Abstract
We report a novel inhibitor that selectively suppresses dengue virus (DENV) by targeting viral NS4B protein. The inhibitor was identified by screening a 1.8-million-compound library using a luciferase replicon of DENV serotype 2 (DENV-2). The compound specifically inhibits all four serotypes of DENV (50% effective concentration [EC50], 1 to 4 μM; and 50% cytotoxic concentration [CC50], >40 μM), but it does not inhibit closely related flaviviruses (West Nile virus and yellow fever virus) or nonflaviviruses (Western equine encephalomyelitis virus, Chikungunya virus, and vesicular stomatitis virus). A mode-of-action study suggested that the compound inhibits viral RNA synthesis. Replicons resistant to the inhibitor were selected in cell culture. Sequencing of the resistant replicons revealed two mutations (P104L and A119T) in the viral NS4B protein. Genetic analysis, using DENV-2 replicon and recombinant viruses, demonstrated that each of the two NS4B mutations alone confers partial resistance and double mutations confer additive resistance to the inhibitor in mammalian cells. In addition, we found that a replication defect caused by a lethal NS4B mutation could be partially rescued through trans complementation. The ability to complement NS4B in trans affected drug sensitivity when a single cell was coinfected with drug-sensitive and drug-resistant viruses. Mechanistically, NS4B was previously shown to interact with the viral NS3 helicase domain; one of the two NS4B mutations recovered in our resistance analysis—P104L—abolished the NS3-NS4B interaction (I. Umareddy, A. Chao, A. Sampath, F. Gu, and S. G. Vasudevan, J. Gen. Virol. 87:2605-2614, 2006). Collectively, the results suggest that the identified inhibitor targets the DENV NS4B protein, leading to a defect in viral RNA synthesis.
INTRODUCTION
The family Flaviviridae consists of three genera: Flavivirus, Pestivirus, and Hepacivirus. Many members of the genus Flavivirus are important human pathogens, including the four serotypes of dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV). These viruses are mainly transmitted by mosquitoes or ticks (8). Infection with the flaviviruses causes fevers, encephalitis, and hemorrhage; some infected individuals develop life-threatening symptoms. Flavivirus infection has increased dramatically over the past 40 years in tropical and subtropical regions of the world, most likely due to reduced mosquito control, an increase in global transportation and travel, and dense urbanization. DENV alone causes about 50 to100 million human infections annually, with 500,000 cases of dengue hemorrhagic fever and 22,000 deaths globally. YFV infects about 200,000 people each year, leading to 30,000 fatalities. Since an outbreak in New York City in 1999, WNV has caused thousands of human infections in North America. In Asia, JEV infection causes approximately 50,000 people to develop Japanese encephalitis, causing about 10,000 deaths (8). Despite the medical need, no clinically approved antiviral therapy is currently available for treatment of any flavivirus infection. Therefore, the search for safe and effective therapeutics is urgent.
The flaviviral genome is a single-stranded, positive-sense RNA about 11 kb in length. It contains 5′ and 3′ untranslated regions (UTR) flanking a single open reading frame. The open reading frame encodes a long polyprotein that is co- and posttranslationally processed by viral and cellular proteases into three structural proteins (capsid [C], premembrane [prM], and envelope [E]) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Structural proteins are involved in virus entry and assembly. Nonstructural proteins are involved in viral RNA replication (17), evasion of innate immune response (9, 18, 22, 23), and virus assembly (11, 19). The glycoprotein NS1 plays a role in viral RNA replication at an early step of viral RNA replication (15, 16). NS3 acts as a viral serine protease with the cofactor NS2B (3, 6), an RNA triphosphatase (38, 39), and an RNA helicase (13). NS5 functions as a methyltransferase (5, 32) and an RNA-dependent RNA polymerase (RdRp) (1, 35). Very little is known about the functions of the small hydrophobic proteins NS2A, NS4A, and NS4B; these transmembrane proteins anchor the viral replication complex to the endoplasmic reticulum (ER) membrane (20, 21).
Antiviral development could target both structural and nonstructural proteins to block the viral infection cycle. The traditional antiviral approach often focuses on viral proteins with enzymatic activities. Indeed, the majority of current anti-HIV drugs in clinical use and anti-hepatitis C virus (HCV) inhibitors in clinical trials or clinical use target viral enzymes, such as protease and polymerase. Due to the emergence of resistance, it is critical to develop inhibitors with novel modes of action that could be used for combination therapy. Multiple strategies are possible to identify inhibitors with new mechanisms. One strategy is to target viral factors with nonenzymatic activity that is essential for viral replication; such strategies have achieved proof of concept in clinics in HCV drug discovery. Gao and colleagues demonstrated that, besides protease and polymerase, viral NS5A, a protein with no enzymatic activity, could also be successfully targeted for HCV therapy (7). The HCV NS5A inhibitor was identified through a cell-based HCV replicon screen, followed by target deconvolution through resistance selection (12). The replicon-based approach should be applicable to flavivirus (e.g., DENV) drug discovery.
In this study, we performed high-throughput screening (HTS) using a DENV replicon cell line. The screening allowed us to identify a novel inhibitor of DENV. The compound specifically inhibited all four serotypes of DENV but did not inhibit other flaviviruses or nonflaviviruses. Mode-of-action studies indicated that the inhibitor functions at the step of viral RNA synthesis. Resistance analysis showed that mutations within viral NS4B were responsible for compound resistance in mammalian cells. Furthermore, we show that DENV NS4B could be trans complemented in cell culture; such trans complementation could affect drug sensitivity when a single cell is coinfected with drug-sensitive and drug-resistant viruses.
MATERIALS AND METHODS
Cells, viruses, and compounds.
A549 human alveolar epithelial cells were maintained in F-12 medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. BHK-21 (baby hamster kidney) and Vero (African green monkey) cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. C6/36 (mosquito) cells were grown in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin. A549, BHK-21, and Vero cells were incubated at 37°C, and C6/36 cells were maintained at 28°C.
The viruses used in this study included DENV serotype 1 (DENV-1) (West Pacific strain), DENV-2 (New Guinea C and TSV01 strains), DENV-3 (H87 strain), DENV-4 (H241 strain), WNV (strain 3356), YFV (17D vaccine strain), Western equine encephalomyelitis virus (WEEV) (strain Cova746), Chikungunya virus (CHIKV) (Ross strain), and vesicular stomatitis virus (VSV) (New Jersey serotype). The sources of these viruses were reported previously (30, 31). The synthesis of compound NITD-618 will be reported elsewhere. The compound was dissolved in 90% dimethyl sulfoxide (DMSO) as a stock for in vitro experiments.
HTS using a luciferase reporter replicon of DENV-2.
A549 cells containing a luciferase reporter replicon of DENV-2 (New Guinea C strain) were used for HTS. Renilla luciferase and puromycin acetyltransferase genes were engineered in the replicon to replace viral structural proteins, as described previously (24). Briefly, A549 DENV-2 replicon cells were seeded at a density of 750 cells per well in a 1,536-well microplate. After incubation at 37°C with 5% CO2 overnight, the cells were treated with compounds. Luciferase activities were measured after 48 h of incubation using Promega's EnduRen live-cell substrate. Following luciferase activity measurement, Promega's CellTiter-Glo reagent was added to each well to determine the cytotoxicity of the compounds. The compounds were screened at a single concentration of 5 μM.
CFI assay.
The cell-based flavivirus immunodetection (CFI) assay was performed as described previously (37). In brief, 2 × 104 A549 cells per well in a 96-well plate were infected with DENV-2 (New Guinea C strain) at a multiplicity of infection (MOI) of 0.3 in the presence of 2-fold serial dilutions of the compound. At 48 h postinfection (p.i.), the infected wells were washed, fixed, and immunoblotted with primary antibody (4G2; American Type Culture Collection) and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Sigma) to determine the yield of viral envelope protein. A dose-response curve was plotted using Prism 5 software (GraphPad Software, La Jolla, CA), and the effective concentration of compound to reduce envelope protein production by 50% (EC50) was calculated using nonlinear regression analysis.
Viral-titer reduction assay.
A viral-titer reduction assay was performed for the following viruses: DENV-1, DENV-2, DENV-3, DENV-4, CHIKV, WNV, YFV, WEEV, and VSV. Approximately 2 × 104 cells (A549, BHK-21, or Vero) or 8 × 104 cells (C6/36) were seeded per well of 96-well plates. At 24 h postseeding, A549 cells were infected with DENV-2 (MOI, 0.3; New Guinea C strain) or CHIKV (MOI, 0.1); Vero cells were infected with WNV, YFV, WEEV, or VSV (MOI, 0.1); and BHK-21 cells were infected with DENV-1 to -4 (MOI, 0.3). The infected cells were immediately treated with series dilutions of compound. Because of the difference in replication kinetics among different viruses, culture fluids were collected at different time points postinfection: CHIKV and VSV infections at 16 h p.i.; WNV, YFV, and WEEV infections at 42 h p.i.; DENV-1 and DENV-2 infections at 48 h p.i.; and DENV-3 and DENV-4 infections at 72 h p.i. C6/36 cells were infected with wild-type (WT) or mutant DENV-2 (strain TSVO1; MOI, 0.3), and culture fluids were collected at 72 h p.i. The viral titers of all samples collected were quantified using a plaque assay (30).
Cytotoxicity assay.
Cell viability was measured using the Cell Counting Kit-8 (CCK-8) (measuring cellular dehydrogenase activity; Dojindo Molecular Technologies) according to the manufacturer's protocol. A549, BHK-21, or Vero cells were seeded at 5 × 103 cells per well in a 96-well plate. After 24 h of incubation at 37°C in 5% CO2, the cells were treated with 2-fold serial dilutions of the compound. At 48 h posttreatment, 4 μl of CCK-8 solution was added to each well. After another 90 min of incubation at 37°C, the absorbance was measured at 450 nm using a microplate reader (Tecan).
Antiviral assay in K562 cells.
A human myelogenous leukemia cell line, K562, was used to study the antiviral activity of NITD-618 against a luciferase reporter DENV-2 (New Guinea C strain) (46). K562 cells were seeded at 1 × 104 cells per well in a 96-well plate. After overnight incubation at 37°C in 5% CO2, the cells were infected with the reporter DENV-2 at an MOI of 1.0 in the presence of serial dilutions of NITD-618. At 48 h p.i., Renilla luciferase activity was quantified using ViviRen Live Cell Substrate (Promega), after which cytotoxicity was measured by the intracellular level of ATP using a CellTiter-Glo luminescent-cell viability assay (Promega) according to the manufacturer's protocol.
Time-of-addition assay.
Approximately 2 × 105 A549 cells were seeded per well of a 24-well plate. At 24 h postseeding, the cells were infected with DENV-2 (New Guinea C strain; MOI, 2.0) for 1 h at 4°C. Subsequently, the viral inocula were removed, and the cells were washed three times with cold phosphate-buffered saline (PBS) to remove unabsorbed viruses. At 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, or 20 h p.i., 5 μM compound was added to the infected cells. As negative controls, 0.9% DMSO was added to the infected cells at 0, 10, and 20 h postinfection. At 24 h p.i., culture fluids were collected, and the viral titer was determined by plaque assay (30).
Selection and sequencing of a DENV-2 replicon resistant to NITD-618.
BHK-21 cells containing DENV-2 replicon (RepPAC-2A-EGFP; New Guinea C strain) were used to select for a replicon resistant to NITD-618. RepPAC-2A-EGFP contained two reporters (a puromycin acetyltransferase [PAC] and enhanced green fluorescent protein [EGFP]) separated by foot-and-mouth disease virus (FMDV) 2A protein (24). The RepPAC-2A-EGFP BHK-21 cells were first cultured in medium containing 6 μM NITD-618 for 3 days (P0). The cells were then passaged in medium containing 6 μM NITD-618 and 10 μg/ml puromycin for three rounds (P1 to P3; 3 days per passage round). The cells were further passaged in medium containing 12 μM NITD-618 and 20 μg/ml puromycin for three rounds (P4 to P6; 3 days per passage round). Cells from P6 were subjected to fluorescence-activated cell sorter (FACS) analysis using a FACSAria flow cytometer (BD Biosciences). The top 10% of the cells with the strongest GFP signal were recovered and expanded. The foci of the sorted cells were cloned and expanded in medium containing 12 μM NITD-618 and 20 μg/ml puromycin. Individually cloned cells were tested for compound sensitivity using FACS analysis. Data analysis was performed using FlowJo software (version 8.2; Tree Star Inc., Ashland, OR). Total cellular RNA of the cloned cells was extracted using RNeasy kits (Qiagen). Viral replicon cDNA was amplified using SuperScript One-Step reverse transcription (RT)-PCR with Platinum Taq (Invitrogen). The RT-PCR products were gel purified and subjected to DNA sequencing.
Construction of recombinant plasmids.
Recombinant plasmids were constructed by using an infectious cDNA clone of DENV-2 (pACYC TSVFL; strain TSV01), a cDNA clone of a Renilla luciferase replicon of DENV-2 (pACYC TSV replicon; strain TSV01), and one shuttle vector, TA TSV-F (containing cDNA from nucleotide 5426 to the 3′ end of the DENV-2 genome; GenBank accession number AY037116). The construction of plasmids pACYC TSVFL and pACYC TSV replicon and the shuttle vector TA TSV-F was reported previously (45). NS4B mutations and an NS5stop mutation (the first amino acid [Gly] of NS5 was mutated to a stop codon) were first engineered into the shuttle vector TA TSV-F using a QuikChange II XL site-directed mutagenesis kit (Stratagene). The fragment containing the mutation(s) was then engineered into the infectious cDNA clone and the replicon cDNA clone using XhoI (nucleotide position 5426) and ClaI (immediately downstream of the 3′ end of the viral genome). The primer pairs used for mutagenesis are listed in Table 1. All constructs were verified by DNA sequencing.
Table 1.
Primers for mutagenesisa
| Primer name | Sequence (5′ to 3′) |
|---|---|
| P104A_F | GTGCTATTCACAAGTCAACGCAATAACTCTCACGGCAGCCC |
| P104A_R | GGGCTGCCGTGAGAGTTATTGCGTTGACTTGTGAATAGCAC |
| P104E_F | GTGCTATTCACAAGTCAACGAAATAACTCTCACGGCAGCCC |
| P104E_R | GGGCTGCCGTGAGAGTTATTTCGTTGACTTGTGAATAGCAC |
| P104R_F | GTGCTATTCACAAGTCAACAGAATAACTCTCACGGCAGCCC |
| P104R_R | GGGCTGCCGTGAGAGTTATTCTGTTGACTTGTGAATAGCAC |
| P104V_F | GTGCTATTCACAAGTCAACGTGATAACTCTCACGGCAGCCC |
| P104V_R | GGGCTGCCGTGAGAGTTATCACGTTGACTTGTGAATAGCAC |
| P104L_F | GCTATTCACAAGTCAACCTCATAACTCTCACGGCAGC |
| P104L_R | GCTGCCGTGAGAGTTATGAGGTTGACTTGTGAATAGC |
| A119T_F | CTTATTGGTAGCACATTATACTATCATAGGGCCAGGAC |
| A119T_R | GTCCTGGCCCTATGATAGTATAATGTGCTACCAATAAG |
| NS5stop_F | CACGGCCAACACAAGAAGGTAGACTGGCAACACAGGAGAG |
| NS5stop_R | CTCTCCTGTGTTGCCAGTCTACCTTCTTGTGTTGGCCGTG |
A pair of forward (F) and reverse (R) primers was used to generate mutant replicons and genome length RNAs (see Materials and Methods for details).
Transient replicon assay.
A Renilla luciferase replicon of DENV-2 was in vitro transcribed using a T7 mMessage mMachine kit (Ambion, Austin, TX) from cDNA plasmids linearized with ClaI, as described previously (6). BHK-21 cells were electroporated with 10 μg of replicon RNA using a GenePulser Xcell system (Bio-Rad, Hercules, CA) and an established protocol (6). For transfection of Vero, A549, and C6/36 cells, 8 × 106 cells in 0.8 ml cold Ingenio electroporation solution (Mirus Bio, Madison, WI) were electroporated with 10 μg of replicon RNA in a 4-mm cuvette at settings of 450 V and 25 μF with three pulses at 3-s intervals. The transfected cells (BHK-21, Vero, and A549) were seeded in a 12-well plate (3 × 105 cells per well). At various time points posttransfection (p.t.), the cells were washed once with PBS and lysed in 200 μl 1× lysis buffer (Promega). The plates containing the lysis buffer were sealed with Parafilm and stored at −80°C. Once samples for all time points had been collected, 20 μl of cell lysates was transferred to a 96-well plate and assayed for luciferase signals in a Clarity luminescence microplate reader (BioTek). For testing compound inhibition of the replicon, the transfected A549 (2 × 104 cells per well), BHK-21 (2 × 104 cells per well), or C6/36 (8 × 104 cells per well) cells were seeded in a 96-well plate. Immediately after seeding, the cells were treated with serial dilutions of NITD-618 or 0.9% DMSO. At 48 h p.t. (A549 cells and BHK-21 cells) or 96 h p.t. (C6/36 cells), the cells were washed once with PBS, lysed in 20 μl 1× lysis buffer, and assayed for luciferase activity as described above.
Production of recombinant viruses, SIA, and IFA.
Recombinant viruses were produced as described previously (45). Briefly, BHK-21 cells were transfected with 10 μg of WT or mutant genome length RNA containing the NS4B P104L, A119T, or P104L plus A119T mutation. The electroporated cells were incubated at 37°C for 24 h and then at 30°C for an additional 96 h. On day 5 p.t., culture fluids were harvested, aliquoted, and stored at −80°C. The titers of recombinant viruses were quantified by plaque assay using BHK-21 cells (30). For specific infectivity assay (SIA), 1 ml of a series of 1:10 dilutions of the transfected cells was seeded onto confluent BHK-21 cell monolayers (6 × 105 cells per well seeded in a six-well plate 2 days in advance). The seeded cells were allowed to attach to the plates for 5 h before addition of 3 ml RPMI 1640 medium containing 0.8% methyl cellulose (Aquacide II; Calbiochem) and 2% FBS. After 5 days of incubation at 37°C with 5% CO2, the cells were fixed in 3.7% formaldehyde (Sigma) and stained with 1% crystal violet. An immunofluorescence assay (IFA) was performed as described previously (34), using anti-E monoclonal antibody 4G2 and Alexa Fluor 488 goat anti-mouse IgG as primary and secondary antibodies, respectively.
Trans complementation analysis.
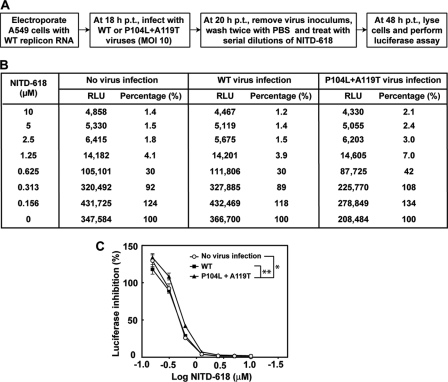
Two types of trans complementation experiment were performed. For trans complementation of an NS4B lethal mutant, BHK-21 cells with or without the WT replicon of DENV-2 (TSV01) were transfected with equal amounts (10 μg) of WT or mutant genome length RNA. At 96 h p.t., the transfected cells were assayed for viral E protein expression using IFA, as described above. For trans complementation between the WT replicon and compound-resistant virus, A549 cells were transfected with 10 μg of WT luciferase replicon RNA of DENV-2. The transfected cells were resuspended in DMEM containing 10% FBS at a density of 2 × 105 cells/ml; 100 μl of the cell suspension was immediately seeded in a 96-well white polystyrene microplate (Greiner, Germany). At 18 h p.t., the culture medium was removed and the cells were infected with WT or P104L plus A119T mutant DENV-2 (MOI = 10) or incubated with 50 μl DMEM supplemented with 2% FBS (as a no-virus infection control). At 20 h p.t., the inocula were removed and the cells were washed twice with PBS and treated with serial dilutions of NITD-618 in fresh medium containing 2% FBS. At 48 h p.t., the cells were lysed in 20 μl 1× lysis buffer, and luciferase activity was measured. A two-way analysis of variance (ANOVA) test was performed to indicate statistical significance among different experimental groups.
RESULTS
Identification of NITD-618.
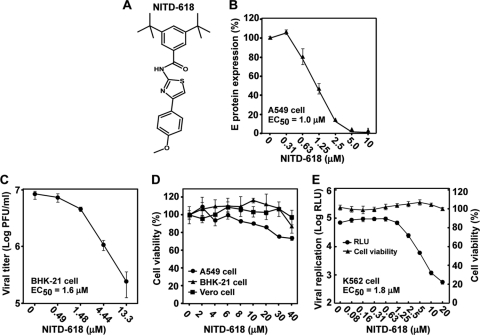
We performed an HTS using an A549 cell line containing a luciferase replicon of DENV-2. The HTS was performed in a 1,536-well format with a signal-to-noise ratio of 5 and a Z′ value of 0.4. Approximately 1.8 million compounds with diverse structures were screened at a single concentration of 5 μM. Compounds inhibiting ≥50% of luciferase activity were defined as “hits.” The HTS had a hit rate of 0.96%. The screening allowed us to identify compound NITD-618 (Fig. 1A), which suppressed 85% of the replicon luciferase activity at 5 μM (data not shown). The antiviral activity of NITD-618 was validated using a DENV CFI assay. The CFI assay is an ELISA-based test that measures the amount of viral E protein in cells infected with DENV-2 (Fig. 1B). The compound reduced viral E protein production in a dose-responsive manner, with an EC50 of 1.0 μM (Fig. 1B). Next, the anti-DENV activity was further confirmed using a viral-titer reduction assay. As shown in Fig. 1C, NITD-618 inhibited virus production, with an EC50 of 1.6 μM. A cytotoxicity assay showed that the compound was not cytotoxic up to 40 μM in Vero, BHK-21, and A549 cells (Fig. 1D), suggesting that the observed antiviral activity was not due to compound-mediated cytotoxicity. The antiviral activity of NITD-618 was further validated using K562 cells (a human myelogenous leukemia cell line) infected with a luciferase reporter DENV-2. The compound showed an EC50 of 1.8 μM with no detectable cytotoxicity up to 20 μM in K562 cells (Fig. 1E).
Fig. 1.
Antiviral activity of NITD-618 against DENV-2. (A) Structure of NITD-618. (B) Effect of NITD-618 on the expression of DENV-2 E protein. A549 cells were infected with DENV-2 (New Guinea C strain; MOI, 0.3) in the presence of 2-fold serial dilutions of NITD-618. After incubation at 37°C for 48 h, the expression of viral E protein was quantified by CFI assay. The E protein expression level is represented as a percentage of the E expression level derived from the infected cells with DMSO treatment. Each data point shows the average and standard deviation (n = 4). (C) Effect of NITD-618 on the growth of DENV-2. BHK-21 cells were infected with DENV-2 (New Guinea C strain; MOI, 0.3). After incubation at 37°C for 48 h, cell culture fluids were harvested for plaque assay in BHK-21 cells. The curve is reported as logarithm (log10) values of average viral titers of triplicates versus the NITD-618 concentration. The error bars represent standard deviations (n = 3). (D) Cytotoxicity of NITD-618. Cytotoxicity was examined by incubation of A549, BHK-21, and Vero cells with the indicated concentrations of NITD-618. After 48 h of incubation, cell viability was determined with a CCK-8 kit and plotted as a percentage of 0.9% DMSO-treated cells. The average results and standard deviations (n = 3) are presented. (E) Antiviral activity of NITD-618 in K562 cells. K562 cells were infected with a Renilla luciferase DENV-1 (MOI, 1.0) in the presence of NITD-618. At 48 h p.i., the cells were measured for Renilla luciferase activity (relative light units [RLU]) to indicate viral replication and the for intracellular level of ATP to indicate cytotoxicity. The average results and standard deviations from quadruplicate data points are presented.
Specific inhibition of DENV.
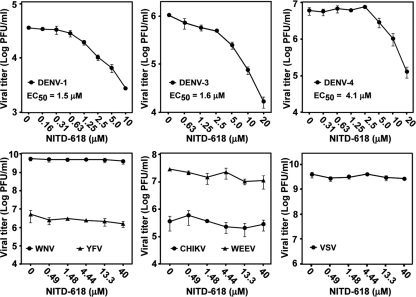
We examined the antiviral spectrum of NITD-618. The compound was tested in a viral-titer reduction assay using three other serotypes of DENV (DENV-1, -3, and -4), two closely related flaviviruses (WNV and YFV), two plus-strand RNA alphaviruses (CHIKV and WEEV), and a negative-strand RNA rhabdovirus (VSV). NITD-618 was active against three other serotypes of DENV, with EC50s of 1.5, 1.6, and 4.1 μM against DENV-1, -3, and -4, respectively (Fig. 2). In contrast, the compound (up to 40 μM) did not suppress titers of any other viruses, including the closely related WNV and YFV. We did not test the compound at concentrations of >40 μM due to potential cytotoxicity (Fig. 1D). These results demonstrate that NITD-618 selectively inhibits all four serotypes of DENV.
Fig. 2.
Antiviral spectrum of NITD-618. BHK-21 cells were infected with DENV-1, DENV-3, and DENV-4 (MOI, 0.3). A549 cells were infected with CHIKV (MOI, 0.1). Vero cells were infected with WNV, YFV, WEEV, and VSV (MOI, 0.1). EC50s were calculated by nonlinear regression analysis using Prism 5 software. The log10 values of average viral titers and standard deviations (n = 3) are presented.
Suppression of viral RNA synthesis.
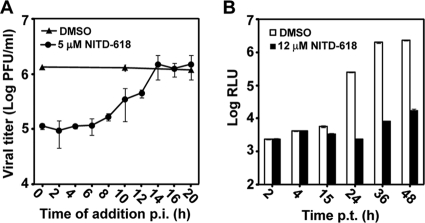
A time-of-addition experiment was performed to determine the stage of inhibition in a viral infection cycle. A549 cells were synchronously infected with DENV-2. NITD-618 (5 μM) was added to the infected cells at various time points after infection, and viral yields were quantified at 24 h postinfection. The inhibition of virus production by NITD-618 gradually diminished when the compound was added at time points later than 12 h p.i. (Fig. 3A), suggesting that the compound blocks a late stage(s) of the viral life cycle.
Fig. 3.
Mode-of-action analysis. (A) Time-of-addition analysis. A549 cells were infected with DENV-2 (New Guinea C strain) at an MOI of 2 at 4°C for 1 h. After three washes with PBS, NITD-618 (5 μM) was added to the infected cells at the indicated time points postinfection. As controls, the infected cells were treated with 0.9% DMSO. At 24 h p.i., the culture fluids were harvested, and viral titers were measured by plaque assay. Average results and standard errors (n = 4) are presented. (B) Transient-transfection assay using a luciferase replicon of DENV-2. A549 cells were transfected with equal amounts of WT and mutant replicon RNAs and immediately treated with 12 μM NITD-618 or 0.9% DMSO (negative control). At the indicated time point p.t., cells were assayed for luciferase signals (RLU). The log10 values of average Renilla luciferase signals (RLU) and standard deviations are presented (n = 4).
Since NITD-618 was identified using replicon screening, the compound should inhibit viral translation and/or RNA synthesis. To differentiate between the two steps, we performed a transient replicon assay using a luciferase replicon of DENV-2. The luciferase replicon was electroporated into A549 cells. The transfected cells were immediately treated with NITD-618 (12 μM) or DMSO (0.9% as a negative control) and assayed for luciferase activity at various time points posttransfection. As shown in Fig. 3B, the compound showed no effect on luciferase signals at 2 and 4 h p.t. (representing translation of input replicon RNA). In contrast, the compound suppressed luciferase activities at >15 h p.t. (indicating replicon RNA synthesis). At 24, 36, and 48 h p.t., NITD-618 blocked the luciferase signals to <1% of those of the DMSO control group. The results indicate that NITD-618 inhibits viral RNA synthesis.
To examine whether the compound inhibits replication through suppression of known viral enzymes, we tested NITD-618 in the DENV protease (2), NTPase (40), methyltransferase (4), and RdRp (25) assays. None of the enzymatic activities were inhibited by the compound at up to 20 μM (data not shown).
Selection and characterization of resistant replicon cells.
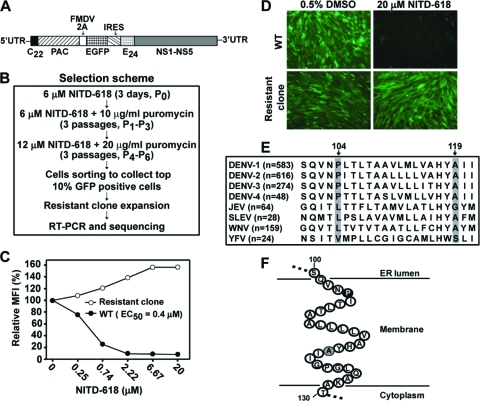
To identify the target of NITD-618, we selected resistant replicons of DENV-2 by using a flow cytometry-based cell-sorting method (33). BHK-21 cells containing a GFP replicon of DENV-2 (Fig. 4A) were continuously passaged in medium with increasing concentrations of NITD-618 (Fig. 4B). After 7 rounds of passaging, the cells with the top 10% of EGFP signal were pooled through live-cell sorting. Individual cells were cloned, expanded, and examined for compound sensitivity by FACS analysis. Figure 4C shows the compound inhibition result for one of the selected replicon cell lines (clone 1). Upon compound treatment, the GFP signals from WT replicon cells were effectively suppressed, with an EC50 of 0.4 μM. In contrast, the GFP signals from selected replicon cells were not reduced but increased in the presence of the compound. Microscopic analysis showed that the EGFP fluorescence from the selected replicon cells was not suppressed by the compound, whereas the EGFP signal from the WT replicon cells was completely inhibited by 20 μM compound (Fig. 4D). Similar resistance phenotypes were observed for two other independently selected replicon cells (clones 2 and 3) (data not shown). These results demonstrate that resistant replicons could be selected in cell culture.
Fig. 4.
Selection and characterization of NITD-618-resistant DENV-2 replicon cells. (A) Schematic diagram of EGFP-expressing DENV-2 replicon (New Guinea C strain). The diagram is not drawn to scale. C22, N-terminal 22 amino acids of the capsid protein; E24, C-terminal 24 amino acids of the E protein; PAC, puromycin acetyltransferase; FMDV 2A, foot-and-mouth disease virus 2A protein; IRES, internal ribosomal entry site from encephalomyocarditis virus. (B) Scheme for selection of resistant replicon cells. See Materials and Methods for details. (C) Resistance analysis of a selected replicon cell line (clone 1). WT and mutant replicon cells were incubated with the indicated concentrations of NITD-618. At 72 h after treatment, the cells were trypsinized, resuspended in PBS containing 2% FBS, and subjected to FACS analysis. The curves were plotted from the mean fluorescence intensity (MFI) versus the concentration of NITD-618. (D) Microscopic analysis of resistance replicon cells. WT and resistant (clone 1) EGFP replicon cells were incubated with 20 μM NITD-618 or 0.5% DMSO (control) for 72 h in the absence of puromycin. Representative microscope images are presented, with EGFP signal in green. (E) Amino acid sequence alignment of NS4B regions. The numbers in parentheses are the numbers of NS4B sequences that were used for the alignment. Sequences were downloaded from the National Center for Biotechnology Information (NCBI) protein database, and alignment was performed using CLC main workbench software (CLC bio). The diagram shows alignment results from amino acid positions 100 to 121 of NS4B. The positions of amino acids in NS4B are numbered according DENV-2 (GenBank accession number AY037116). The identified mutation sites (positions 104 and 119 in DENV-2 NS4B) are shaded. (F) Cartoon diagram of DENV-2 NS4B transmembrane domain 3 (TMD3). The amino acid sequence of TMD3 (residues 100 to 130) of DENV-2 NS4B is shown according to the topology proposed by Miller and colleagues (21). The Pro104 and Ala119 residues identified in this study are highlighted in black and gray, respectively.
We sequenced the complete genomes of three independently selected resistant replicons. The mutations are summarized in Table 2. Replicon from clone 1 accumulated two mutations. (i) A C→U change at nucleotide position 7136 of the viral genome resulted in a Pro→Leu substitution at amino acid position 104 of the NS4B protein (P104L). This mutation was recovered from all three independently selected replicons. (ii) A G→A mutation at nucleotide position 7180 led to an Ala→Thr change at amino acid position 119 of the NS4B protein (A119T). This mutation was recovered from two of the three selected replicons (clones 1 and 2). It should be noted that the P104L and A119T NS4B mutations were not recovered in replicons passaged without compound treatment (negative controls).
Table 2.
Mutations recovered from resistant replicons of DENV-2a
| Clone | Nucleotideb | Amino acidc | Associated protein |
|---|---|---|---|
| 1 | C7136U | P104L | NS4B |
| G7180A | A119T | NS4B | |
| 2 | C7136U | P104L | NS4B |
| G7180A | A119T | NS4B | |
| G7568A | R248K | NS4B | |
| G8848C | E427Q | NS5 | |
| 3 | A5526C | Silent | NS3 |
| C7136U | P104L | NS4B | |
| C10215U | Silent | NS5 | |
| G10255U | A896S | NS5 |
Mutations recovered from all three independently selected resistant replicons are shaded in gray.
The nucleotide acid position in the DENV-2 genome (New Guinea C; GenBank accession number AF038403).
Amino acid positions in mutated genes.
Sequence alignment of NS4B showed that P104 and A119 are absolutely conserved among all four serotypes of DENV but are different among other flaviviruses (Fig. 4E). Notably, the mutated 104Leu residue (recovered from the resistant DENV-2 replicon) exists as the WT amino acid in the JEV, St. Louis encephalitis virus (SLEV), and WNV NS4B proteins. Such differences at amino acid 104 of NS4B may account for the selectivity of NITD-618 in inhibiting DENV (Fig. 2). According to the NS4B topology proposed by Miller and colleagues (21), amino acids P104 and A119 are located in the third transmembrane domain and are embedded in the ER membrane (Fig. 4F).
Replicon analysis of P104L and A119T mutations.
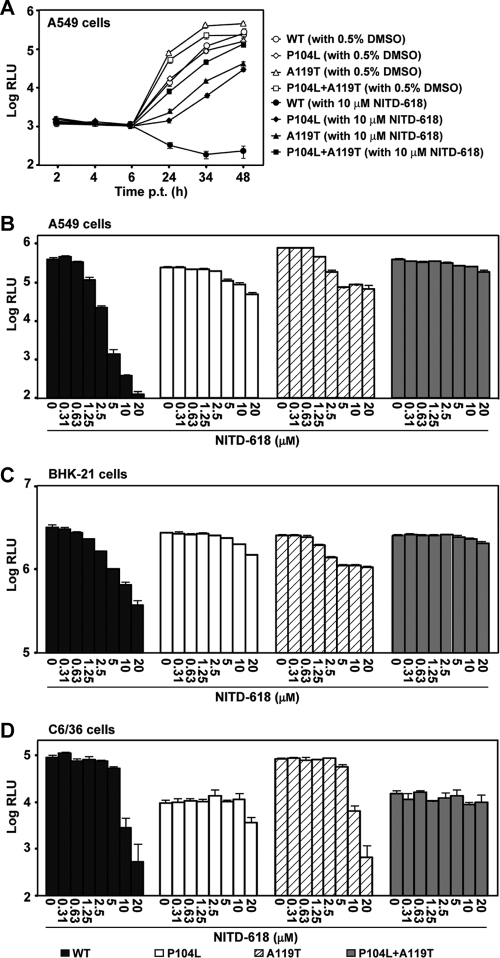
The functions of NS4B mutations were analyzed using a luciferase replicon of DENV-2. We generated three mutant replicons containing mutation P104L alone or A119T alone or the combined mutations P104L plus A119T. Initially, we examined the effects of these mutations on viral replication in the absence of compound. A549 cells were transfected with equal amounts of replicon RNAs and assayed for luciferase activities at various time points after transfection. None of the mutations affected the translation of input RNA at 2, 4, and 6 h p.t. (Fig. 5A). In contrast, luciferase signals at ≥24 h p.t. showed differences in replication efficiencies among various replicons in the following order: A119T > P104L plus A119T > WT ≥ P104L. To examine whether the observed effects on viral replication were cell type specific, we compared the replicon replication in two other cells lines. Figures 5C and D show the luciferase signals from replicon-transfected BHK-21 (at 48 h p.t.) and C6/36 (at 96 h p.t.) cells, respectively. In BHK-21 cells, the replication levels of all three mutant replicons were slightly reduced compared with the WT (Fig. 5C). In C6/36 cells, the A119T mutant replicated to a level comparable to that of the WT replicon, whereas the P104L and P104L plus A119T mutants replicated much less efficiently than the WT replicon (Fig. 5D). Taken together, the results demonstrate that NS4B mutations (P104L and A119T) affect viral replication in a host species-dependent manner.
Fig. 5.
Resistance analysis using a luciferase replicon of DENV-2. (A) A549 cells were transfected with equal amounts (10 μg) of WT, P104L, A119T, or P104L plus A119T mutant replicon RNAs. The transfected cells were immediately treated with NITD-618 (12 μM) or DMSO (0.5%, as a control). Renilla luciferase activities (RLU) were measured at the indicated time points posttransfection. Each data point is a log10 value of the average of luciferase signals from three independent experiments; the error bars indicate standard deviations. (B to D) A549 (B), BHK-21 (C), and C6/36 (D) cells were transfected with WT or mutant replicon RNAs (10 μg). The transfected cells were treated with the indicated concentrations of NITD-618. Luciferase activities were measured at 48 h (A549 and BHK-21 cells) or 96 h (C6/36 cells) posttransfection. Average results and standard deviations from three independent experiments are presented.
Next, we compared compound inhibition between the WT and mutant replicons. The replicon-transfected cells (A549, BHK-21, and C6/36) were treated immediately after transfection with various concentrations of NITD-618 and assayed for luciferase activities at the indicated time points. In A549 (Fig. 5A and B) and BHK-21 (Fig. 5C) cells, mutation P104L or A119T alone conferred partial resistance; the double mutation P104L plus A119T enhanced the resistance in an additive manner. In C6/36 cells (Fig. 5D), the single mutation P104L and the double mutation P104L plus A119T conferred partial resistance, whereas mutant A119T did not show any resistance. Interestingly, the compound inhibited the WT replicon in A549 cells more efficiently that in BHK-21 and C6/36 cells. Nevertheless, these results indicate that both NS4B mutations play roles in conferring resistance in mammalian cells, whereas only mutation P104L (but not mutation A119T) functions in resistance in mosquito cells.
Recombinant virus analysis of P104L and A119T mutations.
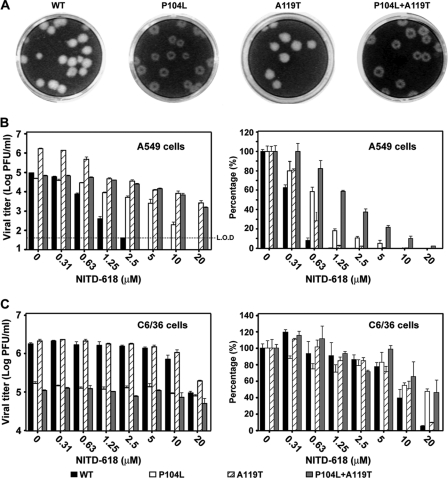
To confirm the above replicon results, we validated the functions of NS4B mutations in the context of genome-length RNA. Three recombinant mutant viruses (P104L, A119T, and P104L plus A119T) were prepared using an infectious cDNA clone of DENV-2. Three mutant viruses produced plaques with sizes comparable to that of the WT virus on BHK-21 cells (Fig. 6A). Interestingly, mutant viruses P104L and P104L plus A119T produced opaque plaques. Sequencing of the recombinant viruses confirmed that the engineered mutations were retained in the recovered viruses without any other changes (data not shown).
Fig. 6.
Resistance analysis using recombinant viruses. (A) Plaque morphologies of WT and P104L, A119T, and P104L plus A119T mutant viruses. The plaques were developed in BHK-21 cells (5 days p.i.) without treatment with NITD-618. (B) Viral-titer reduction assay in A549 cells. The dotted line indicates the limit of detection (L.O.D.) of 40 PFU/ml. (C) Viral-titer reduction assay in C6/36 cells. In panels B and C, the log10 values of viral titers versus compound concentrations are plotted on the left, and the percentages of viral titers from the compound-treated samples versus the viral titers from the DMSO-treated samples are shown on the right; for each replicon, the viral titers from the DMSO-treated samples were set as 100%. Average results and standard deviations (n = 3) are presented.
Next, we examined the sensitivity of mutant viruses to NITD-618 inhibition using a viral-titer reduction assay. In A549 cells (Fig. 6B), the single mutation P104L or A119T alone conferred resistance; the double mutation P104L plus A119T enhanced the resistance. For example, treatment with 5 μM NITD-618 reduced the WT virus titer by ≥2.4 × 103-fold to an undetectable level, whereas treatment with the same concentration of NITD-618 reduced the titers of the P104L, A119T, and P104L plus A119T viruses by 19-, 140-, and 4.6-fold (fold reduction = viral titer without compound treatment/viral titer with compound treatment), respectively. In C6/36 cells (Fig. 6C), the resistance phenotype was much less pronounced, only mutants P104L and P104L plus A119T showed resistance, and the resistance was clearly observed when treated with 20 μM NITD-618. Collectively, the results demonstrate that, in A549 cells, (i) both P104L and A119T mutations in NS4B are responsible for resistance and (ii) the double mutation P104L plus A119T improves resistance in an additive manner, whereas in C6/36 cells, only P104L is responsible for resistance.
Effects of other P104 substitutions on viral replication and resistance.
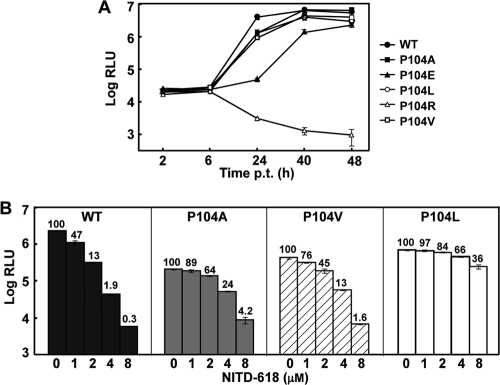
To examine whether amino acid substitutions other than P104L of NS4B could confer resistance to NITD-618, we generated a panel of mutant luciferase replicons, each of which contained a single substitution: P104A, P104V, P104E, or P104R. We first compared the replication efficacies of the WT and mutant replicons in the absence of compound treatment. BHK-21 cells were selected for this experiment because the cell line is incompetent in producing interferon (29). Equal amounts of replicon RNAs were transfected into BHK-21 cells, and luciferase activities were measured at various time points posttransfection. As shown in Fig. 7A, P104R completely abolished viral RNA synthesis; P104E reduced viral RNA replication; and P104A, P104V, and P104L slightly attenuated luciferase activity at 24 h posttransfection.
Fig. 7.
Comparison of DENV-2 replicons containing different substitutions of NS4B P104. (A) Transient replicon transfection assay. BHK-21 cells were electroporated with equal amounts (10 μg) of WT or NS4B mutant (P104A, P104E, P104L, P104R, or P104V) luciferase replicon RNA. After electroporation, the cells were seeded in 12-well plates (2 × 105 cells/well). At the indicated time point p.t., cells were collected, lysed, and assayed for luciferase activity. Each data point represents the log10 value of the average, and the error bars show the standard deviations (n = 3). (B) Resistance analysis of various replicon mutants. A549 cells were transfected with equal amounts (10 μg) of WT or NS4B mutant (P104A, P104V, or P104L) replicon RNA. The transfected cells were seeded in a 96-well plate (2 × 104 cells/well) and immediately treated with serial dilutions of NITD-618 or 0.5% DMSO (as a control). At 48 h p.t., the cells were washed and lysed, and the luciferase signals were quantified. The data are shown as averages and standard deviations from triplicates. The number above each bar shows the percentage of luciferase units in the treated compared to the untreated samples.
Next, we compared drug sensitivities among the mutant replicons by incubating the replicon-transfected cells with NITD-618. A549 cells were used for this experiment because the cell line showed higher sensitivity than BHK-21 and C3/36 cells in compound inhibition (Fig. 5B to D). Luciferase activities at 48 h p.t. showed that mutant replicons were less sensitive to compound inhibition than the WT replicon; the sensitivity to compound inhibition was on the order of WT > P104V > P104A > P104L (Fig. 7B). The mutant P104E replicon was not included in the inhibition assay because the mutation was not stable (see details below). As a positive control, NITD-008, a known nucleoside inhibitor of flavivirus (41), suppressed both WT and mutant replicons with equal potency (data not shown).
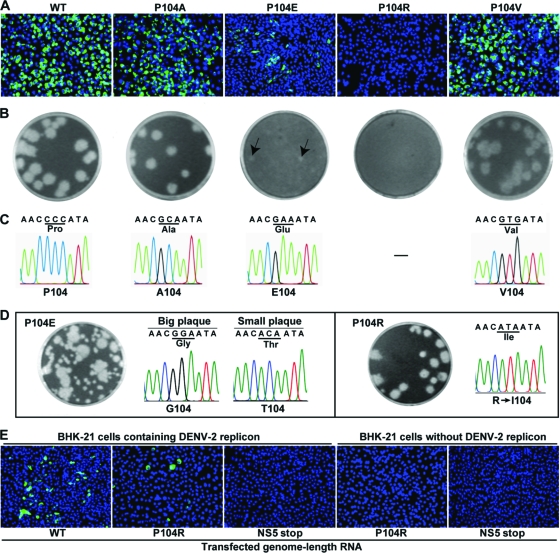
To further analyze the effect of P104 substitution on viral replication, we engineered individual mutation of P104A, P104V, P104E, or P104R into the genome length RNA of DENV-2. Equal amounts of genome length RNAs were transfected into BHK-21 cells and analyzed for their abilities to express viral E protein using an IFA (Fig. 8A). The transfected cells were directly seeded onto monolayers of BHK-21 cells for plaque development (Fig. 8B). (i) For P104A and P104V mutants, the transfected cells generated slightly fewer IFA-positive cells than the WT RNA-transfected cells. Both P104A and P104V RNAs produced infectious viruses with a homogeneous plaque morphology. Sequencing analysis showed that the recovered viruses retained the P104A and P104V mutations without any other changes (Fig. 8C). (ii) For the P104E mutant, the transfected cells yielded far fewer IFA-positive cells than the WT-RNA-transfected cells. The plaques of P104E virus were smaller than those of the WT virus (Fig. 8C). Sequencing of the viruses derived from culture fluids of the transfected cells showed the engineered P104E mutation. Passaging of the recovered viruses on Vero cells for six rounds (4 days per round) generated viruses with mixed plaque morphologies (Fig. 8D). Sequencing of the plaque-purified viruses showed that viruses with big plaques contained a P104E-to-P104G reversion whereas viruses with small plaques accumulated a P104E-to-P104T change (Fig. 8D). These results suggest that the P104E mutation attenuates viral replication and that the P104E mutation could revert to P104G or P104T. (iii) For the P104R mutant, the transfected cells did not generate any IFA-positive cells (Fig. 8A) or plaques (Fig. 8B). Passaging the supernatant from transfected cells on Vero cells for 4 days recovered virus with clear plaque morphology; sequencing of the recovered virus showed a 104R-to-104I reversion (Fig. 8D). These data indicate that the P104R mutation is lethal for viral replication.
Fig. 8.
Analysis of various NS4B P104 mutant viruses. (A) BHK-21 cells were transfected with equal amounts (10 μg) of WT or NS4B mutant genome length RNA of DENV-2. The transfected cells were monitored for viral E protein expression by IFA at 96 h p.t. Anti-E monoclonal antibody 4G2 and Alexa Fluor 488 goat anti-mouse IgG were used as primary and secondary antibodies, respectively. (B) Plaque morphology. Plaques were derived from specific infectivity assays (see Materials and Methods for details). Small plaques of the P104E mutant are indicated by the arrows. (C) Sequencing chromatogram of the mutated NS4B region. Virus RNA recovered from transfected cell supernatant on day 5 p.t. was sequenced for WT, P104A, P104E, and P104V. (D) Revertant analysis of P104E and P104R mutants. For mutant P104E, supernatant from the transfected cells was passaged on Vero cells for six rounds (4 days per round). The passaged viruses yielded mixed plaque morphologies. Plaque purification was performed to isolate viruses exhibiting big plaques and viruses exhibiting small plaques (not shown), and sequencing chromatograms of the plaque-purified viruses are presented. For mutant P104R, supernatant from the transfected cells was passaged on Vero cells for 4 days, and the plaque morphology and sequencing chromatogram of the recovered virus are shown. (E) Trans complementation of the NS4B P104R lethal mutant. BHK-21 cells with or without DENV-2 replicon were transfected with equal amounts (10 μg) of genome length RNA of the WT, NS4B P104R mutant, or NS5stop mutant. The first amino acid (Gly) of NS5 was changed to a UAG stop codon in the NS5stop mutant. At 96 h p.t., the transfected cells were assayed for viral E protein expression using IFA.
We performed full-genome sequencing of the following revertant viruses: P104E-derived virus exhibiting big plaques, P104E-derived virus exhibiting small plaques, and P104R-derived virus. The sequencing results showed that, besides the NS4B mutations presented in Fig. 8D, mutations outside NS4B had accumulated in those revertant viruses (data not shown). Experiments are ongoing to define the functions of the recovered mutations.
Trans complementation of NS4B.
Since NS4B P104R is lethal for viral replication, we asked whether the lethal phenotype could be trans complemented. To address this question, we transfected WT and mutant NS4B P104R genome length RNAs into BHK-21 cells containing the DENV-2 replicon. Since the replicon does not contain viral structural genes, expression of E protein in the transfected cells was used to monitor the replication of genome length RNA. As shown in Fig. 8E, WT genome length RNA yielded E protein-expressing IFA-positive cells. However, the IFA-positive cells were fewer than when naive BHK-21 cells were transfected with the WT RNA (compare Fig. 8A and E); the difference in the number of IFA-positive cells was most likely due to exclusion of homologous virus replication in the replicon-containing cells (47). Interestingly, the NS4B P104R genome length RNA generated IFA-positive signals in the replicon BHK-21 cells (Fig. 8E). As a negative control, transfection of NS5stop RNA (containing a substitution for the first amino acid of NS5 by a stop codon) into the replicon BHK-21 cells did not generate any IFA-positive cells. As expected, no IFA-positive cells were detected when naive BHK-21 cells (without replicon) were transfected with the NS4B P104R or NS5stop RNA (Fig. 8E). The results demonstrate that NS4B could be trans complemented.
Next, we examined whether coinfection of compound-sensitive and compound-resistant viruses would affect antiviral efficacy. To explore this possibility, we transfected A549 cells with WT DENV-2 luciferase replicon. The transfected cells were infected with WT or P104L plus A119T resistant virus. The cells were then treated with NITD-618 and assayed for luciferase activity (derived from the WT replicon). Figure 9A depicts the experimental procedure. The results showed that coinfection with WT virus did not change the sensitivity of the WT replicon to NITD-618 inhibition (Fig. 9B). In contrast, coinfection of P104L plus A119T resistant virus reduced the compound inhibition of the WT replicon (Fig. 9B); such reduction of WT replicon compound inhibition was statistically significant (Fig. 9C). These results indicate that trans complementation of NS4B could affect drug sensitivity when a cell is coinfected with drug-sensitive and drug-resistant viruses.
Fig. 9.
Analysis of trans complementation of resistance. (A) Experimental scheme for trans complementation of resistance. (B) Luciferase activity from the trans complementation experiment. Average luciferase activities (RLU) from quadruplicates are presented. The luciferase activity percentage is equal to the RLU derived from compound-treated sample divided by the RLU derived from the mock-treated control (0.5% DMSO) times 100. (C) Dose-response curve. Prism 5 software was used to plot the data from panel D. The y axis indicates the percent luciferase activity. The x axis indicates the log10 value of the compound concentration. The error bars represent standard deviations (n = 4). The asterisks indicate that the differences are statistically significant (*, P < 0.05; **, P < 0.0001) using a two-way ANOVA test.
DISCUSSION
Both target-based and cell-based approaches have been used to identify small-molecule inhibitors of DENV (27). The target-based approach allows us to identify compounds with known mechanisms. Using this approach, we have identified inhibitors of DENV protease (43, 44), polymerase (26, 41, 42), and methyltransferase (14). The cell-based approach uses viral replication (e.g., replicon or complete virus infection) to screen for compounds that target viral or host factors required for viral replication. Although the cell-based approach has the potential to identify inhibitors of both viral and host targets, this approach rarely reveals inhibitors of viral targets in our HTS campaigns. Such a result is expected, because hundreds of cellular factors are required for a productive viral infection cycle, whereas only 10 viral proteins are produced during flavivirus infection. Therefore, the chances of identifying inhibitors of viral targets are much lower than those of identifying inhibitors of host targets. Indeed, from our DENV replicon “hit” list, NITD-618 is the only identified inhibitor that seems to inhibit a viral protein. To select for inhibitors of viral targets, one could filter the primary screening “hits” using an RNA virus from a different genus (e.g., HCV replicon cell lines). If it inhibits both DENV and HCV replicons, the compound is most likely to target a host factor that is required for both viral replications. If it inhibits only DENV replicon, the compound is likely to target a viral factor or a host factor that is uniquely required for DENV replication. In HCV drug discovery, such a dual-replicon strategy may have contributed to the identification of the NS5A inhibitor (7, 28).
Two lines of evidence indicate that NITD-618 targets DENV NS4B protein. First, the antiviral activity of the compound is specific to DENV. It inhibits all four serotypes of DENV but does not inhibit the closely related flaviviruses (WNV or YFV) or nonflaviviruses. The specificity for DENV inhibition excludes the possibility that the observed antiviral activity is due to compound-mediated cytotoxicity, arguing that NITD-618 suppresses a viral target of DENV. However, although treatment of A549, BHK-21, and Vero cells with NITD-618 (up to 40 μM) for 48 h did not show any dramatic cytotoxicity (Fig. 1D), extensive treatment of HepG2 cells with the compound for 7 days showed cytotoxicity, with a 50% cytotoxic concentration (CC50) value of 4 μM (data not shown). Second, resistance analysis revealed two mutations (P104L and A119T) in NS4B that could confer partial resistance to NITD-618 in mammalian cells. However, only the P104L mutation conferred resistance in C6/36 mosquito cells. Based on the current topology model of NS4B (21), amino acids P104 and A119 are both embedded in the ER membrane. The discrepancy in resistance of the A119T mutation between the mammalian and mosquito cells is likely due to the species-dependent lipid composition of the ER membrane. It should be noted that the current genetic results for the resistance mutation(s) do not prove that NS4B is the target of NITD-618. Therefore, we attempted to establish a biophysical method (e.g., isothermal titration calorimetry or surface plasmon resonance) to show direct binding of NITD-618 to the NS4B protein. Unfortunately, due to the transmembrane nature of NS4B, we were not able to generate soluble recombinant protein of DENV NS4B for the biophysical experiments (data not shown).
The molecular details of how NS4B participates in flavivirus replication remain elusive. NS4B is involved in a replication complex on the ER membrane (17). The current study has provided two lines of evidence to indicate that DENV NS4B could be complemented in trans. First, using a lethal NS4B P104R mutant, we showed that transfection of this mutant RNA into cells containing WT replicating replicon could rescue the replication of the mutant RNA (Fig. 8). Second, when cells were coinfected with drug-resistant and drug-sensitive viruses, the presence of resistant virus reduced the compound inhibition of the drug-sensitive virus (Fig. 9). Interestingly, the presence of resistant virus did not confer full resistance on the drug-sensitive viral RNA. This result suggests that some NS4B molecules in the replication complexes (RCs) are derived from trans; trans complementation of these NS4B molecules from the drug-resistant virus to the drug-sensitive RCs confers partial resistance. On the other hand, some NS4B molecules in the RCs must be cis synthesized; the cis required NS4B molecules in the drug-sensitive RCs could not be swapped with the NS4B molecules derived from the drug-resistant virus.
Besides trans complementation, DENV-2 NS4B was previously shown to bind to the C-terminal helicase domain of NS3 (amino acids 303 to 618). The formation of an NS3-NS4B complex triggers NS3 to release single-stranded RNA and, consequently, enhances the helicase activity of NS3 (36). Remarkably, the P104L mutation in NS4B was previously shown to abolish the NS3-NS4B interaction (36). Because residue P104 of NS4B is embedded in the ER membrane (21), the amino acid is not expected to directly interact with the NS3 protein. Additionally, since Pro has a profound effect on the secondary structure of protein, the P104L change could affect the folding and structure of NS4B. These biochemical results raise the possibility that NITD-618 may interfere with NS3-NS4B complex formation, leading to a reduction of viral RNA synthesis.
In addition to NS3-NS4B interaction, NS4B was also shown to antagonize the host interferon response (9, 18, 23). A mutation that changes the efficiency of NS4B in antagonizing host innate immunity could lead to a difference in virus yields. To test this hypothesis, we compared the efficiencies of the WT and mutant NS4B (P104L) in blocking the interferon response in Vero and 293T cells. The results showed that the P104L mutation does not change the efficiency of NS4B-mediated interferon inhibition (data not shown).
DENV-4 was previously reported to accumulate NS4B P101L mutations after chemical mutagenesis (10). Sequence alignment showed that the DENV-4 P101L mutation is equivalent to the DENV-2 Pl04L mutation described in the current study. In the previous study, the DENV-4 NS4B P101L mutation was found to increase viral replication in Vero cells but to decrease replication in C6/36 cells. The opposing effects of this mutation in mosquito and mammalian cells suggest that the protein is involved in maintaining a balance between efficient replication in the mosquito vector and the human host (10). In agreement with these results, we also found that the DENV-2 NS4B P104L mutation affected viral replication in a host species-dependent manner. The DENV-2 P104L mutation reduced viral replication in C6/36 cells (Fig. 5D); for mammalian cells, the mutation increased viral replication in Vero cells (data not shown) but slightly decreased viral replication in A549 cells (Fig. 5B). The latter results indicate that, even in mammalian cells, the effect of NS4B P104 mutation on viral replication is host species dependent.
In summary, we have identified an inhibitor that specifically blocks DENV RNA replication. Genetic analysis demonstrated that mutations in viral NS4B confer compound resistance. The identified NS4B resistance mutations affect viral replication in a host species-dependent manner. The current study also demonstrates that DENV NS4B could be trans complemented in cell culture. Such NS4B trans complementation could affect drug sensitivity when a single cell is coinfected with drug-sensitive and drug-resistant viruses. The resistance results, together with a previous biochemistry study (36), strongly suggest that the compound targets viral NS4B protein.
ACKNOWLEDGMENTS
We thank Shahul Nilar for analysis of screening hits, Swee Hoe Ong for sequence alignment, and Wouter Schul for critical reading of the manuscript. We also thank colleagues at Novartis Institute for Tropical Diseases for helpful discussions and support during the course of this study.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Ackermann M., Padmanabhan R. 2001. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276:39926–39937 [DOI] [PubMed] [Google Scholar]
- 2. Bodenreider C., et al. 2009. A fluorescence quenching assay to discriminate between specific and nonspecific inhibitors of dengue virus protease. Anal. Biochem. 395:195–204 [DOI] [PubMed] [Google Scholar]
- 3. Chambers T. J., Grakoui A., Rice C. M. 1991. Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J. Virol. 65:6042–6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung K. Y., et al. 2010. Higher catalytic efficiency of N-7-methylation is responsible for processive N-7 and 2′-O methyltransferase activity in dengue virus. Virology 402:52–60 [DOI] [PubMed] [Google Scholar]
- 5. Egloff M. P., Benarroch D., Selisko B., Romette J. L., Canard B. 2002. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 21:2757–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falgout B., Miller R. H., Lai C. J. 1993. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J. Virol. 67:2034–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao M., et al. 2010. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gubler D., Kuno G., Markoff L. 2007. Flaviviruses, p. 1153–1253 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 1 Lippincott William & Wilkins, Philadelphia, PA [Google Scholar]
- 9. Guo J., Hayashi J., Seeger C. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 79:1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanley K., et al. 2003. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology 312:222–232 [DOI] [PubMed] [Google Scholar]
- 11. Kummerer B. M., Rice C. M. 2002. Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J. Virol. 76:4773–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lemm J. A., et al. 2010. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 84:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H., Clum S., You S., Ebner K. E., Padmanabhan R. 1999. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J. Virol. 73:3108–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim S. P., et al. 2011. Small molecule inhibitors that selectively block dengue virus methyltransferase. J. Biol. Chem. 286:6233–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindenbach B., Rice C. 1997. Trans-complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 71:9608–9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindenbach B. D., Rice C. M. 1999. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J. Virol. 73:4611–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindenbach B. D., Thiel H.-J., Rice C. M. 2007. Flaviviridae: the virus and their replication, p. 1101–1152 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 1 Lippincott William & Wilkins, Philadelphia, PA [Google Scholar]
- 18. Liu W., et al. 2005. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 79:1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu W. J., Chen H. B., Khromykh A. A. 2003. Molecular and functional analyses of Kunjin virus infectious cDNA clones demonstrate the essential roles for NS2A in virus assembly and for a nonconservative residue in NS3 in RNA replication. J. Virol. 77:7804–7813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller S., Kastner S., Krijnse-Locker J., Buhler S., Bartenschlager R. 2007. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 282:8873–8882 [DOI] [PubMed] [Google Scholar]
- 21. Miller S., Sparacio S., Bartenschlager R. 2006. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J. Biol. Chem. 281:8854–8863 [DOI] [PubMed] [Google Scholar]
- 22. Munoz-Jordan J. L., et al. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munoz-Jordan J. L., Sanchez-Burgos G. G., Laurent-Rolle M., Garcia-Sastre A. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. U. S. A. 100:14333–14338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng C. Y., et al. 2007. Construction and characterization of a stable subgenomic dengue virus type 2 replicon system for antiviral compound and siRNA testing. Antiviral Res. 76:222–231 [DOI] [PubMed] [Google Scholar]
- 25. Niyomrattanakit P., et al. 2010. A fluorescence-based alkaline phosphatase-coupled polymerase assay for identification of inhibitors of dengue virus RNA-dependent RNA polymerase. J. Biomol. Screen. 16:201–210 [DOI] [PubMed] [Google Scholar]
- 26. Niyomrattanakit P., et al. 2010. Inhibition of dengue virus polymerase by blocking of the RNA tunnel. J. Virol. 84:5678–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noble C. G., et al. 2010. Strategies for development of dengue virus inhibitors. Antiviral Res. 85:450–462 [DOI] [PubMed] [Google Scholar]
- 28. O'Boyle D. R., II, et al. 2005. Development of a cell-based high-throughput specificity screen using a hepatitis C virus-bovine viral diarrhea virus dual replicon assay. Antimicrob. Agents Chemother. 49:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otsuki K., Maeda J., Yamamoto H., Tsubokura M. 1979. Studies on avian infectious bronchitis virus (IBV). III. Interferon induction by and sensitivity to interferon of IBV. Arch. Virol. 60:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puig-Basagoiti F., et al. 2006. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob. Agents Chemother. 50:1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qing M., Liu W., Yuan Z., Gu F., Shi P. Y. 2010. A high-throughput assay using dengue-1 virus-like particles for drug discovery. Antiviral Res. 86:163–171 [DOI] [PubMed] [Google Scholar]
- 32. Ray D., et al. 2006. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J. Virol. 80:8362–8370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robida J. M., Nelson H. B., Liu Z., Tang H. 2007. Characterization of hepatitis C virus subgenomic replicon resistance to cyclosporine in vitro. J. Virol. 81:5829–5840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi P. Y., Tilgner M., Lo M. K., Kent K. A., Bernard K. A. 2002. Infectious cDNA clone of the epidemic West Nile virus from New York City. J. Virol. 76:5847–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan B. H., et al. 1996. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology 216:317–325 [DOI] [PubMed] [Google Scholar]
- 36. Umareddy I., Chao A., Sampath A., Gu F., Vasudevan S. G. 2006. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 87:2605–2614 [DOI] [PubMed] [Google Scholar]
- 37. Wang Q. Y., et al. 2009. A small-molecule dengue virus entry inhibitor. Antimicrob. Agents Chemother. 53:1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wengler G., Wengler G. 1991. The carboxy-terminal part of the NS 3 protein of the West Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology 184:707–715 [DOI] [PubMed] [Google Scholar]
- 39. Wengler G., Wengler G. 1993. The NS3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology 197:265–273 [DOI] [PubMed] [Google Scholar]
- 40. Xu T., et al. 2005. Structure of the Dengue virus helicase/nucleoside triphosphatase catalytic domain at a resolution of 2.4 A. J. Virol. 79:10278–10288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yin Z., et al. 2009. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 106:20435–20439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin Z., et al. 2009. N-sulfonylanthranilic acid derivatives as allosteric inhibitors of dengue viral RNA-dependent RNA polymerase. J. Med. Chem. 52:7934–7937 [DOI] [PubMed] [Google Scholar]
- 43. Yin Z., et al. 2006. Peptide inhibitors of dengue virus NS3 protease. Part 2: SAR study of tetrapeptide aldehyde inhibitors. Bioorg. Med. Chem. Lett. 16:40–43 [DOI] [PubMed] [Google Scholar]
- 44. Yin Z., et al. 2006. Peptide inhibitors of Dengue virus NS3 protease. Part 1: Warhead. Bioorg. Med. Chem. Lett. 16:36–39 [DOI] [PubMed] [Google Scholar]
- 45. Zou G., et al. 2011. Functional analysis of two cavities in flavivirus NS5 polymerase. J. Biol. Chem. 286:14362–14372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zou G., Xu H. Y., Qing M., Wang Q.-Y., Shi P.-Y. 2011. Development and characterization of a stable luciferase dengue virus for high-throughput screening. Antiviral Res. 91:11–19 [DOI] [PubMed] [Google Scholar]
- 47. Zou G., et al. 2009. Exclusion of West Nile virus superinfection through RNA replication. J. Virol. 83:11765–11776 [DOI] [PMC free article] [PubMed] [Google Scholar]