Abstract
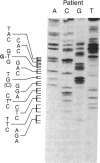
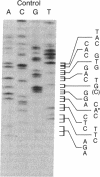
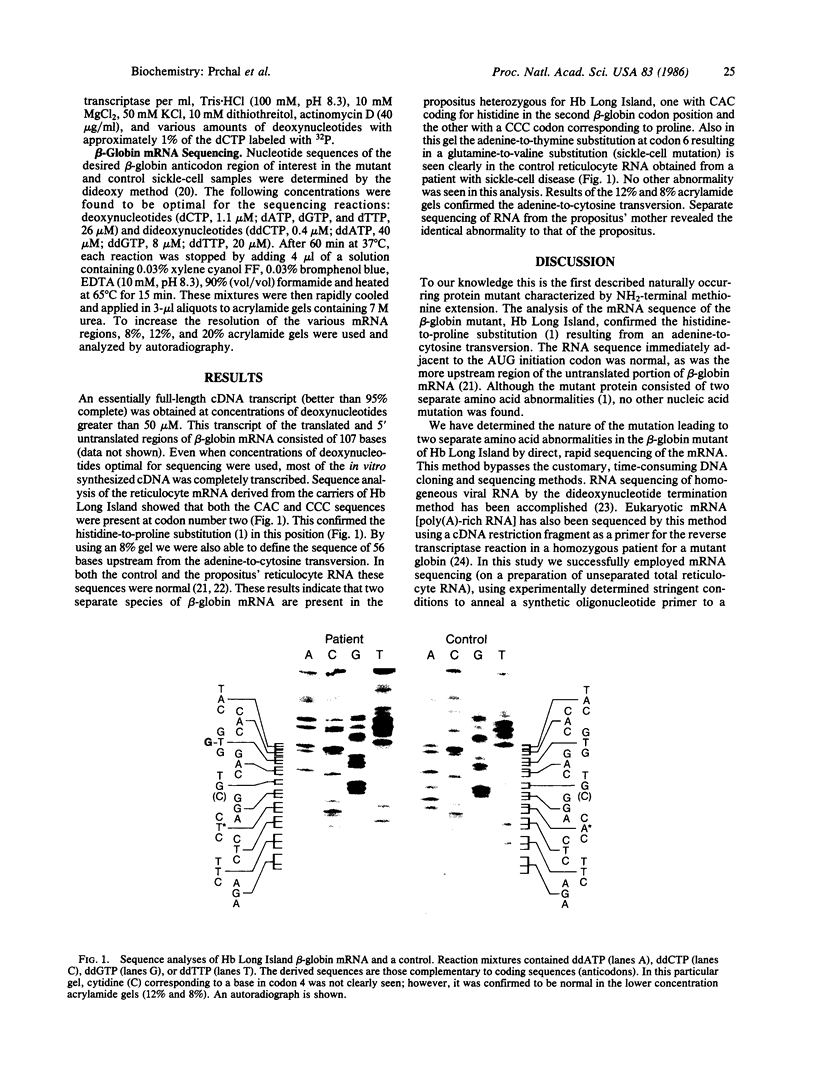
Hemoglobin Long Island has two separate amino acid abnormalities of beta-globin structure: an extension of the NH2 terminus by a methionine residue and a histidine-to-proline substitution at the normal second position. The NH2-terminal methionine residue, the translation product of an AUG initiation codon, is present only transiently in nascent proteins. Because of the general biological implications of this abnormality, we investigated the nature of the genetic defect of this mutant. We determined the sequence of the relevant portion of the beta-globin mRNA by means of dideoxynucleotide chain termination of the complementary DNA (cDNA) in which an oligonucleotide complementary to codons 10-17 was used as a primer for reverse transcriptase. A histidine-to-proline substitution was confirmed in the mutant mRNA by identifying an adenine-to-cytosine transversion in the second codon. However, we were unable to find any other abnormality at either the AUG initiation codon or in the 56 bases upstream from the adenine-to-cytosine transversion (encompassing most of the 5' untranslated region of the mutant beta-globin mRNA). Thus, it appears that this single lesion probably interferes with the poorly understood methionine-cleaving mechanism that modulates most of prokaryotic and eukaryotic proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin D. R., Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B. The amino acid sequence of the gamma chain of bovine fetal hemoglobin. Biochemistry. 1966 Apr;5(4):1297–1310. doi: 10.1021/bi00868a025. [DOI] [PubMed] [Google Scholar]
- Barwick R. C., Jones R. T., Head C. G., Shih M. F., Prchal J. T., Shih D. T. Hb Long Island: a hemoglobin variant with a methionyl extension at the NH2 terminus and a prolyl substitution for the normal histidyl residue 2 of the beta chain. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4602–4605. doi: 10.1073/pnas.82.14.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Temple G. F., Poon R., Neumann K. H., Kan Y. W. The nucleotide sequences of the untranslated 5' regions of human alpha- and beta-globin mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5145–5149. doi: 10.1073/pnas.74.11.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Haynes J. R., Rosteck P., Jr, Lingrel J. B. Unusual sequence homology at the 5-ends of the developmentally regulated beta A-, beta C-, and gamma-globin genes of the goat. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7127–7131. doi: 10.1073/pnas.77.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Adams H. R., Dimmock M. O., Edwards W. E., Wilson J. B. The structure of goat hemoglobins. I. Structural studies of the beta chains of the hemoglobins of normal and anemic goats. J Biol Chem. 1967 May 25;242(10):2534–2541. [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Leclercq F., Schnek A. G., Braunitzer G., Stangl A., Schrank B. Direct reciprocal allosteric interaction of oxygen and hydrogen carbonate sequence of the haemoglobins of the Caiman (Caiman crocodylus), the Nile crocodile (Crocodylus niloticus) and the Mississippi crocodile (Alligator mississippiensis). Hoppe Seylers Z Physiol Chem. 1981 Aug;362(8):1151–1158. [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimenti J. C., Duncan C. H. Ruminant globin gene structures suggest an evolutionary role for Alu-type repeats. Nucleic Acids Res. 1984 Feb 10;12(3):1641–1655. doi: 10.1093/nar/12.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B., Babin D. R. A comparison of amino acid sequences in the beta-chains of adult bovine hemoglobins A and B. Arch Biochem Biophys. 1967 Apr;120(1):124–135. doi: 10.1016/0003-9861(67)90606-6. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Itano H. A. Quantitative differences between N-terminal methionyl nascent globin chains of human and rabbit reticulocytes. Nat New Biol. 1973 Nov 28;246(152):107–109. doi: 10.1038/newbio246107a0. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Stewart J. W., Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985 May 10;260(9):5382–5391. [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle D. T., Dixon G. H. Transient incorporation of methionine at the N-terminus of protamine newly synthesized in trout testis cells. Nature. 1970 Aug 15;227(5259):676–680. doi: 10.1038/227676a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lin M. NH 2 -terminal formylmethionine- and NH 2 -terminal methionine-cleaving enzymes in rabbits. J Biol Chem. 1972 Feb 10;247(3):952–957. [PubMed] [Google Scholar]
- Yoshida A., Watanabe S., Morris J. Initiation of rabbit hemoglobin synthesis: methionine and formylmethionine at the N-terminal. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1600–1607. doi: 10.1073/pnas.67.3.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]