Abstract
In vitro blood-brain barrier (BBB) models often consist of brain microvascular endothelial cells (BMECs) that are co-cultured with other cells of the neurovascular unit, such as astrocytes and neurons, in order to enhance BBB properties. Obtaining primary astrocytes and neurons for co-culture models can be laborious, while yield and heterogeneity of primary isolations can also be limiting. Neural progenitor cells (NPCs), due to their self-renewal capacity and ability to reproducibly differentiate into tunable mixtures of neurons and astrocytes, represent a facile, readily scalable alternative. To this end, differentiated rat NPCs were co-cultured with rat BMECs and shown to induce BBB properties such as elevated trans-endothelial electrical resistance (TEER), improved tight junction continuity, polarized p-glycoprotein efflux, and low passive permeability at levels indistinguishable from those induced by primary rat astrocyte co-culture. An NPC differentiation time of 12 days, with the presence of 10% fetal bovine serum, was found to be crucial for generating NPC-derived progeny capable of inducing the optimal response. This approach could also be extended to human NPC-derived astrocytes and neurons which similarly regulated BBB induction. The distribution of rat or human NPC-derived progeny under these conditions was found to be a roughly 3:1 mixture of astrocytes to neurons with varying degrees of cellular maturity. BMEC gene expression analysis was conducted using a BBB gene panel, and it was determined that 23 of 26 genes were similarly regulated by either differentiated rat NPC or rat astrocyte co-culture while 3 genes were differentially altered by the rat NPC-derived progeny. Taken together, these results demonstrate that NPCs are an attractive alternative to primary neural cells for use in BBB co-culture models.
Introduction
The blood-brain barrier (BBB) is formed by the microvascular endothelial cells (BMECs) which line brain capillaries. BMECs are linked by intercellular tight junction protein complexes and lack fenestrae, thus restricting passive molecular transport between the brain and bloodstream. In addition, using specific transport proteins, the BBB maintains ionic homeostasis for proper neuron function and facilitates nutrient and metabolite import and export. The BBB also prevents toxic substances from penetrating and accumulating in the brain by employing a variety of efflux pumps. It is believed that a complex interplay between endothelial cells, astrocytes, neurons, and pericytes leads to regulation of these specific barrier properties within the neurovascular unit (Lok et al. 2007). Many researchers have attempted to re-create the neurovascular microenvironment in vitro to probe neural/endothelial cell-cell interactions, study neurological diseases, and screen for brain-penetrating pharmaceuticals. Early models focused on astrocytes to help modulate BBB properties in cultured BMECs because astrocytes were shown to be key modulators of BMEC permeability in vivo (Janzer and Raff 1987). Primary astrocytes co-cultured with BMECs can favorably affect BBB properties such as trans-endothelial electrical resistance (TEER) and permeability in vitro (reviewed in (Deli et al. 2005)). Pericyte co-culture with BMECs has been shown to upregulate TEER (Nakagawa et al. 2007; Nakagawa et al. 2009), decrease permeability (Dohgu et al. 2005; Nakagawa et al. 2007; Nakagawa et al. 2009), and cause structural reorganization (Ramsauer et al. 2002). Additionally, co-culture of BMECs with both astrocytes and pericytes was shown to enhance this TEER increase and permeability reduction compared to either cell type alone (Nakagawa et al. 2009). Neurons have been shown to impact the correct localization of the tight junction protein occludin in a BBB model (Savettieri et al. 2000; Schiera et al. 2003) and reduce permeability, an effect that was enhanced by triple co-culture with neurons and astrocytes (Schiera et al. 2005). Neurons can also increase enzymatic activities of γ-glutamyl transpeptidase and Na+-K+ ATPase in BMECs (Tontsch and Bauer 1991). Thus, many cellular components of the neurovascular unit can contribute to BBB properties in vitro, and combinations of these cells have been shown to modulate properties more effectively than the individual cell types alone.
A significant consideration in the set-up of in vitro BBB models is the acquisition of neural cells. Astrocytes, neurons, and pericytes are usually obtained from primary culture of brain tissue. Some disadvantages of primary culture include the low amount and purity of cells obtained and the cellular heterogeneity amongst different isolations. In addition, the ages of animals used for the isolation of BMECs (adult), astrocytes (early postnatal), and neurons (embryonic) are all different, making for a laborious process. Furthermore, limited yield and availability of primary tissue from human sources has restricted the development of a widely employed and robust human in vitro BBB model. To circumvent these challenges characteristic to BBB co-culture models, we have identified neural progenitor cells (NPCs) as an attractive alternative to primary astrocytes and neurons. NPCs proliferate extensively in the presence of specific growth factors due to their stem cell-like properties while maintaining a stable gene expression profile (Wright et al. 2003), and they have the capability to differentiate into both neuronal and glial lineages under a variety of conditions (Ostenfeld and Svendsen 2003). Because a large number of NPCs can be generated from a single primary isolation, concerns regarding yield as well as heterogeneity between isolations are diminished. Differentiating NPCs have also been previously shown to participate in cell-cell interactions with BMECs in vitro (Weidenfeller et al. 2007) and may be crucial for BBB development in vivo (Stenman et al. 2008; Daneman et al. 2009). In this study, embryonic rat NPCs were differentiated to form mixtures of astrocytes and neurons for co-culture with puromycin-purified rat BMECs to test their suitability for BBB models. We demonstrate optimal conditions for generating mixtures of NPC-derived progeny capable of inducing a BBB phenotypic response indistinguishable from that generated by primary astrocytes. The model quality was evaluated further using a previously established BBB gene panel useful for benchmarking in vitro models (Calabria and Shusta 2008). Finally, we show that differentiated human NPCs can induce a TEER response similar to rat NPCs, which suggests this NPC-based system could eventually be translated to a fully human BBB platform.
Materials and Methods
Isolation of rat brain microvascular endothelial cells
All animal work was performed using protocols approved by University of Wisconsin-Madison Animal Care and Use Committees and following NIH guidelines for care and use of laboratory animals. Rat brain capillaries were isolated as previously described (Calabria et al. 2006). Adult male Sprague Dawley rats (Harlan; 220-250 grams) were anesthetized and decapitated. Brain cortices were isolated and the meninges were removed. After being minced and triturated, the tissue was digested at 37 °C for 1.25 h with collagenase type-2 and DNase I (Worthington Biochemical Corporation, Lakewood, NJ, USA) at respective concentrations of 0.7 mg/mL and 39 U/mL in DMEM. The digested material was centrifuged in a 20% (w/v) solution of bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) in DMEM and the purified microvessel pellet was digested further in 1 mg/mL collagenase/dispase (Roche Applied Sciences, Indianapolis, IN, USA) and 39 U/mL DNase I in DMEM for 1 h at 37 °C. Pure capillaries were obtained using a continuous 33% Percoll (GE Healthcare, Piscataway, NJ, USA) gradient and plated onto 1.12 cm2 Transwell-Clear® permeable inserts (0.4 μm pore size) coated with collagen IV/fibronectin (Sigma). Capillaries were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) containing 20% (v/v) platelet-poor plasma-derived serum (PDS; Biomedical Technologies Inc., Stoughton, MA, USA), 1 ng/mL basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA), 1 μg/mL heparin (Sigma), 2 mmol/L L-glutamine (Sigma), and 1% (v/v) antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA, USA). This culture medium was supplemented with 4 μg/mL puromycin (Sigma) for 2 days after seeding to eliminate contaminating cells and puromycin-free medium was used for 2 days thereafter. The cultures were maintained in a 37 °C incubator under humidified 5% CO2/95% air. Co-culture experiments were started when the BMECs reached confluence after 4 days of growth in vitro.
Isolation and culture of rat cortical embryonic NPCs and human NPCs
Rat cortical NPCs were isolated as previously described (Weidenfeller et al. 2007). Cortices were dissected from E14 rat brains in Hanks’ balanced salt solution (HBSS; Sigma), treated with accutase (Invitrogen) for 10 min in a standing water bath, washed with DMEM, and triturated to a single-cell suspension. Cells were seeded in T25 flasks at a density of 2×104 cells/mL in serum-free medium: 70%/30% DMEM/Ham’s F-12 (Invitrogen), supplemented with 2% (v/v) B27 (Invitrogen), 1% (v/v) antibiotic-antimycotic, 20 ng/mL epidermal growth factor (EGF; Sigma), 20 ng/mL bFGF, and 5 μg/mL heparin. Cells were grown as free-floating neurospheres for 4-5 days, then passaged to a single-cell suspension with accutase and subsequently frozen in liquid nitrogen as stock vials for co-culture experiments. Typical yields from pre-frozen NPCs were 4-5×106 cells/flask and cells were frozen at 1×106 cells/vial. Typical yields from pre-frozen NPCs were 4-5×106 cells/flask and cells were frozen at 1×106 cells/vial. Human NPCs derived from the frontal cortex of 8 week old embryo’s (a kind gift of Dr. Guido Nikkhah) prepared as described previously (Svendsen et al. 1998) and maintained in T75 flasks containing 20 mL of the same serum-free medium as rat NPCs plus 10 ng/mL leukemia inhibitory factor (LIF; Millipore, Billerica, MA, USA). 10 mL of spent medium was removed every 2-3 days and replaced with fresh medium. Human NPCs were passaged every 7-10 days using standard chopping methods (Svendsen et al. 1998).
Isolation and culture of primary astrocytes
Astrocytes were isolated as previously described (Weidenfeller et al. 2007). Cortices were isolated from P6 neonatal rats and minced in a Petri dish containing HBSS. This tissue was digested first in HBSS containing 0.5 mg/mL trypsin (Mediatech Inc., Manassas, VA, USA) in a 37 °C shaker bath for 25 min, followed by digestion in HBSS containing 114 U/mL DNase I in a 37 °C shaker bath for 5 min. The resulting cell mixture was triturated and filtered with 70-μm mesh. Cells were plated at a density of 2.5×104 cells/cm2 in flasks or culture wells coated with 50 μg/mL collagen I (Sigma) and cultured in DMEM containing 10% (v/v) qualified heat-inactivated fetal bovine serum (FBS; Invitrogen), 10% (v/v) heat-inactivated horse serum (Sigma), 2 mmol/L L-glutamine, and 1% (v/v) antibiotic-antimycotic. After 1-2 weeks, astrocytes were either used in co-culture experiments as “fresh” astrocytes or trypsinized and frozen in liquid nitrogen for subsequent use in co-culture experiments as “thawed” astrocytes.
Co-culture of BMECs with differentiated NPCs or astrocytes
Frozen NPCs were thawed at a density of 2×104 cells/mL. After 3 days of growth, free-floating neurospheres were dissociated with accutase to form a single-cell suspension and plated onto poly-L-lysine/laminin-coated 12-well plates at a density of 5×105 cells/well. Per-vial yields ranged from 10-20 million cells. Plated NPCs were differentiated in 70%/30% DMEM/Ham’s F-12, 2% (v/v) B27, 1% (v/v) antibiotic-antimycotic, and concentrations of FBS ranging from 1% to 10% (v/v). Human NPCs were similarly plated, except at a density of 2.5×105 cells/well. Figure 1 outlines the differentiation schedule and variables with respect to BMEC co-cultures. Frozen astrocytes were thawed and seeded onto 12-well plates coated with collagen I at a density of 2.5×104 cells/cm2 in astrocyte culture medium and grown an additional 1-2 weeks prior to co-culture with BMECs. Fresh astrocytes were also used for co-culture 1-2 weeks after isolation. Cells were treated with 0.25 mmol/L dibutyryl cAMP for 3 days prior to co-culture. Co-culture was initiated in NPC differentiation media containing 1% or 10% FBS. Negative controls were either monoculture BMECs or BMECs co-cultured with embryonic mouse fibroblasts (3T3s; ATCC).
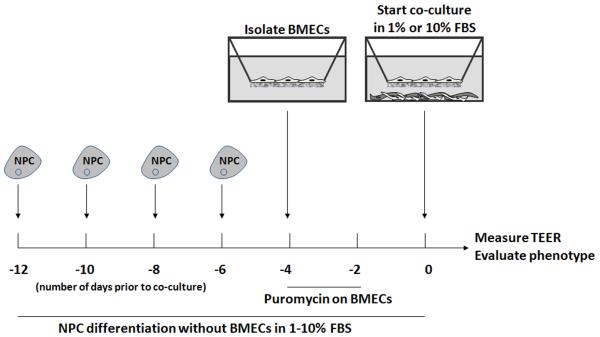
Figure 1. Experimental set-up for NPC differentiation and co-culture with BMECs.
NPCs were grown as free-floating neurospheres, passaged with accutase, and plated in media containing serum to facilitate differentiation. NPCs were plated at two-day intervals for 6 to 12 days prior to co-culture. BMECs were isolated 4 days prior to co-culture, purified for 2 days using puromycin, and cultured an additional 2 days. BMEC-containing filters were added to the differentiated NPC wells and fresh medium was added to both the filter and the well, and TEER was measured immediately following and every 24 hours thereafter.
Resistance measurements
Transendothelial electrical resistance measurements were performed using an EVOM voltohmmeter (World Precision Instruments, Sarasota, FL, USA). The resistance value (Ω × cm2) of an empty filter coated with collagen/fibronectin was subtracted from each measurement. Three measurements were taken per filter, and each culture condition was performed with triplicate filters to obtain average TEER and standard deviation.
Permeability measurements
Permeability measurements were conducted similar to previous experiments (Calabria et al. 2006). BMECs were co-cultured with 12-day differentiated NPCs, astrocytes, or mouse 3T3s in medium containing 10% FBS. After 72 h of co-culture, fresh medium was added to the top and bottom of each filter, with the media in the upper chamber containing 1 μM sodium fluorescein (376 Da; Sigma). 200 μL aliquots were taken from the bottom chamber at time points of 0, 15, 30, 45, and 60 min, and pre-warmed medium was immediately added to replace the removed volume. Based on the influx of fluorescein to the bottom chamber, permeability coefficients were calculated. Triplicate filters were used for each condition of interest to calculate average and standard deviation.
P-glycoprotein activity/polarization assay
P-glycoprotein activity was assayed essentially as previously described (Perriere et al. 2007). BMECs were co-cultured with 12-day differentiated NPCs or astrocytes in medium containing 10% FBS for 72 h. BMECs were then pre-incubated with or without 5 μM cyclosporin A (Sigma) for 30 min on a rocking platform. Fresh medium was then added to the apical and basolateral chambers, with either the apical or basolateral chamber containing 10 μM rhodamine 123 (Sigma) with or without 5 μM cyclosporin A. After 1 h (apical to basolateral transport) or 3 h (basolateral to apical transport) of incubation, 200 μL of medium was extracted from the opposite chamber and fluorescence was measured. Results were normalized to transport in the absence of inhibitor. TEER was measured at the culmination of the experiment to confirm the integrity of the BMEC monolayer.
Immunocytochemistry
All immunolabeling steps were performed at room temperature. Cells were washed three times with 0.01 M phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde (2% w/v in PBS) for 15 min. Cells were then blocked and permeabilized for 30 min using a mixture of 0.1% (v/v) Triton X-100 (Fisher) and 40% (v/v) goat serum (Sigma) in PBS (PBSG). Primary antibodies for astrocyte, neuron, oligodendrocyte, pericyte, and NPC detection were anti-glial fibrillary acidic protein (GFAP, 1:500 dilution; Dako, Carpinteria, CA, USA), anti-βIII tubulin (1:1000 dilution; Sigma), anti-O4 (1:100 dilution; Millipore), anti-α-smooth muscle actin (1:100 dilution; American Research Products, Belmont, MA, USA), anti-NG2 (1:100 dilution; Millipore) and anti-nestin (mouse anti-rat monoclonal, 1:1000 dilution, BD Biosciences, San Jose, CA, USA; rabbit anti-rat polyclonal, 1:500 dilution, Sigma; mouse anti-human, 1:500 dilution, Millipore), respectively. BMECs were labeled with anti-zonula occluden-1 (ZO-1, 1:100 dilution; Invitrogen), anti-occludin (1:100 dilution; Invitrogen), or anti-claudin-5 (1:100 dilution; Invitrogen) antibodies. Primary antibody labeling was performed for 1 h in PBSG. After three washes in PBS, cells were labeled with secondary antibodies in PBSG for 1 h (Texas Red goat anti-rabbit IgG or Alexa Fluor 488 goat anti-mouse IgG at 1:1000 dilution; Invitrogen). Cell nuclei were labeled with 4′,6-Diamidino-2-pheny-lindoldihydrochloride (DAPI) at a concentration of 300 nM for 10 min. Cells were washed an additional three times in PBS and visualized using an Olympus fluorescence microscope (Melville, NY, USA). Photographs were taken using a Diagnostic Instruments camera (Sterling Heights, MI, USA) run by MetaVue software (Molecular Devices Corp., Sunnyvale, CA, USA). Negative controls were rabbit IgG (Sigma) and mouse IgG1κ (BD Biosciences) at the concentrations of the primary antibodies. For quantitative analysis of BMEC integrity, the percentage of cells expressing frayed tight junctions was counted using BMECs immunolabeled for ZO-1. Cells were defined as having frayed tight junctions if greater than 10% of the immunolabeled tight junction protrusions were not parallel to the cell-cell border. A minimum of three separate frames and 250 total cells were counted to obtain an average percentage of frayed tight junctions. To quantify O4 expression, three separate frames totaling a minimum of 1500 cells were counted.
Flow cytometry
NPCs or astrocytes were washed once with 37 °C pre-warmed PBS and incubated with 0.25% trypsin/EDTA for 5 min. The reaction was quenched with NPC medium and cells were pipetted gently from the surface of the plate. Cells were resuspended in PBS, counted on a hemacytometer, and fixed in 2% paraformaldehyde for 20 min at room temperature. Cells were then incubated in PBSG containing 0.1% Triton X-100 for 20 min at room temperature. Primary antibodies were added to the cells for 30 min at the following dilutions in PBSG: GFAP (1:5000), βIII tubulin (1:3000), and nestin (1:3000) with control rabbit IgG and control mouse IgG at matching concentrations. Cells were then washed three times in PBS containing 1% FBS and 0.1% sodium azide. Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 647 goat anti-mouse IgG were added to the cells at a dilution of 1:200 in PBSG and incubated for 30 min. Cells were washed three times and analyzed using a FACScaliber (BD, Franklin Lakes, NJ, USA). The rabbit and mouse IgG samples were used to set gates for GFAP+/nestin+, GFAP+-only, βIII tubulin+/nestin+, βIII tubulin+-only, and nestin+-only events (Supplemental Fig. 1).
Quantitative analysis of gene expression
BMECs were co-cultured with 12-day differentiated NPCs, fresh astrocytes, or mouse 3T3s for 72 h in 10% FBS. Total RNA was isolated from BMECs using the RNeasy kit (Qiagen, Valencia, CA, USA). Genomic DNA was removed during RNA isolation using an RNase-free DNase kit (Qiagen) and RNA concentration was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop products, Wilmington, DE, USA). 60 ng of each RNA sample was reverse-transcribed using the Omniscript RT kit (Qiagen). 2 ng of cDNA was added to each quantitative PCR sample (iQ SYBR Green kit, Biorad, Hercules, CA, USA), and each reaction was carried out on a Bio-Rad iCycler thermal cycler. Primer sequences were used from a previously established gene panel (Supplemental Table 1) and relative gene expression was calculated as previously described (Calabria and Shusta 2008). Briefly, the cycle number (Ct) of β-actin was subtracted from the Ct of each gene of interest to normalize gene expression within a biological sample (ΔCt) and these ΔCt values were subtracted between comparative samples to determine delta-delta cycle threshold values (ΔΔCt) and differential gene expression. Triplicate data points were used to determine mean ΔΔCt values and associated standard deviation. RNA from two separate co-culture experiments was used to verify biological reproducibility.
Results
Impact of differentiation time and serum concentration on the ability of NPCs to induce a TEER response in BMECs
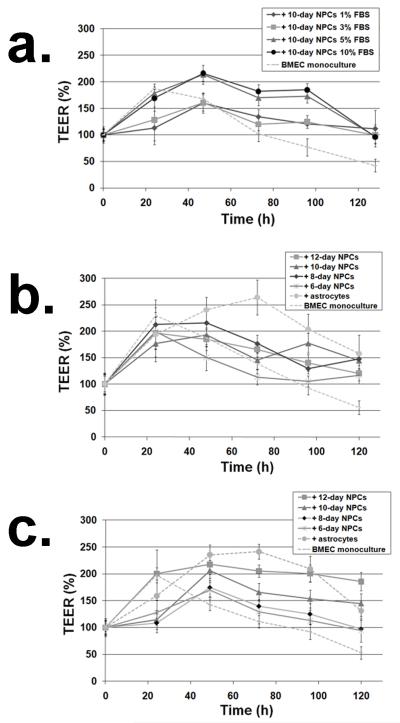
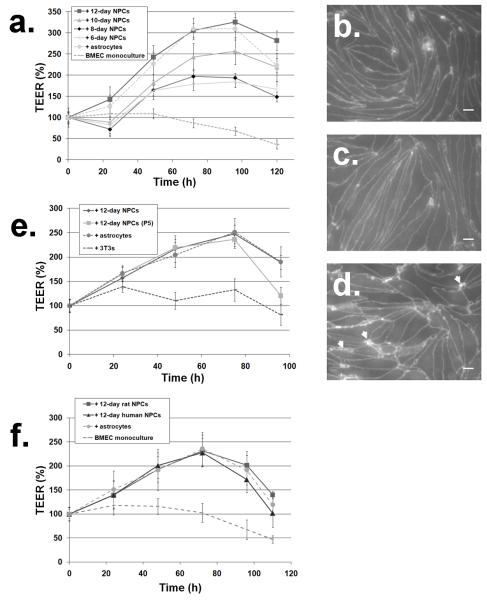
The first necessary step in using NPC-derived cells in BBB modeling was to determine the appropriate conditions for the differentiation of NPCs into astrocyte and neuron mixtures capable of inducing BBB properties. It has previously been demonstrated that by varying serum concentration during human NPC differentiation, the lineage fate of NPC-derived progeny can be tuned (Ostenfeld and Svendsen 2003). However, the appropriate ratio of astrocytes to neurons that would most effectively induce BBB properties was unknown. Thus, to determine the optimum serum concentration for differentiation of rat NPCs prior to BMEC co-culture, NPCs were initially differentiated for 10 days in FBS concentrations of 1%, 3%, 5%, or 10% and then co-cultured with BMECs in 1% FBS (Fig. 1). As a noninvasive metric of BBB induction, TEER measurements were taken at the start of co-culture and approximately every 24 hours thereafter (Fig. 2a). NPCs differentiated in 5% and 10% FBS were shown to enhance both the maximal TEER response and duration of this response in BMECs compared to a BMEC monoculture control (213±18% and 216±15% compared to 168±10% after 48 hours; p<0.02). In contrast, the NPCs differentiated in a low serum concentration (1% and 3% FBS) only outperformed the monoculture after extended co-culture times (>96 hours). Since differentiation time can also influence the maturation of NPC-derived progeny (Svendsen et al. 1998), NPCs were differentiated for 6-12 days in either 1% FBS (Fig. 2b) or 10% FBS (Fig. 2c) prior to co-culture. In these experiments, primary astrocytes were used as a comparative control for TEER induction. It was shown that, regardless of differentiation time, NPCs differentiated in 1% FBS did not outperform the monoculture control, similar to the result observed in Figure 2a (10 days, 1% FBS), while astrocytes yielded TEER that was significantly different from the monoculture control after 48 hours and reached a maximum TEER of 264±33% at 72 hours. In contrast, NPCs differentiated in 10% FBS demonstrated an impressive capability to induce TEER that correlated well with differentiation time prior to co-culture (Fig. 2c). NPCs pre-differentiated for 6 and 8 days only exhibited a statistically significant but modest increase in TEER from 48-120 hours of co-culture. However, 10 and 12-day pre-differentiated NPCs helped co-cultured BMECs achieve a substantially elevated TEER over the course of the experiment, with 12-day differentiated NPCs inducing TEER similar to that induced by primary astrocytes from 48-120 hours. Collectively, these results indicate that both NPC differentiation time and serum concentration during differentiation were important variables for TEER induction, with differentiation for 12 days in 10% FBS generating the NPC-derived progeny that most effectively enhanced BBB properties.
Figure 2. Serum and timing effects on the capability of differentiated NPC to induce response in cultured BMECs.
TEER in co-cultures was monitored for 0-120 hours and normalized to the time zero TEER measurement for each experimental sample. (a) NPCs were differentiated for 10 days in medium containing 1-10% FBS prior to co-culture with BMECs. Monocultured BMECs were used as a control. (b) NPCs were differentiated for 6-12 days in 1% FBS or (c) 10% FBS. For panels (b) and (c), primary astrocytes were also used as a comparative control. During the co-culture phase, medium containing 1% FBS was used for all samples. Each data point is the average and standard deviation of triplicate filters and statistical significance was calculated using the student’s unpaired t-test.
Assessment of NPC-derived progeny distribution and maturity
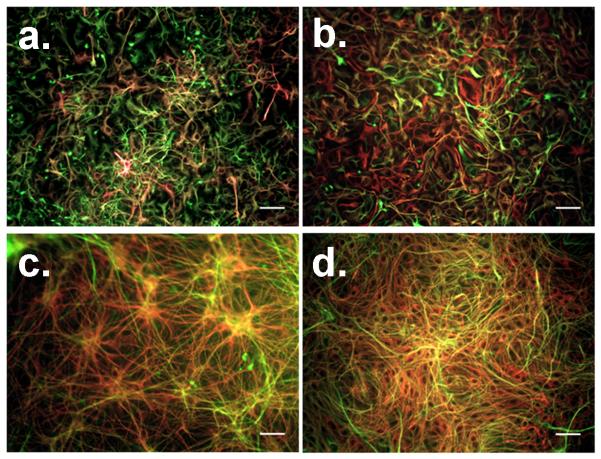
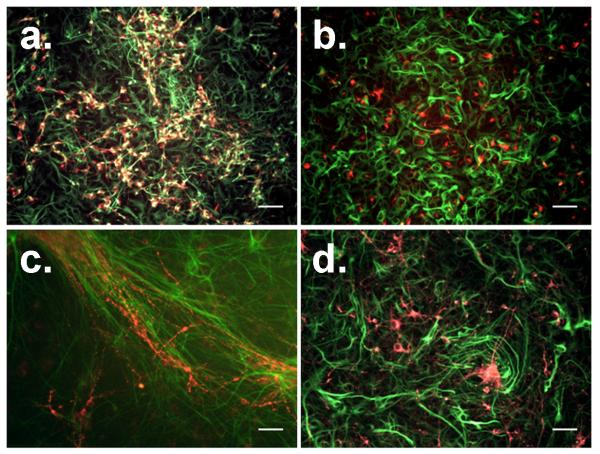
The cellular distribution and maturity of NPC-derived progeny were evaluated to determine why NPCs differentiated in 1% FBS did not yield an inductive response when co-cultured with BMECs, while NPCs differentiated in 10% FBS could generate an inductive response similar to primary astrocytes. First, differentiated NPCs were probed with antibodies against the intermediate filaments GFAP (astrocytes), βIII tubulin (neurons), and nestin (progenitor cells). Immunocytochemical analysis of GFAP- and nestin-labeled cultures at 6 days of differentiation showed the NPC-derived progeny as having an immature morphology consisting of small processes and flattened precursor-like cells regardless of FBS concentration (Fig. 3a and 3b). In contrast, at 12 days of differentiation, individual cells had acquired multiple extended processes, characteristic of maturing astrocytes in vitro (Fig. 3c and 3d). Moreover, many cells continued to express the progenitor cell marker nestin under all conditions, even in combination with GFAP, indicating some level of remaining immaturity. Qualitative analysis of βIII tubulin- and nestin-labeled cultures at 6 days of differentiation revealed a reduced number of βIII tubulin+ cells in 10% FBS compared to the 1% FBS differentiation condition (Fig. 4a and 4b). The βIII tubulin+ cells in 10% FBS also appeared to largely lack nestin expression, while many of the βIII tubulin+ cells were also nestin+ in the presence of 1% FBS. At 12 days of differentiation, the βIII tubulin+ cells in 1% FBS had organized into large tracts of fibrous bundles (Fig. 4c), while the NPC-progeny in 10% FBS remained sparse and unorganized (Fig. 4d). Thus, generally speaking, NPC-derived astrocytes and neurons were acquiring a more mature morphological phenotype from 6 to 12 days of differentiation regardless of serum concentration. In addition, qualitative differences in cellular distribution and nestin expression were readily observable as a function of serum concentration and differentiation time (Figs. 3 and 4). To better assess these differences, flow cytometry was employed to quantify the percent of GFAP+, βIII tubulin+, and nestin+ cells under all tested differentiation conditions (Supplemental Fig. 1 and Table 1). Interestingly, it was observed that the absolute number of astrocytes (including both GFAP+/nestin+ and GFAP+-only cells) was nearly identical between the two differentiated NPC cultures, but the ratio of astrocytes to neurons was strikingly different for the two differentiated NPC cultures (~1:3 in 1% FBS versus ~3:1 in 10% FBS). This 3:1 astrocyte/neuron ratio, which yielded TEER induction, more closely resembles the in vivo ratio of glial cells to neurons in the adult rodent and human cortex (Herculano-Houzel and Lent 2005; Azevedo et al. 2009).
Figure 3. Immunofluorescent examination of NPC-derived astrocytes during differentiation.
NPCs were differentiated for 6 days in either 1% FBS medium (a) or 10% FBS medium (b). Alternatively, NPCs were differentiated for 12 days in 1% FBS medium (c) or 10% FBS medium (d). Cellular distribution and morphology were probed by immunolabeling for GFAP (red) or nestin (green) expression. Scale bars indicate 50 μm.
Figure 4. Immunofluorescent examination of NPC-derived neurons during differentiation.
NPCs were differentiated in for 6 days in either 1% FBS medium (a) or 10% FBS medium (b). Alternatively, NPCs were differentiated for 12 days of in 1% FBS medium (c) or 10% FBS medium (d). Cellular distribution and morphology were probed by immunolabeling for βIII tubulin (red) or nestin (green) expression. Scale bars indicate 50 μm.
Table 1. Quantitative analysis of NPC-derived progeny and primary astrocyte cultures.
| Differentiated NPC distributions | |||||||
|---|---|---|---|---|---|---|---|
| Days of differentiation |
Cell density (106 cell/well) |
GFAP+ onlya |
βIII tubulin+ onlya |
GFAP+/nestin+ co-label |
βIII tubulin+/nestin+ co-label |
Nestin+ onlyb |
Astrocyte to neuron ratioc |
| Rat NPCs differentiated in 1% FBS | |||||||
| 6 days | 0.94 ± 0.01 | 3 ± 1 | 8 ± 3 | 32 ± 11 | 61 ± 10 | 0 | 1:2 |
| 8 days | 1.15 ± 0.01 | 7 ± 3 | 18 ± 4 | 22 ± 11 | 58 ± 11 | 0 | 1:2.6 |
| 10 days | 1.46 ± 0.25 | 13 ± 3 | 38 ± 4 | 11 ± 1 | 33 ± 8 | 0 | 1:3 |
| 12 days | 1.21 ± 0.1 | 12 ± 3 | 40 ± 6 | 12 ± 2 | 38 ± 6 | 0 | 1:3.3 |
| Rat NPCs differentiated in 10% FBS | |||||||
| 6 days | 0.56 ± 0.16 | 8 ± 1 | 16 ± 2 | 66 ± 0 | 14 ± 3 | 0 | 2.5:1 |
| 8 days | 0.61 ± 0.06 | 6 ± 2 | 19 ± 4 | 48 ± 4 | 8 ± 1 | 19 ± 1 | 2:1 |
| 10 days | 0.63 ± 0.01 | 6 ± 6 | 14 ± 4 | 46 ± 2 | 7 ± 2 | 27 ± 2 | 2.5:1 |
| 12 days | 0.62 ± 0.04 | 6 ± 0 | 11 ± 1 | 42 ± 2 | 4 ± 0 | 38 ± 2 | 3.2:1 |
| Human NPCs differentiated in 1% FBS | |||||||
| 12 days | 0.78 ± 0.1 | 5 ± 1 | 16 ± 4 | 64 ± 1 | 5 ± 1 | 11 ± 0 | 3.3:1 |
| Primary astrocyte distributions | ||||
|---|---|---|---|---|
| Cell density (106 cell/well) |
GFAP+ only | GFAP+/nestin+ co-label | Nestin+ only | Unlabeled |
| Fresh astrocytes | ||||
| 0.77 ± 0.14 | 6 ± 2 | 31 ± 6 | 41 ± 8 | 22 ± 1 |
| Frozen/thawed astrocytes | ||||
| 0.61 ± 0.12 | 0 | 18 ± 2 | 62 ± 2 | 21 ± 4 |
Labeled populations are presented as a percentage of the total population. Two samples were used to calculate averages and standard deviation, with each sample being generated from a separate primary isolation of NPCs or astrocytes to confirm biological reproducibility.
As described in the text, a large number of βIII tubulin+ cells and GFAP+ cells in the 12 day, 1% FBS condition also express O4.
Nestin+-only percentage was calculated by subtracting GFAP+/nestin+ and βIII tubulin+/nestin+ percentages from the total nestin+ percentage.
Ratio is expressed as the total number of GFAP+ (GFAP+/nestin+ and GFAP+-only) to βIII tubulin+ (βIII tubulin+/nestin+ and βIII tubulin+-only) cells.
In addition, although oligodendrocytes have not yet been linked to BBB induction, we next tested for NPC differentiation along the oligodendrocyte lineage by assaying for the O4 glycolipid. O4+ cells were not detected at day 6 of differentiation regardless of serum concentration. However, at 12 days of differentiation, small numbers of O4+ oligodendroglial cells (3-4%, O4+/nestin−/GFAP−/βIII tubulin−) were detected in the 10% FBS condition (Supplemental Figs. 2a and 2b). In contrast, for the 12 day, 1% FBS condition, O4 expression was detected in ~90% of the total cell population and was predominantly expressed by cells that co-expressed βIII tubulin (O4+/βIII tubulin+; Supplemental Fig. 2d) and some that co-expressed GFAP (O4+/GFAP+; Supplemental Fig. 2c). This unexpected co-expression of multiple lineage markers in the 1% FBS condition likely indicates the majority of NPC-derived cells are not fully mature and represent some sort of multipotent progenitor (Trotter and Schachner 1989; Dore-Duffy et al. 2006), which may also help explain why this condition was unable to effectively induce TEER.
Also of note, inductive NPCs differentiated in 10% FBS for 12 days yielded a large percentage of cells that were nestin+ (38%, Table 1) but lacking GFAP, βIII tubulin, or O4. Pericytes have been recently described as a potential multipotent progenitor cell type in the brain that co-expresses nestin and NG2 chondroitin sulfate proteoglycan (Dore-Duffy et al. 2006) and pericytes have been shown to elicit BBB properties in vivo (Armulik et al.; Daneman et al.) and in vitro (Nakagawa et al. 2007; Nakagawa et al. 2009). Thus, we tested the possibility that the nestin+-only population might be such nestin+/NG2+ pericyte progenitors in the 12 day, 10% FBS cultures. NG2 expression was not detected, nor did we detect any maturing pericytes expressing alpha-smooth muscle actin. However, while the nestin+-only population did not co-express the chosen subset of markers characteristic of maturing neural cells or pericytes, they were acquiring a more mature morphology (Figs. 3d and 4d), likely pointing to this nestin+-only population as immature progenitors in the early stages of differentiation. Because the 12-day, 10% FBS differentiation condition induced TEER similar to primary astrocytes, we decided to also probe the primary astrocyte cultures for nestin expression. Interestingly, whether astrocytes were cultured fresh after primary isolation or thawed from frozen stock, a large percentage of cells were nestin+-only with no co-expression of GFAP (Supplemental Figs. 3a and 3b and Table 1), which is consistent with previous reports (Sergent-Tanguy et al. 2006). Moreover, fresh or frozen/thawed astrocytes generated indistinguishable TEER profiles when co-cultured with BMECs (Supplemental Fig. 3c), indicating their capability to induce BBB properties even in a so-called nestin+ immature state. In fact, the relative numbers of GFAP+-only and GFAP+/nestin+, and nestin+-only cells in the optimal 12-day, 10% FBS NPC condition and fresh primary astrocytes were very similar (Table 1). Taken together, for the NPC-derived system, there exists an optimum ratio of astrocytes to neurons and a role for cellular maturity that lead to robust TEER induction. In addition, the presence of immature nestin+ cells in the NPC and astrocyte cultures may have an important impact on TEER.
Optimization and phenotypic characterization of the differentiated NPC model
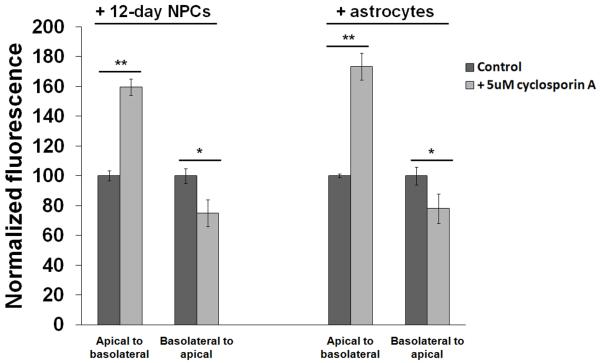
We first chose to use medium containing 1% FBS for the actual BMEC co-culture phase (Fig. 1) because of our previous study employing the same medium condition to study the effects of co-differentiating NPCs on BMEC properties (Weidenfeller et al. 2007). However, previous models using the puromycin-purified BMEC platform have also conducted neural cell co-cultures in higher serum concentrations (Nakagawa et al. 2007; Perriere et al. 2007; Nakagawa et al. 2009). For this reason, we next explored the use of co-culture medium containing 10% FBS. NPCs that had been differentiated in 10% FBS for 6-12 days were used as the co-cultured cell in these experiments given their success in previous experiments. The maximum TEER reached by both NPC-based and primary astrocyte-based co-culture in 10% FBS medium were substantially increased (Fig. 5a) compared to co-culture in 1% FBS (Fig. 2c). NPCs differentiated for 6 or 8 days yielded indistinguishable TEER in the cultured BMECs, while 10- and 12-day differentiated NPCs yielded a progressively enhanced TEER response (Fig. 5a). In particular, 12-day differentiated NPCs in 10% FBS co-culture yielded TEER values that were much higher than those generated in 1% FBS co-culture (326±22% vs. 218±19%). With this fully optimized condition (12-day differentiation in 10% FBS medium, co-culture in 10% FBS medium), differentiated NPCs induced a maximum absolute TEER (246±19 Ω×cm2) indistinguishable from primary astrocytes (252±38 Ω×cm2) (Table 2). To further ensure the observed TEER inductive response was dependent on NPC-derived progeny, mouse 3T3s were used as a non-inductive cell control and yielded an absolute TEER of 121±33 Ω×cm2 (Table 2), which was indistinguishable from the monoculture control under these same conditions (115±33 Ω×cm2; Fig. 5a). NPCs were also differentiated for up to 16 days prior to co-culture, but the absolute TEER (244±15 Ω×cm2, data not shown) was identical to the TEER induced by 12-day differentiated NPCs, indicating no advantage for longer differentiation times. We also investigated whether the observed TEER increases were a result of improved junctional integrity as had been observed previously using the puromycin-purified BMEC model with either hydrocortisone, co-differentiating NPC, or astrocyte/pericyte influences (Calabria et al. 2006; Weidenfeller et al. 2007; Nakagawa et al. 2009). As such, tight junction integrity was probed using an antibody against the tight junction accessory protein ZO-1 (Figs. 5b-5d). It was determined that BMECs co-cultured with 12-day differentiated NPCs or primary astrocytes had significantly fewer “frayed” tight junctions than BMECs co-cultured with mouse 3T3s, correlating with the TEER results (Table 2). To assess the barrier properties in terms of passive diffusion, sodium fluorescein permeability was measured during co-culture with 12-day differentiated NPCs, primary astrocytes, or mouse 3T3s and was not found to be statistically different (Table 2), indicating a fairly uniform tightness to the 376 Da molecule even though ionic permeability as measured by TEER was significantly different. These data are in agreement with a previous study that indicated when TEER exceeds a threshold of approximately 150 Ω×cm2, no difference in permeability is observed (Gaillard and de Boer 2000). To assess functional barrier properties, we compared p-glycoprotein function between the 12-day differentiated NPC and astrocyte co-culture models using the p-glycoprotein substrate rhodamine 123 and inhibitor cyclosporin A. Rhodamine 123 transport from the apical to basolateral chamber was significantly increased in the presence of inhibitor regardless of the co-culture condition as a result of inhibited efflux in the basolateral to apical direction (160±5% for NPCs and 173±9% for astrocytes, p<0.0001 compared to normalized controls in the absence of inhibitor; Fig. 6). Conversely, transport from the basolateral to apical chamber was significantly decreased in the presence of cyclosporin A as a consequence of loss of p-glycoprotein extrusion at the apical membrane (75±11% for NPCs and 78±12% for astrocytes, p<0.05 compared to normalized controls in the absence of inhibitor; Fig. 6). These data indicate that p-glycoprotein is active and polarized in the NPC-derived co-culture system. To evaluate the potential advantage of the self-renewal capacity of NPCs for generating very large cell stocks, model reproducibility was assessed as a function of NPC passage. NPCs were maintained in an undifferentiated state for three weeks (corresponding to five passages) prior to differentiation and co-culture with BMECs. TEER measurements indicate indistinguishable maximum TEER values for the extended passage NPCs compared to low passage NPCs and primary astrocytes (Fig. 5e). This result, along with previous data compiled over multiple NPC isolations, confirms the model fidelity, reproducibility, and scalability.
Figure 5. Optimization of co-culture conditions and analysis of tight junction integrity.
(a) NPCs were differentiated in medium containing 10% FBS and subsequent co-culture with BMECs was also in medium containing 10% FBS, and the TEER response was monitored. (b-d) Immunofluorescent analysis of ZO-1 localization for BMECs co-cultured with 12-day differentiated NPCs (b), primary astrocytes (c) or mouse 3T3s (d) to quantify the level of discontinuous or “frayed” junctions (arrowheads highlight several of these areas). Cells were defined as having frayed tight junctions if greater than 10% of the immunolabeled tight junction protrusions were not parallel to the cell-cell border. Frayed tight junction percentages are summarized in Table 2. Scale bars indicate 20 μm. Occludin and claudin-5 were also found at the cell-cell junctions with distributions matching that shown for ZO-1 (data not shown). (e) NPCs expanded and maintained in an undifferentiated state for five passages over the course of three weeks prior to differentiation were co-cultured with BMECs and compared to primary astrocytes, mouse 3T3s, and differentiated NPCs that had not been serially passaged. Differentiation (12 days) and co-culture were again conducted in medium containing 10% FBS. (f) Human NPCs were differentiated for 12 days in medium containing 1% FBS and co-cultured with rat BMECs in medium containing 10% FBS. The TEER profile induced by human NPCs was indistinguishable from optimally-differentiated rat NPCs and primary rat astrocytes. Each data point is the average and standard deviation calculated from triplicate filters. The TEER profile is representative of two biological replicates.
Table 2.
Properties of BMECs co-cultured with 12-day differentiated NPCs, primary astrocytes, or mouse 3T3s.
| Co-cultured cella | Average TEER (Ω × cm2)b | Permeability coefficient (10−6 cm/s)c |
Percent frayed tight junctionsd |
|---|---|---|---|
| 12-day differentiated NPCs | 246 ± 19 | 0.17 ± 0.05 | 20 ± 1 |
| primary astrocytes | 252 ± 38 | 0.16 ± 0.03 | 20 ± 1 |
| mouse 3T3s | 121 ± 33 | 0.22 ± 0.07 | 30 ± 5 |
Co-cultures were carried out under optimized conditions, where NPCs were differentiated for 12 days in 10% FBS and co-cultured with puromycin-purified BMECs in medium containing 10% FBS. Primary astrocytes and mouse 3T3s were also co-cultured with BMECs in 10% FBS medium.
TEER was measured at 72 hours of co-culture. Mean ± standard deviation calculated from 6 individual experiments across 2 separate NPC isolations, with NPC and astrocyte co-culture TEER being significantly different from 3T3s in each experiment (student’s unpaired t-test; p<0.0001).
Permeability coefficient averages and standard deviations were calculated from three separate co-culture experiments. Values were not found to be significantly different (student’s unpaired t-test; p>0.05).
Percentages were quantified after 72 hours of co-culture. 12-day differentiated NPCs and astrocytes yielded significantly fewer frayed tight junctions than 3T3s (student’s unpaired t-test; p<0.002).
Figure 6. P-glycoprotein polarization assays.
Transport of rhodamine 123 from the apical to basolateral chamber was significantly increased in the presence of 5 μM cyclosporin A (student’s unpaired t-test, **; p<0.0001). Transport of rhodamine 123 from the basolateral to apical chamber was significantly decreased in the presence of 5 μM cyclosporin A (*; p<0.05). Transport of Rhodamine is expressed as fluorescence normalized to the control in each co-culture condition. No significant difference in polarization was observed between the 12-day differentiated NPC and astrocyte co-cultures.
Next, to demonstrate the potential utility of the NPC-based co-culture system for a human BBB model, human NPCs were employed as a source for co-culture experiments with rat BMECs. First, human NPCs were differentiated for 12 days in medium containing 1% FBS. The decision to use 1% FBS for the differentiation of human NPC compared with the optimal 10% FBS rat NPC system was based on previous studies that demonstrated human NPCs differentiate primarily to astrocytes even at very low serum concentration (Ostenfeld and Svendsen 2003). Indeed, after 12 days of differentiation in 1% FBS, human NPCs generated a mixture comprised of mainly GFAP+/nestin+ astrocytes (64±1%; Supplemental Figs. 4a and 4c), mature βIII tubulin+/nestin− neurons (16±4%; Supplemental Figs. 4b and 4c), and nestin+-only cells (11±1%) (Table 1). Unlike the rat NPC system, no O4+ cells were detected. The relative ratio of astrocytes to neurons (3.3:1, Table 1), was nearly identical to the distribution of rat NPC-derived progeny in the optimized condition (12 days, 10% FBS). The presence of neurons that were largely nestin− along with large numbers of GFAP+/nestin+ cells was also very similar, while the nestin+-only population was somewhat decreased (Table 1). When co-cultured with rat BMECs, the differentiated human NPCs generated a TEER response nearly identical to both differentiated rat NPCs and primary rat astrocytes, with appropriate tight junction localization to cell-cell borders (Fig. 5f and Supplemental Fig. 4d). Thus, human NPC-derived astrocytes and neurons are capable of eliciting TEER induction in rat BMECs, with a cellular distribution in the human NPC cultures similar to that identified for rat NPCs.
Differential gene expression in BMECs due to astrocyte or NPC co-culture
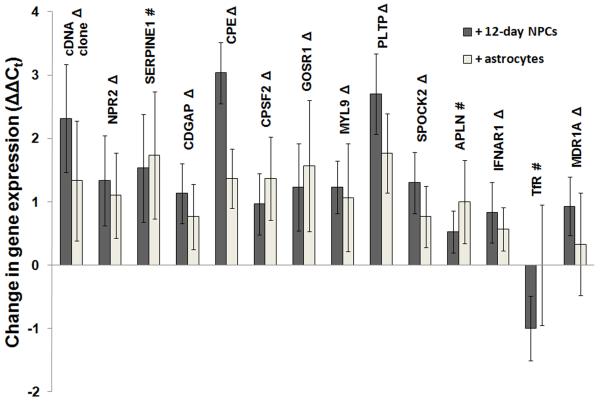
Although it was demonstrated that NPC-derived astrocyte/neuron mixtures could induce positive BBB phenotypic changes in cultured BMECs, molecular level events cannot be evaluated using these approaches. It has previously been shown that many genes are differentially regulated when BMECs are removed from the in vivo environment and cultured in vitro (Calabria and Shusta 2008; Lyck et al. 2009). In particular, our laboratory has used genomics to identify a 26-member gene panel capable of assessing the in vivo-like qualities of in vitro BBB models, and we previously demonstrated that the BBB-inducing agent hydrocortisone was capable of shifting many of these genes towards a more in vivo-like level (Calabria and Shusta 2008). Thus, using this gene panel in concert with quantitative PCR, we determined the comparative ability of both primary astrocytes and 12-day differentiated NPCs to regulate the molecular fingerprint of co-cultured BMECs. Fresh astrocytes were chosen for this analysis because their GFAP and nestin expression levels most closely resembled NPC-derived astrocytes (Table 1). Using ΔΔCt gene expression analysis, it was found that 12 of the 26 genes in the panel were upregulated by a statistically significant amount (ΔΔCt values of 0.5-3 PCR cycles corresponding to fold differences of 1.4-8) as a result of co-culture with either 12-day differentiated NPCs or astrocytes (cDNA clone, NPR2, SERPINE1, CDGAP, SPOCK2, MYL9, APLN, PLTP, GOSR1, CPSF2, IFNAR1, and CPE; Fig. 7). Of these genes, all were shifted closer to in vivo expression levels except APLN and SERPINE1, and CPE was expressed with significant difference between the astrocyte and differentiated NPC co-culture (ΔΔCt of 3.0 ± 0.5 for differentiated NPC co-culture vs. 1.4 ± 0.5 for astrocyte co-culture). In addition, MDR1A was upregulated (shifted towards in vivo expression) by differentiated NPC but not astrocyte co-culture and TfR was downregulated by NPC but not astrocyte co-culture (shifted away from in vivo expression). The remaining 12 genes in this panel (FLT1, RECK, LHK14, CST3, ITM2B, NDRG2, SEMA3G, ATP1A2, OATP14, CCL7, TSP1, and GLUT1) were unchanged by co-culture with differentiated NPCs or astrocytes. Taken together, co-culture with either primary astrocytes or NPC-derived astrocyte/neuron/oligodendroglia mixtures drives cultured BMECs to a more in vivo-like BBB model, with the NPC-derived system yielding potential molecular advantages over primary astrocytes alone.
Figure 7. Genomic analysis of BMECs co-cultured with differentiated NPCs or astrocytes.
BMECs were co-cultured with 12-day pre-differentiated NPCs, primary astrocytes, or mouse 3T3s in medium containing 10% FBS for 72 hours. TEER values due to differentiated NPCs (246±21 Ω×cm2) and astrocytes (266±30 Ω×cm2) were significantly greater than values generated by 3T3s (98±18 Ω×cm2) as also described in the experiments of Table 2. Twelve genes were upregulated (ΔΔCt compared to 3T3 co-culture) by a statistically significant amount (student’s unpaired t-test; p<0.05) due to either differentiated NPC or astrocyte co-culture (cDNA clone, NPR2, SERPINE1, CDGAP, CPSF2, GOSR1, MYL9, PLTP, SPOCK2, APLN, IFNAR1). CPE was significantly more upregulated by differentiated NPCs compared to astrocytes (p<0.01). MDR1A was significantly upregulated by differentiated NPC but not astrocyte co-culture, while TfR was downregulated significantly by differentiated NPC but not astrocyte co-culture (p<0.05). cDNA clone, NPR2, CDGAP, CPSF2, GOSR1, MYL9, PLTP, SPOCK2, MDR1A, and IFNAR1 were shifted towards in vivo expression levels (denoted by Δ) while SERPINE1, APLN, and TfR were shifted away from in vivo expression levels (denoted by #). Values are presented as mean ± standard deviation for triplicate biological samples and are representative of two separate co-culture experiments. Within the gene panel, FLT1, RECK, LHK14, CST3, ITM2B, NDRG2, SEMA3G, ATP1A2, OATP14, CCL7, TSP1, and GLUT1 were not significantly altered by differentiated NPC or astrocyte co-culture (see ref. (Calabria and Shusta 2008) for details regarding gene panel and gene function).
Discussion
In an effort to properly mimic the in vivo BBB, the design of in vitro BBB models has become increasingly complex. The main goal of this study was to investigate NPCs as a potential facile, scalable replacement for primary astrocytes and neurons in multicellular BBB co-culture models. We identified optimal differentiation conditions to generate a 3:1 mixture of astrocytes to neurons that was able to induce a BBB phenotype in cultured BMECs. This phenotype was shown to be virtually indistinguishable from that induced by primary astrocyte cultures in terms of functional permeability (TEER, permeability coefficients, tight junctions, and p-glycoprotein activity). Gene panel analysis was also used to further benchmark the molecular attributes of each model to the in vivo BBB and demonstrated some differences in gene regulation between primary astrocytes and multicellular mixtures derived from NPCs. Finally, human NPCs differentiated to yield similar cellular distributions as found in the rat NPC optimization were also shown capable of inducing BBB properties.
The optimized NPC-derived cultures were able to increase TEER in puromycin-purified BMEC monolayers up to 246±19 Ω×cm2, while primary astrocytes induced TEER up to 252±38 Ω×cm2. This TEER magnitude correlates well with previous work in our lab using hydrocortisone to induce TEER in the puromycin-purified BMEC model (218±66 Ω×cm2) (Calabria et al. 2006). Another recent study using the puromycin-purified rodent BMEC platform in co-culture with primary astrocytes and in the presence of hydrocortisone and cAMP demonstrated a TEER of 270±119 Ω×cm2, and a third study using this platform reached TEER values of 354±15 Ω×cm2 when co-cultured with both astrocytes and pericytes (Perriere et al. 2007; Nakagawa et al. 2009). In our study, we observed no significant difference in permeability values to sodium fluorescence for co-culture with the NPC-derived progeny, primary astrocytes, or mouse 3T3 control (ranging from 0.17-0.22 × 10−6 cm/s). This is altogether unsurprising since several studies have indicated there are minimal changes in permeability when TEER increases above a certain “tightness” threshold of approximately 150 Ω×cm2 (Gaillard and de Boer 2000; Perriere et al. 2007; Nakagawa et al. 2009). Our absolute permeability coefficient values also compare favorably with those achieved using similar models (0.75±0.03 and 3.9±0.2 × 10−6 cm/s) (Perriere et al. 2005; Nakagawa et al. 2009) as well as those determined in our previous efforts with hydrocortisone induction (1.1±0.3 × 10−6 cm/s) (Calabria et al. 2006). Further, p-glycoprotein functionality assays using rhodamine 123 demonstrated similar efflux activity and polarization between the NPC-based and astrocyte co-culture models. The increases in apical to basolateral transport in the presence of cyclosporin A (160±5% for differentiated NPCs and 173±9% for astrocytes) closely mirror the results of Perriere et al (170±34% increase of rhodamine 123 transport in the presence of p-glycoprotein inhibitor PSC833) (Perriere et al. 2007). Thus, the barrier properties of the NPC-based model compare favorably with other models that also use the puromycin-purified rodent BMEC platform along with soluble inducing agents or primary cell co-culture.
Throughout our study we identified serum concentration as a key variable for producing NPC-derived progeny that could induce BBB properties. The underlying decision to initially test increased serum concentration during NPC differentiation was made due to previous reports that higher serum concentrations can both direct NPC-derived progeny towards the astrocytic lineage and induce molecular properties of mature astrocytes (Ostenfeld and Svendsen 2003; Brunet et al. 2004). The optimum ratio of astrocytes to neurons necessary to induce BBB properties in vitro was not known a priori, but several studies have shown that in the cerebral cortex, the region from which BMECs were obtained in this study, glial cells outnumber neurons in vivo: the adult rat cortex was found to have a glial:neuron ratio of 1.5:1 (Herculano-Houzel and Lent 2005) while the human cortex has a glial:neuron ratio of 3.76:1 (Azevedo et al. 2009). We found that NPC differentiation in 1% FBS for 12 day produced a 1:3.3 ratio of astrocyte to neurons unable to enhance TEER induction, while differentiation in 10% FBS for 12 days yielded the opposite ratio of approximately 3.2:1 that along with the human NPC-based system (3.3:1) yielded substantial TEER induction. Thus, a neural cell ratio more closely resembling the ratio found in vivo was essential for induction of BBB properties. Maturity of the NPC-derived progeny also played a key factor in regulating BBB properties. The ratio of NPC-derived astrocytes to neurons in 10% FBS was always at least 2:1, but 10- and 12-day pre-differentiated NPCs were much more effective at increasing TEER in BMECs (Fig. 5a). This differentiation time correlated to the NPC-derived astrocytes acquiring a “mature” morphology as indicated by multiple extended processes compared to the flattened precursor morphology present in the 6-8 day cultures. In contrast, the 12-day, 1% FBS condition that also exhibited morphological maturation resulted in a large number of immature multipotent progenitors as defined by O4+/βIII tubulin+ and O4+/βIII GFAP+, incapable of eliciting a TEER response. We have also noted that many cells in the human and rodent NPC cultures as well as in the primary astrocyte cultures were nestin+-only. Nestin is an intermediate filament protein that was first characterized in neuroepithelial stem cells and is found in many other embryonic and developing tissues (Wiese et al. 2004). In the adult brain, nestin expression is mostly restricted to areas of active cell proliferation and neurogenesis, such as the subventricular zone (Doetsch et al. 1999), dentate gyrus (Seri et al. 2001; Fukuda et al. 2003), and olfactory bulb (Liu and Martin 2003), or at sites of injury (Duggal et al. 1997; Shibuya et al. 2002; Kim et al. 2003). The nestin+-only population in the 12 day rat and human NPC-derived systems did gain some level of morphological maturity although the cells lacked co-expression of maturing neural cell markers, indicating that this cell population is likely an immature progenitor in the early stages of differentiation. We have previously shown in our lab that NPCs in very early stages of differentiation, where nestin+ cells lacking GFAP or βIII tubulin comprised >60% of the culture, can marginally induce TEER in cultured BMECs (Weidenfeller et al. 2007), and others have demonstrated the in vivo contribution of neuroepithelial cells to the onset of BBB-specific properties (Stenman et al. 2008; Daneman et al. 2009). Combined with the results reported here, it appears that immature nestin+ cells can play a supporting role in the BBB induction process. While some O4+ oligodendroglia were also observed in the 10% FBS differentiation condition, they were not observed in the human NPC-derived progeny or primary astrocyte cultures and thus are not required to achieve the phenotypic induction response. However, this does not exclude the possibility that the presence of oligodendrocytes would be beneficial to the BBB response, particularly on a molecular level, and these cellular interactions warrant further study.
It was also desired to gain insight into the molecular role of NPC-derived cell mixtures in BBB regulation versus astrocytes alone and to compare such in vitro qualities to the in vivo BBB. To this end, we benchmarked the NPC-based model against the primary astrocyte model on a genomic level using the gene panel discussed above in the Results section. It was discovered that 10 of the 26 panel genes (cDNA clone, NPR2, CDGAP, SPOCK2, MYL9, PLTP, GOSR1, CPSF2, IFNAR1, and CPE) were upregulated and thus shifted towards a more in vivo-like expression levels by a statistically significant amount (1.4- to 8-fold) as a result of either differentiated NPC or primary astrocyte co-culture while 12 genes (FLT1, RECK, LHK14, CST3, ITM2B, NDRG2, SEMA3G, ATP1A2, OATP14, CCL7, TSP1, and GLUT1) were not altered by the presence of either cell type. Two genes (APLN and SERPINE1) were also upregulated by a significant amount due to co-culture with NPC-derived progeny or primary astrocytes, which resulted in a shift away from in vivo expression. SERPINE1 has previously been shown to be upregulated in BMECs co-cultured with primary astrocytes (Tran et al. 1998) or treated with hydrocortisone (Calabria and Shusta 2008) and its protein product, plasminogen activator inhibitor 1, can induce angiogenesis at physiological concentrations (Devy et al. 2002). APLN, which encodes for apelin, induces angiogenesis in retinal endothelial cells (Kasai et al. 2004). The upregulation of these genes may reflect the in vitro proliferation of the cultured BMECs compared to the fully differentiated in vivo adult brain vasculature represented by the gene panel. Of the genes shifted towards in vivo expression, a point of interest was the ability of differentiated NPCs to produce a more significant change in the gene CPE than astrocytes alone, suggesting a direct molecular contribution of nestin+-progenitors, neurons, or oligodendroglia to BBB regulation that could not be detected by phenotypic analysis alone. CPE encodes for carboxypeptidase E, a membrane protein involved in neuropeptide processing, such as regulated BDNF secretion (Lou et al. 2005). Neurons and astrocytes both secrete BDNF and other neurotrophins and an increase in CPE expression in BMECs may reflect an increased concentration of such peptides due to the presence of neurons. In addition, MDR1A (encoding for p-glycoprotein) was upregulated (shifted towards in vivo expression) by differentiated NPCs but not astrocytes, and TfR was downregulated (shifted away from in vivo expression) only by differentiated NPCs. Lyck and co-workers have noted a small but significant decrease of MDR1A mRNA in cultured mouse BMECs upon co-culture with astrocytes (Lyck et al. 2009), while Perriere and co-workers observed an increase (not statistically significant) in MDR1A expression due to astrocyte co-culture, but also in combination with hydrocortisone and cAMP treatment (Perriere et al. 2007). Like the Lyck et al study, we did not observe an increase in MDR1A expression due to astrocyte co-culture, but observed a small, statistically significant increase in MDR1A expression due to differentiated NPC co-culture (as compared to 3T3 co-culture control), perhaps indicating that nestin+-progenitors, neurons or oligodendroglia may contribute to increasing p-glycoprotein expression. However, the difference in MDR1A expression when directly comparing astrocyte and NPC co-culture was not statistically significant, matching the indistinguishable p-glycoprotein activity results detailed above. Several of the genes shifted towards in vivo expression by differentiated NPCs have also been previously shown in our lab to be similarly shifted by hydrocortisone treatment (cDNA clone, CDGAP, MDR1A, SPOCK2, and NPR2), while the remaining genes affected by NPC-based co-culture did not change in response to hydrocortisone (Calabria and Shusta 2008). Important to the benchmarking of in vitro BBB models, these results indicate different molecular roles in BBB maintenance offered by different cellular co-culture systems and/or exogenous chemical mediators that may all give a similar phenotypic readout.
In conclusion, we have demonstrated that neural cell mixtures derived from NPCs can induce the same positive phenotypic changes in cultured BMECs as astrocytes alone. By increasing the complexity of a co-culture model to include multiple neural cells instead of astrocytes alone, additional genes in a BBB gene panel were shifted to a more in vivo-like expression level. Because NPCs have extensive self-renewing capabilities, are easy to use, and can be maintained over long periods of time with no loss in model viability, they are an excellent candidate to replace primary astrocytes and neurons in rodent BBB models. In addition, because we have provided evidence that differentiated human NPCs can also induce BBB properties in rodent BMECs, we believe the methods described in this study may serve as the basis for creating mixtures of human NPC-derived cells for use in fully human BBB models.
Supplementary Material
Acknowledgments
This work was funded in part by National Institutes of Health grants NS052649 and AA020476. Ethan Lippmann is a recipient of a National Institutes of Health Chemistry Biology Interface Traineeship (T32 GM008505) and Christian Weidenfeller was a recipient of a Deutsche Forschungsgemeinschaft post-doctoral fellowship (DFG, We 4172/1-1).
List of abbreviations
- BBB
Blood-brain barrier
- NPC
Neural progenitor cell
- BMEC
Brain microvascular endothelial cell
- TEER
Transendothelial electrical resistance
- GFAP
Glial fibrillary acidic protein
Footnotes
The authors declare no competing financial interests regarding the work presented in this manuscript.
References
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. The Journal of comparative neurology. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Grollimund L, Chatton JY, Lengacher S, Magistretti PJ, Villemure JG, Pellerin L. Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia. 2004;46:8–17. doi: 10.1002/glia.10348. [DOI] [PubMed] [Google Scholar]
- Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2008;28:135–148. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria AR, Weidenfeller C, Jones AR, de Vries HE, Shusta EV. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. Journal of neurochemistry. 2006;97:922–933. doi: 10.1111/j.1471-4159.2006.03793.x. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cellular and molecular neurobiology. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Noel A, Foidart JM. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. Faseb J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain research. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain research. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard PJ, de Boer AG. Relationship between permeability status of the blood-brain barrier and in vitro permeability coefficient of a drug. Eur J Pharm Sci. 2000;12:95–102. doi: 10.1016/s0928-0987(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochemical and biophysical research communications. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Kim DH, Heo SD, Ahn MJ, Sim KB, Shin TK. Activation of embryonic intermediate filaments contributes to glial scar formation after spinal cord injury in rats. Journal of veterinary science. 2003;4:109–112. [PubMed] [Google Scholar]
- Liu Z, Martin LJ. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. The Journal of comparative neurology. 2003;459:368–391. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell signaling in the neurovascular unit. Neurochemical research. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, Makrides V, Verrey F. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29:1491–1502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cellular and molecular neurobiology. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochemistry international. 2009;54:253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Svendsen CN. Recent advances in stem cell neurobiology. Advances and technical standards in neurosurgery. 2003;28:3–89. doi: 10.1007/978-3-7091-0641-9_1. [DOI] [PubMed] [Google Scholar]
- Perriere N, Demeuse P, Garcia E, Regina A, Debray M, Andreux JP, Couvreur P, Scherrmann JM, Temsamani J, Couraud PO, Deli MA, Roux F. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. Journal of neurochemistry. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Perriere N, Yousif S, Cazaubon S, Chaverot N, Bourasset F, Cisternino S, Decleves X, Hori S, Terasaki T, Deli M, Scherrmann JM, Temsamani J, Roux F, Couraud PO. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain research. 2007;1150:1–13. doi: 10.1016/j.brainres.2007.02.091. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. Faseb J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D’Agostino S, Schiera G, De Caro V, Giandalia G, Giannola LI, Cestelli A. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- Schiera G, Bono E, Raffa MP, Gallo A, Pitarresi GL, Di Liegro I, Savettieri G. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. Journal of cellular and molecular medicine. 2003;7:165–170. doi: 10.1111/j.1582-4934.2003.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiera G, Sala S, Gallo A, Raffa MP, Pitarresi GL, Savettieri G, Di Liegro I. Permeability properties of a three-cell type in vitro model of blood-brain barrier. Journal of cellular and molecular medicine. 2005;9:373–379. doi: 10.1111/j.1582-4934.2005.tb00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent-Tanguy S, Michel DC, Neveu I, Naveilhan P. Long-lasting coexpression of nestin and glial fibrillary acidic protein in primary cultures of astroglial cells with a major participation of nestin(+)/GFAP(−) cells in cell proliferation. Journal of neuroscience research. 2006;83:1515–1524. doi: 10.1002/jnr.20846. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 2002;114:905–916. doi: 10.1016/s0306-4522(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science (New York, N.Y. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. Journal of neuroscience methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain research. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- Tran ND, Schreiber SS, Fisher M. Astrocyte regulation of endothelial tissue plasminogen activator in a blood-brain barrier model. J Cereb Blood Flow Metab. 1998;18:1316–1324. doi: 10.1097/00004647-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Trotter J, Schachner M. Cells positive for the O4 surface antigen isolated by cell sorting are able to differentiate into astrocytes or oligodendrocytes. Brain Res Dev Brain Res. 1989;46:115–122. doi: 10.1016/0165-3806(89)90148-x. [DOI] [PubMed] [Google Scholar]
- Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. Journal of neurochemistry. 2007;101:555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PubMed] [Google Scholar]
- Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN. Gene expression in human neural stem cells: effects of leukemia inhibitory factor. Journal of neurochemistry. 2003;86:179–195. doi: 10.1046/j.1471-4159.2003.01826.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.