Abstract
Pathogen-triggered activation of the inflammasome complex leading to caspase-1 activation and IL-1β production involves similar sensor proteins between mouse and human. However, the specific sensors used may differ between infectious agents and host species. In mice, Francisella infection leads to seemingly exclusive activation of the Aim2 inflammasome with no apparent role for Nlrp3. Here we examine the IL-1β response of human cells to Francisella infection. Francisella strains exhibit differences in IL-1β production by influencing induction of IL-1β and ASC transcripts. Unexpectedly, our results demonstrate that Francisella activates the NLRP3 inflammasome in human cells. Francisella infection of THP-1 cells elicits IL-1β production, which is reduced by siRNA targeting of NLRP3. Moreover, in reconstituted 293T cells, Francisella triggers assembly of the NLRP3 inflammasome complex. In addition, inhibitors of reactive oxygen species, cathepsin B, and K+ efflux pathways, known to specifically influence NLRP3, substantially but not completely impair the Francisella-elicited IL-1β response, suggesting the involvement of another inflammasome pathway. Finally, shRNA targeting of NLRP3 and AIM2 reveals that both pathways contribute to the inflammasome response. Together these results establish NLRP3 as a cytosolic sensor for Francisella in human cells, a role not observed in mouse.
Keywords: Bacteria, Inflammation, Innate Immunity, Macrophages, Pattern Recognition Receptor, Inflammasome, Nucleotide Binding Domain and Leucine-rich Receptor (NLR)
Introduction
Innate immune responses to pathogens are initiated by recognition of pathogen-associated molecular patterns by both extracellular and intracellular sensors (1–3). Pathogen-associated molecular pattern recognition by members of the Toll-like receptor (TLR)3 family result in the activation of the NF-κB, MAPK, and/or interferon regulatory factor signaling pathways depending on the specific TLR engaged (1, 4–8). Intracellular receptors of the MAVS/RIG-I family act similarly and are involved in recognition of viral nucleic acids (9, 10). Thus, production of inflammatory cytokines and chemokines such as TNFα, IL-6, and MCP-1 in response to infection generally follows activation via these receptors. The inflammatory cytokines IL-1β and IL-18, however, require processing by caspase-1 to produce their active forms (11). Pathogen-associated molecular patterns and danger-associated molecular patterns activate various members of the nucleotide binding leucine-rich receptor (NLR) family in the cytoplasm, resulting in the assembly of an NLR containing, multiprotein complex (the inflammasome) that recruits and activates caspase-1 leading to IL-1β processing (12, 13). Although CARD domain-containing NLRs (e.g. Ipaf) can directly interact with caspase-1, most inflammasomes are assembled by Pyrin domain containing NLRs (NLRPs), which recruit caspase-1 indirectly through the adapter molecule ASC (14).
Francisella tularensis is the causative agent of tularemia and a potential bioweapon (15). Pulmonary infection with even a single, virulent F. tularensis bacterium is potentially lethal if untreated (16, 17). For humans, the type A strain, SchuS4 (F. tularensis sp. tularensis) results in the highest mortality, whereas neither the attenuated type B live vaccine strain LVS (F. tularensis sp. holarctica) or the U112 strain (Francisella tularensis sp. novicida) are virulent. In mice however, the SchuS4, LVS and U112 strains are lethal (18). Because of its importance in controlling bacterial infection and promoting adaptive immunity, the innate immune response to F. tularensis has been an area of recent interest. In mouse models of tularemia, the macrophage response to F. tularensis LVS is heavily reliant upon TLR2 as TLR2-deficient macrophages fail to produce TNFα, IL-6, and other NF-κB dependent proinflammatory cytokines (19, 20). Mouse macrophages infected with U112 produce IL-1β in an ASC and caspase-1-dependent fashion, indicating the likely involvement of an NLRP inflammasome (21, 22). The Pyrin domain containing non-NLR protein, Aim2, a member of the Hin200 family of DNA-binding proteins, has recently been demonstrated to mediate the vast majority, if not all, of the IL-1β produced by mouse macrophages upon infection with U112 or LVS (23, 24). Importantly, Asc−/−, Casp1−/−, or Aim2−/− mice infected with U112 have increased susceptibility to subcutaneous tularemia, further highlighting the importance of ASC/caspase-1 pathway in protective immune responses against Francisella infection (21).
Listeria monocytogenes, another intracellular bacterium, elicits inflammasome activity via three intracellular protein sensors in mice, Aim2, Nlrp3, and Nlrc4/Ipaf (25). However, although a role for mouse Aim2 has recently been recognized in the detection of Francisella (23, 24), deletion of Nlrp3 or Nlrc4/Ipaf had little or no effect on mouse macrophage IL-1β responses (23). The life cycle of Francisella within the macrophage, like Listeria, involves invasion and replication within the cytosol after “escape” by disruption of endosomal membranes (26, 27), events implicated as being upstream of Nlrp3 activation. Thus it is surprising that mouse Nlrp3 does not sense Francisella.
Differences between mouse and human cells in the inflammatory response, including IL-1β, have been recently reported (28, 29). Of note, in human macrophages NLRP3 appears to be the principle sensor of Listeria (30). Furthermore, Pyrin has been suggested as a Francisella sensor in human cells (31) but plays no role in mouse macrophages (23). Thus, the differences between human and mouse in inflammatory responses are incompletely understood.
In this report we demonstrate the surprising finding that NLRP3 is sufficient to form a functional IL-1β-generating inflammasome after Francisella infection. Additionally, in accordance with known differences affecting virulence, the capacity of the U112 and LVS strains of Francisella to do so differs considerably. Our findings demonstrate that these differences are influenced by variations in the induction of inflammasome components, the activation state of the monocyte-macrophage, and the triggering of NLRP3 inflammasome assembly/activation. Finally, although NLRP3 senses Francisella in infected 293T and THP-1 cells, mouse Nlrp3-deficient bone marrow-derived macrophages are fully sufficient in their IL-1β response to Francisella. These data demonstrate that the use of NLRP3 in response to Francisella differs between mouse and human.
EXPERIMENTAL PROCEDURES
Cell Culture, Macrophages
Human monocytic cell lines THP-1 and the epithelial cell line HEK293T were cultured in RMPI-1640 or DMEM, respectively, with 10% FBS, 1% l-glutamine, and 0.1% penicillin/Streptomycin. Mouse macrophages were isolated from bone marrow as previously described (32).
Bacteria, Infection, and Macrophage Invasion Assay
F. tularensis LVS, F. novicida U112, and F. tularensis SchuS4 were obtained from the Albany Medical College Microbiology Core Facility. Bacteria were cultured on modified Mueller-Hinton (MH) agar plates or in modified MH broth (Difco) with ferric pyrophosphate and IsoVitalex (BD Biosciences) and maintained as described (19). For invasion assays, 2.5 × 105 THP-1 or 293T cells were seeded in 24-well plates and infected with LVS or U112 (100 m.o.i.) for 2 h followed by gentamicin (50 μg/ml) treatment to kill extracellular bacteria. Cells were lysed with 0.1% sodium deoxycholate at the indicated time points, and bacterial colonies were enumerated on chocolate-agar plates.
Expression Constructs, DNA Transfection, and Inflammasome Reconstitution
Expression plasmids encoding human NLRP1, NLRP2, caspase-1, and pro-IL-1β were all obtained from OpenBioSystems. Human NLRP3 (33), NLRP12 (34), and myc-ASC (35) have been described previously. Transfections were performed using FuGENE 6 (Roche Applied Science) at 2.5 μl of FuGENE 6:1 μg of DNA. For inflammasome reconstitution, 293T cells were seeded (2.5 × 105) in 24-well plates and, after overnight culture, transfected with plasmids encoding pro-caspase1 (50 ng), pro-IL1β (200 ng), and ASC (10 ng) with or without an NLR (100 ng). At 4 h post-transfection, cells were infected with Francisella (100 m.o.i.). After 24 h, culture supernatants were collected by centrifugation, and secreted IL-1β was measured by ELISA (eBiosciences) as per the manufacturer's instructions.
Immunofluorescence Microscopy
293T cells (5 × 104) were seeded in two-well chamber slides (Nunc). Transient transfection and Francisella infection was performed as described above for inflammasome reconstitution. Immunofluorescence staining for myc-ASC was performed essentially as described (36) using mouse anti-myc IgG1 mAb (1:300, clone 4A6, Millipore) for 1.5 h at room temperature and R-phycoerythrin-conjugated goat anti-mouse Ab (1:500) for 1 h and visualized using an Axio Observer.Z1 fluorescence microscope (Zeiss). For myc-ASC and NLRP3 co-localization, rabbit anti-NLRP3 (1:100, Santa Cruz) and mouse anti-myc (1:300, Millipore) antibodies were used and detected with either goat anti-rabbit-Alexa488 (1:400, Invitrogen) or goat anti-mouse R-phycoerythrin (1:400, Invitrogen), respectively. ASC-positive cells containing a speck were counted manually using randomly selected fields imaged at 20× or 40×. Percent speck formation was calculated as the number of ASC-positive cells containing a speck divided by the number containing no speck.
Real-time Quantitative PCR, RT-PCR, and siRNA
RNA was isolated using the RNeasy method (Qiagen) and treated with DNase I. Quantitative PCR was performed in triplicate using the SuperScript III Platinum SYBR Green One-Step quantitative real-time-PCR kit (Invitrogen). Ct values were normalized to β-actin as internal control, and relative copy numbers were calculated by the standard 2−ΔΔCt method. The following primer pairs were used: CASP1, 5′-TGG GAC TCT CAG CAG ATC AA-3′ and 5′-CTG CCG ACT TTT GTT TCC AT-3′; pro-IL1B, 5′-AGC TTG GTG ATG TCT GGT CC-3′ and 5′-TCC AGC TGT AGA GTG GGC TT-3′; ASC, 5′-CCC TCC TCA GTC GGC AG-3′ and 5′-AGG CTG GTG TGA AAC TGA A-3′; NLRP3, 5′-GGC ATA TCA CAG TGG GAT TC-3′ and 5′-GAT CTT CGC TGC GAT CAA C-3′; AIM2, 5′-GTT TGA GAC CCA AGA AGG CA-3′ and 5′-CAC ACG TGA GGC GCT ATT TA-3′; β-actin, 5′-CCC CCA TGC CAT CCT GCG TCT G-3′ and 5′-CTC GGC CGT GGT GGT GAA GC-3′. For NLRP3 siRNA experiments, 2.5 × 105 THP-1 cells/well were seeded in 24-well plates. After overnight culture, cells were transfected with either NLRP3-specific or -scrambled siRNA using FuGENE 6 (Roche Applied Science) as per the manufacturer's instructions (NLRP3, 5′-ACC GCG GUG UAC GUC UUC UUC CUU U-3′ and 5′-AAA GGA AGA AGA CGU ACA CCG CGG U-3′; NLRP1, 5′-GGC AUC AAC CAC ACG UUU CCU AUU-3′ and 5′-AAU AGG AAA CGU GUG GUU GAU GCC C-3′; scrambled control, 5′-AUU CUC UCA UGA CGU UUC C-3′ and 5′-GAA ACG UCA UGA GAG AAU-3′).
shRNA Knockdown Experiments
Transient shRNA-mediated knockdown experiments in THP-1 were performed using pRS-shGFP retroviral expression vectors targeting ASC, NLRP3, and AIM2 (OriGene). Briefly, 293T cell-based Ampho packaging cells (Orbigen) were transfected with 1 μg of the nonspecific control 29-mer or gene-specific pRS-shGFP vector using FuGENE 6 as described above. Culture medium was changed the next day, at which point >80% of each set of transfected cells expressed GFP. Virus-containing supernatants were collected at 48 h post-transfection. Equal volumes of virus-containing supernatant were added to 2 × 106 THP-1 cells in the presence of 8 μg/ml Polybrene (Invitrogen) and incubated for 36 h. Cells were then washed and counted, and 2.5 × 105 cells were used for infection with U112 (m.o.i. = 100) as described above.
Western Blot and Cytokine Measurements
Cells were lysed in 1% Nonidet P-40 lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40 (v/v), 2 mm EDTA, 2 mm DTT with protease inhibitors (Roche Applied Science)). Proteins (20–30 μg) were resolved by SDS-PAGE (4–20%, Bio-Rad) and transferred to nitrocellulose (0.2 μm). After blocking with 3% milk, PBS, the membrane was probed with anti-NLRP3 (Nalpy-3a, Abcam) or anti-GAPDH (Chemicon). To detect active IL-1β (p17), supernatants were concentrated using a 10-kDa filter (Millipore), mixed with cell lysates, and immunoblotted with anti-IL1β (Cell Signaling). Human TNFα, IL-6, and IL-1β were assayed in cell-free supernatants by Cytometric bead array analysis (BD Pharmingen). Data were analyzed using FCAP Array software, Version 1.0 (BD Biosciences).
Inhibitor Studies
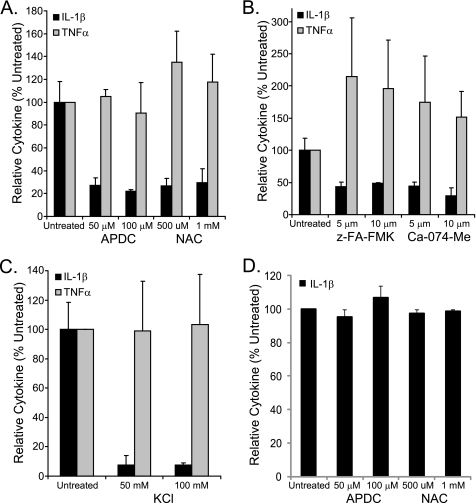
THP-1 cells plated in antibiotic-free RPMI at 2.5 × 105 cells per ml in 24-well dishes were treated with 100 nm phorbol 12-myristate 13-acetate (PMA) overnight and subsequently treated with inhibitors diluted in DMSO 30 min before infection with U112 at an m.o.i. of 100. Culture supernatants were collected 24 h inoculation and analyzed in triplicate for IL1-β and TNFα by ELISA and for cytotoxicity by LDH (Promega). Inhibitors used include ammonium pyrrolidinedithiocarbamate (Enzo Life Sciences), N-acetylcysteine (Sigma), benzyloxycarbonyl-FA-fluoromethyl ketone (BD Biosciences), Ca-074-Me (Calbiochem), and KCl.
Statistical Analysis
All experiments represent the results of at least three independent experiments unless otherwise indicated. Statistical significance were determined using Student's t test (p < 0.05 considered significant).
RESULTS
Response of THP-1 Cells to Infection with Francisella
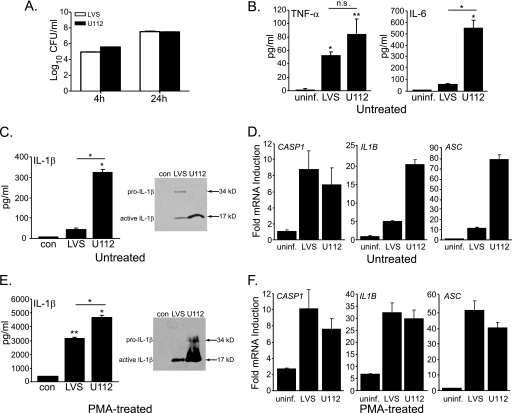
TLR and ASC/caspase-1 pathways play a critical role in the response of mouse macrophages to Francisella infection. However, the mouse and human macrophage cytokine response differs after Francisella infection (28). As a prelude to molecular studies and to better understand how human macrophages respond to Francisella infection, the cytokine profile of the commonly used human monocytic cell line, THP-1, infected with the LVS and U112 strains of F. tularensis was examined. Both strains infect and replicate similarly in THP-1 cells (Fig. 1A). Infected THP-1 cells produced TNFα, IL-6, and IL-1β at 24 h post-infection (Fig. 1, B and C). Overall, although the cytokine response of THP-1 to U112 infection is fairly robust, the response to LVS is weaker. This is especially true for IL-1β where the LVS response is very modest compared with U112. Furthermore, in contrast to U112 infection, where the pro-form of IL-1β (p34) is completely converted into active form (p17), LVS exhibits lower pro-IL1β processing (Fig. 1C). These data demonstrate a difference in the IL-1β response between the U112 and LVS strains in THP-1 cells as previously demonstrated for primary human monocytes (29).
FIGURE 1.
Francisella infection induces IL-1β production in THP-1 cells. A, colony counts after infection at the times are indicated. CFU, colony-forming units. B, secretion of TNFα and IL-6 at 24 h in THP-1 is shown. uninf, uninfected. C, IL-1β (left panel) was measured at 24 h post-infection, and a Western blot (right panel) of IL-1β from cell lysate combined with concentrated culture supernatants (34, pro-IL-1β; 17, active IL-1 β) in THP-1 is shown. D, quantitative real-time PCR of the indicated mRNAs at 24 h post-infection in THP-1 is shown. E and F, conditions were as for C and D but with PMA-treated THP-1. Data are shown as the mean ± S.E. for panels B, C, and E (n = 3–4) and the mean ± S.D. for one representative experiment for panels D and F. *, p < 0.05; **, p < 0.005.
Failure of LVS to sufficiently induce expression of inflammasome components could explain the observed differences in IL-1β production; therefore, expression of caspase-1, ASC, and pro-IL-1β genes was examined by real-time quantitative PCR (Fig. 1D). Infection with LVS or U112 increased caspase-1, ASC, and pro-IL-1β mRNA expression in THP-1 cells. However, LVS infection resulted in markedly lower induction of ASC and pro-IL-1β message than that seen with U112, which may largely account for the modest LVS-induced IL-1β response. Caspase-1 message induction was comparable. Because monocyte responses may differ from those of macrophages (31, 37), THP-1 cells were stimulated with PMA before infection. PMA treatment induces a more macrophage-like morphology and enhances the proinflammatory cytokine response of THP-1 cells to LPS stimulation (38). PMA-treated THP-1 cells infected with Francisella produce ∼10-fold more IL-1β than untreated cells (Fig. 1E). A similar response to Francisella infection is also seen with U937 cells treated with PMA (data not shown). Besides IL-1β, TNFα and IL-6 production were also higher in PMA-treated THP-1 or U937 cells after Francisella infection (data not shown). Both the LVS and U112 strains of Francisella induced comparable levels of caspase-1, ASC, and pro-IL-1β mRNAs in PMA-treated THP-1 cells (Fig. 1F). Thus, the robust IL-1β response observed in LVS-infected, THP-1, or U937 cells stimulated with PMA suggests that the low IL-1β response using non-PMA-treated monocytic cells is not due to a lack of responsiveness or a cell type-specific response but, rather, may depends upon intrinsic differences between bacterial strains. However, in PMA-treated cells, LVS infection still results in ∼25% less IL-1β than infection with U112 (Fig. 1E). This suggests that LVS may be less stimulatory (or possesses inhibitory properties). These data suggest that differences in THP-1 responsiveness to Francisella strains result in part from a dichotomy in ASC and pro-IL-1β gene induction between the strains that PMA treatment overcomes (Fig. 1, D and F). Furthermore, the similar response of PMA-treated THP-1 cells to LVS and U112 infection suggests that the underlying mechanism leading to inflammasome triggering by Francisella is likely similar between strains.
Francisella Infection Induces Assembly of the NLRP3-Inflammasome Complex
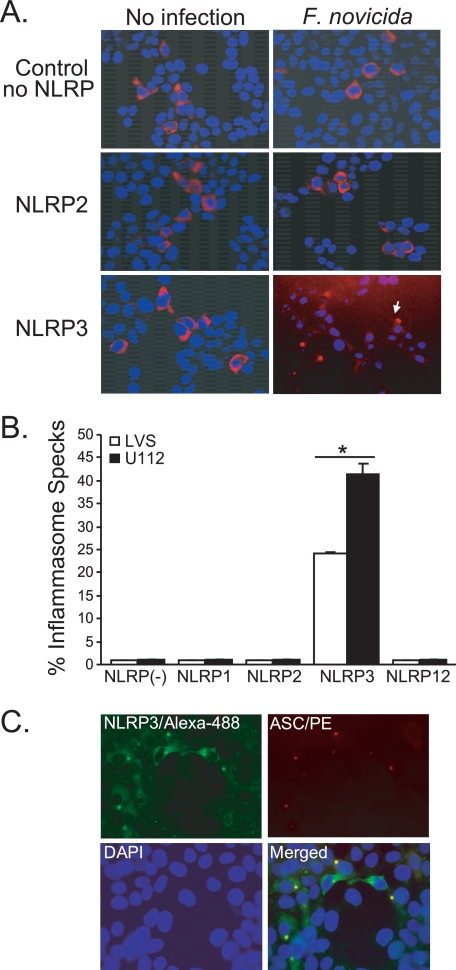
In mice, Francisella infection activates caspase-1 in an ASC-dependent fashion (21) as a consequence of DNA-mediated activation of Aim2 (23). However, the ability of any NLRP to respond after Francisella infection has yet to be demonstrated. Recently, a number of groups have shown the formation of a perinuclear speck-like, punctate structure (inflammasome speck; ASC pyroptosome) analogous to the Pyrin:ASC “speck” after exposure to an appropriate stimulus (39–41). This structure is widely believed to represent the active inflammasome complex. To screen for the NLRP(s) capable of responding to Francisella, 293T cells were reconstituted with plasmids encoding pro-IL-1β, pro-caspase1, ASC, and various individual NLRPs and then examined for the recruitment of ASC to inflammasome specks by immunofluorescence (Fig. 2A). Because overexpression of PYD or CARD domain-containing proteins can lead to self-oligomerization, limiting amounts of plasmid DNA were used to avoid forced oligomerization of ASC and/or caspase-1. In the absence of an exogenous NLRP or in the presence of NLRP1, NLRP2, or NLRP12, neither LVS nor U112 infection induces the formation of an ASC-containing speck (Fig. 2, A and B). However, in NLRP3 transfectants, both LVS and U112 infection resulted in the recruitment of ASC to perinuclear specks. These data demonstrate that NLRP3 interacts with ASC to form an inflammasome-associated speck-like structure in response to Francisella infection but do not conclusively rule out NLRP1, -2, or- 12. Notably, LVS induced ∼40% fewer inflammasome specks compared with U112 infection (Fig. 2B). To further demonstrate the co-localization of ASC and NLRP3 to an inflammasome speck after Francisella infection, similar experiments staining for both NLRP3 and ASC were performed. Consistently, ASC and NLRP3 co-localized together in the perinuclear speck after U112 infection (Fig. 2C). Thus, LVS and U112 are both capable of initiating inflammasome assembly via NLRP3. Moreover, NLRP3 is a sufficient sensor of live Francisella. The differing capacity of the LVS and U112 strains to induce NLRP3 specks raises the possibility that LVS is a weaker agonist of the NLRP3 inflammasome compared with U112.
FIGURE 2.
Francisella infection induces assembly of the NLRP3-inflammasome complex. A and B, HEK293T cells were transfected with plasmids encoding human procaspase-1, pro-IL-1β, ASC, and the indicated NLR followed by infection with either LVS or U112. At 24 h post-infection, cells were fixed and stained for myc-ASC to monitor the recruitment of ASC to inflammasome specks. A, shown are images (20× magnification) representative for each condition. B, the percentage of ASC-positive cells (red) with formation of a single perinuclear speck (ASC-aggregates) is shown as the mean ± S.E. for two-three independent experiments. More than 500 cells were manually counted for each experimental condition (*, p < 0.05). C, co-localization of human myc-ASC (red) and NLRP3 (green) is shown. One representative image of two-three independent transfections is shown for NLRP3 inflammasome after F. novicida U112 infection.
Human NLRP3 Is Sufficient to Assemble a Functional Inflammasome Complex after Francisella Infection
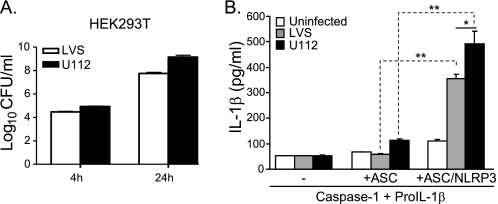
Because Nlrp3 has been reported to be dispensable for caspase-1 activation and IL-1β production after F. tularensis infection of mouse macrophages (21), we considered the possibility that the Francisella-induced NLRP3 inflammasome speck might not be an active inflammasome. To establish whether NLRP3 activates caspase-1, leading to cleavage of IL-1β upon exposure to live Francisella, 293T cells were transiently reconstituted with NLRP3, ASC, caspase-1, and pro-IL-1β as above. This approach is a well established technique for exploring inflammasome activity (12, 13). 293T cells, like type II lung epithelial cells (42), can be infected with Francisella and support intracellular replication (Fig. 3A). No significant IL-1β response to Francisella infection was observed in cells transfected with plasmids encoding human ASC, caspase-1, and pro-IL-1β, demonstrating that 293T cells lack a sufficient, endogenous, Francisella-specific, inflammasome-activating sensor (Fig. 3B). However, in the presence of NLRP3, infection with either the LVS or U112 strains led to robust IL-1β production (Fig. 3B). These results demonstrate that NLRP3 is sufficient to assemble a functional inflammasome complex after F. tularensis infection. Notably, in all these experiments, U112 infection induced higher levels of IL-1β compared with LVS, consistent with the degree of NLRP3:ASC aggregate formation (Fig. 2B). This result suggests that in addition to their differential capacity to induce ASC and IL-1β gene expression (Fig. 1D), strain differences are also likely responsible for the observed dichotomy in inflammasome formation and activation.
FIGURE 3.
Human NLRP3 inflammasomes are responsive to Francisella infection. A, an invasion and replication assay of HEK293T cells infected with Francisella LVS or U112 strains is shown. CFU, colony-forming units. B, HEK293T cells were transiently transfected with plasmids encoding human procaspase1, pro-IL-1β, ASC, and NLRP3. At 4 h post-transfection, cells were infected with LVS or U112, and the level of IL-1β secretion was measured at 24 h post-infection. Data shown are the mean ± S.E. of duplicate samples from 3–4 independent experiments (*, p < 0.05; **, p < 0.01).
The IL-1β Response of Francisella-infected THP-1 Cells Requires NLRP3
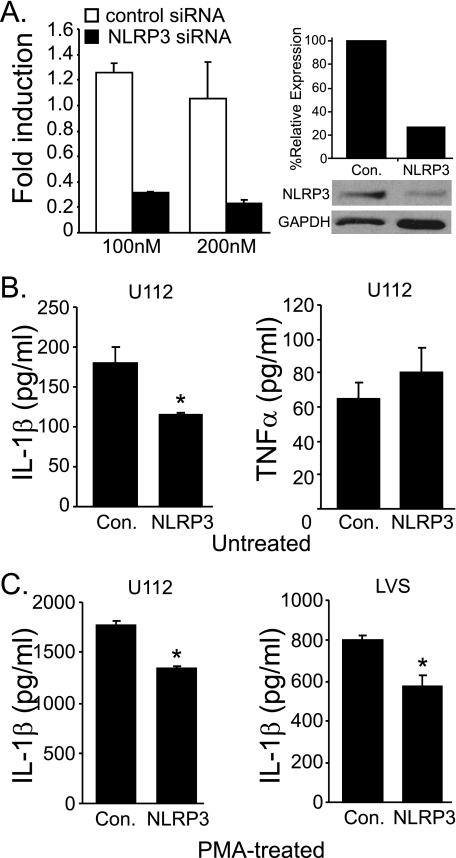
Because it has been demonstrated previously that Francisella activation of caspase-1 in mouse macrophages is Nlrp3-independent (22–24), it could also be argued that the requirement for NLRP3 is cell type-specific. Thus, although functional in epithelial cells, NLRP3 might be dispensable in human macrophages. After transfection of NLRP3-specific siRNA into THP-1 cells, NLRP3 mRNA expression is decreased by 75–80% relative to a nonspecific scrambled siRNA control (Fig. 4A). Correspondingly, NLRP3 protein level in siRNA-treated cells is reduced by ∼75% (Fig. 4A). This reduction in NLRP3 mRNA and protein correlates with decreased IL-1β production, but not TNFα secretion, after U112 infection in undifferentiated THP-1 cells (Fig. 4B), demonstrating that NLRP3 is involved in the IL-1β response of human macrophages to Francisella infection. This result confirms that the 293T reconstitution experiments reflect the involvement of NLRP3. In PMA-treated THP-1 cells, siRNA knockdown of NLRP3 was similarly effective at reducing the IL-1β response to both U112 (∼20%) and LVS (∼30%) (Fig. 4C). These results support three conclusions; first, that in addition to NLRP3, human macrophages likely have at least one additional inflammasome-initiating molecule responsive to Francisella (e.g. AIM2); second, that NLRP3 may contribute similarly to the inflammasome response in both untreated (monocyte-like) and PMA-treated (macrophage-like) THP-1 cells; finally, that the usage and/or activation of Francisella-responsive inflammasome sensors likely differs between mice and humans.
FIGURE 4.
NLRP3 is involved in the IL-1β response to Francisella infection. A, PMA-treated THP-1 cells were transfected with the indicated siRNA and incubated for 36 h, and NLRP3 mRNA was measured by quantitative PCR (left panel). NLRP3 protein expression was determined by a Western blot using 100 nm NLRP3 siRNA, and band intensities were compared with ImageJ, normalized to GAPDH (% relative expression) (right panel). B, PMA-treated THP-1 cells were transfected with the indicated siRNA (100 nm) followed by infection with F. novicida U112 strain. At 24 h post-infection, IL-1β (left panel) and TNFα levels (right panel) were measured in culture supernatants. C, PMA-treated THP-1 cells were transfected with indicated siRNA followed by infection with either U112 (left panel) or LVS (right panel) strains of Francisella. At 24 h post-infection IL-1β was measured in culture supernatants. Data are shown as the mean ± S.E. of duplicate samples from at least three independent experiments (*, p < 0.05).
ROS, Cathepsin B, and K+ Efflux Inhibitors Impair Francisella Activation of the NLRP3 Inflammasome
Our knockdown results suggest the presence of an NLRP3-independent inflammasome complex responsive to Francisella. To further explore this idea, we reasoned that inhibition of NLRP3 inflammasome activation should further reveal the contribution of this other pathway. ROS generated in response to infection has been demonstrated to activate the NLRP3 inflammasome (43, 44) but have not been shown to contribute to Aim2 activation, which instead requires interaction of dsDNA with the HIN200 domain of Aim2 (40, 41). In addition, PMA-treated human macrophages infected with U112 have been shown to produce ROS despite the presence of antioxidant virulence factors, such as superoxide dismutase and catalase, produced during infection by Francisella (45). Infecting PMA-treated cells with U112 in the presence of ROS scavengers ammonium pyrrolidinedithiocarbamate and N-acetylcysteine reduced IL-1β production by ∼70% (Fig. 5A). However, neither treatment reduced IL-1β production after introduction of plasmid DNA to activate the AIM2 inflammasome (Fig. 5D), consistent with the recent demonstration that these ROS scavengers have no effect upon the AIM2 inflammasome (46). These results indicate that inhibiting ROS produced by Francisella infection partially reduces the activation of the IL-1β-producing inflammasomes, consistent with the identified role of NLRP3. These data further support the involvement of another Francisella-elicited inflammasome activation pathway insensitive to ROS scavengers (e.g. AIM2).
FIGURE 5.
ROS, cathepsin B, and K+ efflux inhibitors reduce IL-1β production after Francisella infection. A–C, PMA-treated THP-1 cell were infected with U112 after a 30-min preincubation with inhibitors at the indicated concentrations. Secretion of IL-1β and TNFα was measured at 24 h post-infection. Cytokine production is shown as a percentage of the untreated control. A, ROS inhibitors. B, cathepsin B inhibitors. z-FA-FMK, benzyloxycarbonyl-FA-fluoromethyl ketone. C, K+ efflux inhibitor. D, PMA-treated THP-1 cell were transfected with endotoxin-free plasmid DNA (1 μg) after a 30-min preincubation with ROS inhibitors at the indicated concentrations. IL-1β secretion was measured at 24 h post-transfection. TNFα was also measured in two experiments with a decrease of ∼60% for ammonium pyrrolidinedithiocarbamate (APDC) and 0–30% for N-acetylcysteine (NAC) relative to untreated DNA-transfected controls (data not shown). For all panels, the mean ± S.E. for three independent experiments is shown.
Lysosomal membrane disruption leads to release of cathepsin B, and inhibition of cathepsin B is known to prevent activation of NLRP3 inflammasomes (47, 48). Because Francisella escapes the phagolysosomal compartment (49), and this escape is necessary for generation of IL-1β (50), it is likely that the lysosome is damaged and releases cathepsin B. We, therefore, utilized the cathepsin B inhibitors benzyloxycarbonyl-FA-fluoromethyl ketone and Ca-074-Me to inhibit activation of NLRP3. The cathepsin B inhibitors reduced Francisella-elicited IL-1β production in PMA-treated THP-1 cells by ∼60% (Fig. 5B), again supporting the involvement of both NLRP3 and another Francisella-sensitive, cathepsin B inhibitor-insensitive inflammasome. NLRP3 inflammasome formation can also be blocked by the addition of extracellular KCl (51), but KCl has also been demonstrated to prevent Aim2-dependent activation in mouse macrophages through inhibition of ASC oligomerization (23). IL-1β production was reduced by ∼90% with KCl (Fig. 5C), suggesting that all Francisella-activated inflammasomes are sensitive to blockade of K+ efflux and/or blockade of ASC oligomerization. Although IL-1β production was reduced with inhibitor treatment, TNFα was not (Fig. 5, A–C). Cytotoxicity as measured by LDH release was low (15% or less) with all inhibitors (data not shown).
As mentioned above, Aim2 is the major, if not exclusive, trigger for Francisella-activated inflammasomes in mice (23, 24). Thus it is likely that the ROS scavenger- and cathepsin B inhibitor-insensitive IL-1β response in our system results from the action of AIM2. Consistent with this notion, more complete inhibition was observed with KCl.
Francisella Infection Up-regulates AIM2 Expression and shRNA against AIM2, and NLRP3 Reduces IL-1β Production Equivalently in THP-1
As the Aim2 inflammasome is relevant in mouse bone marrow-derived macrophages, we examined the contribution of AIM2 in THP-1 infected with Francisella. Both AIM2 and NLRP3 are expressed constitutively in THP-1 cells (estimated copies per 100 of GADPH are ∼1.5/100 for NLRP3 and 0.7/100 for AIM2). After PMA treatment, THP-1 cells show some increase (∼2–3-fold) in both NLRP3 and AIM2 mRNA expression, but infection with LVS or U112 for 24 h does not lead to any further increase (data not shown).
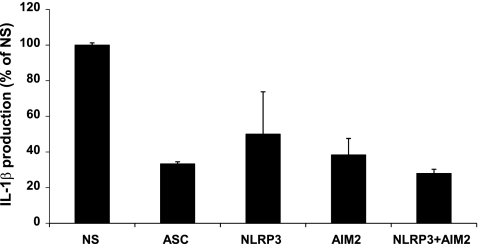
To examine the relative contributions of NLRP3 and AIM2 to IL-1β production in THP-1 cells, we used viral transduction of shRNA to transiently reduce expression of inflammasome components. IL-1β production was reduced by about 70% with ASC shRNA, about 50% with NLRP3 shRNA, about 60% with AIM2 shRNA, and about 70% with both NLRP3 and AIM2 shRNA after infection with U112 (Fig. 6). The differences in IL-1β reduction between the ASC, NLRP3, AIM2, and the combined AIM2/NLRP3 shRNA transductions were not statistically significant (p > 0.05). These data suggest that both NLRP3 and AIM2 are responsive to Francisella in humans.
FIGURE 6.
Silencing of either AIM2 or NLRP3 reduces IL1β secretion. PMA-treated THP-1 cells were transiently transduced with retroviral shRNA constructs targeting the indicated gene or a nonspecific (NS), 29-mer control. After infection with U112 (m.o.i. = 100), production of IL-1β was measured at 24 h post-infection. Results are the means ± S.E. for triplicate samples from three experiments. None of the differences observed between specific shRNAs reached statistical significance (p < 0.05).
Recent studies demonstrate that the inflammasome response of mouse macrophages to U112 is Aim2-dependent but independent of Nlrp3. Taken together, our observation that NLRP3 and AIM2 are responsive to Francisella in human cells suggests that human NLRP3 is responsive to Francisella, whereas mouse Nlrp3 is not. Thus, species differences in NLR usage and/or activation requirements are likely important.
DISCUSSION
To understand the molecular mechanisms underlying IL-1β response to Francisella, recent attention has focused on dissecting the role of inflammasome components utilizing various knock-out mice. Not surprisingly, both ASC and caspase-1 are required, and thus, NLRs were postulated as the critical sensor (21). In mice, however, the non-NLR, Pyrin domain-bearing Aim2 protein, which recognizes dsDNA, is likely the sole inflammasome-activating protein triggered after Francisella infection (23, 24, 52). Realizing that the NLR family is complex, diverse, and disparate between human and mouse and that different strains of Francisella are not equivalent in virulence or in eliciting in vitro cytokine responses, we explored the role of human NLRPs as a sensor of Francisella. Through a multi-pronged approach, we demonstrate that human NLRP3 is one of at least two sensors mediating the inflammasome dependent IL-1β response to Francisella infection in humans. Furthermore, differences in the inflammasome response between commonly used strains of Francisella (LVS and U112) likely result from strain differences and disparity in NLRP3 inflammasome activation.
Using inflammasome reconstitution, visualization of inflammasome formation, and siRNA knockdown, we have firmly established that human NLRP3 is sufficient to mediate inflammasome-dependent production of IL-1β in response to both LVS and U112 infection. In initial experiments using the highly pathogenic F. tularensis tularensis (SchuS4) strain, NLRP3-dependent inflammasome activation was also observed in the HEK293T reconstitution system (data not shown). NLRP3 was also necessary for a complete IL-1β response against Francisella in both untreated and PMA-treated THP-1 cells (Fig. 4, B and C). Thus, NLRP3 appears to respond similarly to the presence of live Francisella in both epithelial cells and monocyte/macrophages, establishing human NLRP3 as one of the sensors for Francisella infection.
In light of the distinct pathogenesis of LVS, U112, and SchuS4 strains of Francisella, there has been recent interest in comparing strain-dependent variations in vivo and between mouse and human cells in vitro. The LVS strain elicits significantly higher IL-1β production from primary human monocytes and monocyte-derived macrophages than mouse bone marrow-derived macrophages (28). Furthermore, infection of primary human monocytes with U112 elicited higher levels of IL-1β compared with those infected with LVS strain (29). Similarly, we find that untreated monocyte-like or PMA-treated macrophage-like THP-1 cells produce more IL-1β upon infection with U112 than with LVS. The difference in IL1β production with PMA treatment cannot be attributed to PMA alone, as PMA treatment does not increase IL1β levels (Ref. 53 and data not shown). TNFα and IL-6 cytokine responses are also concordant in the contrast between strains, similar to previously published data for IL-8 (29), further establishing that the U112 and LVS strains differ in their capacity to stimulate proinflammatory cytokines. These differences are likely not a consequence of Francisella lipopolysaccharide (LPS). Although initial studies pointed to a stimulatory U112 LPS and a non-stimulatory LVS LPS (16, 45) as a potential explanation for the above differences, later work clearly demonstrated that highly purified LPS from the U112 strain was non-stimulatory (46). Consistently, differences in the IL-1β response between Francisella strains were evident in all the experimental systems, including those using inflammasome-reconstituted epithelial cells. It is plausible that the U112 strain may engage innate signaling pathways other than TLR2. Alternatively, LVS may possess a component that can suppress the inflammatory response that F. novicida (U112) lacks, as suggested by the increased Aim2 response upon transposon mutagenesis of mviN in LVS (48).
Mouse Nlrp3 responds to heat-killed F. novicida when ATP is provided (54); however, during infection with F. novicida, Nlrp3 is dispensable (22–24). Given our results and to further exclude a possible role for mouse Nlrp3, we have confirmed this observation using a range of m.o.i. and various time points post-infection (supplemental Fig. 1). Our data demonstrate that human cells employ NLRP3 in the inflammasome response after infection, leading to the conclusion that human NLRP3 is functionally distinct from mouse Nlrp3 in this respect.
This result is not surprising as the two proteins have abundant differences (55) and 83% identity. A variety of single point mutations within the nucleotide binding domain or leucine-rich repeats alter the function of NLRP3 (56). Yet very little is known about the molecular requirements for NLRP3 inflammasome activation; thus, it is plausible that differences confer a Francisella-responsive state to NLRP3 that Nlrp3 lacks. For example, the nucleotide binding domain-leucine-rich repeat region of NLRP3 differs from Nlrp3 in the distribution of potential serine/threonine phosphorylation sites, oxidant-sensitive cysteine residues, charged residues, and lysines that may serve as ubiquitination sites (data not shown). However, our results cannot rule in a critical function difference based on amino acid sequence, thus both the possible contribution of specific residues and potential differences acting in an indirect fashion such as species differences in reactive oxygen generation (53, 57). Sensitivity to apoptotic/pyroptotic death signals and potential ligand adapter proteins (58) should be considered. Notably, Francisella-infected mouse macrophages die within ∼6 h post-infection, whereas THP-1 and primary human macrophages do not undergo significant death until much later (>24 h) (Refs. 21 and 31 and data not shown).
Human AIM2 and mouse Aim2 are also not identical (55% identity) (59). Although it has been suggested that AIM2 protein expression is absent in primary human monocytes (60), THP-1 cells express AIM2 mRNA after either U112 or LVS infection. Furthermore, it has been suggested that AIM2 expression in THP-1 requires IFNβ exposure (61), but F. novicida does not induce IFNβ production by infected monocytes (62).
It has also been reported that the human Pyrin protein is important for the IL-1β response in Francisella-infected cells (31) and may represent an independent inflammasome or a potential necessary partner for either NLRP3 or AIM2. But, like Nlrp3, Pyrin is also not involved in the mouse response to the U112 strain of Francisella (23), again highlighting another potential disparity in the response of mouse and human macrophages to specific pathogens.
This study highlights one difference in NLRP sensing of pathogens between mouse and human. Undoubtedly others exist and will be important to our understanding of host-pathogen interactions. Our data also raise some immediate questions. For example, although recent studies highlight the role of ROS in activating NLRP3, how human NLRP3 senses Francisella and why mouse Nlrp3 is not similarly activated remains puzzling. Moreover the ability of NLRP3 to distinguish between or respond differently to LVS and U112 warrants further investigation.
Acknowledgments
We thank Dr. Timothy J. Sellati and the Microbiology Core Facility of the Center for Immunology and Microbial Disease for assistance in the use of Francisella strains and helpful discussion, Dr. Fayyaz Sutterwala (University of Iowa) for providing Nlrp3−/− and wild type mouse femurs, the Albany Medical College Immunology Core Facility and Yili Lin for cytokine analyses, and Dr. James R. Drake for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R21AI075250 (to M. M.) and P01AI056320 and R01AI072259 (J. A. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- TLR
- Toll-like receptor
- ROS
- reactive oxygen species
- NLR
- nucleotide binding and leucine-rich repeat receptor
- NLRP
- NLR Pyrin
- LVS
- live vaccine strain
- PMA
- phorbol 12-myristate 13-acetate
- m.o.i.
- multiplicity of infection.
REFERENCES
- 1. Janeway C. A., Jr., Medzhitov R. (2002) Annu. Rev. Immunol. 20, 197–216 [DOI] [PubMed] [Google Scholar]
- 2. Kopp E. B., Medzhitov R. (1999) Curr. Opin. Immunol. 11, 13–18 [DOI] [PubMed] [Google Scholar]
- 3. Kanneganti T. D., Lamkanfi M., Núñez G. (2007) Immunity 27, 549–559 [DOI] [PubMed] [Google Scholar]
- 4. Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr. (1997) Nature. 388, 394–397 [DOI] [PubMed] [Google Scholar]
- 5. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 6. Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr. (1998) Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 7. Medzhitov R. (2001) Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 8. Jiang Z., Mak T. W., Sen G., Li X. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 10. Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. (2004) Nat. Immunol. 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 11. Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. (1992) Nature 356, 768–774 [DOI] [PubMed] [Google Scholar]
- 12. Martinon F., Burns K., Tschopp J. (2002) Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 13. Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. (2004) Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 14. Srinivasula S. M., Poyet J. L., Razmara M., Datta P., Zhang Z., Alnemri E. S. (2002) J. Biol. Chem. 277, 21119–21122 [DOI] [PubMed] [Google Scholar]
- 15. Oyston P. C., Sjostedt A., Titball R. W. (2004) Nat. Rev. Microbiol 2, 967–978 [DOI] [PubMed] [Google Scholar]
- 16. Conlan J. W., Vinogradov E., Monteiro M. A., Perry M. B. (2003) Microb. Pathog. 34, 39–45 [DOI] [PubMed] [Google Scholar]
- 17. Conlan J. W., Chen W., Shen H., Webb A., KuoLee R. (2003) Microb. Pathog. 34, 239–248 [DOI] [PubMed] [Google Scholar]
- 18. Shen H., Chen W., Conlan J. W. (2004) Vaccine 22, 2116–2121 [DOI] [PubMed] [Google Scholar]
- 19. Malik M., Bakshi C. S., Sahay B., Shah A., Lotz S. A., Sellati T. J. (2006) Infect. Immun. 74, 3657–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole L. E., Shirey K. A., Barry E., Santiago A., Rallabhandi P., Elkins K. L., Puche A. C., Michalek S. M., Vogel S. N. (2007) Infect. Immun. 75, 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mariathasan S., Weiss D. S., Dixit V. M., Monack D. M. (2005) J. Exp. Med. 202, 1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 23. Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C. P., Alnemri E. S. (2010) Nat. Immunol. 11, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O'Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., Dixit V. M., Monack D. M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9771–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J., Fernandes-Alnemri T., Alnemri E. S. (2010) J. Clin. Immunol. 30, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corr S. C., O'Neill L. A. (2009) Cell. Microbiol. 11, 703–709 [DOI] [PubMed] [Google Scholar]
- 27. Santic M., Al-Khodor S., Abu Kwaik Y. (2010) Cell. Microbiol. 12, 129–139 [DOI] [PubMed] [Google Scholar]
- 28. Bolger C. E., Forestal C. A., Italo J. K., Benach J. L., Furie M. B. (2005) J. Leukoc. Biol. 77, 893–897 [DOI] [PubMed] [Google Scholar]
- 29. Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., Wewers M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meixenberger K., Pache F., Eitel J., Schmeck B., Hippenstiel S., Slevogt H., N'Guessan P., Witzenrath M., Netea M. G., Chakraborty T., Suttorp N., Opitz B. (2010) J. Immunol. 184, 922–930 [DOI] [PubMed] [Google Scholar]
- 31. Gavrilin M. A., Mitra S., Seshadri S., Nateri J., Berhe F., Hall M. W., Wewers M. D. (2009) J. Immunol. 182, 7982–7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noah C. E., Malik M., Bublitz D. C., Camenares D., Sellati T. J., Benach J. L., Furie M. B. (2010) Infect. Immun. 78, 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Connor W., Jr., Harton J. A., Zhu X., Linhoff M. W., Ting J. P. (2003) J. Immunol. 171, 6329–6333 [DOI] [PubMed] [Google Scholar]
- 34. Williams K. L., Taxman D. J., Linhoff M. W., Reed W., Ting J. P. (2003) J. Immunol. 170, 5354–5358 [DOI] [PubMed] [Google Scholar]
- 35. Bedoya F., Sandler L. L., Harton J. A. (2007) J. Immunol. 178, 3837–3845 [DOI] [PubMed] [Google Scholar]
- 36. Harton J. A., O'Connor W., Jr., Conti B. J., Linhoff M. W., Ting J. P. (2002) Hum. Immunol. 63, 588–601 [DOI] [PubMed] [Google Scholar]
- 37. Kahlenberg J. M., Dubyak G. R. (2004) J. Leukoc. Biol. 76, 676–684 [DOI] [PubMed] [Google Scholar]
- 38. Schwende H., Fitzke E., Ambs P., Dieter P. (1996) J. Leukoc. Biol. 59, 555–561 [PubMed] [Google Scholar]
- 39. Bryan N. B., Dorfleutner A., Rojanasakul Y., Stehlik C. (2009) J. Immunol. 182, 3173–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. (2009) Nature 458, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D. R., Latz E., Fitzgerald K. A. (2009) Nature 458, 514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall J. D., Craven R. R., Fuller J. R., Pickles R. J., Kawula T. H. (2007) Infect. Immun. 75, 1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2010) Nat. Immunol. 11, 136–140 [DOI] [PubMed] [Google Scholar]
- 44. Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 45. Mohapatra N. P., Soni S., Rajaram M. V., Dang P. M., Reilly T. J., El-Benna J., Clay C. D., Schlesinger L. S., Gunn J. S. (2010) J. Immunol. 184, 5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. (2011) J. Virol. 85, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Golovliov I., Baranov V., Krocova Z., Kovarova H., Sjöstedt A. (2003) Infect. Immun. 71, 5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cole L. E., Santiago A., Barry E., Kang T. J., Shirey K. A., Roberts Z. J., Elkins K. L., Cross A. S., Vogel S. N. (2008) J. Immunol. 180, 6885–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 52. Rathinam V. A., Jiang Z., Waggoner S. N., Sharma S., Cole L. E., Waggoner L., Vanaja S. K., Monks B. G., Ganesan S., Latz E., Hornung V., Vogel S. N., Szomolanyi-Tsuda E., Fitzgerald K. A. (2010) Nat. Immunol. 11, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carta S., Tassi S., Pettinati I., Delfino L., Dinarello C. A., Rubartelli A. (2011) J. Biol. Chem. 286, 27069–27080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. (2007) Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 55. Ting J. P., Davis B. K. (2005) Annu. Rev. Immunol. 23, 387–414 [DOI] [PubMed] [Google Scholar]
- 56. Church L. D., Cook G. P., McDermott M. F. (2008) Nat. Clin. Pract. Rheumatol 4, 34–42 [DOI] [PubMed] [Google Scholar]
- 57. Schneemann M., Schoeden G. (2007) J. Leukoc. Biol. 81, 579. [DOI] [PubMed] [Google Scholar]
- 58. Dangl J. L., Jones J. D. (2001) Nature 411, 826–833 [DOI] [PubMed] [Google Scholar]
- 59. Choubey D., Duan X., Dickerson E., Ponomareva L., Panchanathan R., Shen H., Srivastava R. (2010) J. Interferon Cytokine Res. 30, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gavrilin M. A., Wewers M. D. (2011) Front. Microbiol. 2:11, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bürckstümmer T., Baumann C., Blüml S., Dixit E., Dürnberger G., Jahn H., Planyavsky M., Bilban M., Colinge J., Bennett K. L., Superti-Furga G. (2009) Nat. Immunol. 10, 266–272 [DOI] [PubMed] [Google Scholar]
- 62. Butchar J. P., Cremer T. J., Clay C. D., Gavrilin M. A., Wewers M. D., Marsh C. B., Schlesinger L. S., Tridandapani S. (2008) PLoS One 3, e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]