Abstract
α-Synuclein is central to the pathogenesis of Parkinson disease (PD). Mutations as well as accumulation of α-synuclein promote the death of dopaminergic neurons and the formation of Lewy bodies. α-Synuclein is monoubiquitinated by SIAH, but the regulation and roles of monoubiquitination in α-synuclein biology are poorly understood. We now report that the deubiquitinase USP9X interacts in vivo with and deubiquitinates α-synuclein. USP9X levels are significantly lower in cytosolic fractions of PD substantia nigra and Diffuse Lewy Body disease (DLBD) cortices compared to controls. This was associated to lower deubiquitinase activity toward monoubiquitinated α-synuclein in DLBD cortical extracts. A fraction of USP9X seems to be aggregated in PD and DLBD, as USP9X immunoreactivity is detected in Lewy bodies. Knockdown of USP9X expression promotes accumulation of monoubiquitinated α-synuclein species and enhances the formation of toxic α-synuclein inclusions upon proteolytic inhibition. On the other hand, by manipulating USP9X expression levels in the absence of proteolytic impairment, we demonstrate that monoubiquitination controls the partition of α-synuclein between different protein degradation systems. Deubiquitinated α-synuclein is mostly degraded by autophagy, while monoubiquitinated α-synuclein is preferentially degraded by the proteasome. Moreover, monoubiquitination promotes the degradation of α-synuclein, whereas deubiquitination leads to its accumulation, suggesting that the degradation of deubiquitinated α-synuclein by the autophagy pathway is less efficient than the proteasomal one. Lower levels of cytosolic USP9X and deubiquitinase activity in α-synucleinopathies may contribute to the accumulation and aggregation of monoubiquitinated α-synuclein in Lewy bodies. Our data indicate that monoubiquitination is a key determinant of α-synuclein fate.
Parkinson disease (PD) is mainly characterized by the death of dopaminergic neurons in the substantia nigra and presence of inclusions called Lewy bodies. Even though Lewy bodies are composed predominantly of α-synuclein, the mechanisms by which Lewy bodies are formed and their role in the death of dopaminergic neurons remain elusive (1, 2).
α-Synuclein purified from Lewy bodies is monoubiquitinated (3–5). Although some ubiquitin-ligases can polyubiquitinate α-synuclein (6–8), the E3 ubiquitin-ligase responsible for α-synuclein monoubiquitination has remained obscure. We and others have recently shown that α-synuclein is monoubiquitinated by the E3 ubiquitin-ligase SIAH both in vitro and in vivo (9–11). SIAH is present in Lewy bodies (9) and monoubiquitinates α-synuclein at the same lysine residues that are monoubiquitinated in α-synuclein immunopurified from Lewy bodies (5, 10). Most importantly, we found that in the presence of proteolytic inhibitors, monoubiquitination of α-synuclein promoted by SIAH leads to a marked increase in the aggregation of α-synuclein and formation of inclusions, which are toxic to cells (10–12). Therefore, our data indicate that monoubiquitination by SIAH might play a primary role in the aggregation and toxicity of α-synuclein and possibly in the formation of Lewy bodies.
Despite the data linking monoubiquitination to α-synuclein aggregation, the physiological role of α-synuclein monoubiquitination is not known. Furthermore, it is not even clear whether α-synuclein monoubiquitination is a dynamically regulated process or constitutes a dead-end posttranslational modification seen in the aggregated protein.
In order to clarify the role of α-synuclein monoubiquitination, we have now sought to identify its specific deubiquitinase. We found that the deubiquitinase USP9X interacts and deubiquitinates α-synuclein in vitro and in vivo. Knockdown of USP9X expression in conditions of proteolytic inhibition leads to the accumulation of monoubiquitinated α-synuclein and increases the aggregation of α-synuclein into toxic inclusions, strengthening the connection between monoubiquitination, inclusion formation, and toxicity of α-synuclein. USP9X cytosolic levels are lower in DLBD and PD tissues, which may contribute to the accumulation and aggregation of monoubiquitinated α-synuclein. Surprisingly, we found that, in the absence of proteolytic impairment, monoubiquitination promotes the degradation of α-synuclein through the proteasome, while deubiquitinated α-synuclein is almost entirely targeted to the autophagy pathway. Drugs that modulate USP9X activity, together with enhancers of autophagy or proteasomal activity, may help decrease the levels of α-synuclein and provide a novel therapeutic strategy to treat α-synucleinopathies.
Results
USP9X Deubiquitinates α-Synuclein.
We have previously shown that endogenous SIAH monoubiquitinates α-synuclein at lysines that are monoubiquitinated in Lewy bodies (10). We now sought to determine if monoubiquitination of α-synuclein is a dynamically regulated process. For this, we added lysates of dopaminergic SH-SY5Y cells to in vitro monoubiquitinated α-synuclein and monitored changes in the levels of α-synuclein monoubiquitination. We found that small amounts of cell lysates significantly decreased α-synuclein monoubiquitination (Fig. 1A). This activity was inhibited by a deubiquitinase inhibitor, NEM but not by the proteasome inhibitor MG132 (Fig. 1B), suggesting that a deubiquitinase enzyme present in the cell lysate removes α-synuclein monoubiquitin chains.
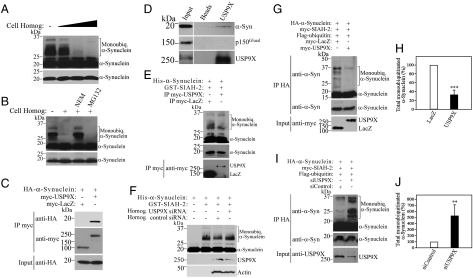
Fig. 1.
USP9X interacts with and deubiquitinates α-synuclein. (A) His-α-synuclein was monoubiquitinated in vitro as described in Materials and Methods. Monoubiquitinated α-synuclein was further incubated for 30 min in the presence of increasing amounts of SH-SY5Y cell homogenates (1 to 10 μg). (B) In vitro monobiquitinated α-synuclein was incubated for further 30 min with 5 μg of SH-SY5Y cell homogenate, in the absence or in the presence of the deubiquitinase inhibitor NEM (1 mM) or the proteasome inhibitor MG132 (30 μM). (C) SH-SY5Y cells were transfected with HA-α-synuclein and myc-USP9X or myc-LacZ as control. Myc-proteins were immunoprecipitated and samples were analyzed for coimmunoprecipitation with anti-HA antibody (Upper). (D) USP9X was immunoprecipitated from rat brain lysate using anti-USP9X antibody and coimmunoprecipitation was determined using anti-α-synuclein or anti-p150Glued antibody. (E) In vitro monobiquitinated α-synuclein was incubated for further 30 min with immunopurified myc-USP9X or myc-LacZ, obtained by immunoprecipitation with anti-myc antibody and high-stringency washes. (F) In vitro monobiquitinated α-synuclein was incubated for further 30 min with 5 μg lysates of SH-SY5Y cells transfected with siRNA control or siRNA to USP9X. Levels of endogenous USP9X were monitored with anti-USP9X antibody. (G) SH-SY5Y cells were transfected and treated for 16 h with 10 μM lactacystin, 25 mM ammonium chloride and 10 mM 3-MA. HA-α-synuclein was immunoprecipitated with anti-HA antibody. (H) Percent of α-synuclein monoubiquitination in SH-SY5Y cells transfected as in G; mean ± SEM of three experiments. (I) Transfected SH-SY5Y cells were treated, immunoprecipitated and detected as in G. Lower panel depicts the expression levels of USP9X in the cell lysates with anti-USP9X antibody. (J) Percent of α-synuclein monoubiquitination in SH-SY5Y cells transfected as in I; mean ± SEM of four experiments. The levels of α-synuclein monoubiquitination in A, B, E, F, G, and I were determined with anti-α-synuclein antibody. Lower (A, B) and middle panels (E, G, and I) depict the levels of α-synuclein under a short exposure. **, Significantly different from control at p < 0.01; ***, p < 0.002.
In order to identify the deubiquitinase responsible for α-synuclein deubiquitination, we focused on brain-enriched deubiquitinases that act on monoubiquitin linkage. One strong candidate is USP9X that controls the monoubiquitination of the TGF-β-related protein Smad4 (13, 14). Thus, we investigated if USP9X regulates the levels of monoubiquitinated α-synuclein. We carried out coimmunoprecipitation experiments of transfected SH-SY5Y cells and found that α-synuclein coimmunoprecipitates with USP9X but not with a control protein (Fig. 1C), indicating that α-synuclein and USP9X interact in a specific manner. We then carried out coimmunoprecipitation experiments using rat brain tissues and found that endogenous α-synuclein coimmunoprecipitates with USP9X (Fig. 1D), indicating that α-synuclein and USP9X interact in vivo.
Subsequently, we investigated the ability of USP9X to modulate α-synuclein monoubiquitination. We immunopurified USP9X and incubated it with in vitro monoubiquitinated α-synuclein. Immunopurified USP9X, but not a control protein, decreased the levels of monoubiquitinated α-synuclein (Fig. 1E). In addition, lysates of cells transfected with siRNA to USP9X showed less deubiquitinase activity compared to lysates of cells transfected with siRNA control (Fig. 1F).
To confirm the relevance of USP9X as a deubiquitinase for α-synuclein, we next investigated whether USP9X deubiquitinates α-synuclein in vivo. For this, we transfected SH-SY5Y cells and found that the expression of USP9X, but not of a control protein, decreased the monoubiquitination of α-synuclein that is promoted by the E3 ligase SIAH (Fig. 1 G and H). Moreover, knockdown of endogenous USP9X expression by siRNA significantly increased α-synuclein monoubiquitination levels in SH-SY5Y cells (Fig. 1 I and J), indicating that USP9X is a physiological deubiquitinase of α-synuclein.
USP9X Levels Are Decreased in α-Synucleinopathies.
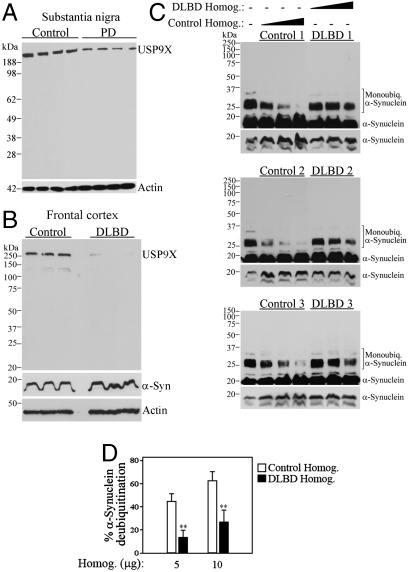
In order to further determine the relevance of USP9X to PD, we investigated its levels in the substantia nigra. We found that USP9X levels are significantly lower in cytosolic fractions of PD substantia nigra when compared to controls (Fig. 2A). We also observed a drastic decrease in USP9X levels in cytosolic fractions of DLBD frontal cortex (Fig. 2B). This was associated to a decrease in the deubiquitinase activity in DLBD lysates toward previously monoubiquitinated α-synuclein (Fig. 2 C and D). The data indicate that dysfunctional deubiquitinase activity may be important to the pathogenesis of α-synucleinopathies and that the decrease of USP9X levels could account for the reduced deubiquitinase activity we observe in the disease brains.
Fig. 2.
α-Synucleinopathies brains have lower levels of cytosolic USP9X and lower deubiquitinase activity. (A and B) Homogenates prepared from PD substantia nigra (A) and from DLBD frontal cortex (B) were probed with anti-USP9X antibody. (C) His-α-synuclein was monoubiquitinated in vitro and further incubated for 30 min in the presence of frontal cortex homogenates (5 to 15 μg) of DLBD and control cases. Levels of His-α-synuclein monoubiquitination were determined with anti-α-synuclein antibody. Each of the panels represents a different control and DLBD case, equally processed and analyzed. The three lower panels depict the levels of α-synuclein under a short exposure. (D) Percent of remaining α-synuclein monoubiquitination after the addition of control and DLBD homogenates. **, Significantly different from control at p < 0.01; mean ± SEM of four experiments.
USP9X Regulates the Formation of Toxic α-Synuclein Inclusions.
We and others recently reported that inhibition of proteolytic pathways promotes the accumulation of monoubiquitinated α-synuclein, leading to aggregation and formation of α-synuclein inclusions in cells (10, 11). However, α-synuclein monoubiquitination was not thought to be under dynamic regulation. We now investigated the effect of USP9X in the formation of monoubiquitinated α-synuclein inclusions. We carried out immunocytochemistry experiments in SH-SY5Y cells, in the presence of proteolytic inhibition, and found that USP9X decreased the amount of α-synuclein inclusions (Fig. 3A), whereas the knockdown of endogenous USP9X expression by siRNA increased the amount of α-synuclein inclusions (Fig. 3B). The data indicate that monoubiquitination and deubiquitination processes are major determinants of α-synuclein inclusion formation. This also highlights the dynamic nature of the inclusion formation that is regulated by USP9X.
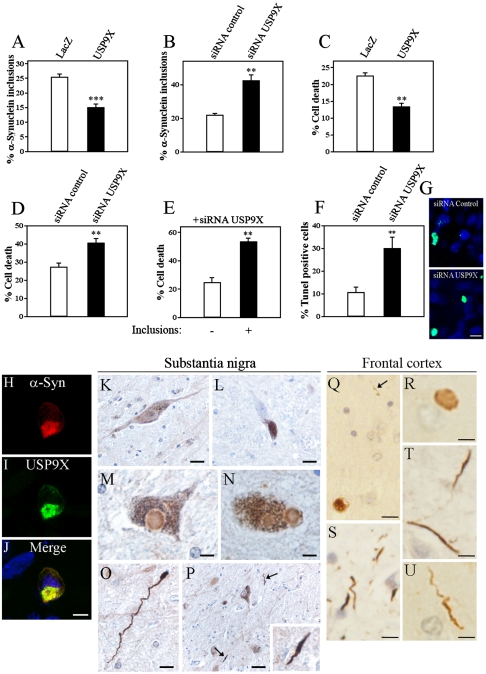
Fig. 3.
USP9X regulates α-synuclein inclusion formation and toxicity, and is present in Lewy bodies and Lewy neurites. (A) Percent of α-synuclein inclusions in SH-SY5Y cells transfected with HA-α-synuclein, myc-SIAH-2, and myc-USP9X or myc-LacZ. Cells were treated for 16 h with 10 μM Lactacystin, 25 mM NH4Cl and 10 mM 3-MA, and immunocytochemistry was carried out with anti-HA antibody. (B) Percent of α-synuclein inclusions in SH-SY5Y cells transfected with HA-α-synuclein, myc-SIAH-2, and siRNA control or siRNA to USP9X. Cells were treated and processed as in A. (C) Percent of cell death by Hoechst 33342 in SH-SY5Y cells transfected and treated as in A. (D) Percent of cell death by Hoechst 33342 in SH-SY5Y cells transfected and treated as in B. (E) Comparison of cell death by Hoechst 33342 between transfected cells containing inclusions with those lacking inclusions. SH-SY5Y cells were transfected and treated as in B. (F and G) Percent (F) and depicting image (G) of Tunel labeling in SH-SY5Y cells transfected and treated as in B. (H–J) SH-SY5Y cells were transfected with HA-α-synuclein, myc-SIAH-2, Flag-USP9X, and treated as in A. Immunocytochemistry was carried out with anti-HA (red, H) and anti-myc antibodies (green, I). Scale bar, 25 μm. (K–P) Immunohistochemistry of substantia nigra of control (K and L) and PD patients (M–P) using anti-USP9X antibody. K depicts the widespread distribution of USP9X in a dopaminergic neuron while L confirms the specificity of anti-USP9X antibody as shown by the lack of immunoreactivity when preabsorbed with antigen. Brown grains represent remaining neuromelanin (L). The presence of USP9X-immunostaining in Lewy bodies is depicted in M and N. Strong USP9X immunoreactivity in Lewy neurites is shown in O and P. Arrows and inset in P depict Lewy neurites. (Q–U) Immunohistochemistry of frontal cortex of DLBD patients using anti-USP9X antibody. The presence of USP9X-immunostaining in cortical Lewy bodies is depicted in Q and R. USP9X immunoreactivity in Lewy neurites is shown in Q (arrow), S, T and U. K–U sections were counterstained with hematoxylin. Scale bars, 30 μm in P; 20 μm in K, L; 10 μm in O, Q; 5 μm in M, N; 2 μm in P inset and R–U. **, Significantly different from control at p < 0.02; ***, p < 0.002. The values in A–F correspond to the mean ± SEM of 3–4 experiments.
We subsequently tested whether changing the levels of α-synuclein monoubiquitination by altering the levels of USP9X affect α-synuclein toxicity. In conditions of proteolytic inhibition, we found that analogous to the decrease in the formation of α-synuclein inclusions (Fig. 3A), USP9X decreased α-synuclein toxicity (Fig. 3C). In addition, knocking down endogenous USP9X expression significantly increased α-synuclein toxicity (Fig. 3 D, F, and G), which paralleled the increase in α-synuclein inclusion formation (Fig. 3B). This suggests that, when proteolysis is prevented, an increase in α-synuclein monoubiquitination by means of USP9X knockdown promotes the formation of toxic α-synuclein inclusions. We further analyzed cells transfected with siRNA to USP9X and observed that cells containing α-synuclein inclusions had higher rates of death when compared to cells devoid of inclusions (Fig. 3E). No significant cell death was observed when USP9X was suppressed in SH-SY5Y cells in the absence of α-synuclein (Fig. S1A). Thus, the increase in α-synuclein toxicity is not due to the depletion of USP9X per se but rather to higher levels of monoubiquitinated α-synuclein and inclusions. Moreover, overexpression of α-synuclein did not change the endogenous levels of USP9X (Fig. S1B). Taken together, these results support the toxic role of α-synuclein inclusions in conditions of increased monoubiquitination and proteolytic inhibition.
Interestingly, USP9X colocalizes with α-synuclein inclusions that were still formed (Fig. 3 H–J). Accordingly, immunohistochemistry experiments of PD and DLBD brain sections revealed that USP9X is present in Lewy bodies and Lewy neurites of midbrain and cortical sections (Fig. 3 M–U). USP9X immunoreactivity was particularly robust in Lewy neurites, both in substantia nigra of PD as well as in frontal cortex of DLBD cases (Fig. 3 O, P, and S–U), indicating that USP9X may coaggregate with α-synuclein, especially in less organized structures like Lewy neurites.
α-Synuclein Fate Is Determined by its Monoubiquitination Levels.
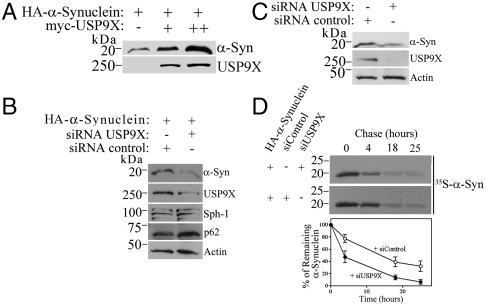
We next investigated how USP9X affects the biology of α-synuclein in the absence of proteolytic inhibition. By analyzing the effects of USP9X on α-synuclein in SH-SY5Y cells, we found that the expression of USP9X robustly increased the steady-state levels of α-synuclein (Fig. 4A). Conversely, knockdown of endogenous USP9X expression by siRNA led to a significant decrease in the steady-state levels of both overexpressed (Fig. 4B) and endogenous α-synuclein (Fig. 4C). In pulse-chase analysis, we observed that knockdown of USP9X significantly shortened the half-life of α-synuclein (Fig. 4D), indicating that lowering the levels of USP9X accelerates α-synuclein degradation. Taken together, the data indicate that monoubiquitination promotes α-synuclein degradation.
Fig. 4.
USP9X regulates the degradation rate of α-synuclein. (A and B) SH-SY5Y cells were transfected and HA-α-Synuclein was detected from total cell lysates using anti-HA antibody. USP9X was revealed with anti-myc antibody. In B, endogenous synphilin-1 and p62 were determined using specific antibodies. (C) SH-SY5Y cells were transfected only with siRNA control or siRNA to USP9X. Endogenous α-synuclein and USP9X from total cell lysates were detected using anti-α-synuclein and anti-USP9X antibodies. (D) SH-SY5Y cells were transfected, labeled, chased, and α-synuclein was immunoprecipitated with anti-HA. Graph shows the quantification of remaining [35S]HA-α-synuclein, in the presence of siRNA to USP9X (●) or control (○) at indicated time points. Mean ± SEM of three experiments.
In order to rule out the possibility that the USP9X-modulated degradation of α-synuclein is due to an unrelated effect of USP9X on the proteasomal or autophagic pathway efficiencies, we determined if USP9X affects the steady-state levels of the proteasome substrate synphilin-1 (9) and the bona fide autophagy substrate p62 (15). In cells transfected with siRNA to USP9X, we found that the levels of synphilin-1 and p62 were not significantly changed relative to the actin levels (Fig. 4B). Therefore, the effects of USP9X on the degradation of α-synuclein are not due to global changes in the proteasomal and autophagic activities but rather reflect its deubiquitination activity toward α-synuclein.
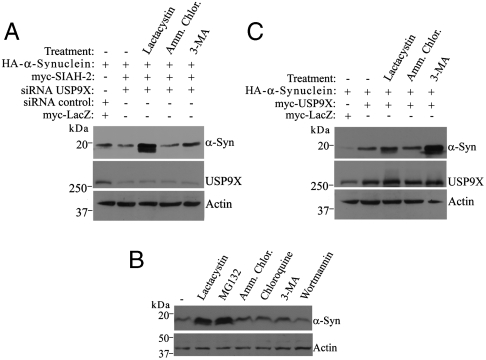
α-Synuclein is degraded by different pathways that include the autophagy, the lysosomal, and the proteasomal system (10, 16). It is not known, however, how the partition of α-synuclein between these pathways is regulated. Therefore, we next determined the pathway(s) responsible to degrade monoubiquitinated α-synuclein. We carried out experiments in SH-SY5Y cells transfected with siRNA to USP9X to maximize the monoubiquitination of α-synuclein by preventing its deubiquitination and treated cells with inhibitors of the different proteolytic pathways. We observed a decrease in the steady-state levels of α-synuclein promoted by USP9X knockdown (Fig. 5A; compare lanes 1 and 2). Under this condition, lactacystin promoted the largest accumulation of α-synuclein (Fig. 5A, lane 3) while inhibition of autophagy by 3-MA had only a small effect (Fig. 5A, lane 5). This indicates that monoubiquitinated α-synuclein species are preferentially targeted to the proteasome for degradation. Likewise, the proteasome inhibitor MG132, but not inhibitors of other proteolytic pathways, prevented the degradation of α-synuclein when its monoubiquitination is maximal by means of USP9X knockdown (Fig. 5B), strengthening the notion that monoubiquitinated α-synuclein is preferentially targeted to the proteasome. Overexpression of USP9X promoted a large increase in α-synuclein steady-state levels (Fig. 5C; compare lanes 1 and 2). However, a different pattern of sensitivity to proteolytic inhibitors was observed when USP9X was overexpressed to deubiquitinate α-synuclein (Fig. 5C). Under this condition, the autophagy inhibitor 3-MA promoted a marked increase in the steady-state levels of α-synuclein (Fig. 5C, lane 5), while proteasome inhibition had much less effect (Fig. 5C, lane 3). This indicates that deubiquitinated α-synuclein is preferentially degraded by the autophagy pathway.
Fig. 5.
Monoubiquitination partitions α-synuclein between different proteolytic pathways. (A) SH-SY5Y cells were transfected and treated for 16 h with DMSO, 10 μM Lacatacystin, 25 mM NH4Cl, or 10 mM 3-MA. HA-α-Synuclein and USP9X from total cell lysates were detected using anti-HA antibody and anti-USP9X antibody. (B) SH-SY5Y cells were transfected with HA-α-synuclein, myc-SIAH-2 and siRNA to USP9X. Cells were treated for 16 h with DMSO, 10 μM lactacystin, 30 μM MG132, 25 mM NH4Cl, 50 μM chloroquine, 10 mM 3-MA or 100 nM wortmannin. HA-α-synuclein from total cell lysates was detected using anti-HA antibody. (C) Transfected SH-SY5Y cells were treated and analyzed as in A.
Monoubiquitinated α-Synuclein Is Degraded by the 26S Proteasome.
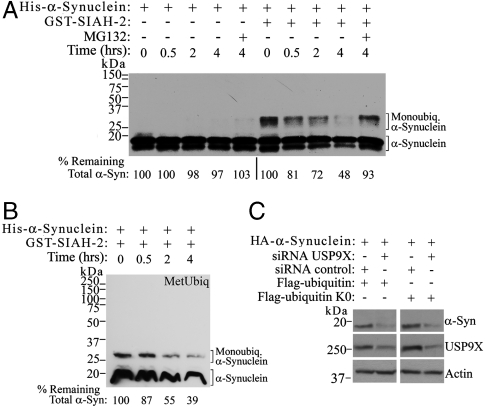
In order to confirm the role of monoubiquitination in the proteasomal degradation of α-synuclein, we employed direct in vitro experiments. We incubated α-synuclein monoubiquitinated in vitro by SIAH with purified 26S proteasomes and found that monoubiquitinated α-synuclein was significantly degraded by the 26S proteasome (Fig. 6A). In contrast, no degradation by 26S of α-synuclein was observed in the absence of SIAH (Fig. 6A). We did not observe any polyubiquitination of α-synuclein by SIAH, even after very long exposures. In addition, 26S proteasome was still able to significantly degrade monoubiquitinated α-synuclein even if the reaction was carried out with methylated ubiquitin instead of ubiquitin (Fig. 6B). Because methylated ubiquitin prevents the formation of polyubiquitin chains (17), it further supports the finding that monoubiquitination is sufficient for α-synuclein degradation.
Fig. 6.
Monoubiquitinated α-synuclein is required for proteasomal degradation. (A) His-α-synuclein was incubated with E1, UbcH5b, ubiquitin, in the absence or in the presence of recombinant SIAH-2. Subsequently, 26S proteasome was added to the reaction mixtures for the indicated times. Levels of remaining α-synuclein were determined using anti-α-synuclein antibody and quantified by the chemiluminescent signal. (B) His-α-synuclein was in vitro monoubiquitinated in the presence of SIAH-2 and methylated ubiquitin. Subsequently, 26S proteasome was added to the reaction mixtures and levels of remaining α-synuclein were determined as in A. (C) SH-SY5Y cells were transfected and HA-α-synuclein from total cell lysates was detected using anti-HA antibody. The presence of USP9X was monitored with anti-USP9X antibody.
We monitored the rate of α-synuclein degradation using CHIP, an E3 ubiquitin-ligase that also ubiquitinates α-synuclein (18). Under our conditions, we found that ubiquitination by CHIP was much less efficient than SIAH in promoting the degradation of α-synuclein by the 26S proteasome (Fig. S2).
Cells expressing lysineless ubiquitin, which reduces polyubiquitination levels, display increased degradation of α-synuclein upon USP9X knockdown (Fig. 6C). Thus, data obtained both in vitro and in cell systems indicate that monoubiquitination is sufficient for the proteasomal degradation of α-synuclein.
Discussion
We found that α-synuclein interacts with the deubiquitinase USP9X in vivo and in vitro. Knockdown of USP9X leads to less deubiquitinase activity toward α-synuclein both in in vitro assays and SH-SY5Y cells, supporting the idea that USP9X is the endogenous deubiquitinase of α-synuclein. USP9X regulates the partition of α-synuclein between the proteasomal and autophagy compartments, favoring the latter. By suppressing endogenous USP9X expression, we found that monoubiquitination leads to α-synuclein degradation, which was prevented mainly by proteasomal inhibition, indicating that monoubiquitination targets α-synuclein for degradation by the proteasome. In agreement, in vitro monoubiquitinated α-synuclein was significantly degraded by purified 26S proteasome. Conversely, its deubiquitination shifts the preferred degradation pathway of α-synuclein and targets it to the autophagic pathway. Our data indicate that USP9X dynamically regulates α-synuclein monoubiquitination and specifies its degradation pathway.
We observed a drastic decrease in the levels of USP9X in cytosolic extracts of DLBD cortex and PD substantia nigra. The deubiquitinase activity toward previously monoubiquitinated α-synuclein is about 70% lower in DLBD cortex, which is compatible with lower levels of USP9X in the disease. USP9X is present in Lewy bodies and Lewy neurites in both PD and DLBD brains, indicating that USP9X aggregates along with α-synuclein, which may contribute to its inactivation. These findings provide a mechanistic rationale for accumulation and aggregation of monoubiquitinated α-synuclein in PD and DLBD. It is noteworthy that inhibition of USP9X by the small-molecule WP1130 leads to the accumulation of ubiquitinated proteins and the formation of aggresomes in cells (19). Thus, it is possible that decreased USP9X activity may be important to the pathogenesis of α-synucleinopathies and may contribute to the accumulation of monoubiquitinated α-synuclein and its aggregation.
The mechanisms that trigger the formation of α-synuclein inclusions and whether they are toxic to cells are still unclear. We found that under proteolytic inhibition, monoubiquitination by SIAH promotes the formation of α-synuclein inclusions that are toxic to cells (10, 11). We now found that the formation of α-synuclein inclusions is regulated by deubiquitination because altering USP9X expression levels further modulates this process. USP9X knockdown increases α-synuclein monoubiquitination levels and promotes the formation of α-synuclein inclusions. This indicates that USP9X is the endogenous deubiquitinase of α-synuclein, and also supports the notion that monoubiquitination facilitates α-synuclein aggregation when its degradation is impaired by proteasomal inhibition.
Higher levels of α-synuclein monoubiquitination observed upon USP9X knockdown were correlated with increased cell toxicity in conditions where proteolysis was prevented. This is in agreement with recent data using cultured cells and in vivo models where α-synuclein inclusions were shown to be toxic to cells (20, 21). Nevertheless, the data do not exclude the possibility that the apparent inclusion toxicity may be caused by formation of toxic α-synuclein oligomers as well.
Although different proteolytic pathways are known to process α-synuclein (10, 16), it has been unclear how α-synuclein degradation is regulated. We found that, in the absence of proteolytic impairment, knockdown of USP9X expression increases α-synuclein monoubiquitination and leads to a decrease in its steady-state levels and half-life. This supports the idea that monoubiquitination promotes the degradation of α-synuclein and that deubiquitination by USP9X counteracts this process. Moreover, in conditions of USP9X knockdown, the degradation of monoubiquitinated α-synuclein was prevented by proteasome inhibitors, indicating that monoubiquitination targets α-synuclein for proteasomal degradation. This finding is in line with several reports showing the accumulation of ubiquitinated inclusions positive for α-synuclein in the brain and the appearance of PD phenotype in mice mutated for proteasome subunits or injected with proteasome inhibitors (22, 23).
Monoubiquitinated proteins are generally degraded by the lysosomal pathway (24). However, we found that monoubiquitinated α-synuclein is preferentially targeted for degradation by the proteasome. This finding seems to be an exception to the rule, because proteasomal degradation is thought to require polyubiquitination composed of at least four ubiquitin chains (25). We have previously shown that the ubiquitination pattern of α-synuclein is identical with wild-type or lysineless ubiquitin, supporting the occurrence of monoubiquitin modifications rather than polyubiquitination. Accordingly, mass spectrometry analysis demonstrated at least seven monoubiquitination sites at α-synuclein (10). The data are consistent with the existence of multiple monoubiquitin modifications of α-synuclein. In this framework, it is conceivable that the attachment of multiple monoubiquitin chains to α-synuclein is sufficient for its proteasomal degradation. Monoubiquitination-driven proteasomal degradation has been also reported for phospholipase D1 and Pax3 (26, 27). The finding that monoubiquitination can target α-synuclein to the proteasome constitutes a previously undescribed mechanism and has implications for the accumulation of α-synuclein in PD.
A previous report has shown that purified proteasomal particles support the degradation of nonubiquitinated α-synuclein (28). In contrast, we found that α-synuclein can only be degraded by the 26S proteasome when it is monoubiquitinated by SIAH. The reason for this discrepancy is not clear.
α-Synuclein is also degraded by macroautophagy, referred in this work as autophagy. While autophagy inhibitors, such as 3-MA, promote the accumulation of α-synuclein (10, 16), activators of this pathway, such as rapamycin, decrease α-synuclein levels (16). We now demonstrated that the degradation of α-synuclein by autophagy is negatively modulated by its monoubiquitination levels. Deubiquitination of α-synuclein by USP9X leads to a marked increase in the sensitivity to 3-MA, indicating that deubiquitinated α-synuclein is targeted preferentially for degradation by autophagy.
We also observed that deubiquitination by USP9X leads to accumulation of α-synuclein in the absence of proteolytic inhibition, suggesting that the degradation of α-synuclein by autophagy may be less efficient than the degradation of monoubiquitinated α-synuclein species by the proteasome. However, when the proteasomal activity is impaired, USP9X diminishes α-synuclein aggregation and toxicity by decreasing the accumulation of monoubiquitinated α-synuclein that has enhanced aggregatory activity. In addition, because proteasomal activity may be compromised in the substantia nigra of PD patients (29), activators of autophagy are promising candidates to prevent α-synuclein accumulation. Compounds that activate USP9X deubiquitinase activity or inhibit α-synuclein monoubiquitination will facilitate its autophagic degradation and might be useful to reduce α-synuclein levels along with general autophagy activators.
Materials and Methods
Further experimental details are provided in SI Text.
In Vitro Deubiquitination Assays.
In vitro monoubiquitination assays for α-synuclein were done as described in SI Text but in the absence of ubiquitin aldehyde. Reactions were incubated at 37 °C for 1 h, and cell lysates (5 μg) or USP9X-immunoprecipitates were added to the reactions and further incubated at 37 °C for 30 min to allow deubiquitination. Levels of ubiquitinated α-synuclein were determined by Western blot.
In Vitro Degradation Assays.
In vitro ubiquitination assays were carried out by incubating recombinant α-synuclein in reaction medium containing 40 mM Tris-HCl (pH 7.6), 5 mM MgCl2, 2 mM DTT, 1 mM ATP, 10 mM phosphocreatine, 0.1 mg/mL phosphocreatine kinase, 7.5 μg ubiquitin or methylated ubiquitin, 1 μM ubiquitin aldehyde, 100 ng E1 and 200 ng UbcH5b, in the presence or absence of 500 ng SIAH-2 or CHIP. After incubation at 37 °C for 1 h, 20 nM 26S proteasome (Boston Biochem) were added and further incubated for indicated times. Remaining ubiquitinated and nonubiquitinated α-synuclein wa detected using α-synuclein antibody.
Supplementary Material
Acknowledgments.
We are indebted to Dr. Ariel Stanhill (The B. Rappaport Faculty of Medicine, Israel) for helpful discussions and Prof. Kozo Kaibuchi (Nagoya University, Japan) for the USP9X cDNA. This work was supported by Israel Academy of Sciences, Ministry of Health, The B. Rappaport Institute Foundation, and the Technion Research funds to S.E.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105725108/-/DCSupplemental.
References
- 1.Engelender S. Ubiquitination of alpha-synuclein and autophagy in Parkinson’s disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- 2.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa M, et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 4.Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 7.Shimura H, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: Implications for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 8.Mulherkar SA, Sharma J, Jana NR. The ubiquitin ligase E6-AP promotes degradation of alpha-synuclein. J Neurochem. 2009;110:1955–1964. doi: 10.1111/j.1471-4159.2009.06293.x. [DOI] [PubMed] [Google Scholar]
- 9.Liani E, et al. Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:5500–5505. doi: 10.1073/pnas.0401081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rott R, et al. Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem. 2008;283:3316–3328. doi: 10.1074/jbc.M704809200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JT, Wheeler TC, Li L, Chin LS. Ubiquitination of alpha-synuclein by Siah-1 promotes alpha-synuclein aggregation and apoptotic cell death. Hum Mol Genet. 2008;17:906–917. doi: 10.1093/hmg/ddm363. [DOI] [PubMed] [Google Scholar]
- 12.Szargel R, et al. Synphilin-1A inhibits seven in absentia homolog (SIAH) and modulates alpha-synuclein monoubiquitylation and inclusion formation. J Biol Chem. 2009;284:11706–11716. doi: 10.1074/jbc.M805990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont S, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Region-specific protein abundance changes in the brain of MPTP-induced Parkinson’s disease mouse model. J Proteome Res. 2010;9:1496–1509. doi: 10.1021/pr901024z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorkoy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 17.Hershko A, Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985;128:1079–1086. doi: 10.1016/0006-291x(85)91050-2. [DOI] [PubMed] [Google Scholar]
- 18.Kalia LV, et al. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5) PLoS ONE. 2011;6:e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapuria V, et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 20.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedford L, et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008;28:8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie W, et al. Proteasome inhibition modeling nigral neuron degeneration in Parkinson’s disease. J Neurochem. 2010;115:188–199. doi: 10.1111/j.1471-4159.2010.06914.x. [DOI] [PubMed] [Google Scholar]
- 24.d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 25.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 26.Yin H, Gui Y, Du G, Frohman MA, Zheng XL. Dependence of phospholipase D1 multi-monoubiquitination on its enzymatic activity and palmitoylation. J Biol Chem. 2010;285:13580–13588. doi: 10.1074/jbc.M109.046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 28.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett. 2001;297:191–194. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.