Abstract
TREK-1 is a member of the two-pore domain potassium channel family that is known as a leak channel and plays a key role in many physiological and pathological processes. The conformational transition of the selectivity filter is considered as an effective strategy for potassium channels to control the course of potassium efflux. It is well known that TREK-1 is regulated by a large volume of extracellular and intracellular signals. However, until now, little was known about the selectivity filter gating mechanism of the channel. In this research, it was found that Ba2+ blocked the TREK-1 channel in a concentration- and time-dependent manner. A mutagenesis analysis showed that overlapped binding of Ba2+ at the assumed K+ binding site 4 (S4) within the selectivity filter was responsible for the inhibitory effects on TREK-1. Then, Ba2+ was used as a probe to explore the conformational transition in the selectivity filter of the channel. It was confirmed that collapsed conformations were induced by extracellular K+-free and acidification at the selectivity filters, leading to nonconductive to permeable ions. Further detailed characterization demonstrated that the two conformations presented different properties. Additionally, the N-terminal truncated isoform (ΔN41), a product derived from alternative translation initiation, was identified as a constitutively nonconductive variant. Together, these results illustrate the important role of selectivity filter gating in the regulation of TREK-1 by the extracellular K+ and proton.
Keywords: Membrane Proteins, Neurobiology, pH Regulation, Potassium Channels, Protein Conformation, Ba2+, K2p, TREK-1, Gate Mechanism, Selectivity Filter
Introduction
Potassium channels are ubiquitous pore-forming transmembrane proteins that transport K+ ions selectively and rapidly across the biological membranes. The efflux of K+ ions is controlled not only by the electrochemical gradient, but also by the gating mechanism. Along the ion conduction pathway of potassium channels, three structures are arranged from intercellular to extracellular: the lower activation gate, the selectivity filter (SF)2, and the upper inactivation gate (also termed the C-type inactivation gate). Correspondingly, there are mainly two kinds of mechanisms controlling K+ ion passage. Manipulation of the lower activation gate controls the transition between the open and close state of the channel. The upper inactivation gate, which is characterized by slow kinetics, controls the transition between conduction and nonconduction of the pore. The selectivity filter of the K+ channels, formed by the highly conserved sequence TV(I)GY(F)G, plays a pivotal role in both mechanisms. Accumulating evidence shows that the selectivity filter itself has the ability to act as the inactivation gate (1–4). High resolution crystallographic analysis has revealed detailed structural changes in the selectivity filter associated with the activation gating and inactivation gating (5, 6). The carbonyl oxygens together with the side chain hydroxyl oxygen of the threonine define four equally spaced ion-binding sites that are commonly termed S1-S4, from the extracellular to the intracellular region (7). The rearrangement of the selectivity filter is well coordinated with the alteration of K+ ion-binding sites, providing a built-in mechanism for adjusting channel gating (5, 8, 9).
The two-pore domain potassium channels (K2P) family, a branch discovered over a decade ago, has attracted increasing interest in its unique structure and function. In mammals, these channels are divided into six subfamilies on the basis of sequence similarity and function resemblance. All the members of this family are characterized by a distinguishing topology. That is, each subunit contains four transmembrane domains and two pore-forming domains (P1 and P2). Accordingly, K2P subunits dimerize (in contrast to tetramerization in other potassium subunits) to constitute the functional selectivity filter containing four pore loop domains, a structure common to all known potassium channels (10–12). The current produced by these channels is outwardly rectifying and is insensitive to classic potassium channel blockers such as 4-aminopyridine and tetraethylammonium. K2P channels are strongly implicated in the background or leak conductance that regulates the resting membrane potential and excitability of many kinds of cells.
TREK-1 (also called K2P2.1), one of the best studied members of K2P family, is expressed at high levels in excitable tissues such as the nervous system (13) and heart (14). Functionally, the channel is involved in many diverse physiological or pathological processes, including neuroprotection, cerebrovascular vasodilatation, regulation of aldosterone production and secretion, tumorigenesis, depression, chemoreception, and pulmonary vasoconstriction (15, 16). The functional versatility of the channel is highly associated with its sensitivity to a large volume of chemical and physical signals. Mechanical stretch, temperature, polyunsaturated fatty acids, intra-and extracellular pH, G-protein coupled receptors, and volatile general anesthetics constitute the large TREK-1 regulatory machine (16). Particular efforts have been made to explain the relationship between these regulations and gating mechanism of TREK-1 (17, 18). It has been confirmed that the regulation of phosphatidylinositol 4,5-biphosphate to TREK-1 involves alteration of gating patterns (19). Extracellular acid could depress the TREK-1 current by facilitating C-type inactivation, in which conformational modification of the pore region could be involved (20). However, up to now, no clues have been found about how these signals interact with the SF in TREK-1. As the Ba2+ ion has a similar radius to the K+ ion, the divalent ion is able to bind into the SF of several classes of potassium channels (21–23). Because of its greater charge, Ba2+ tends to dwell a long time on its binding site, leading to a block of the K+ efflux. Thus, Ba2+ has been exploited extensively to decipher the SF properties of potassium channels (24–29). However, it has long been believed that the TREK-1 channel is Ba2+ insensitive or resistant (13, 30–33). Here, we report for the first time that Ba2+ inhibits the TREK-1 current dramatically by binding to the assumed K+ binding site 4 (S4). Then, using Ba2+ as a probe, we demonstrate that the regulation of extracellular potassium ions and protons involves conformational modification in the SF of TREK-1. Particularly, the N terminus truncated isoform of TREK-1 (ΔN41) possesses a constitutively nonconductive SF.
EXPERIMENTAL PROCEDURES
Molecular Biology
cDNA encoding the 411-amino acid isoform of human TREK-1 used in this work was amplified from a TREK-1 plasmid (a generous gift from Dr. Florian Lesage, Institut de Pharmacologie Moléculaire et Cellulaire, Valbonne, France) using PCR. To investigate whether the isoform was under the regulation of alternative translation initiation (ATI) mechanism, TREK-1 containing the translation initial sequence (UGA AUA AGA) preceding the first start codon and the N-terminal truncated isoform (ΔN41) were inserted into pcDNA3.1 vector (Invitrogen). To get a high expression level in the Xenopus laevis oocyte, TREK-1 was subcloned into the pGH19 vector as follows: the pGH19-HERG (human ether-àgo-go-related gene) plasmid (a generous gift of Dr. Gail Robertson, University of Wisconsin-Madison Medical School, Madison, WI) was first cut with BamHI and HindIII to get rid of its initial inserted HERG, and then TREK-1 was inserted into the matched sites.
Mutants were generated using the MutanBEST kit (TaKaRa) according to the manufacturer's manual. All mutations were confirmed by DNA sequencing. cRNA was transcribed in vitro using the RiboMAXtm large-scale RNA production systems kit (Promega).
Cell Culture, Protein Expression, and Western Blot Analysis
HEK 293 cells were cultured in DMEM supplemented with 10% fetal bovine serum and 2 mm l-glutamine and held at 37 °C in humidified air with 5% CO2. cDNAs encoding the wild-type TREK-1, the mutant M42I, and ΔN41 were transiently transfected into HEK 293 cells using Lipofectamine 2000 (Invitrogen). Two days after transfection, the cells were harvested. Protein extracts were prepared by solubilization in buffer X (50 mm Tris (pH 7.4), 270 mm NaCl, 1% Triton X) for 1 h and clarified by centrifugation at 12,000 × g for 30 min. Then the lysates were subjected to SDS-PAGE on 10% gel and wet-transferred onto a PVDF membrane. The anti-myc (ZSGB-BIO, 1:1000) and anti-actin (ZSGB-BIO, 1:5000) primary antibodies were used in Western blotting. Secondary antibodies were used at 1:5000 dilutions.
Electrophysiology
X. laevis oocytes were isolated and injected with 10 ng of cRNA (46 nl in volume) per cell. Whole-cell currents were measured 1–3 days after injection by the two-electrode voltage clamp technique using an Axoclamp2B amplifier (Axon Instruments, Union City, CA). For two-electrode voltage clamp experiments, the electrodes were filled with 3 m KCl and had a tip resistance of 0.1–1 MΩ. Recordings were performed under constant perfusion at room temperature. Data were sampled at 2 kHz and filtered at 0.5 kHz with the Clampex 10.0 software (Axon Instruments). K+ currents through the TREK-1 channel were elicited either by the Ramp protocol (voltage ramps from −120 to +60 mV, with 1 s in duration) or by the Pulse protocol (a 100-ms pulse from −80 to +20 mV from a holding potential of −80 mV, with a 2 s interpulse interval). The normalized current was the average recorded current divided by the original control current. The standard physiological extracellular solution contained the following unless noted otherwise: 5 mm KCl, 93 mm NaCl, 1 mm MgCl2, 1.8 mm CaCl2, 5 mm HEPES (pH 7.4), adjusted with NaOH (standard solution). HEPES buffer was replaced by Tris in pH 8.5 solutions and MES in pH 6.5 solutions. When required, potassium ions in bath solutions were isotonically replaced by sodium ions. BaCl2 was diluted from a 1 m stock and added to the various solutions as indicated.
Data Analysis
Concentration-response curves were fitted to the Hill equation according to the following parameters: I = 1/(1+ ([X]o/IC50)h), where I is the measured current, IC50 is the concentration of extracellular ions ([X]o, X represents H+ or Ba2+ in the research) required to achieve 50% inhibition, and h is the Hill coefficient. Time constants were calculated by fitting the data to the following equation: I = Io+A*e-T/τ, where I is the measured current, Io is the blocked current at equilibrium, T is the elapsed time after Ba2+ application, τ is the apparent forward time constant in seconds, and A is a constant. The fitting was separately performed for each experiment using Origin 7.5 software (OriginLab). Values were presented as mean ± S.E.
Statistical significance was assessed with Student's t test using GraphPad Prism 5.0 (GraphPad Software, Inc.) software. Multiple comparisons were performed with one-way analysis of variance followed by Dunnett post-testing. A significant difference was considered for p < 0.05.
RESULTS
Ba2+ Blocks TREK-1 in a Concentration- and Time-dependent Manner
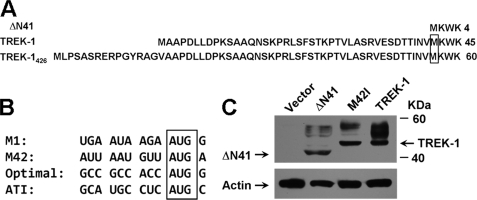
It has been reported that the 426 amino acid of TREK-1 (TREK-1426) is subjected to the ATI mechanism because of the suboptimal sequence context flanking the first start codon compared with the second one (34). Therefore, a mixture composed of the full-length TREK-1426 and an N-terminal truncated isoform (termed ΔN41 in this study) will be produced by its mRNA (Fig. 1A). The 411-amino acid of TREK-1 (TREK-1), which may be originated from alternative splicing, displayed a very different sequence context surrounding the start site compared with TREK-1426 (Fig. 1B). To investigate whether TREK-1 was under the control of the ATI mechanism, a mutant containing the second methionine-to-isoleucine mutation (M42I, no ATI function) was constructed. Protein expression analysis revealed that only one specific band was yielded by wild-type TREK-1 and M42I mutant, whereas the product of ΔN41 was a little smaller than anticipated (Fig. 1C). These results indicate that there is very little, if any, ΔN41 variant that was produced by TREK-1. In other words, the currents produced by TREK-1 would exclusively represent functional expression of TREK-1 rather than a mixture containing ΔN41. Thus, TREK-1 was used in the followed experiments to explore the gate mechanism of SF.
FIGURE 1.
Characterization of the TREK-1 isoform. A, comparison of protein sequences of ΔN41, TREK-1, and TREK-1426. The second methionine in TREK-1 and TREK-1426 are boxed. B, comparison of mRNA sequences flanking the first (M1) and second (M42) start codon of the TREK-1, the optimal Kozak sequence (Optimal), and the first start codon (ATI) of TREK-1426. The start codons are boxed. C, protein expression analysis of ΔN41, the M42I mutant, and wild-type TREK-1 by Western blot analysis with anti-myc antibody. The internal control (Actin) was visualized by an anti-actin antibody.
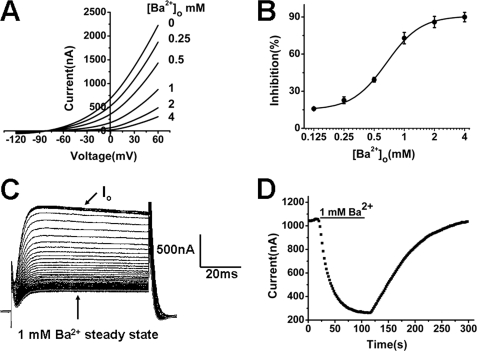
Currents recorded from a representative Xenopus oocyte expressing TREK-1 were shown in Fig. 2A in the absence and presence of different concentrations of extracellular Ba2+ ([Ba2+]o). When [Ba2+]o was up to 4 mm, a drastic current reduction was observed compared with the control current. For currents recorded at +20 mV, the inhibition was concentration-dependent (Fig. 2B). The fitting to the Hill equation revealed a half block (IC50) at 0.56 ± 0.03 mm with an apparent Hill coefficient of 1.48 ± 0.18 (n = 7).
FIGURE 2.
TREK-1 channels are inhibited by externally applied Ba2+ in a concentration- and time-dependent manner. A, representative current-voltage relationship for TREK-1 expressed in Xenopus oocytes in the absence and presence of different [Ba2+]o. Currents were elicited by the ramp protocol. The concentration of K+ in extracellular solution is 5 mm. B, concentration-response for Ba2+ inhibition (%) of TREK-1 channels at 20 mV with the protocol in A. Data points were mean ± S.E. of seven cells. The solid line is a fit of the data to the Hill equation. C, Ba2+ block of representative TREK-1 current amplitude during application 1 mm Ba2+. Currents were recorded with the pulse protocol. Io is the original current before application of Ba2+. D, plot of current-time recorded from the currents recorded in C. The following withdrawal of Ba2+ was also included.
Then the time-dependence of the Ba2+ inhibition was investigated. In the presence of 1 mm Ba2+, the currents evoked by depolarizing from the holding potential of −80 to +20 mV were decreased gradually as the time elapsed until the steady-state blockade was obtained (Fig. 2C). The process was plotted in Fig. 2D, and the current recovery by immediately following Ba2+-free solution exchange was also included. The time courses for the block and complete recovery were well fitted by a single exponential equation, with a τblock of 20.28 ± 1.95 s and a τunblock of 45.68 ± 4.80 s (n = 6), respectively.
External K+ Impedes the Blocking Effect of Ba2+ on TREK-1 Currents
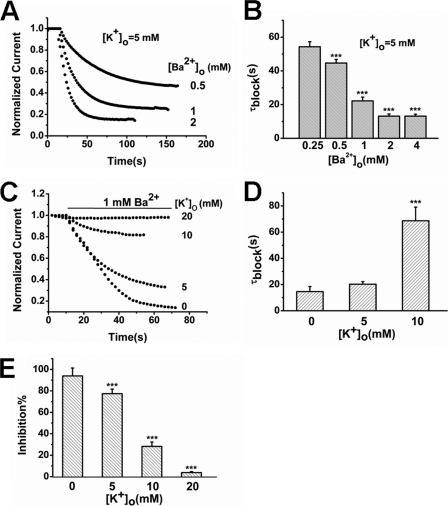
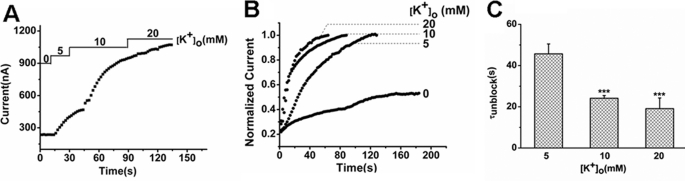
Ba2+ inhibition of some potassium channels resulted from its docking properties in the SF (21–23). If that is also the case in TREK-1, competitive binding between Ba2+ and K+ would occur. To demonstrate the situation, the access rate of Ba2+ to the channel was firstly assessed. It was found that the inhibition rate (τblock, s) was accelerated as [Ba2+]o increased. The τ value declined from 54.32 ± 3.00 s at the 0.25 mm level to 13.18 ± 1.12 s at the 4 mm level (Fig. 3, A and B, n = 6). Then the effects of extracellular K+ on Ba2+ access were evaluated. When [K+]o was increased from 0 mm to 10 mm, the block time constant at 1 mm [Ba2+]o was prolonged from 14.63 ± 3.88 s to 68.69 ± 10.34 s (p < 0.001, n = 6). Very little access of Ba2+ was observed in the presence of 20 mm [K+]o (Fig. 3, C and D). Accordingly, the blocking effects of Ba2+ were also alleviated as [K+]o increased. The inhibition was decreased from 93.91 ± 7.41% at the 0 mm [K+]o level to 3.80 ± 0.97% at 20 mm [K+]o level (Fig. 3E, n = 6, p < 0.001). These results reveal the competitive binding between Ba2+ and K+, which may result from closing binding sites within the SF of TREK-1.
FIGURE 3.
Effects of different [K+]o on Ba2+ inhibition of TREK-1 currents. A, the time course of inhibition to TREK-1 channels by different [Ba2+]o from a representative TREK-1-expressing oocyte. B, the time constant (τblock, s) of inhibition to TREK-1 current by different [Ba2+]o (mean ± S.E., n = 6 cells). ***, p < 0.001 versus 0.25 mm [Ba2+]o. C, effects of different [K+]o on the time course of blockade by 1 mm Ba2+ externally applied to a representative oocyte expressing TREK-1 channels. D, the block rate (τblock) (mean ± S.E., n = 6) of Ba2+ in different [K+]o external solutions as indicated. ***, p < 0.001 versus 0 mm [K+]o. The block rate was not significantly different at 0 and 5 mm [K+]o (p > 0.05). E, the inhibition percentage to TREK-1 current by Ba2+ in different [K+]o. Data represent mean ± S.E.(n = 6). ***, p < 0.001 versus 0 mm [K+]o.
Ba2+ Blocks TREK-1 by Overlapped Binding at the S4 Site within the Selectivity Filter
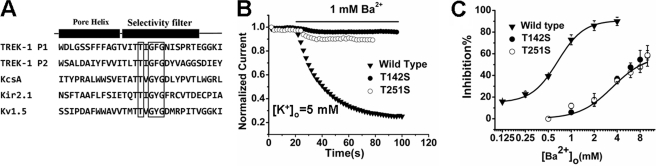
Structural insight into the Streptomyces lividans K+ channel, KcsA (potassium channels from S. lividans), provided by crystallographic studies has demonstrated that the binding sites of Ba2+ is located in the innermost SF, very close to the K+ S4 (9, 35). S4 is composed of four main chain carbonyl oxygen atoms and four threonine side chain hydroxyl oxygen atoms in most potassium channels. The threonine (positions 142 and 251) were conserved in the TREK-1 Pore I and Pore II domains (Fig. 4A). We presumed that Ba2+ might occupy a similar position in TREK-1. To test our hypothesis, mutants containing the threonine-to-serine transition at positions 142 (T142S) and 251 (T251S) were constructed. In the presence of 1 mm [Ba2+]o, the T142S and T251S mutants displayed very little blockade (5.97 ± 0.64% and 11.93 ± 2.46% inhibition, respectively, n = 6), whereas the current of the wild-type TREK-1 was decreased dramatically (72.90 ± 4.63% inhibition, n = 7) (Fig. 4B). As the concentration-response curve shows in Fig. 4C, the IC50 of externally applied Ba2+ to wild-type TREK-1 was 0.56 ± 0.03 mm, whereas to T142S and T251S it was 3.60 ± 0.19 mm and 3.28 ± 1.20 mm, respectively (n = 6). Obviously, the threonine mutants increase the IC50 for Ba2+ by approximately 6 fold, suggesting that Ba2+ depresses the TREK-1 current by occupying the K+ binding site at the SF.
FIGURE 4.
Ba2+ inhibits TREK-1 current through binding at the location occupied by Threonine 142 and Threonine 251 in the SF. A, multiple sequence alignment of the SF regions of TREK-1 (P1 and P2), KcsA, Kir2.1, and Kv1.5. The positions of the conserved threonine residues among them are boxed. The SF GY/FG signature sequence is also boxed. B, comparison of the relative 1 mm Ba2+ inhibitory effects on wild-type TREK-1, the T142S mutant, and the T251S mutant. The currents recorded from representative oocytes expressing the above channels were recorded, respectively. C, concentration-response curves of the wild-type TREK-1 and the mutants. Data points were mean ± S.E. for six to seven cells. The data were fitted with the Hill equation.
The Ba2+ Exit Rate Uncovers Conformational Alterations within the Selectivity Filter Induced by Different Levels of [K+]o
The SF of KcsA has two distinct conformations associated with low and high concentrations of K+. In low-K+ solutions, the filter adopts a nonconductive conformation, which is pinched closed at S2 and S3, and K+ binds at the ends of the filter (S1 and S4) (36). In high K+ solutions, the existence of four fully dehydrated K+ ion sites endows the filter in a conductive conformation. According to the well established rule, the alteration of coordinated K+ sites would influence the exit of barium from its binding site (35). Therefore, the change of the Ba2+ exit pattern would correspond to the conformational alteration of the SF. In our experiments, it was found that the macroscopic currents of TREK-1 were enhanced by increasing [K+]o from 0 to 20 mm (Fig. 5A), implying a conformational transition of the SF from nonconductive to conductive. Then, after the currents were depressed and reached a stable blockade in the presence of 1 mm Ba2+, different levels of [K+]o were used to facilitate the exit of Ba2+ from its binding site. As shown in Fig. 5, B and C, the exit rate of Ba2+ was strongly speeded up as [K+]o increased. The decreased τ values were 45.68 ± 4.80 s, 24.13 ± 1.33 s, and 19.09 ± 5.20 s at 5 mm, 10 mm, and 20 mm [K+]o, respectively (n = 6, p < 0.001). Particularly, the incomplete exit of Ba2+ was observed in the K+-free external solution (Fig. 5B). Therefore, alteration of [K+]o evokes conformational transitions in the SF of TREK-1, and the off rate of Ba2+ is highly associated with the transitions.
FIGURE 5.
Effects of different [K+]o on the dissociation of Ba2+ from the TREK-1 channel. A, effects of different [K+]o on the macroscopic currents of TREK-1 recorded from a representative oocyte. B, effects of different [K+]o on the time course of recovery from the blockade by 1 mm Ba2+ in a representative TREK-1-expressing oocyte. C, the time constant (τunblock, s) (mean ± S.E., n = 6) of Ba2+ inhibition in different [K+]o as indicated. ***, p < 0.001 versus 5 mm [K+]o.
External H+ Traps Ba2+ Inside the Selectivity Filter of TREK-1
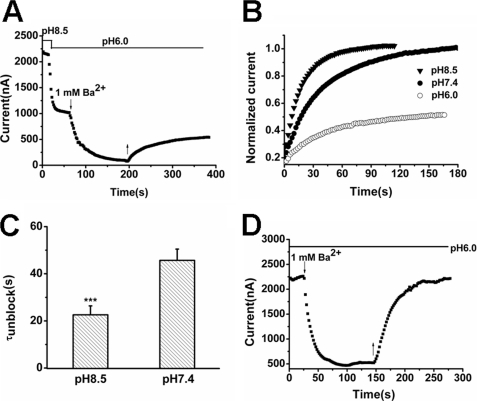
TREK-1 is very sensitive to external protons, and acidification strongly inhibits the channel via a mechanism resembling C-type inactivation in voltage-dependent potassium channels (20, 37). The key property of this mechanism is conformational transition of the SF to the nonconductive state. Because structural transitions of the SF occurred between low-K+ and high-K+ extracellular solution in TREK-1 (Fig. 5), we speculated that the alteration of extracellular pH values (pHo) might also influence the behavior of Ba2+ in response to conformational modifications. In our experiment, the currents of TREK-1 fell quickly after the extracellular solution was switched from pH 8.5 to pH 6.0. Subsequent application of 1 mm Ba2+ at pH 6.0 caused a further decrease. However, the washout with pH 6.0 solution achieved only a limited recover of the current (Fig. 6A). High pHo accelerated Ba2+ dissociation with decreased τunblock values from 45.68 ± 4.80 s at pH 7.4 to 22.59 ± 3.80 s at pH 8.5 (Fig. 6, B and C, n = 6, p < 0.001). H126A, a pH-insensitive mutant containing a histidine-to-alanine transition (20, 37), was used to eliminate the pH sensitivity of TREK-1. External application of 1 mm Ba2+ still blocked the currents quickly, but the currents of H126A recovered completely from Ba2+ blockade in pH 6.0 external solutions (Fig. 6D). These data suggest that the conformational transition in TREK-1 occurs in the course of the pHo switch and that the SF structure evoked by acid tends to trap Ba2+ because of its collapsed state.
FIGURE 6.
Effects of different pHo on the exit of Ba2+ from the TREK-1 channel. A, acidic pHo inhibits Ba2+ unbinding from TREK-1. Experiments were started in pH 8.5 bath solution. The external solution was switched between pH 8.5 and pH 6.0, as indicated. ↓ and ↑ indicate the time of Ba2+ (1 mm) fast application and withdrawal, respectively. B, the time course of Ba2+ exit from TREK-1 in different pHo solutions. The representative TREK-1 currents were depressed by 1 mm Ba2+ in standard solution and then subjected to washout by different pHo solutions. C, time constant (τunblock) of Ba2+ exit from TREK-1 channels in different pHo. Data represent mean percentage ± S.E. (n = 6). ***, p < 0.001 versus pH 7.4. D, Ba2+ dissociates completely from the H126A mutant in pH 6.0 external solutions. Currents were recorded from a representative H126A-expressing oocyte. ↓ and ↑ indicated the time of Ba2+ (1 mm) fast application and withdrawal, respectively.
The Conformational Transitions Induced by Extracellular H+ and K+-free Are Different in TREK-1
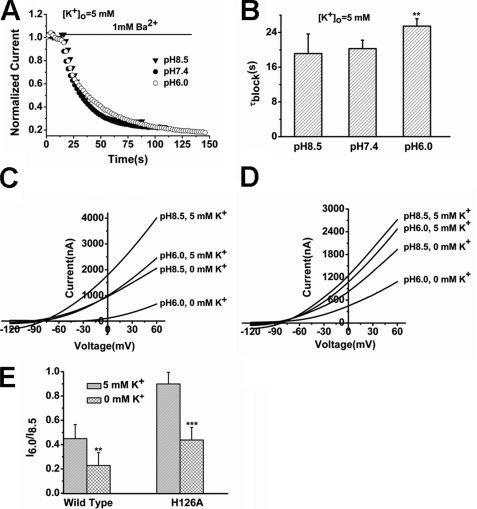
Most recently, it has been demonstrated that the SF conformation caused by low pH is very similar to that caused by low K+ in KcsA, of which S2 and S3 are lost (5). In TREK-1, incomplete recovery from Ba2+ inhibition was observed in the two conformations evoked by low-pH (acid conformation) and K+-free (K+-free conformation) conditions. We asked whether the SF structures of TREK-1 caused by K+-free and acid conditions are also similar. Our results demonstrate that they are different from each other. Firstly, although the K+-free conformation promoted the access of Ba2+ (Fig. 3, C and D), the acid conformation tended to impede the process (Fig. 7, A and B). The τblock was prolonged from 19.14 ± 4.52 s at pH 8.5 to 25.48 ± 1.69 s at pH 6.0 (n = 6, p < 0.01). Next, the interaction between the two conformations was investigated. As shown in Fig. 7C, currents recorded from a representative Xenopus oocyte expressing TREK-1 were subjected to a pH drop from 8.5 to 6.0 in the absence and in the presence of K+. Unequivocally, the K+-free conformation was more sensitive to the pH drop (I6.0/I8.5 of 0.22 ± 0.11 at 20 mV) than the conformation in standard solution (I6.0/I8.5 of 0.45 ± 0.12 at 20 mV) (Fig. 7E, n = 5, p < 0.01). Furthermore, the pH-insensitive mutant H126A also showed inhibition by acid in K+-free solution (I6.0/I8.5 is 0.44 ± 0.10 at 20 mV), compared with that in standard solution (I6.0/I8.5 is 0.90 ± 0.10 at 20 mV) (Fig. 7, D and E, n = 6, p < 0.001). Therefore, it seems that the K+-free conformation potentiates the formation of acid conformation.
FIGURE 7.
Characterization of the conformational modifications induced by K+-free and acid conditions in the selectivity filter of TREK-1. A, the time course of Ba2+ access to TREK-1. Currents were recorded from a representative TREK-1-expressing oocyte and were subjected to 1 mm Ba2+ perfusion in different pHo solutions. B, time constant (τblock) of Ba2+ access to TREK-1 channels in different pHo solutions. Data represent mean percentage ± S.E. (n = 6). **, p < 0.01 versus pH 8.5. τblock was not significantly different at pH 8.5 and 7.4 (p > 0.05). C, pHo effects on the representative TREK-1 currents in the standard and K+-free external solutions. D, pHo effects on the representative currents for H126A mutant expressed in Xenopus oocytes in the standard and K+-free external solutions. E, comparative analysis of I6.0/I8.5 in wild-type TREK-1 and the H126A mutant in different [K+]o. Data represent mean fraction ± S.E. (n = 5–6) at 20 mV. **, p < 0.01 versus wild-type at 5 mm [K+]o. ***, p < 0.001 versus H126A at 5 mm [K+]o.
ΔN41 Possesses a Constitutively Collapsed Selectivity Filter
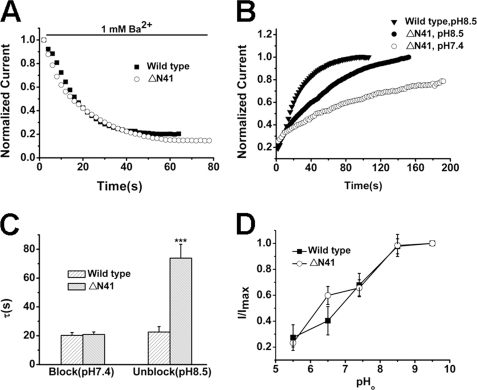
ΔN41, the product of ATI, is permeable to sodium under physiological conditions leading to membrane depolarization (34). The decreased macroscopic current, reduced open probability, along with the alteration of permeability of the isoform reminded us that conformational alteration to nonconductive state at its SF may happen. According to our results, Ba2+ ions were trapped in the nonconductive SF induced by low pHo and K+-free conditions in TREK-1. We then employed the blocker to test the possibility. No significant difference was found in the blockade ratio and access rate of Ba2+ to wild-type TREK-1 and ΔN41 (Fig. 8, A and C), but the behavior of Ba2+ exit displayed a big divergence. Ba2+ could not dissociate completely from ΔN41 in standard solution (pH 7.4). In alkali solution (pH 8.5), the time constant (τunblock) of ΔN41 was 73.9 ± 10.74 s (n = 7), much bigger than that of the wild type (22.59 ± 3.80 s, n = 6, p < 0.001, Fig. 8, B and C).
FIGURE 8.
Identification of the selectivity filter status in ΔN41. A, the time course of Ba2+ access to the representative wild-type TREK-1 and ΔN41 channels. B, the time course of Ba2+ dissociation from wild-type TREK-1 and ΔN41 channels. C, comparative analysis of time constants (τ, s) of Ba2+ blocking and unblocking in wild-type TREK-1 and ΔN41 channels. Data are mean ± S.E. from five to seven cells. τblock was not significantly different between the wild type and ΔN41 at pH 7.4 (p > 0.05). ***, p < 0.001 versus the wild type at pH 8.5. D, comparison of response to different pHo between wild-type TREK-1 and ΔN41. Data points represent mean fraction ± S.E. (n = 5) at 20 mV.
To further learn about the conformations of ΔN41, the pH sensitivity of wild-type TREK-1 and ΔN41 was examined next. As shown in Fig. 8D, for the steady-state currents recorded at +20 mV, the IC50 of acid inhibition was 7.29 ± 0.07 and 7.02 ± 0.51 (pH) for wild-type TREK-1 and ΔN41 (n = 5), respectively. No significant difference was found between them (p > 0.05), indicating that the location of conformational alteration induced by acid was similar in wild-type TREK-1 and ΔN41. Meanwhile, the pinched location in ΔN41 was not accordance with that in the wild type induced by acid.
DISCUSSION
Over the past decade, great progress has been made in the study of function and regulation in TREK-1. However, relatively little is known in the field of its gate mechanism, which may be due to the complex structure in the pore region compared with its voltage-gated brethren. The alkaline metal Ba2+ has a similar radius as K+ and is able to prevent the rapid flow of K+ effectively in several types of potassium channels through fitting into the SF by binding at S4 (21, 35, 38). Because of the inhibitory properties, Ba2+ has been used as a probe to learn about the pore structure of potassium channels (21–23). However, several studies have reported that TREK-1 is a Ba2+-insensitive or resistant channel (13, 30–33). In the current study, it was firstly demonstrated that Ba2+ could inhibit TREK-1 channel expressed in X. laevis oocytes in a concentration- and time-dependent manner (Fig. 2). Moreover, from the competitive binding between Ba2+and K+ (Fig. 3), we could speculate that the docking site for the blocker may be very closing to K+ coordinated sites in the SF of TREK-1. Through further mutagenesis study, it was elucidated that Ba2+ inhibits TREK-1 current via binding at the location occupied by T142 and T251, the assumed S4 site of the channel (Fig. 4). These results indicated that the interactive pattern between Ba2+ and TREK-1 was very similar to other studied potassium channels. This is less surprising because the SF is highly conserved among all the potassium channels. Only methyl groups in Thr-142 and Thr-251 were deleted in the Ba2+ insensitive mutants, and therefore the methyl groups were responsible for the Ba2+ binding site in the SF of TREK-1. This phenomenon is also found in Kcv and Kir2.1 potassium channels (21, 23). On the other hand, the similarly retained Ba2+ sensitivity in the two mutants implies that in the SF of TREK-1 either another low affinity binding site exists, as in the case of KCNQ1 (39), or that Thr-142 and Thr-251 are only partly composed of the binding site, and the neighbored threonines carry the retained binding ability, as in the case of Kir2.1 (21, 23).
The interaction between K+ and Ba2+ follows the rule established previously: The presence of K+ at the external lock-in site (S1 and/or S2) would impede the outward movement of barium, and occupancy of both the lock-in site and the enhanced site (S3) by K+ would destabilize Ba2+ and promote its exit (35). Interestingly, conformational transition occurs in the switch of low [K+] to high [K+]. According to the data from the crystallography analysis, the low K+ conformation of KcsA represents a nonconductive state in which only S1 and S4 are retained. That is, the external lock-in site is present. The high K+ conformation of KcsA represents a conductive state in which all four sites are present that means the lock-in site and enhance site are retained. Our presented results reveal that a low [K+]o prevented Ba2+ exit from the pore and that a higher [K+]o enhanced its exit (Fig. 5). Therefore, we can speculate that the conformational transition in a different [K+]o may be similar to that of KcsA. In a K+-free structure, the external lock-in site is occupied by K+ (probably S1) whereas the enhanced site is vacant (probably S2 and/or S3). According to the data from a large-conductance Ca2+-activated channel, this kind of conformation may lead to a longer dock time of Ba2+ (27). In the high-K+ conformation, both the external lock-in site and the enhanced site are occupied by K+ (S1-S3), and Ba2+ ions are repelled mainly by electrostatic repulsion.
In KcsA, the structure of C-type inactivation is very similar to that resulting from low concentration of K+, of which the SF is collapsed at S2 and S3 (5). However, data from some other potassium channels seem not to agree with the view. C-type inactivation conformation evoked by a low pHo in TASK1 (TWIK-related acid-sensitive), another member of the K2P family, displayed S0 lost (40). TASK2, a close relative of TASK1, shows another pattern of C-type inactivation in which the permeating ions are trapped at S1 and/or S2 (41). In addition, it was believed that conformational alterations happened at the outer pore of KCNK0 during C-type inactivation (42). In the acid conformation of TREK-1, Ba2+ was not able to dissociate completely from its binding site, indicating that collapse occurs in the SF. What about the collapsed location in the C-type inactivation (acid conformation) in TREK-1? Our results support the view that the position is located at the outer pore (S0 and/or S1) rather than at the middle location corresponding to S2 and S3. Three reasons may explain the situation. 1) As discussed above, it seems that evidence from the K2P channels supports this hypothesis. The pore structure of TREK-1 is more homologous with K2P members than with KcsA. The composition of the pore structure in K2P channels is heterogeneous, whereas Kcs A is homogeneous. 2) From our data, the access rate of Ba2+ to TREK-1 is probably associated with two factors: the concentration of itself and the SF conformation of TREK-1. If [Ba2+]o is fixed, the change of the access rate would reflect the conformational alteration. As shown in Figs. 3 and 7, the K+-free conformation caused an increased access rate, whereas the acid conformation caused a decreased tendency, indicating that the two conformations are different in the collapsed location. 3) The decreased access rate in acid conditions may reflect constriction in the outer pore. This phenomenon was also observed in Kv 1.5 (24). The interaction between K+-free and acid conformations reveals that the K+-free structure can facilitate the formation of the low-pHo structure. It has been revealed previously that an increased [K+]o can alleviate C-type inactivation resulting from acid in TREK-1 (20). Clearly, the alleviation originated from the conformation transition to the conductive state, according to our results.
In light of the above results, we also identified ΔN41 as a constitutively nonconductive variant in the SF. Compared with wild-type TREK-1, ΔN41 showed a similar blocking rate of Ba2+ but a slower unblocking rate. These results imply that the large difference in current density between the two isoforms may originate from their conformational divergence of SF rather than trafficking properties of the channels. TREK2, the closest relative of TREK-1 in K2P family, also uses the ATI mechanism to yield an N-terminal truncated isoform that displays a large conductance and sodium permeability (43). TREK-2 isoforms are similarly activated and inhibited by various modulators. We also found that TREK-1 isoforms showed similar response to pHo alteration, which implied that the collapsed location in the SF induced by acid in both isoforms is essentially identical. Clearly, other collapsed or pinched locations lead to the constitutive phenotype in ΔN41. Given the similar access rate of Ba2+ between wild-type TREK-1 and ΔN41, the collapsed site should be relatively far from S4. Because Ba2+ ions are also trapped inside the SF of ΔN41in the standard solutions and the N terminus locates at the intracellular side, we propose that the internal lock-in site may be occupied in the truncated isoform, but further evidence still needs to support the view.
Taken together, we have demonstrated the contribution of SF and the coordinated K+ to the conformation transition of TREK-1 channel using Ba2+, a qualified tool that could inhibit current robustly by preferably docking on the K+ binding site 4 of TREK-1. Accordingly, our results throw new light on the gate mechanism of TREK-1, a representative member of K2P class.
Acknowledgments
We thank Dr. Florian Lesage (Institut de Pharmacologie Moléculaire et Cellulaire) for the 411-amino acid TREK-1 plasmid and Dr. Gail Robertson for the pGH19-HERG plasmid (University of Wisconsin-Madison Medical School). We also thank Dr. Ying-Xia Tan (Beijing Institute of Transfusion Medicine) for reading the manuscript.
This work was supported by National Program on Key Basic Research Project of China Grant 2007CB512307.
- SF
- selectivity filter
- K2P
- two-pore-domain potassium channels
- ATI
- alternative translation initiation
- KcsA
- potassium channels from S. lividans.
REFERENCES
- 1. Wang D. T., Hill A. P., Mann S. A., Tan P. S., Vandenberg J. I. (2011) Nat. Struct. Mol. Biol. 18, 35–41 [DOI] [PubMed] [Google Scholar]
- 2. Domene C., Klein M. L., Branduardi D., Gervasio F. L., Parrinello M. (2008) J. Am. Chem. Soc. 130, 9474–9480 [DOI] [PubMed] [Google Scholar]
- 3. Cordero-Morales J. F., Jogini V., Lewis A., Vásquez V., Cortes D. M., Roux B., Perozo E. (2007) Nat. Struct. Mol. Biol. 14, 1062–1069 [DOI] [PubMed] [Google Scholar]
- 4. Cordero-Morales J. F., Cuello L. G., Zhao Y., Jogini V., Cortes D. M., Roux B., Perozo E. (2006) Nat. Struct. Mol. Biol. 13, 311–318 [DOI] [PubMed] [Google Scholar]
- 5. Cuello L. G., Jogini V., Cortes D. M., Perozo E. (2010) Nature 466, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuello L. G., Jogini V., Cortes D. M., Pan A. C., Gagnon D. G., Dalmas O., Cordero-Morales J. F., Chakrapani S., Roux B., Perozo E. (2010) Nature 466, 272–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernèche S., Roux B. (2001) Nature 414, 73–77 [DOI] [PubMed] [Google Scholar]
- 8. Bhate M. P., Wylie B. J., Tian L., McDermott A. E. (2010) J. Mol. Biol. 401, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lockless S. W., Zhou M., MacKinnon R. (2007) PLoS Biol. 5, e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kollewe A., Lau A. Y., Sullivan A., Roux B., Goldstein S. A. (2009) J. Gen. Physiol. 134, 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuill K. H., Stansfeld P. J., Ashmole I., Sutcliffe M. J., Stanfield P. R. (2007) Pfluegers Arch. 455, 333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niemeyer M. I., González-Nilo F. D., Zúñiga L., González W., Cid L. P., Sepúlveda F. V. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fink M., Duprat F., Lesage F., Reyes R., Romey G., Heurteaux C., Lazdunski M. (1996) EMBO J. 15, 6854–6862 [PMC free article] [PubMed] [Google Scholar]
- 14. Joon Kim S., Earm Y. E. (2006) Cardiovasc. Res. 69, 13–14 [DOI] [PubMed] [Google Scholar]
- 15. Es-Salah-Lamoureux Z., Steele D. F., Fedida D. (2010) Trends Pharmacol. Sci. 31, 587–595 [DOI] [PubMed] [Google Scholar]
- 16. Enyedi P., Czirják G. (2010) Physiol. Rev. 90, 559–605 [DOI] [PubMed] [Google Scholar]
- 17. Mathie A., Al-Moubarak E., Veale E. L. (2010) J. Physiol. 588, 3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen A., Ben-Abu Y., Zilberberg N. (2009) Eur. Biophys. J. 39, 61–73 [DOI] [PubMed] [Google Scholar]
- 19. Chemin J., Patel A. J., Duprat F., Lauritzen I., Lazdunski M., Honoré E. (2005) EMBO J. 24, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen A., Ben-Abu Y., Hen S., Zilberberg N. (2008) J. Biol. Chem. 283, 19448–19455 [DOI] [PubMed] [Google Scholar]
- 21. Chatelain F. C., Gazzarrini S., Fujiwara Y., Arrigoni C., Domigan C., Ferrara G., Pantoja C., Thiel G., Moroni A., Minor D. L., Jr. (2009) PLoS ONE 4, e7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang H. K., Marton L. J., Liang K. K., Shieh R. C. (2009) Biochim. Biophys. Acta 1788, 500–506 [DOI] [PubMed] [Google Scholar]
- 23. Chatelain F. C., Alagem N., Xu Q., Pancaroglu R., Reuveny E., Minor D. L., Jr. (2005) Neuron 47, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y. M., Fedida D., Kehl S. J. (2008) Biophys. J. 95, 4456–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibor G., Yakubovich D., Rosenhouse-Dantsker A., Peretz A., Schottelndreier H., Seebohm G., Dascal N., Logothetis D. E., Paas Y., Attali B. (2007) Biophys. J. 93, 4159–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruening-Wright A., Lee W. S., Adelman J. P., Maylie J. (2007) J. Gen. Physiol. 130, 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vergara C., Alvarez O., Latorre R. (1999) J. Gen. Physiol. 114, 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shieh R. C., Chang J. C., Arreola J. (1998) Biophys. J. 75, 2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris R. E., Larsson H. P., Isacoff E. Y. (1998) Biophys. J. 74, 1808–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inglis S. K., Brown S. G., Constable M. J., McTavish N., Olver R. E., Wilson S. M. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meadows H. J., Benham C. D., Cairns W., Gloger I., Jennings C., Medhurst A. D., Murdock P., Chapman C. G. (2000) Pfluegers Arch. 439, 714–722 [DOI] [PubMed] [Google Scholar]
- 32. Patel A. J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. (1998) EMBO J. 17, 4283–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou M., Xu G., Xie M., Zhang X., Schools G. P., Ma L., Kimelberg H. K., Chen H. (2009) J. Neurosci. 29, 8551–8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson A. N., Posson D. J., Parsa P. V., Nimigean C. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6900–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang Y., MacKinnon R. (2000) J. Gen. Physiol. 115, 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Y., Morais-Cabral J. H., Kaufman A., MacKinnon R. (2001) Nature 414, 43–48 [DOI] [PubMed] [Google Scholar]
- 37. Sandoz G., Douguet D., Chatelain F., Lazdunski M., Lesage F. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14628–14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Connell A. D., Morton M. J., Sivaprasadarao A., Hunter M. (2005) J. Physiol. 562, 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibor G., Yakubovich D., Peretz A., Attali B. (2004) J. Gen. Physiol. 124, 83–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stansfeld P. J., Grottesi A., Sands Z. A., Sansom M. S., Gedeck P., Gosling M., Cox B., Stanfield P. R., Mitcheson J. S., Sutcliffe M. J. (2008) Biochemistry 47, 7414–7422 [DOI] [PubMed] [Google Scholar]
- 41. Zúñiga L., Márquez V., González-Nilo F. D., Chipot C., Cid L. P., Sepúlveda F. V., Niemeyer M. I. (2011) PLoS ONE 6, e16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zilberberg N., Ilan N., Goldstein S. A. (2001) Neuron 32, 635–648 [DOI] [PubMed] [Google Scholar]
- 43. Simkin D., Cavanaugh E. J., Kim D. (2008) J. Physiol. 586, 5651–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]