Abstract
Th17 cells have been implicated in the pathogenesis of colitis; however, a cellular mechanism by which colitogenic Th17 immunity arises in vivo remains unclear. In this study, we report that a subset of IL-17+γδ T cells plays a crucial role in enhancing in vivo Th17 differentiation and T cell-mediated colitis. TCRβ-/- mice were highly susceptible to T cell-mediated colitis, while TCRβδ-/- mice were resistant to the disease. Importantly, cotransfer of IL-17+ but not of IL-17-γδ T cells with CD4 T cells was sufficient to enhance Th17 differentiation and induce full-blown colitis in TCRβδ-/- recipients. Collectively, our results provide a novel function of IL-17+γδ T cell subsets in supporting in vivo Th17 differentiation and possibly in fostering the development of intestinal inflammation.
Keywords: colitis, γδ T cells, IL-17, Th17 CD4 T cells
Introduction
A mechanism underlying Th17 differentiation has been an area of extensive investigation. Factors involved in this process including cytokines and transcription factors have been identified (1); however, a cellular mechanism by which Th17 immunity arises in vivo still remains unclear.
γδ T cells are rare T cell subsets primarily found in epithelial cell-rich tissues and in lymphoid tissues (2). γδ T cells in naïve animals express activated phenotypes and produce effector cytokines upon stimulation (2). They have been the targets of recent investigation because of their spontaneous IL-17A (referred to as IL-17 hereafter) expression without a differentiation process (3, 4). We have recently reported that IL-17 acquisition in γδ T cells occurs in the thymus via a TGFβ1-dependent mechanism (5). It was demonstrated that γδ T cells amplify Th17 immunity, thus exacerbating autoimmune encephalomyelitis (6). However, whether γδ T cell mediated regulation also takes place in other Th17 associated inflammatory settings such as IBD remains unexplored.
Naïve CD4 T cells transferred into immunodeficient mice can differentiate into colitogenic effector cells in response to antigens derived from commensal microorganisms (7, 8). Effector CD4 T cells producing proinflammatory cytokines, especially IL-17, have been implicated to play a key role in the pathogenesis of colitis and the inflammatory bowel disease (IBD) (9-11). A cellular mechanism involved in the generation of colitogenic Th17 T cells in vivo is not clear.
In this study, we report that γδ T cells support in vivo Th17 differentiation and the subsequent development of colitis induced by T cell transfer into immunodeficient animals. Interestingly, γδ T cell IL-17 production appears to be associated with Th17 differentiation and colitogenicity of CD4 T cells. Our results suggest that IL-17+ γδ T cells may represent potential targets to intervene inflammatory disorders in the intestine seen in patients with IBD.
Materials and Methods
Mice
C57BL/6, B6 Ly5.1, B6 Thy1.1, B6 TCRβ-/-, B6 Rag1-/- mice were purchased from the Jackson Laboratory (Bar Harbor, ME). IL-17F-RFP Tg mice were previously described (12). IFNγ-YFP Yeti mice (13) were kindly provided from Dr. Markus Mohrs (Trudeau Institute). TCRβδ-/- mice were bred at the animal facility of the Lerner Research Institute. All animal procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee.
Cell sorting and adoptive transfer
Lymph node naive CD4 T cells were obtained as previously reported (14). CD25neg CD44low naive T cells were further sorted using a FACSAria cell sorter (BD Bioscience, San Jose, CA). 2.5 × 105 naive T cells were transferred alone or in combination with sorted γδ T cells to TCRβ-/- or TCRβ/δ-/- mice. After T cell transfer, mice were weighed weekly and monitored for signs of disease.
FACS Analysis
Cells were stained with anti-CD4 (RM4-5), anti-γδ TCR (GL3), anti-IL-17A (17B7), anti-IFN-γ (XMG1.2), anti-Thy1.1 (HIS51), anti-CD45.1 (A20) (all Abs from eBioscience) and anti-CCR6 (140706, BD Pharmingen, San Diego, CA). Cells were acquired using a FACSCalibur or LSR II (BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, OR).
Ex vivo stimulation
Tissue cells were ex vivo stimulated with PMA (10ng/ml) and Ionomycin (1μM) for 4 hrs in the presence of 2 μM monensin (Calbiochem, San Diego, CA) during the last 2 hrs. Cells were immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence conjugated antibodies. Isotype matched control Ab was used for a staining control.
Lamina Propria cell Isolation
Recipients were sacrificed at the indicated time points after T-cell transfer. Colons were isolated and cleaned in HBSS. Colons were cut into small pieces and resuspended in HBSS with 0.5μM EDTA and 15μg/mL Dithiothreitol (DTT) and shaken for 15 min twice at room temperature. Colons were then resuspended in cRPMI with 400μg/mL DNase I (Roche) and 1mg/mL collagenase D (Roche) and shaken at 37°C for 90 min. Supernatant and colon was passed through a 70μM strainer and washed. Cells were resuspended in a 33% Percoll gradient and spun at room temperature for 20 min. Pellet was collected, washed and used for further experiments.
Real time PCR
Colon tissue was disrupted using a TissueLyser II (Qiagen, Valencia, CA). Total RNA was extracted using an RNeasy column (Qiagen). cDNA was subsequently obtained using a SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). Real time PCR was performed using gene specific primers and probe sets (Applied Biosystem, Foster City, CA) and ABI 7500 PCR machine (Applied Biosystem).
Histology
For H&E staining colon tissue was fixed in 10% Acetic Acid/60% Methanol. Slides were stained with hematoxylin and eosin. Colon tissues were scored in a blinded fashion as previously reported (15). For immunohistochemistry paraffin embedded colon sections were incubated overnight at 4°C in primary anti-CD3 (ab5690, Abcam, Cambridge, MA) followed by secondary biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Vectastain Elite ABC Kit and DAB (Vector Laboratories, Burlingame, CA) were used for color development, and processed for mounting using Clarifier I, Bluing Solution, Clear-Rite 3 and Mounting Media (all Richard-Allan, Kalamazoo, MI).
Statistical analysis
Results represent the mean ± SD. Statistical significance was determined by the Student's t-test (unpaired two-tailed) using the Prism 4 software (GraphPad, La Jolla, CA). A p value of <0.05 was considered statistically significant.
Results and Discussion
TCRβ-/- mice are highly susceptible but TCRβδ-/- mice are fully resistant to CD4-mediated colitis
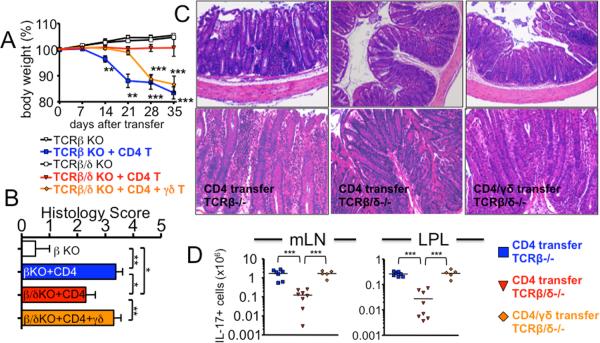
To examine a role of γδ T cells in a CD4-mediated colitis model, naïve CD4 cells were transferred into TCRβ-/- and TCRβδ-/- mice. TCRβ-/- recipients developed severe weight loss with a massive cellular infiltration in the colon tissues, whereas TCRβδ-/- recipients displayed no sign of weight loss only with mild inflammation in the colon (Fig. 1A-1C). Consistent with the disease severity and histopathology, donor T cell accumulation in the lymphoid and gut tissues was markedly elevated in TCRβ-/- compared to TCRβδ-/- mice (data not shown). A dramatic increase in CD4 T cells that produce IL-17 was noticed in TCRβ-/- recipients (Fig. 1D and S1). Moreover, CD4 T cells producing IL-17F were also higher in TCRβ-/- than in TCRβδ-/- recipients (data not shown). Although the proportion of IFNγ+ CD4 T cells was found to be very high in resistant TCRβδ-/- mice (Fig. S1), the absolute numbers of IFNγ-producing CD4 T cells was much higher in colitic TCRβ-/- mice (data not shown). Therefore, severe colitis seen in TCRβ-/- mice is associated with enhanced Th17 differentiation as reported in IBD patients (16), strongly suggesting that the presence of γδ T cells in TCRβ-/- mice may promote Th17 differentiation and colitogenicity of CD4 T cells. Of note, T cell-deficient mice, particularly TCRβ-/-, are known to spontaneously develop colitis starting at ~4 months of age (17). However, both strains of mice used in these experiments were ~2 months of age and continuously gained weight without T cell transfer (Fig. 1A), demonstrating that the spontaneous colitis is not involved.
Figure 1. TCRβ-/- recipients of naïve CD4 T cells develop exacerbated colitis while TCRβδ-/- recipients are fully resistant to the colitis, and adoptive transfer of γδ T cells restores colitis in TCRβδ-/- recipients.
(A) FACS purified CD4+CD25-CD44low Thy1.1 naïve T cells (2.5 × 105) were transferred into TCRβ-/- or TCRβδ-/- mice. In some experiments, FACS sorted γδ T cells (2.5 × 105) were cotransferred with CD4 T cells into TCRβδ-/- mice. Body weight was weekly monitored and presented as a percentage of the initial weight at day 0. ** p<0.01; *** p <0.001. Data shown are from 5-8 individually tested mice. (B) Colitis score of each group. (C) Representative H&E staining of colon 5 weeks post transfer. 20× original magnification. (D) Total donor cell recovery of IL-17+ cells were calculated. Each symbol represents individually tested mouse. *** p<0.001.
γδ T cell cotransfer renders TCRβδ-/- recipients susceptible to colitis
To directly test if γδ T cells are responsible for the enhanced pathology seen in TCRβ-/- mice, purified γδ T cells were cotransferred with CD4 T cells into TCRβδ-/- recipients. γδ T cell cotransfer rendered resistant TCRβδ-/- mice highly susceptible to colitis, thus the recipients developed a weight loss (Fig. 1A) and severe colitis (Fig. 1B and 1C). Donor CD4 T cell accumulation in the lymphoid and gut tissues was dramatically elevated to a level seen in TCRβ-/- recipients (data not shown). Furthermore, CD4 T cells producing IL-17 significantly increased in all tested tissues after γδ T cell cotransfer (Fig. 1D and S1), strongly supporting the hypothesis that Th17 differentiation and colitogenicity of CD4 T cells is greatly enhanced by γδ T cells in vivo. γδ T cell transfer alone without CD4 T cells did not result in any noticeable weight loss (data not shown), further supporting the notion that γδ T cells amplify Th17 differentiation, aggravating colitis. Notably, the onset of weight loss in TCRβ-/- recipients was substantially faster compared to that TCRβδ-/- mice receiving γδ T cells (Fig. 1A), which is probably attributed to high levels of γδ T cells generated in TCRβ-/- mice (17).
IL-17+ but not IL-17-γδ T cells promote T cell mediated colitis
To examine a mechanism underlying γδ T cell-mediated Th17 differentiation, we next measured γδ T cell cytokine production in TCRβ-/- mice before and after CD4 T cell transfer. Interestingly, γδ T cells that produce IL-17 significantly increased following T cell transfer (Fig. 2). The increase was specific for IL-17+ γδ T cell subsets since IFNγ+ γδ T cells remained unchanged. Moreover, a noticeable increase was observed even 2 weeks after transfer, suggesting that IL-17+ γδ T cells may play a role in altering the inflammatory responses elicited by T cells.
Figure 2. Increased IL-17+ γδ T cells after CD4 T cell transfer.
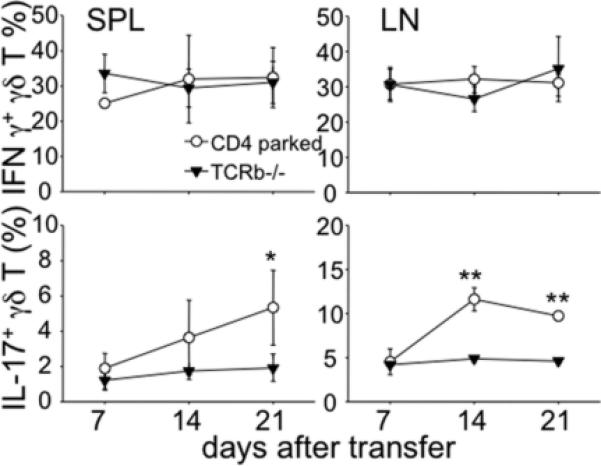
TCRβ-/- mice were transferred with naïve CD4 T cells as described above. Spleen and LN cells were harvested every week post transfer and ex vivo stimulated and γδ T cell cytokine expression was examined. The results shown represent the mean ± SD of 4-5 individually tested mice. * p<0.05; ** p<0.01.
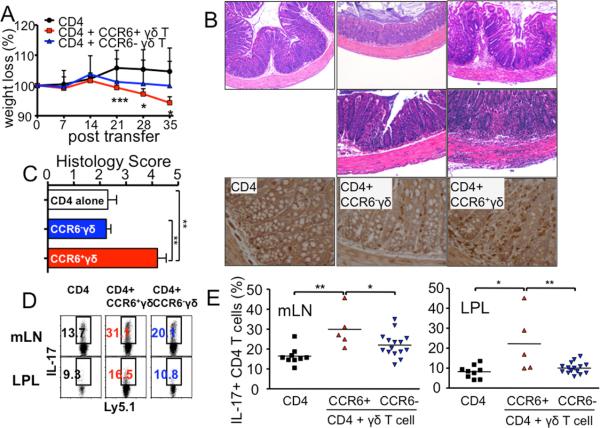
Phenotypic dichotomy associated with IFNγ- or IL-17-producing γδ subsets was recently reported: IFNγ-producing γδ T cells are CD27+ CCR6-, and IL-17-producing γδ T cells are CD27- CCR6+ (18, 19). When CCR6+ and CCR6- γδ T cells were sorted and examined for gene expression, the expression of il17a and il17f genes was primarily associated with CCR6+ γδ T cells, while ifng expression was primarily found in CCR6- γδ T cells (Fig. S2). To test if IL-17-producing and IFNγ-producing γδ T cells play different roles in T cell differentiation and colitis induction, FACS sorted CCR6+ or CCR6- γδ T cells were transferred into TCRβδ-/- recipients with CD4 T cells. Cotransfer of CCR6+ (i.e., IL-17+) γδ T cells resulted in a significant weight loss (Fig. 3A). By stark contrast, weight loss resulted from CCR6- γδ T cell cotransfer was relatively mild (Fig. 3A). Colitis and CD3+ cell infiltration was dramatically pronounced in mice that received CD4 plus CCR6+ γδ T cells compared to recipients of CD4 plus CCR6- γδ T cells or CD4 T cells alone (Fig. 3B and 3C). Notably, a difference in relative weight loss in CCR6+ γδ T cell recipients compared to the recipients of CD4 T cell alone was already noticeable 21 days post transfer (Fig. 3A). Moreover, the cellular infiltration in the colon of CCR6+ γδ T cell recipients was often extended into the outer muscular layer of the colon (Fig. 3B), suggesting more severe colitis after transfer of γδ T cells enriched for IL-17-producing cells. Importantly, both γδ T cell subsets were equally accumulated in the lamina propria of immunodeficient recipients when measured 5 weeks post transfer (data not shown), suggesting that enhanced susceptibility of CCR6+ γδ T cell recipients is not attributed to differential migration of γδ T cells in vivo. Consistent with colitis, IL-17 expression in CD4 T cells was significantly elevated in mice that received CCR6+ γδ compared to those received CCR6- γδ T cells (Fig. 3D and 3E), while IFNγ expression was comparable in all tested groups (data not shown). Moreover, the absolute numbers of IL-17+ CD4 T cells were significantly greater in CD4/CCR6+ γδ T cell recipients than those in CD4/CCR6- γδ T cell or CD4 T cell recipients (data not shown). Therefore, the presence of IL-17+ γδ T cells greatly enhance Th17 differentiation and subsequent development of T cell-mediated colitis.
Figure 3. CCR6+ γδ T cells support Th17 differentiation and colitogenicity of CD4 T cells in vivo.
CD4+CD25-CD44low Ly5.1 naïve T cells (2.5 × 105) were transferred into TCRβδ-/- recipients with sorted CCR6- γδ T cells or CCR6+ γδ T cells (2.5 × 105). (A) Weight was weekly monitored. * p<0.05; *** p<0.001 (compared with CD4 alone group). (B) Representative H&E staining and immunohistochemistry for CD3 staining of colon 5 weeks post transfer of CD4 alone, CD4/CCR6- γδ T cell co-transfer, and CD4/CCR6+ γδ T cell co-transfer. 20X original magnification. (C) Colitis score of each group. (D and E) mLN and LP cells were isolated 5 weeks post transfer. The percentage of IL-17 producing Ly5.1 CD4 T cells from TCRβδ-/- recipients of CD4 alone, CD4/CCR6- γδ T cell co-transfer, and CD4/CCR6+ γδ T cell cotransfer is shown. Each symbol represents individually tested mouse. * p<0.05; ** p<0.01.
Even though CCR6 expression marks IL-17+ γδ T cells, γδ T cells may acquire or lose IL-17-producing potentials after transfer into TCRβδ-/- recipients, where they undergo homeostatic proliferation (20). To verify this possibility purified CCR6+ and CCR6- γδ T cells were separately transferred into Rag-/- mice, allowed to proliferate, and examined for cytokine expression 2 weeks after transfer. Surprisingly, CCR6+ γδ T cells remained IL-17+ cells, while CCR6- γδ T cells failed to convert into IL-17+ cells and remain IFNγ-producing cells (Fig. 4). Furthermore, gene expression profiles measured by qPCR remained unchanged (data not shown). Therefore, producing γδ T cell subsets are not reversible in vivo even during homeostatic proliferation; thus enhanced Th17 differentiation and colitis seen in CCR6+ γδ T cell recipients appears to be strongly associated with IL-17 produced by γδ T cells.
Figure 4. Cytokine profiles of γδ T cells are stable in vivo.
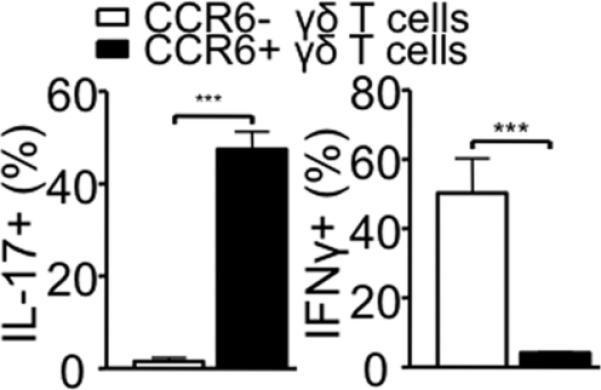
Peripheral LN γδ T cells from Ly5.1 mice were sorted based on CCR6 expression and separately transferred into Rag2-/- mice (2.5×105). Spleen cells were harvested 14 days post transfer and ex vivo stimulated for cytokine expression. Shown are IFNγ and IL-17 expression of Ly5.1 gated γδ T cells. The results shown represent the mean ± SD from two independent experiments.
How IL-17-producing γδ T cells play such proinflammatory roles? Inflammatory IL-6/IL-23-producing DCs support Th17 immunity and T cell mediated colitis (21). Since IL-17 can induce those cytokines from DCs (22), it is possible that IL-17 produced by γδ T cells may induce factors that favor Th17 differentiation from DCs. Although IL-17-/- (or IL-17F-/-) CD4 T cells were shown to equally induce colitis (23, 24), the fact that RORγ-/- CD4 T cells fail to induce Th17 immunity and colitis strongly suggests both the importance of IL-17 and the redundancy between IL-17 and IL-17F in inducing colitis. Since IL-17-producing γδ T cells also express IL-17F (data not shown), it will be important to examine if IL-17 and IL-17F derived from γδ T cells play redundant (or synergistic) roles in supporting Th17 differentiation and colitis. We are currently investigating this issue.
γδ T cell functions are highly divergent in vivo. γδ T cells mediate a regulatory effect in preventing generation of autoantigen-specific Th17 CD4 T cells in an autoimmune uveitis model (25). In contrast, γδ T cells promote the generation of myelin-specific Th17 differentiation, exacerbating the development of EAE (26). In the context of intestinal inflammation, intestinal resident γδ T cells have been proposed to play exacerbating roles in colitis developed in TCRα-/- mice (27). It was recently reported that γδ T cells may augment autoimmune responses by suppressing Treg functions as well as preventing the generation of inducible Treg cells (26). Whether γδ T cell-mediated suppression of Treg functions plays a role in the current model system remains to be determined.
Collectively, our results suggest that IL-17+ γδ T cells promote in vivo Th17 differentiation and may contribute to subsequent development of intestinal inflammation. IL-17+ γδ T cell subsets may represent a potential target for treating chronic inflammatory disorders associated with Th17 effector cells such as IBD.
Supplementary Material
Acknowledgement
We thank Dr. Nina Volokh and Ms. Jennifer Powers for technical assistance in carrying out immunohistochemistry and for cell sorting, respectively.
This work was supported by NIH grant AI074932 (to B.M.).
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 3.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 4.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Powrie F, Uhlig H. Animal models of intestinal inflammation: clues to the pathogenesis of inflammatory bowel disease. Novartis Found Symp. 2004;263:164–174. discussion 174-168, 211-168. [PubMed] [Google Scholar]
- 8.Brimnes J, Reimann J, Nissen M, Claesson M. Enteric bacterial antigens activate CD4(+) T cells from scid mice with inflammatory bowel disease. Eur J Immunol. 2001;31:23–31. doi: 10.1002/1521-4141(200101)31:1<23::aid-immu23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784–5788. doi: 10.3748/wjg.15.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do JS, Min B. Differential requirements of MHC and of DCs for endogenous proliferation of different T-cell subsets in vivo. Proc Natl Acad Sci U S A. 2009;106:20394–20398. doi: 10.1073/pnas.0909954106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 18.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 20.French JD, Roark CL, Born WK, O'Brien R L. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc Natl Acad Sci U S A. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. IL-17A-Producing {gamma}{delta}T Cells Promote CTL Responses against Listeria monocytogenes Infection by Enhancing Dendritic Cell Cross-Presentation. J Immunol. 2010 doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi D, Wakita D, Tajima M, Ashino S, Iwakura Y, Zhang Y, Chamoto K, Kitamura H, Nishimura T. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a T(h)17-independent manner. Int Immunol. 2007;19:1431–1440. doi: 10.1093/intimm/dxm114. [DOI] [PubMed] [Google Scholar]
- 24.Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Nian H, Shao H, Zhang G, Born WK, O'Brien RL, Kaplan HJ, Sun D. Regulatory effect of gammadelta T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010;51:4661–4667. doi: 10.1167/iovs.09-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi-Miyashita M, Shimada S, Kurosu H, Kato-Nagaoka N, Matsuoka Y, Ohwaki M, Ishikawa H, Nanno M. An accessory role of TCRgammadelta (+) cells in the exacerbation of inflammatory bowel disease in TCRalpha mutant mice. Eur J Immunol. 2001;31:980–988. doi: 10.1002/1521-4141(200104)31:4<980::aid-immu980>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.