Abstract
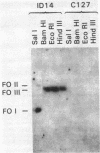
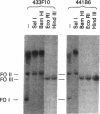
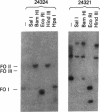
The viral DNA sequences in mouse C127 cells transformed by bovine papillomavirus type 1 (BPV-1) virions, by full-length linear BPV-1 DNA, or by a defined transforming subgenomic DNA segment of BPV-1 were examined by reassociation kinetics and blot hybridization. In all cases, the transformed cells contained multiple copies of BPV-1 DNA, present exclusively as supercoiled or nicked circular extrachromosomal molecules or as a slowly migrating complex of circular viral DNA molecules. In the transformed cell lines established from cells transfected with full-length linear BPV-1 DNA, there was recircularization of the input DNA which in some cases resulted in the loss of the restriction site used in the linearization of the DNA. In the transformed cell lines established with the defined subgenomic segment there was circularization of the DNA accompanied by the acquisition of new sequences or duplication and rearrangement of the BPV-1 sequences. In contrast to other well-studied virus transformation systems, no integration of the BPV-1 genome into the host chromosome could be detected under conditions sensitive enough to detect 0.1-0.2 viral genome equivalent. It was concluded that maintenance of transformation may be mediated by nonintegrated viral DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., HARTLEY J. W., ROWE W. P., HUEBNER R. J. TRANSFORMATION OF BOVINE TISSUE CULTURE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1963 Sep 7;199:1016–1018. doi: 10.1038/1991016a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., LEVY J. P., THOMAS M., FRIEDMANN J. C., BERNARD J. SOME PROPERTIES OF BOVINE PAPILLOMA VIRUS. Nature. 1964 Jan 25;201:423–424. doi: 10.1038/201423a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., THOMAS M., CHENAILLE P. A BIOLOGICAL PROPERTY OF DEOXYRIBONUCLEIC ACID EXTRACTED FROM BOVINE PAPILLOMA VIRUS. Virology. 1965 May;26:150–153. doi: 10.1016/0042-6822(65)90037-1. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birg F., Dulbecco R., Fried M., Kamen R. State and organization of polyoma virus DNA sequences in transformed rat cell lines. J Virol. 1979 Feb;29(2):633–648. doi: 10.1128/jvi.29.2.633-648.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenciner N., Grossi M. P., Meneguzzi G., Corallini A., Manservigi R., Barbanti-Brodano G., Milanesi G. State of viral DNA in BK virus-transformed rabbit cells. Virology. 1980 May;103(1):138–148. doi: 10.1016/0042-6822(80)90132-4. [DOI] [PubMed] [Google Scholar]
- Chenciner N., Meneguzzi G., Corallini A., Grossi M. P., Grassi P., Barbanti-Brodano G., Milanesi G. Integrated and free viral DNA in hamster tumors induced by BK virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):975–979. doi: 10.1073/pnas.77.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury K., Gruss P., Waldeck W., Sauer G. Action of S1 nuclease on nicked circular simian virus 40 DNA. Biochem Biophys Res Commun. 1975 May 19;64(2):709–716. doi: 10.1016/0006-291x(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L., Monier R. Presence of free viral DNA in simian virus 40-transformed nonproducer cells. J Virol. 1978 Aug;27(2):307–312. doi: 10.1128/jvi.27.2.307-312.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN J. C., LEVY J. P., LASNERET J., THOMAS M., BOIRON M., BERNARD J. INDUCTION DE FIBROMES SOUS-CUTAN'ES CHEZ LE HAMSTER DOR'E PAR INOCULATION D'EXTRAITS ACELLULAIRES DE PAPILLOMES BOVINS. C R Hebd Seances Acad Sci. 1963 Oct 14;257:2328–2331. [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Heilman C. A., Law M. F., Israel M. A., Howley P. M. Cloning of human papilloma virus genomic DNAs and analysis of homologous polynucleotide sequences. J Virol. 1980 Nov;36(2):395–407. doi: 10.1128/jvi.36.2.395-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson A. B., Rosenthal J. D., Olson C., Pass F., Lancaster W. D., Shah K. Immunologic relatedness of papillomaviruses from different species. J Natl Cancer Inst. 1980 Mar;64(3):495–500. [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Bovine papilloma virus: presence of virus-specific DNA sequences in naturally occurring equine tumors. Proc Natl Acad Sci U S A. 1977 Feb;74(2):524–528. doi: 10.1073/pnas.74.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D. Physical maps of bovine papillomavirus type 1 and type 2 genomes. J Virol. 1979 Nov;32(2):684–687. doi: 10.1128/jvi.32.2.684-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lancaster W. D., Howley P. M. Conserved polynucleotide sequences among the genomes of papillomaviruses. J Virol. 1979 Oct;32(1):199–207. doi: 10.1128/jvi.32.1.199-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meischke H. R. In vitro transformation by bovine papilloma virus. J Gen Virol. 1979 Jun;43(3):473–487. doi: 10.1099/0022-1317-43-3-473. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Allison A. C., Butel J. S., Eckhart W., Eddy B. E., Kit S., Levine A. J., Miles J. A., Pagano J. S., Sachs L. Papovaviridae. Intervirology. 1974;3(1-2):106–120. doi: 10.1159/000149746. [DOI] [PubMed] [Google Scholar]
- Olson C., Gordon D. E., Robl M. G., Lee K. P. Oncogenicity of bovine papilloma virus. Arch Environ Health. 1969 Dec;19(6):827–837. doi: 10.1080/00039896.1969.10666938. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puget A., Favre M., Orth G. Induction de tumeurs fibroblastiques cutanées ou sous-cutanées chez l'Ochotone afghan (Ochotona rufescens rufescens) par inoculation du virus du papillome bovin. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jun 23;280(24):2813–2816. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- THOMAS M., BOIRON M., TANZER J., LEVY J. P., BERNARD J. IN VITRO TRANSFORMATION OF MICE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1964 May 16;202:709–710. doi: 10.1038/202709a0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nonoyama M. Latent DNA of Epstein-Barr virus: separation from high-molecular-weight cell DNA in a neutral glycerol gradient. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4658–4661. doi: 10.1073/pnas.71.12.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- ter Schegget J., Voves J., van Strien A., van der Noordaa J. Free viral DNA in BK virus-induced hamster tumor cells. J Virol. 1980 Aug;35(2):331–339. doi: 10.1128/jvi.35.2.331-339.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]