H., Lee S., Prag G. (2006) Biochem. J. 399, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang B. S., Cooper D. R., Jelen F., Devedjiev Y., Derewenda U., Dauter Z., Otlewski J., Derewenda Z. S. (2003) Structure 11, 459–468 [DOI] [PubMed] [Google Scholar]
- 23. Koroll M., Rathjen F. G., Volkmer H. R. (2001) J. Biol. Chem. 276, 10646–10654 [DOI] [PubMed] [Google Scholar]
- 24. Ko J., Yoon C., Piccoli G., Chung H. S., Kim K., Lee J. R., Lee H. W., Kim H., Sala C., Kim E. (2006) J. Neurosci. 26, 963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young A. R., Chan E. Y., Hu X. W., Köchl R., Crawshaw S. G., High S., Hailey D. W., Lippincott-Schwartz J., Tooze S. A. (2006) J. Cell Sci. 119, 3888–3900 [DOI] [PubMed] [Google Scholar]
- 26. Hurley J. H. (2010) Crit. Rev. Biochem. Mol. Biol. 45, 463–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizuno E., Kobayashi K., Yamamoto A., Kitamura N., Komada M. (2006) Traffic 7, 1017–1031 [DOI] [PubMed] [Google Scholar]
- 28. Kang R. S., Daniels C. M., Francis S. A., Shih S. C., Salerno W. J., Hicke L., Radhakrishnan I. (2003) Cell 113, 621–630 [DOI] [PubMed] [Google Scholar]
- 29. Prag G., Misra S., Jones E. A., Ghirlando R., Davies B. A., Horazdovsky B. F., Hurley J. H. (2003) Cell 113, 609–620 [DOI] [PubMed] [Google Scholar]
- 30. Bilodeau P. S., Winistorfer S. C., Allaman M. M., Surendhran K., Kearney W. R., Robertson A. D., Piper R. C. (2004) J. Biol. Chem. 279, 54808–54816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komander D., Clague M. J., Urbé S. (2009) Nat. Rev. Mol. Cell Biol. 10, 550–563 [DOI] [PubMed] [Google Scholar]
- 32. Drag M., Mikolajczyk J., Bekes M., Reyes-Turcu F. E., Ellman J. A., Wilkinson K. D., Salvesen G. S. (2008) Biochem. J. 415, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reyes-Turcu F. E., Horton J. R., Mullally J. E., Heroux A., Cheng X., Wilkinson K. D. (2006) Cell 124, 1197–1208 [DOI] [PubMed] [Google Scholar]
- 34. Carvallo L., Muñoz R., Bustos F., Escobedo N., Carrasco H., Olivares G., Larraín J. (2010) J. Biol. Chem. 285, 29546–29555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Juan-Sanz J., Zafra F., Lopez-Corcuera B., Aragon C. (September 12, 2011) Traffic 10.1111/j.1600-0854.2011.01278.x [DOI] [PubMed] [Google Scholar]
- 36. Martinez-Moczygemba M., Huston D. P., Lei J. T. (2007) J. Leukoc. Biol. 81, 1137–1148 [DOI] [PubMed] [Google Scholar]
- 37. Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. (2005) Nature 436, 78–86 [DOI] [PubMed] [Google Scholar]
- 38. Zhou X., Babu J. R., da Silva S., Shu Q., Graef I. A., Oliver T., Tomoda T., Tani T., Wooten M. W., Wang F. (2007) Proc. Nat. Acad. Sci. U.S.A. 104, 5842–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan E. Y., Kir S., Tooze S. A. (2007) J. Biol. Chem. 282, 25464–25474 [DOI] [PubMed] [Google Scholar]
- 40. Ganley I. G., Lam du H., Wang J., Ding X., Chen S., Jiang X. (2009) J. Biol. Chem. 284, 12297–12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.