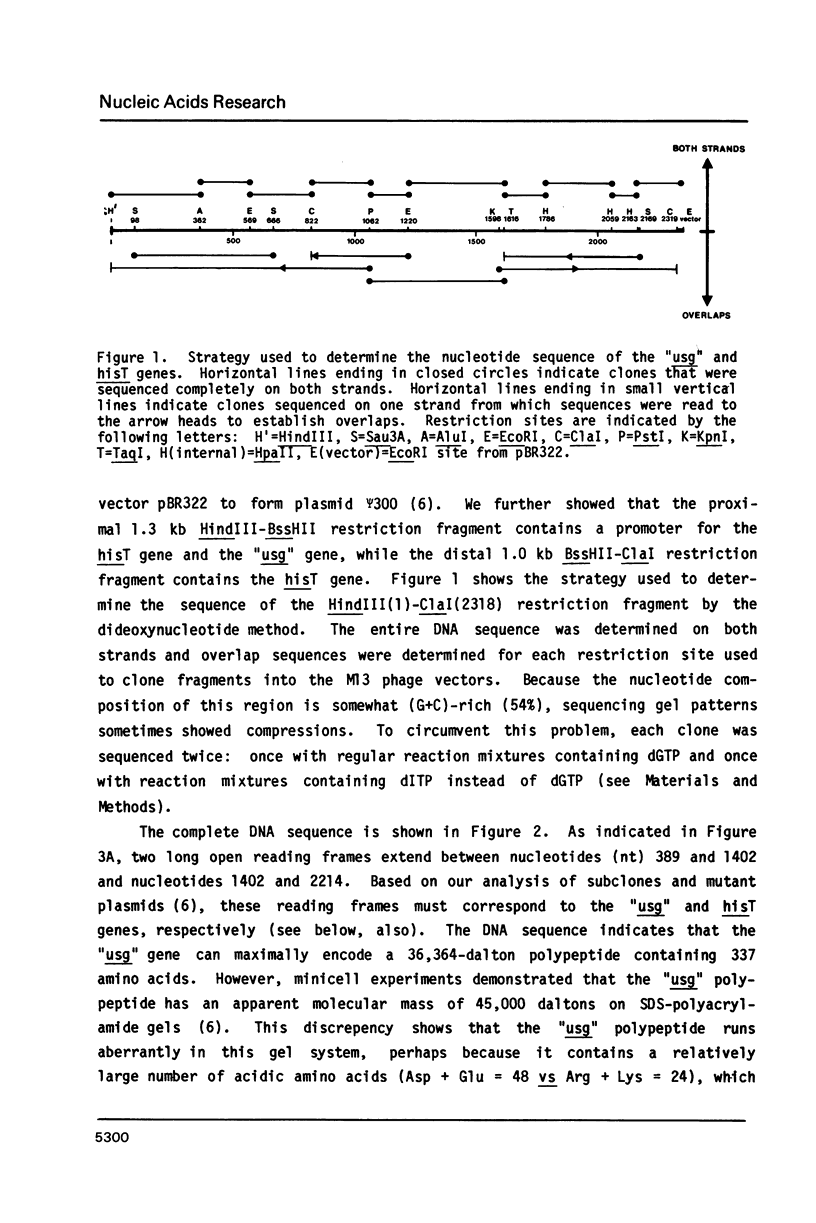
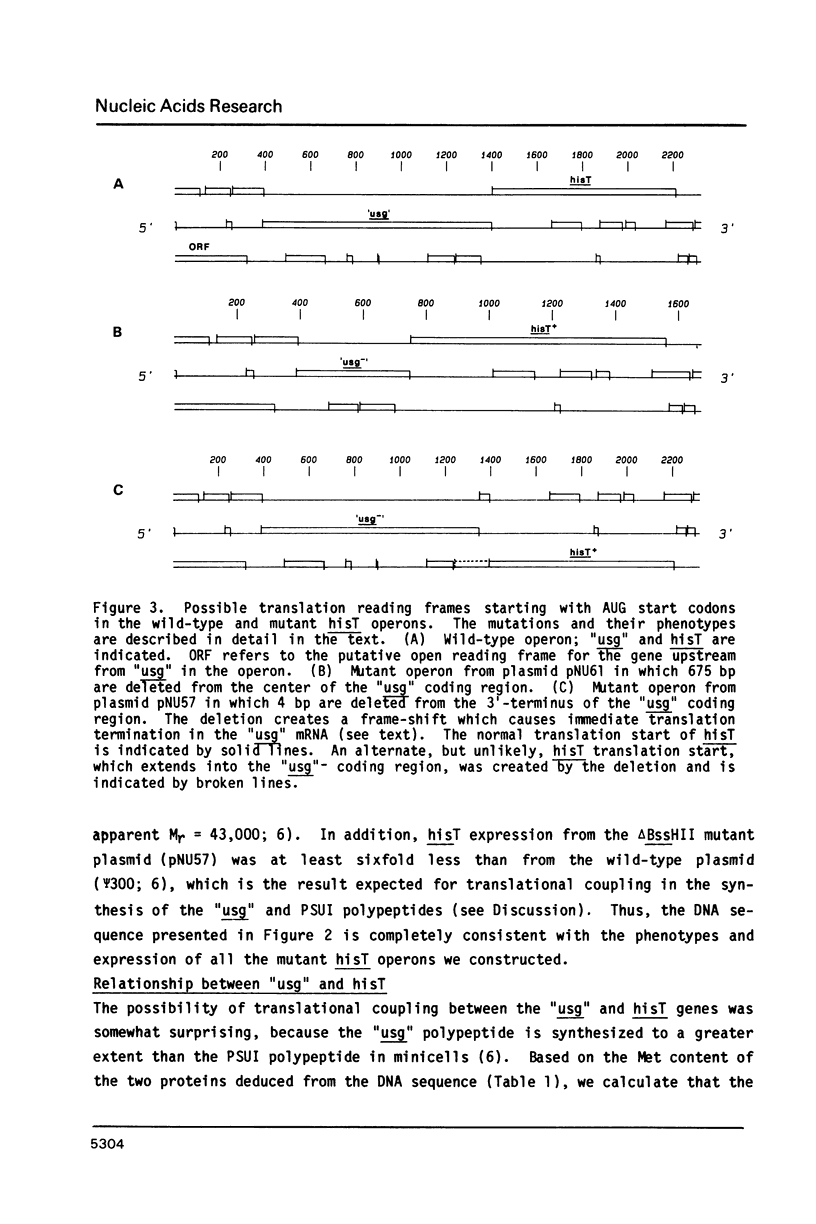
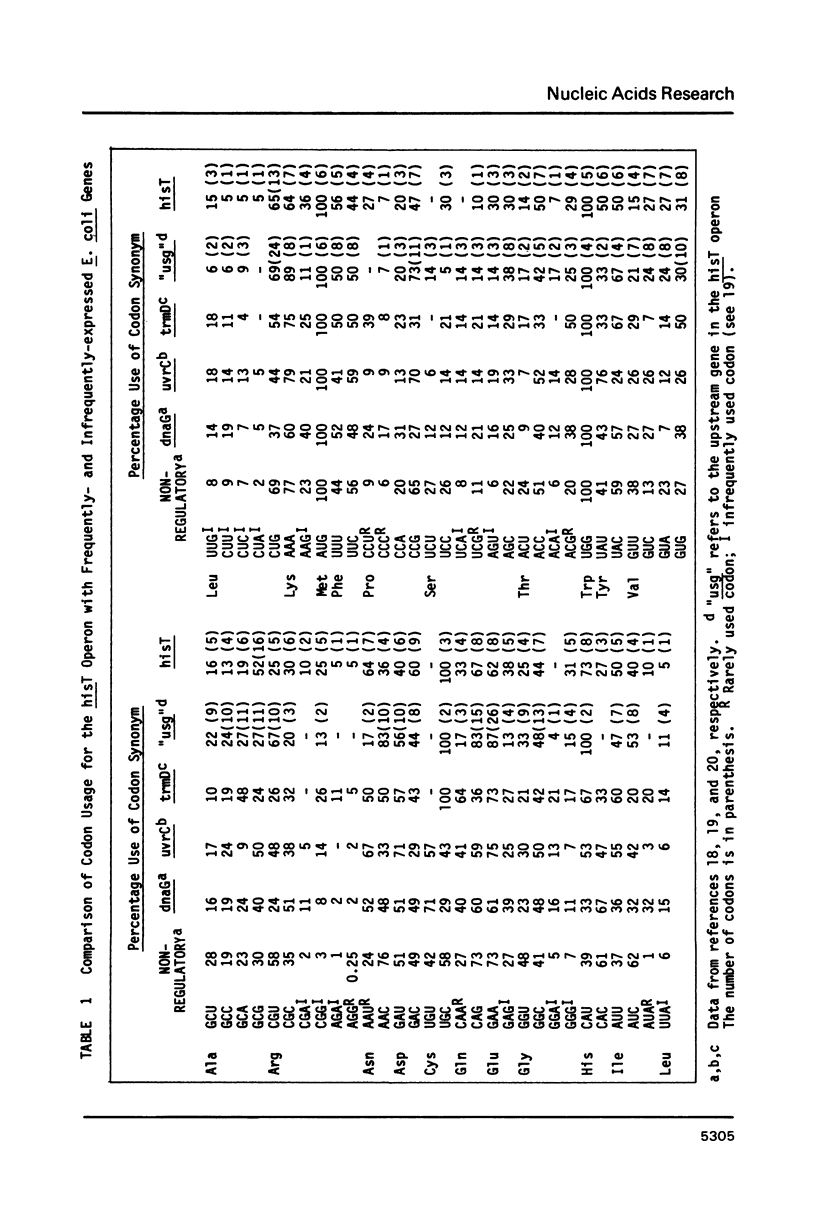
Abstract
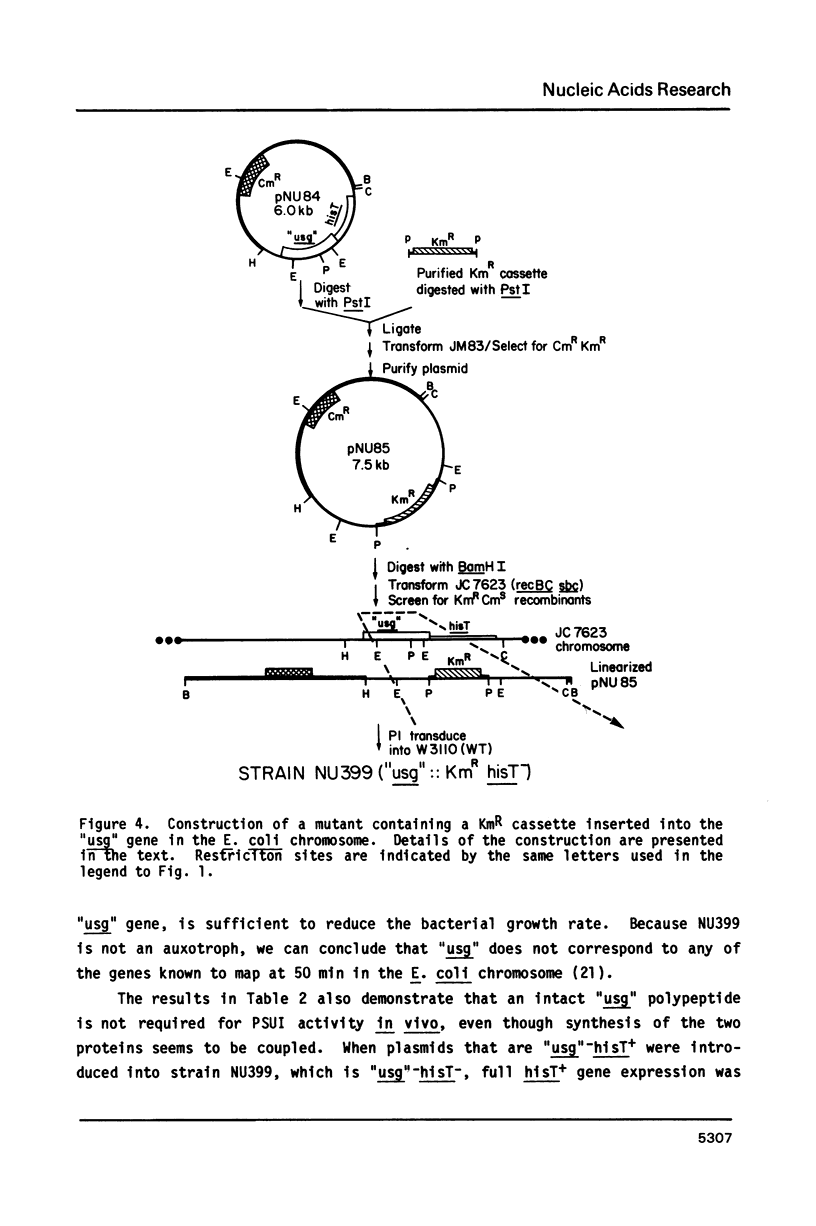
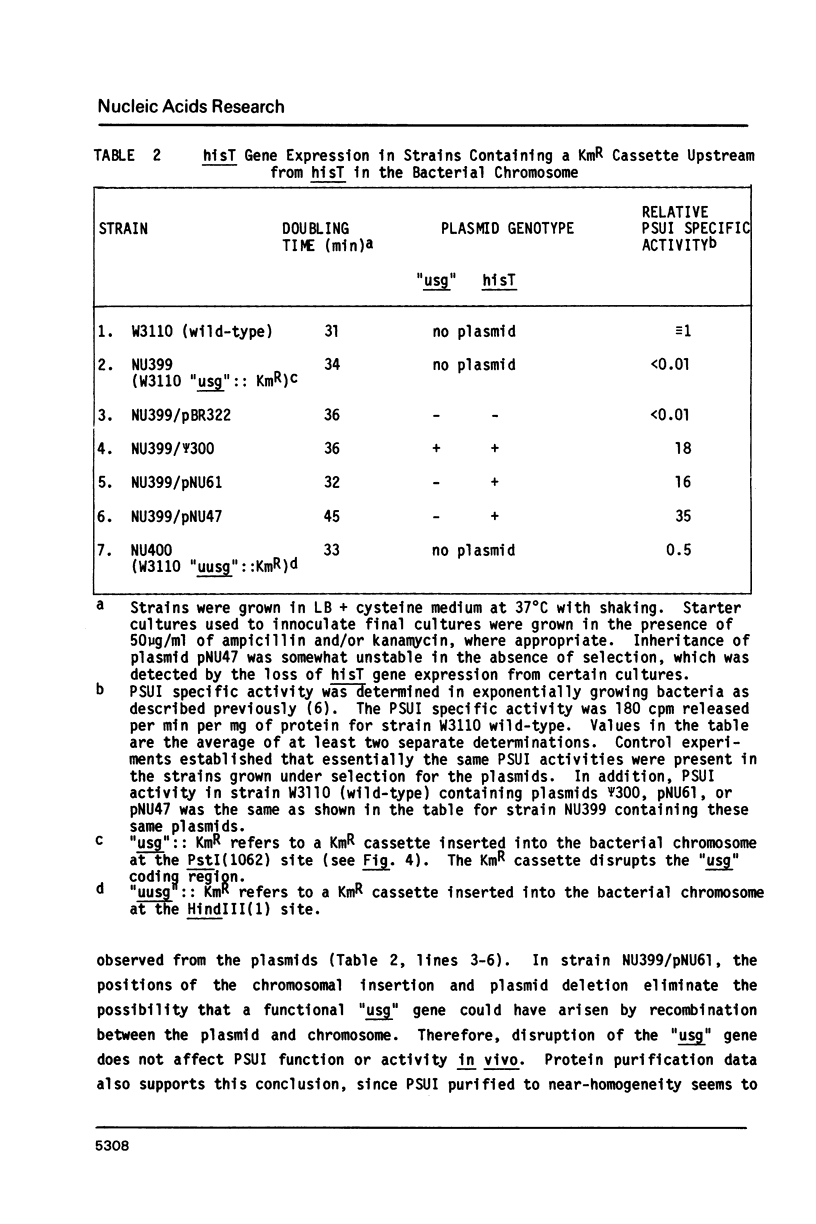
The DNA sequence of a 2,3-kilobase segment of the E. coli hisT operon was determined. Analysis of the sequence indicated that the upstream gene in the operon encodes a 36,364-dalton polypeptide, which runs aberrantly on SDS-polyacrylamide gels. The distal hisT gene encodes the tRNA modification enzyme, pseudouridine synthase I, which was shown to have a polypeptide molecular mass of 30,399 daltons. The DNA sequence was consistent with the phenotypes and hisT expression of mutant operons. Analysis of the sequence and genetic complementation experiments demonstrated that the upstream and hisT genes are evolutionarily, structurally, and functionally unrelated; however, translation signals for the two genes overlap, which is consistent with genetic evidence suggesting translational coupling. Codon usage in the upstream gene is radically different from the hisT gene and may underlie the differential expression observed from the operon. Gene-inactivation experiments and S1-mapping of in vivo transcripts indicated that the operon contains an additional upstream gene. S1-mapping experiments also confirmed the presence of an internal promoter, which might be stringently controlled. Taken together, these results show that the structure of the hisT operon is complex and suggest that the operon might be regulated at several levels.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell. 1985 Feb;40(2):319–326. doi: 10.1016/0092-8674(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Marvel C. C., Arps P. J., Rubin B. C., Kammen H. O., Penhoet E. E., Winkler M. E. hisT is part of a multigene operon in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):60–71. doi: 10.1128/jb.161.1.60-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwerth B. J., Lin V. K., Lewis J., Wang R. J. Lysine tRNA and cell division: a G1 cell cycle mutant is temperature sensitive for the modification of tRNA5Lys to tRNA4Lys. Nucleic Acids Res. 1984 Dec 11;12(23):9009–9023. doi: 10.1093/nar/12.23.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Karels M. J., Navre M., Schachman H. K. Genes encoding Escherichia coli aspartate transcarbamoylase: the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4020–4024. doi: 10.1073/pnas.79.13.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G. B., Sancar A., Rupp W. D. Sequences of the E. coli uvrC gene and protein. Nucleic Acids Res. 1984 Jun 11;12(11):4593–4608. doi: 10.1093/nar/12.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C. Processed pseudogenes for rat cytochrome c are preferentially derived from one of three alternate mRNAs. Mol Cell Biol. 1984 Nov;4(11):2279–2288. doi: 10.1128/mcb.4.11.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. E. Ribosomal ribonucleic acid isolated from Salmonella typhimurium: absence of the intact 23S species. J Bacteriol. 1979 Sep;139(3):842–849. doi: 10.1128/jb.139.3.842-849.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]