Abstract
The stages of development leading up to the formation of mature B-1 cells have not been identified. As a result, there is no basis for understanding why various genetic defects, and those in the classical or alternative NF-κB pathways in particular, differentially affect the B-1 and B-2 B cell lineages. Here, we demonstrate that B-1 B cells are generated from transitional cell intermediates that emerge in a distinct neonatal wave of development that is sustained for approximately two weeks after birth and then declines as B-2 transitional cells predominate. We further show that, in contrast to the dependence of B-2 transitional cells on the alternative pathway, the survival of neonatal B-1 transitional cells and their maturation into B-1 B cells occurs as long as either alternative or classical NF-κB signaling is intact. Based on these results, we have generated a model of B-1 development that allows the defects in B-1 and B-2 cell production observed in various NF-κB deficient strains of mice to be placed into a coherent cellular context.
Introduction
Following production in the bone marrow, immature surface IgM+ (sIgM+) B lymphocytes migrate to the spleen where they transit through distinct transitional cell stages, termed T1, T2, and T3, before maturing into mature follicular (FO) or marginal zone (MZ) B-2 B cells (1-4). MZ B cells are localized to the margins of splenic follicles and respond to blood borne pathogens (5), while FO B cells are the predominant B cell population in adult spleen (SPL) and lymph nodes and participate in adaptive immunity (1, 6, 7). The events involved in transitional cell survival and maturation are increasingly well defined and depend upon interactions between antigen and the BCR, and binding of B cell activating factor (BAFF) to the BAFF-receptor (BAFF-R; TNFRSF13C). Engagement of the BAFF-R in turn results in activation of the alternative NF-κB pathway (8-11).
The analysis of mice with deficient expression of genes known to affect transitional cell survival and maturation has revealed puzzling differences between the formation of mature B-2 cells and a second population of B lymphocytes referred to as B-1 cells. B-1 cells are involved in innate immune responses and preferentially localize to serous cavities wherein sIgM+ CD11b+ CD5+ B-1a and sIgM+ CD11b+ CD5– B-1b subsets can be distinguished (12, 13). In particular, defects in the expression of either BAFF (14), BAFF-R (15), or NFκ-B2 (16), the key transcriptional regulatory protein in the alternative NF-κB pathway, result in a significant deficiency in transitional cell numbers, a block at the T1 to T2 stage of development, and a severe depletion of mature B-2 celIs (9, 17). However, the number of mature B-1 cells is not affected (10, 14, 16). In contrast to these observations, mice with defective expression of various intermediates in the classical NF-κB pathway, such as Btk, Bcl-10 (18, 19), MALT-1 (20, 21), Card11/CARMA1 (22, 23), or NF-κB1 (24), have a reduced number of B-1 cells, and B-1a cells in particular, while the size of their B-2 cell compartment is not significantly affected.
These observations indicate that the stages of development leading up to the formation of mature B-1 and B-2 cells must be differentially regulated. However, while the signals necessary for the formation of mature B-2 cells and the stages of transitional cell development at which they act have been defined, a comparable understanding of the events resulting in the generation of mature B-1 cells has not been attained. It has been proposed that B-1 cells also develop from transitional cell intermediates (25), but such populations have not been identified. As a result, there is no basis for understanding why deletion of specific genes in one or the other NF-κB pathways differentially affects the formation of mature B-1 and B-2 cells.
The layered immune system hypothesis (26) proposes that B-1 and B-2 cells belong to separate lineages that derive from distinct progenitors that emerge at different times during development. Our demonstration that B-1 cells are generated from lineage negative (Lin–) CD45R–/low CD19+ B-1 restricted progenitors that arise in the embryo before B-2 committed progenitors provided support for this view. B-1 progenitor numbers decline by young adulthood as B-2 production establishes (27-29). The emergence of B-1 progenitors before B-2 progenitors suggested that intermediate stages of development between B-1 progenitors and mature B-1 cells would be most abundant in the newborn. In order to test this possibility, we undertook a detailed examination of the kinetics of B cell emergence in neonates.
In this study, we show that B-1 cells are generated from transitional B cell intermediates that emerge in a distinct wave of development in the neonate. This B-1 transitional cell wave is sustained for approximately two weeks after birth and then wanes as B-2 transitional cell production becomes dominant. We further demonstrate that, in contrast to the dependence of B-2 transitional cells on alternative NF-κB signaling, the survival of B-1 transitional cells and their maturation into B-1 B cells occurs as long as either the alternative or classical NF-κB pathway is intact. In view of these observations, we propose a model in which B-1 and B-2 cells are produced in staggered waves from lineage specific transitional cells that are differentially regulated. This new developmental scheme allows the defects in B-1 and B-2 cell production observed in various NF-κB deficient strains of mice to be placed into a coherent cellular context.
Materials and Methods
Mice
Swiss Webster (SW, Igh-6a), CB17.SCID (Igh-6b, SCID) and RAG-2/SJL (B6.SJL(129S6)-Ptprca/BoCrTac-Rag2tm1, CD45.1) mice were obtained from Taconic Farms. BAFF-R—/— (B6(Cg)-Tnfrsf13ctm1Mass/J, CD45.2) mice and their B6 (C57BL/6J) controls, NF-κB1—/— (B6;129P2-Nfkb1tm1Bal/J) mice and their wild type controls ((B6129F1/J Aw-J/AW)F2, WT), and XID (CBA/CaHN-Btkxid/J) mice and their CBA controls (CBA/CaJ) were obtained from The Jackson Laboratory. All animals were housed and/or bred in the vivarium of the University of California, Los Angeles Division of Laboratory Animal Medicine. All animal procedures, including housing, were conducted according to the guidelines of the Institutional Animal Care and Use Committee. Tissues of neonates up to 15 days of age were pooled. PerC cells from neonates up to 21 days of age were pooled, but their SPL were processed individually.
Cell transplantations
0.1-1 × 106 T1 or T2/T3 transitional cells, purified by flow cytometry from the SPL of SW, BAFF-R—/— and NF-κB1—/— mice at the ages indicated in the figures, were transplanted intra-peritoneally into immunodeficient recipients. SW cells were transplanted into SCIDs, while BAFF-R—/— and NF-κB1—/— cells were transplanted into RAG-2/SJL mice. Only cells enriched at more than 95% were used. Recipient mice were sacrificed 4 to 6 days following injection and tested for the presence of mature donor sIgMa+ (SW) or CD45.2+ IgM+ (BAFF-R—/—, NF-κB1—/—) B cells in their peritoneal cavities by immunostaining.
Immunostaining and flow cytometry
Spleen cell suspensions were prepared by crushing tissue between frosted slides in Ca++, Mg++ free PBS. Peritoneal B cell suspensions were prepared by lavage with Ca++, Mg++ free PBS. When necessary, red blood cells were lysed with Tris-ammonium chloride, pH 7.2, before immunostaining. Cells were incubated with anti-CD16/CD32 (FcγRII-III, clone 2.4G2, ebiosciences) to reduce non-specific staining. Transitional T1 and T2/T3 cells, follicular, marginal zone, B-1, B-1a and B-1b cells were resolved by flow cytometry following incubation with the appropriate combination of the following antibodies: polyclonal rat anti-mouse IgD and goat anti-Mouse IgM (Southern Biotech), anti-Mouse IgDa (clone AMS-9.1, Biolegends), IgMa (clone DS-1, Igh-6a, Pharmingen), CD5 (Ly-1, clone 53-7.3), CD11b (clone M1/70), CD21/CD35 (clone CR2/CRI), CD23 (clone FcεRII), CD45.2 (clone 104) and CD93 (AA4.1, clone C1qRp; all from ebiosciences). Sample analyses were performed on a LSRII (Becton Dickinson) located in the Broad Stem Cell Research Center (UCLA). Cell purifications were performed on a FACSAria (Becton Dickinson) located in the Flow Cytometry Core at the Jonsson Comprehensive Cancer Center (UCLA). Values indicated on the FACS plots indicate the population frequencies within the gated cells.
cDNA preparation and PCR reactions
Total mRNA was extracted from flash frozen cell pellets of purified neonatal or adult transitional T1 and T2/T3 cells with the RNeasy Plus micro kit (Qiagen) and cDNA was prepared with the RT2 First Strand kit (Super Arrays) according to manufacturer instructions. Igh VH11 gene rearrangements were assessed by nested PCR as described (27).
Statistical analyses
Data are presented as mean ± s.d. Differences in values between groups were tested using a two-tailed unpaired Student's t test.
Results
Transitional B Cells Predominate in Neonatal Spleen
Our strategy for detecting cellular intermediates between B-1 progenitors and mature B-1 cells is based on the hypothesis that B-1 development would dominate the interval immediately after birth. We therefore determined the kinetics with which mature B-1 and B-2 cells emerged in the SPL of three to 21 days old Swiss Webster (SW) neonates by flow cytometry, as described in Supplemental Fig. 1, in order to determine if this is the case.
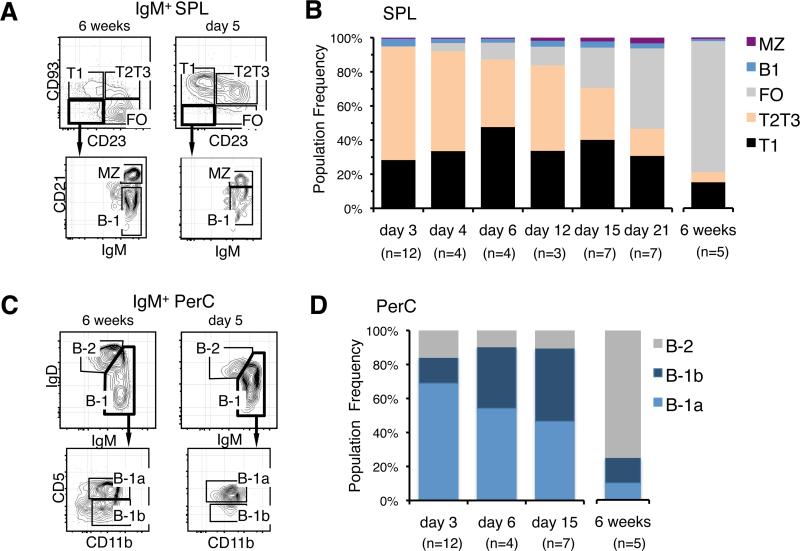
The results showed that sIgM+ CD23+ CD21int CD93— FO and sIgM+ CD23— CD21hi CD93— MZ B cells remained low in that tissue for the first 2 weeks of life (Fig. 1, A and B). In contrast, sIgM+ CD23— CD21lo CD93— B-1 cells were observed in neonatal SPL (Fig. 1, A and B) and sIgMhi sIgDlo CD11b+ CD5+ B-1a and sIgMhi sIgDlo CD11b+ CD5— B-1b cells were readily detected in neonatal peritoneal cavity (PerC) during the first week after birth (Fig. 1, C and D). These results support the view that the majority of mature B cells present immediately after birth are B-1 cells.
FIGURE 1.
Most sIgM+ B cells in neonatal SPL are immature transitional cells. A, Representative FACS analyses showing T1 and T2/T3 transitional, follicular (FO), marginal zone (MZ), and B-1 cells in the spleen (SPL) of 6 week and 5 day old SW mice. B, Age related changes in the relative frequency of B cell populations within sIgM+ cells in SPL. T1 and FO cells were defined according to their levels of sIgM and CD21 expression as illustrated in Supplementary Fig. 1 (on line). C, Representative FACS analyses of B-2, B-1a and B-1b cells in the peritoneal cavity (PerC) of 6 week and 5 day old SW mice. D, Age related changes in the relative frequency of B cell populations within sIgM+ cells in the PerC. n indicates the number of animals processed at the indicated time points. Average frequency and s.d. values for older animals are provided in Supplementary Table I (on line).
However, we also observed that the total number of sIgM+ cells in the SPL of 3 day old neonates exceeded the number of mature B cells (Table I). Further analysis revealed that the majority of neonatal sIgM+ cells were CD23— CD21int CD93+ T1 and sIgM+ CD23+ CD21int CD93+ T2/T3 transitional cells (Fig. 1, A and B). This high frequency of transitional cells in the neonatal SPL contrasts with observations in adult SPL where the transitional cell pool constitutes 5 to 10% of total B cells (Fig. 1B).
Table I.
sIgM+ frequencies and numbers in the SPL of SW, BAFF-R—/—, NF-κB1—/—, XID mice and their respective controls, SW, B6, WT and CBA at the indicated ages.
| Strains | age | n* | sIgM+ cell frequency ± s.d. [ % ] | sIgM+ cells × 106 ± s.d. | |
|---|---|---|---|---|---|
| SW | Neonates | 3 days‡ | 12 | 19.5 | 0.9 |
| 8 days‡ | 7 | 33.1 | 10.0 | ||
| 12 days‡ | 3 | 33 | 18.8 | ||
| 17 days | 4 | 53.9 ± 1.3 | 32.7 ± 3.0 | ||
| 21 days | 8 | 35.4 ± 4.7 | 26.1 ± 4.4 | ||
| Adults | 5 weeks | 5 | 44.7 ± 1.9 | 40.4 ± 8.6 | |
| BAFF-R—/— | Neonates | 5 days‡ | 5 | 6.74 | 1.1 |
| 7 days‡ | 5 | 17.4 | 5.2 | ||
| 10 days‡ | 3 | 28.9 | 7.9 | ||
| Adults | 11 weeks | 2 | 5.6 ± 2.9 | 3.5 ± 0.9 | |
| NF-κB1—/— | Neonates | 6 days‡ | 3 | 19.8 | 1.1 |
| 9 days‡ | 5 | 17.6 | 1.6 | ||
| 12 days‡ | 5 | 43.4 | 5.1 | ||
| Adults | 8 weeks | 2 | 22.1 ± 8.1 | 23.8 ± 7.2 | |
| XID | Neonates | 10 days | 9 | 35.0 | 5.8 |
| Adults | 12 weeks | 2 | 15.9 ± 0.9 | 7.2 ± 2.0 | |
| B6 | Adults | 10 weeks | 3 | 37.3 ± 1.9 | 28.1 ± 2.9 |
| WT | Adults | 8 weeks | 3 | 38.0 ± 3.4 | 39.6 ± 15.2 |
| CBA | Adults | 12 weeks | 2 | 31.2 ± 7.6 | 20.4 ± 3.8 |
n = number of animals
neonates spleens were pooled for immunofluorescence analyses
values provided for older animals indicate average ± s.d.
Neonatal Transitional Cells Primarily Generate B-1 Cells
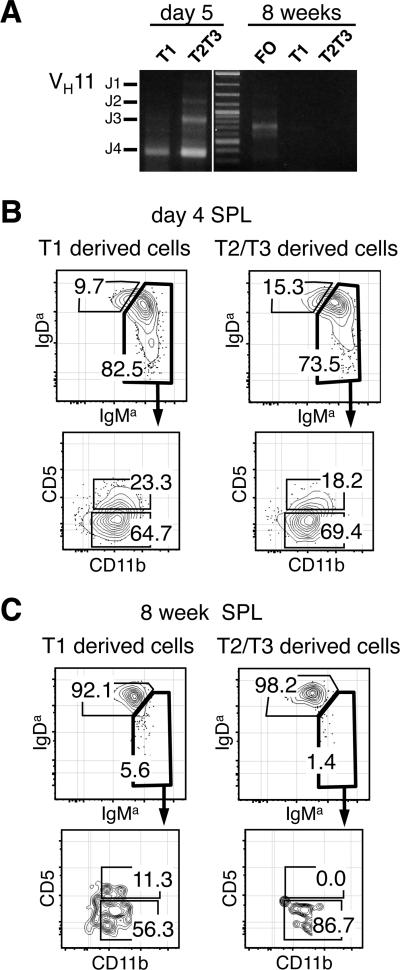
The high frequency of transitional cells in neonatal SPL, combined with the fact that most mature B cells detected in that organ and the PerC are B-1 cells, raised the possibility that the first transitional cells to arise in the newborn might be B-1 specified. This hypothesis was further substantiated by the fact that transitional cells isolated from 5 day old neonates utilized Ig genes of the VH11 family (Fig. 2A), which is a characteristic of B-1 cells (30).
FIGURE 2.
Neonatal T1 and T2/T3 cells preferentially mature into B-1 cells. A, Immunoglobulin Variable Heavy chain 11 (VH11) usage in T1, T2/T3 and FO cells isolated from 5 day and 8 week old SW mice. Representative FACS analyses of PerC cells from SCID mice transplanted with T1 and T2/T3 SPL cells isolated from 4 day (B) and 8 week old (C) donors. Plots show populations gated on donor sIgMa+ sIgDa+ cells.
To test this hypothesis, we examined the potential of purified neonatal transitional cells to differentiate into B-1 or B-2 cells following intra-peritoneal injection into SCID mice. In initial experiments, we compared sIgM+ CD93+ CD23– T1 and sIgM+ CD93+ CD23+ T2/T3 B cells isolated from the SPL of 4 day and 8 week old SW mice. Examination of the recipients 4 to 6 days later showed that neonatal T1 and T2/T3 B cells preferentially generated donor sIgMa/hi sIgDa/lo cells that included both CD11b+ CD5+ B-1a and CD11b+ CD5— B-1b cells (Fig. 2B). In contrast, transitional cells isolated from adult SPL preferentially generated sIgMa/hi sIgDa/lo B-2 cells, and the few B-1 cells produced were mostly CD5— CD11b+ B-1b cells (Fig. 2C). Similar results were obtained when transitional cells were injected intravenously, except that donor cell yields were reduced (data not shown).
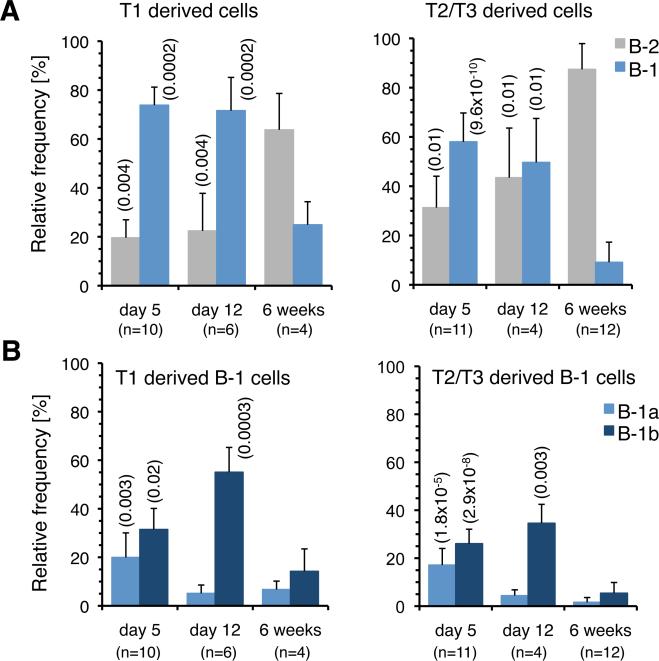
Having established that neonatal transitional cells preferentially generate B-1 cells, we tested how this potential evolved with age. Transitional cells from SW mice up to 12 days of age retained robust B-1 potential, although the capacity of transitional cells isolated from older neonates to generate B-1a cells had declined (Fig. 3, A and B). Furthermore, as B-1 potential waned, B-2 potential increased and transitional cells from 6 week old SW mice primarily generated B-2 cells (Fig. 3A). Consistent with these data, the number of B-1 cells generated per transitional cell transplanted was higher for neonatal than for adult cells, while the reverse was true for B-2 cell generation (unpublished observation).
FIGURE 3.
Age related changes in the B-1 potential of T1 and T2/T3 neonatal SPL cells. A, Relative frequency of B-1 and B-2 cells within donor sIgMa+ cells in the PerC of SCID mice transplanted with T1 and T2/T3 transitional cells isolated from the SPL of 5 and 12 days and 6 week old SW mice. B, Relative frequencies of B-1a and B-1b cells within the donor B-1 cells shown in panel (A). Note that neonatal transitional cells generate B-1a cells more efficiently than adult transitional cells. Differences in reconstitution between adult and neonatal populations were tested by two-tailed unpaired Student's t test (a=0.05), P values are indicated in parentheses. n indicates the number of animal tested at each time point. n indicates the number of recipients processed in each group.
These results indicate that B-1 cells mature through transitional cell intermediates and that, in contrast to adult cells, the majority of transitional cells in neonatal SPL are committed to the B-1 lineage. In addition, the fact that B-1 potential declines with age as B-2 transitional cells arise demonstrates that B-1 and B-2 cells are generated in two sequential, overlapping waves.
B-1 Transitional Cells develop in BAFF-R—/—, NF-κB1—/— and XID mice
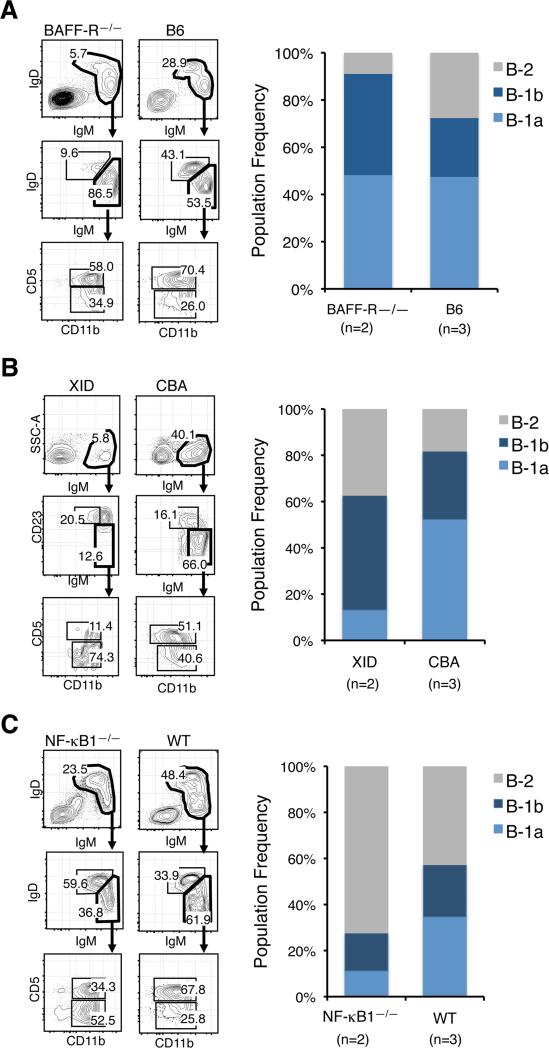
Simultaneous inactivation of the classical and alternative NF-κB pathways results in a severe block in B cell development at the immature B cell stage, and as a result neither transitional nor mature B cells develop (31, 32). However, selective inactivation of one or the other pathway has distinct effects on the mature B-1 and B-2 compartments. For example, B-1 B cells are present in the PerC of adult BAFF-R—/— mice, which are deficient in alternative NF-κB signaling, but there is a pronounced deficiency in B-2 cells (Fig. 4A). On the other hand, defects in classical NF-κB signaling result in a reduced number of B-1 cells in the PerC, although it is important to emphasize that B-1 cells are still present. For example, the number of mature B-1 cells, and B-1a cells in particular, is reduced in Btk deficient XID (33) and NF-kB1—/— mice (34), while B-2 cells are readily detected (Fig. 4, B and C).
FIGURE 4.
Distribution of B-1a, B-1b and B-2 cells in the PerC of adult BAFF-R–/–, XID and NF-κB1–/–Mice. Representative FACS analyses and relative frequency of B-1a, B-1b and B-2 cells in the PerC of BAFF-R—/— (A), XID (B) and NF-κB1—/— (C) mice are shown. n indicates the number of animals processed in each group.
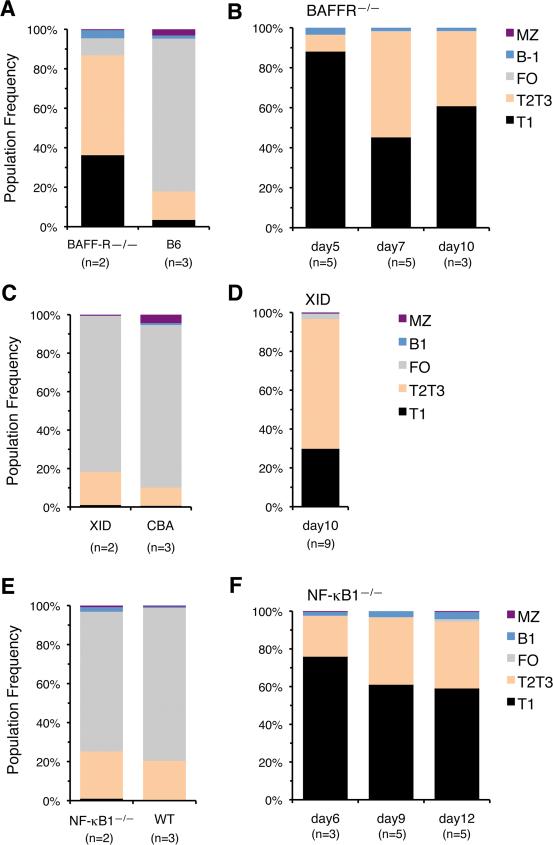
We next examined how these defects in alternative or classical NF-κB signaling affect B-1 transitional cells. Adult BAFF-R–/– mice had a profound deficiency in the frequency of sIgM+ FO and MZ B-2 cells in their SPL (Table I), and the few sIgM+ cells present were mostly transitional B cells (Fig. 5A). In contrast, sIgM+ cells were present in the SPL of BAFF-R—/— neonates at levels only slightly lower than in wild type mice during the first 10 days after birth, and these were mostly T1 and T2/T3 cells (Table 1 and Fig. 5B).
FIGURE 5.
Neonatal B-1 Transitional Cells Emerge in BAFF-R—/—, XID and NF-kB1—/— Mice. Age related changes in the relative frequency of B cell populations within sIgM+ cells in the SPL of adult (A) and neonatal (B) BAFF-R—/— mice, adult (C) and neonatal (D) XID mice and adult (E) and neonatal (F) NF-kkB1—/—mice. n indicates the number of animals processed in each group. Average frequency and s.d. values for older animals are provided in Supplementary Table II (on line).
Comparable analyses of adult Btk and NF-κB1 deficient mice showed no major alterations in the frequencies of sIgM+, T1, T2/T3 transitional cells and FO cells in their SPL (Table I and Fig. 5, C and E). In addition, analyses of Btk and NF-κB1—/— neonates up to 12 days of age showed that they had normal frequencies of sIgM+ cells in their SPL (Table I) and that these mostly consisted of T1 and T2/T3 transitional cells (Fig. 5, D and F).
Together, these observations indicate that the emergence of the B-1 transitional wave in the neonate is not affected by deficiencies in either BAFF-R, Btk or NF-κB1 signaling.
B-1 Transitional Cells From BAFF-R—/— and NF-κB1—/— Mice Mature Normally
We next determined the B-1 developmental potential of T1 and T2/T3 transitional cells isolated from the SPL of BAFF-R—/— mice and NF-κB1—/— neonates by transplanting them into RAG-2/SJL recipients. Doing so was particularly important with the latter strain, because the reduced number of B-1 cells observed in those mice might have reflected a requirement for NF-κB1 in transitional cell maturation into B-1 cells. Recipient mice were examined 4 to 6 days following transplantation.
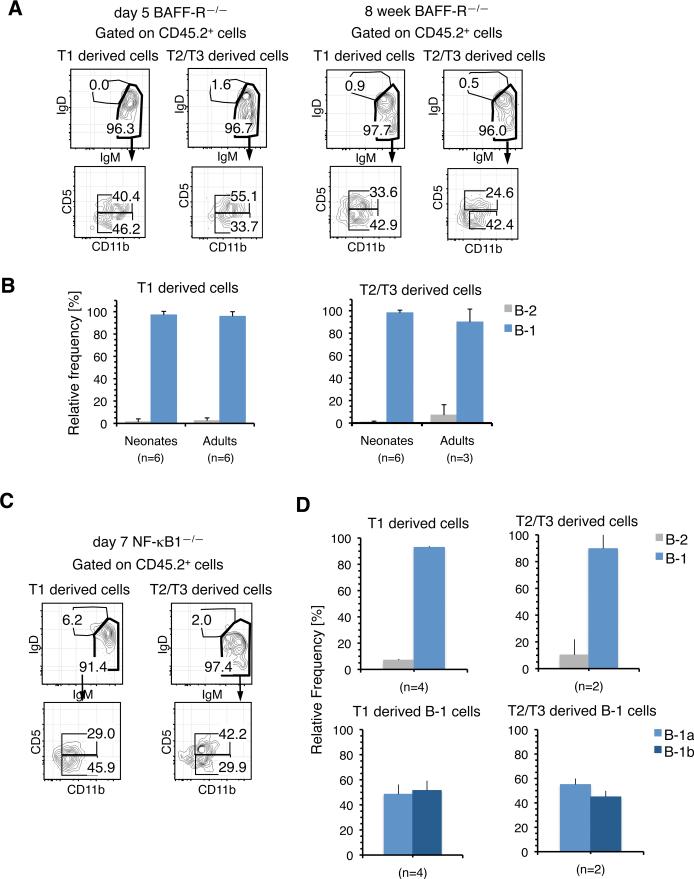
T1 and T2/T3 B cells isolated from the SPL of 5 day old BAFF-R—/— neonates, as well as from 8 week old adults, preferentially generated donor CD45.2+ sIgMhi sIgDlo cells that included both CD11b+ CD5+ B-1a and CD11b+ CD5— B-1b cells, but not B-2 cells (Fig. 6, A and B). In addition, T1 and T2/T3 transitional cells isolated from 7 day old NF-κB1—/— mice also reconstituted B-1 cells following transplantation in vivo (Fig. 6, C and D), and notably, these cells showed no deficiency in their ability to generate B-1a B cells (Fig. 6D).
FIGURE 6.
B-1 Transitional Cells From BAFF-R—/— and NF-κB1—/— Mice Mature into B-1 cells. A, Representative FACS analyses of the PerC cells from RAG-2/SJL mice transplanted with T1 and T2/T3 SPL cells from 5 day and 8 week old BAFF-R—/— donors. B, Relative frequency of B-1 and B-2 cells within donor CD45.2+ sIgM+ cells in the PerC of recipients of BAFF-R–/– transitional cells from 5 day (Neonates) and 8 week (Adults) old donors. C, Representative FACS analyses of the PerC cells from RAG-2/SJL mice transplanted with T1 and T2/T3 SPL cells from 7 day old NF-κB1—/— donors. D, B-1 and B-2 potential of 7 day old NF-kB1—/— T1 and T2/T3 SPL cells. The upper panel shows the frequency of B-1 and B-2 cells reconstituted and the lower panel shows the frequency of B-1a and B-1b cells within the B-1 populations in the upper panels. The data in all panels represent relative frequencies within donor CD45.2+ sIgM+ cells. n indicates the number of recipients processed in each group.
These results, together with the observation in the previous section, indicate that the emergence, survival, and maturation of B-1 transitional cells into B-1a and B-1b cells are not affected by a deficiency in BAFF-R, Btk or NF-κB1 expression.
DISCUSSION
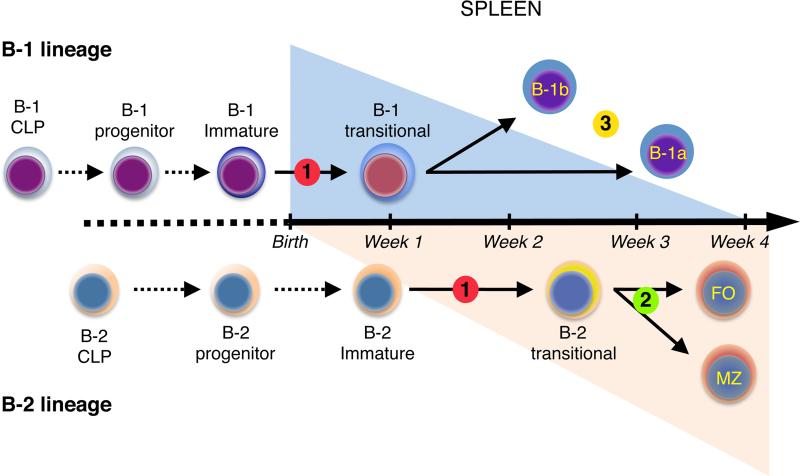
In this study we demonstrate that mature B-1 cells are produced through sIgM+ CD93+ CD23+/– transitional cell intermediates that predominate in neonatal SPL during the first two weeks after birth. B-1 transitional cell production occurs in a single wave that tapers in young adults as B-2 transitional cell production emerges and ultimately predominates. Based on these results, we have formulated a new, layered model of peripheral B-1 and B-2 maturation shown in Fig. 7. We further demonstrate how this scheme allows the reconciliation of our data and a puzzling series of observations showing that defects in the classical and alternative NF-κB pathways have differential effects on the formation of mature B-1 and B-2 B cells.
FIGURE 7.
Model of layered B-1 and B-2 development. In a first neonatal wave, B-1 CLPs generate B-1 progenitors that in turn differentiate into immature B-1 cells. The latter cells then migrate to the SPL where they mature into B-1a and B-1b cells through B-1 transitional cell intermediates. In a second wave, B-2 CLPs generate immature B-2 cells that mature into B-2 transitional cells in the SPL. This B-2 transitional cell wave becomes predominant in the adult. Emergence of B-1 and B-2 transitional cells is blocked when both the classical and alternative NF-κB pathways are inactivated (Step 1). However, selective defects in one or the other pathway have differential effects on the formation of mature B-1 and B-2 cells. While the survival and maturation of B-2 transitional cells is dependent on alternative NF-κB signaling (step 2), B-1 transitional cells emerge and mature in the absence of these signals. The number of mature B-1 cells is reduced in mice with defects in classical NF-κB signaling. However, this does not compromise the emergence or maturation of B-1 transitional cells into B-1a or B-1b cells. Instead, classical signaling is required for maintenance of the mature B-1 pool, and for B-1a cells in particular, following their formation (step 3).
This model integrates the herein described neonatal B-1 transitional cells with the recent identification of B-1 specified common lymphoid progenitors (CLPs) (35) and B-1 specified progenitors (27). In this model, B-1 CLPs generate B-1 progenitors that in turn differentiate into immature B-1 cells. The latter cells then migrate to the SPL where they mature into B-1a and B-1b cells through B-1 transitional cell intermediates. This B-1 pathway is most robust at the earliest stages of immune system development, because all of these populations are present at highest levels in the fetus and neonate and decline in number in the adult. The existence of this transient, neonatal wave of B-1 development distinct from the adult B-2 transitional cell wave is particularly well illustrated in BAFF-R–/– mice. The neonatal B-1 wave appeared in normal numbers and kinetics in this strain, but the subsequent B-2 transitional cell wave did not emerge, and the few transitional cells present in adults were B-1 specified.
The application of this model to the analysis of various NF-κB deficient strains of mice, along with a retrospective review of the literature, allowed us to identify the stages of B-1 development that are dependent or independent on various NF-κB signals. First, when all NF-κB signaling is abrogated, as in NF-κB1—/— × NF-κB2—/— mice, the maturation of immature B-1 cells into B-1 transitional cells is completely blocked (Fig. 7, step 1). This conclusion is based on the fact that B cell development in these double knockout mice does not proceed beyond the immature sIgM+ sIgD— stage of development and few if any CD23 expressing transitional B cells are detected in the SPL of two week old double knockout mice (32), an age at which B-1 transitional cell numbers are expected to be at their peak. This study also shows that abrogation of all NF-κB signaling blocks the emergence of the adult B-2 transitional and mature B-2 cells (32), indicating that regulation of the immature B cell to transitional B cell progression is similarly regulated in the B-1 and B-2 lineages (Fig. 7, step 1).
However, selective deficiencies in one or the other NF-κB pathway have distinct effects on the formation of mature B-1 and B-2 cells. For example, while our data confirm a deficiency of mature B-2 cells but normal numbers of B-1 cells in mice with defects in the alternative pathway (16), why this is the case has not been clear. The results demonstrating that BAFF-R expression is not required for the emergence, survival, or maturation of B-1 transitional cells, provides a framework for understanding why mice with genetic defects in the expression of the BAFF-R and downstream mediators such as NF-κB inducing kinase (NIK) (36) or NF-κB2 (16) do not have defects in the number of mature B-1 cells. We have also observed in preliminary studies that adult p100—/— mice (37) have normal frequencies and numbers of B-1 cells. Thus, while activation of the BAFF/BAFF-R/NF-κB2 cascade is critical for B-2 transitional cell survival and maturation (Fig. 7, step 2), this is not the case for B-1 transitional cells.
The conventional view is that classical NF-κB signaling is required for the formation of mature B-1 cells. In fact, it is often concluded that disruption of this pathway results in a complete block in B-1 development (11). However, it is important to note that the analysis of mature B-1 numbers in many studies of mice with defects in the expression of classical NF-κB components is often incomplete and has focused solely on the B-1a compartment (19, 22, 24). As we show, a different picture emerges when a comprehensive analysis of PerC B-1a and B-1b populations is conducted. In this case, it is clear that deficiencies in classical pathway intermediates such as Btk and NF-κB1—/— result in reduced frequencies of B-1 cells, and B-1a B cells in particular, but not their complete absence.
The analysis of neonatal Btk and NF-κB1—/— mice showed that the neonatal B-1 transitional cell wave arose in both strains. Furthermore, B-1 transitional cells harvested from NF-κB1—/— mice matured normally into both B-1a and B-1b cells. In view of these results, it seems most likely that intact classical NF-κB signaling is required for the homeostatic maintenance of the mature B-1 cell pool following its formation (Fig. 7, step 3). In this regard, B-1 cells are distinguished by their potential to self-renew (38). Therefore, we propose that in the absence of NF-κB1 or Btk, this potential is compromised. A similar mechanism may explain why mice with defects in other classical pathway intermediates such as BCL-10 (18, 19), MALT-1 (20, 21), and Card11/CARMA1 (22, 23) have reduced numbers of mature B-1 cells.
Taken together, the analyses of BAFF-R, Btk, and NF-κB1 knockout mice indicate that the emergence, survival, and maturation of B-1 transitional cells can occur as long as one of the NF-κB pathways is intact, suggesting that classical and alternative NF-κB signaling have redundant roles at the B-1 transitional cell stage of development. This view provides an explanation for why defective expression of Btk or NF-κB1 has no obvious effect on B-1 transitional cells. Only when this redundancy is eliminated, such as in NF-κB1—/— × NF-κB2—/— mice or in strains with defects that block crosstalk between the two pathways (17, 39), are effects at the B-1 transitional cell stage manifest.
In summary, we propose that the majority of B-1 cells are generated through committed B-1 transitional cell intermediates. However, the possibility that other routes into the B-1 pool exist cannot be excluded. For example, committed B-2 transitional cells could be selected into the B-1 population by the strength of BCR signaling following antigenic exposure in the spleen. However, the efficiency with which such an event takes place in a physiological setting remains an open question. That issue aside, our identification of B-1 transitional cells has provided the first opportunity to reconcile a series of puzzling observations made in various NF-κB deficient strains of mice. Our data also provide additional support for the layered immune system hypothesis and demonstrate the implications of viewing B cell development from that perspective.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health grant AI021256 (to K.D) and the flow cytometry core is supported by the National Institute of Health grant CA16042.
Abbreviations used in this article
- BAFF-R
B cell activating factor receptor
- FO
mature follicular B-2 B cells
- MZ
marginal zone B cells
- T1
T1 transitional B cells
- T2/T3
T2 and T3 transitional B cells
- PerC
peritoneal cavity
- SPL
spleen
Footnotes
Disclosures
The authors have no financial conflicts of interests.
E.M.R. performed the experiments; E.M.R. and K.D. designed experiments, analyzed the data, and wrote the manuscript.
Supplemental data: A supplemental Figure illustrating the flow cytometry strategy used for the cell analyses described in this study, as well as tables providing the average frequency and s.d. for adult data presented in the main figures are available on line.
References
- 1.Allman D, Pillai S. Peripheral B Cell Subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J, Sillverman M, Monroe J. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–349. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol. Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell Immunol. 2006;239:92–102. doi: 10.1016/j.cellimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 6.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv. Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 7.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Figgett W, Saulep D, Lepage M, Hibbs M. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol. Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F, Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 10.Gerondakis S, Siebenlist U. Roles of the NF-κB pathway in lymphocyte development and funciton. Cold Spring Harb. Perspect. Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurosaki T, Shinobara H, Baba Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr. Opin. .Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu. Rev. Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 14.Schiemann B, Gommerman J, Vora K, Cachero T, Shulga-Morskaya S, Dobles M, Frew E, Scott M. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 15.Thompson J, Bixler S, Qian F, Vora K, Scott M, Cachero T, Hession C, Schneider P, Sizing I, Mullen C, Strauch K, Zafari M, Benjamin C, Tschopp J, Browing J, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 16.Miosge L, Blasioli J, Blery M, Goodnow C. Analysis of an ethylnitrosourea-generated mouse mutation defines a cell intrinsic role of nuclear factor κB2 in regulating circulating B cell numbers. J. Exp. Med. 2002;196:113–1119. doi: 10.1084/jem.20020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan W. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J. Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 18.Ruland J, Duncan G, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, Millar D, Bouchard D, Wakeham A, Ohashi P, Mak T. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 19.Xue L, Morris S, Orihuela C, Tuomanen E, Cui X, Wen R, Wang D. Defective development and function of Bcl10-deficient follicular, marginal zone, and B1 B cells. Nat. Immunol. 2003;4:857–865. doi: 10.1038/ni963. [DOI] [PubMed] [Google Scholar]
- 20.Ruefli-Brasse A, French D, Dixit V. Regulation of NF-κB dependent lymphocye activation and deveoopment by paracaspse. Science. 2003;302:1581–1584. doi: 10.1126/science.1090769. [DOI] [PubMed] [Google Scholar]
- 21.Ruland J, Duncan G, Wakeham A, Mak T. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity. 2003;19:749–758. doi: 10.1016/s1074-7613(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 22.Hara H, Wada T, Bakal C, Kozieradzki I, Suzuki S, Suzuki N, Nghiem M, Griffiths E, Krawczyk C, Bauer B, D'Acquisto F, Ghosh S, Yeh W, Baier G, Rottapel R, Pennigner J. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–775. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 23.Egawa T, Albrecht B, Favier B, Sunshine M, Mirchandani K, O'Brien W, Thome M, Littman D. Requirement for CARMA1 in antigen receptor-induced NF-kB activation and lymphocyte proliferation. Curr. Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 24.Pohl T, Gugasyan R, Grumont R, Strasser A, Metcalf D, Tarlington D, Sha W, Baltimore D, Gerondakis S. The combined absence of NF-κB1 and c-Rel reveals that overlapping roles for these transcription fctors in the B cel lineage are restricted to activation and function of mature cells. Proc. Natl. Acad. Sci. U S A. 2002;99:4514–4519. doi: 10.1073/pnas.072071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casola S. Control of peripheral B-cell development. Curr Opin Immunol. 2007;19:143–149. doi: 10.1016/j.coi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Herzenberg LA, Herzenberg L. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 27.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 28.Ghosn E, Sadate-Ngatchou P, Yang Y, Herzenberg L, Herzenberg L. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc. Natl. Acad. Sci. U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimoto M, Montecino-Rodriguez E, Prashanth P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. B-1 and Marginal Zone B progenitor cells emerge from yolk sac hemogenic endothelium. Proc. Natl. Acad. Sci. U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmack CE, Shinton SA, Hayakawa K, Hardy RR. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J. Exp. Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev. Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Franzoso G, Carlson L, Xing L, Poljak L, Shores E, Brown K, Leonardi A, Tran T, Boyce B, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes Develop. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 34.Sha W, Liou H, Tuomanen E, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 35.Barber C, Montecino-Rodriguez E, Dorshkind K. Reduced Production of B-1 Specified Common Lymphoid Progenitors Results in Diminished Potential of Adult Marrow to Generate B-1 Cells. Proc. Natl. Acad. Sci. U S A. 2011 doi: 10.1073/pnas.1107172108. doi:10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagarasan S, Shinkura R, Kamata T, Nogaki F, Ikuta K, Tashiro K, Honjo T. Alymphoplasia (aly)-type nuclear factor κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J. Exp. Med. 2000;191:1477–1486. doi: 10.1084/jem.191.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caamaño J. Nuclear Factor (NF)-κB2 (p100/p52) Is Required for Normal Splenic Microarchitecture and B Cell-mediated Immune Responses. J. Exp. Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. e. al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantor A, Stall A, Adams S, Watanbe K, Herzenberg L. De novo development and replenishment of B cells. Int. Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–239. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.