Introduction
In the last decade, virus specific CTL manufacture has become a more widespread and reproducible technology. A number of clinical grade techniques have been described. Here we outline the approach used at Baylor College of Medicine (BCM) since 2004 to treat over 35 patients with EBV-positive lymphoma using T cells specific for the latent membrane proteins (LMP1 and LMP2) of EBV in phase I/II clinical trials. Clinical outcomes have been described, but briefly objective tumor responses were achieved in 11/16 patients and complete remissions in 8. [1], [2]Here we outline the CTL manufacturing process. We have made the detailed SOPs required available with appropriate URL links throughout the manuscript. These should facilitate the creation of protocols suitable for regulatory approval and provide the basis for GMP manufacture of LMP1- and LMP2-specific T cells.
Application
In EBV associated Lymphomas and lymphoproliferative disorders that develop in the immunocompetent host, the EBV antigens LMP1, LMP2 and EBNA1 are expressed in malignant cell populations. These include the Hodgkin Reed Sternberg cells in Hodgkin’s lymphoma and B or NK/T cells in B or NK/T cell non-Hodgkin lymphomas respectively. These antigens therefore represent a potential source of target antigens for adoptive T-cell immunotherapy. In our Phase I dose-escalation proof of principle studies, we have demonstrated the anti-tumor efficacy of autologous LMP1- and LMP2-specific cytotoxic T lymphocytes (CTLs) for patients with type II latency EBV-positive lymphomas, who had failed standard therapies for lymphoma.[3],[4] CTLs have minimal toxicity, indeed they have reduced B symptoms and provide an effective strategy to treat tumors without the devastating side effects of standard chemoradiotherapies.
Patient Eligibility
LMP-specific CTLs are generated from patients with relapsed EBV positive lymphoma expressing type II latency. For each patient, expression of EBER and/or LMP is first determined by in situ hybridization and immunohistochemistry respectively, performed on paraffin-embedded diagnostic biopsy specimens. For generation of CTL lines, blood is procured at the earliest appropriate time usually immediately before a chemotherapy cycle. The protocol allows for multiple samples to be drawn, so that we can obtain sufficient T-cells for the generation of dendritic cells (DCs), EBV-transformed lymphoblastoid cell lines (LCLs) and CTLs, all of which may be generated from fresh or frozen aliquots. This is important because patients may have low lymphocyte counts and it allows for reinitiation of cell lines in the case of failure. The protocol is first discussed with eligible patients and informed consent required for participation in the study, is obtained for the generation of the autologous cell lines. The protocol is approved by the Recombinant DNA Advisory Committee (RAC), the Food and Drug Administration (FDA) and the Baylor College of Medicine’s Institutional Review Board (IRB). In addition, for patients who have received allogeneic hematopoietic stem cell transplant the CTL product is manufactured from the stem cell donor. In this setting consent is obtained from the allogeneic donor to procure blood for CTL manufacture. The National Marrow Donor Program (NMDP) have a IRB protocol and consent form that is used when obtaining blood for cell line preparation from an unrelated donor.
Manufacturing LMP-specific cytotoxic T cells using good manufacturing practices (GMP)
All cell culture and gene transfer manipulations are carried out in the Center for Cell and Gene Therapy GMP facility using current Standard Operating Procedures (SOPs) (available online).
Blood Procurement for CTL and antigen-presenting cell (APC) generation
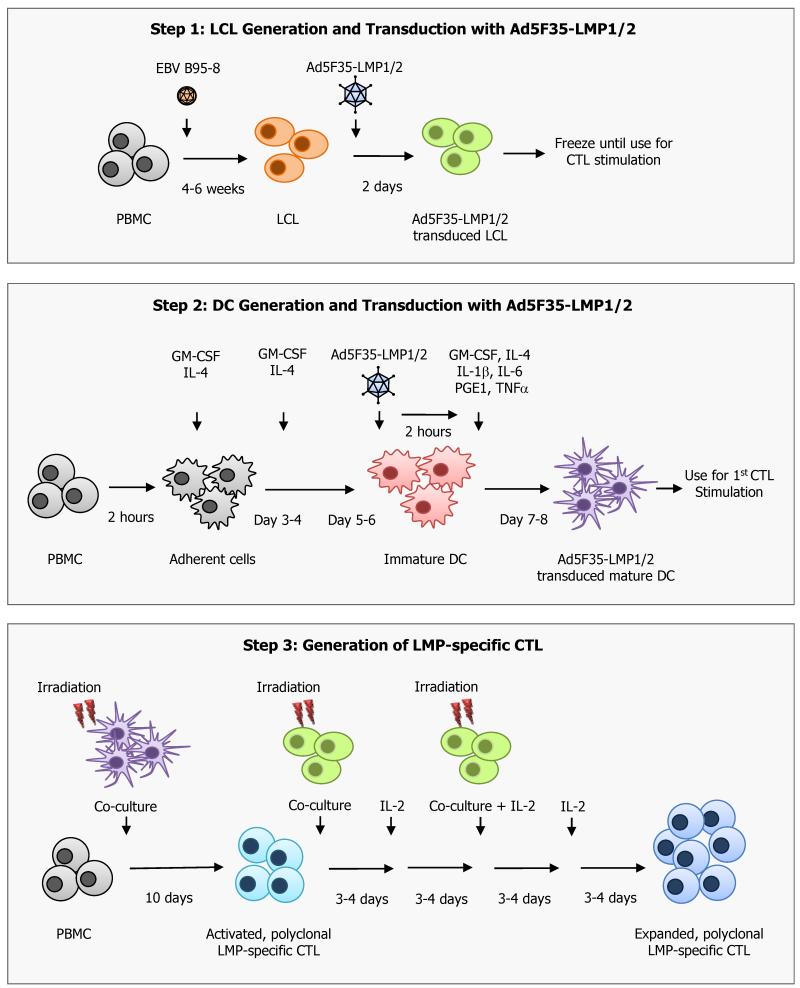
Generation of LMP-specific CTL lines requires the generation of several different components from peripheral blood mononuclear cells (PBMC). The CTL line is initiated from patient (or donor) PBMCs, by stimulation with antigen-presenting cells (APCs) expressing the LMP1 and LMP2 antigens in the presence of IL-15 followed by expansion with interleukin-2 (IL-2). The APCs used to stimulate and expand the LMP1-specific T cells are dendritic cells (DCs) or monocytes and EBV-transformed B lymphoblastoid cell lines (LCLs) derived from patient (or donor) mononuclear cells and B lymphocytes (Figure 1).
Figure 1. Generation of LMP-specific CTL.
Step 1. LCL generation and transduction with Ad5F35-ΔLMP1-I-LMP2 vector. An LCL line is generated by infecting patient PBMC with a laboratory strain of EBV (B95-8). The resultant LCL are then transduced with the Ad5F35-ΔLMP1-I-LMP2 (Ad5F35-LMP1/2) vector and cultured for 2 days at which time they are either frozen for future CTL stimulations or used fresh to stimulate and expand LMP-specific CTL.
Step 2. DC generation and transduction with Ad5F35-ΔLMP1-I-LMP2 vector. DCs are generated from fresh patient PBMCs in the presence of GM-CSF and IL-4 for 5 days. These immature DCs are then transduced with an Ad5F35-ΔLMP1-I-LMP2 vector (Ad5F35-LMP1/2) and matured in the presence of GM-CSF, TNF-α, IL-6, IL-1b, IL-4 and PGE1. After 2 days the Ad5F35LMP1-I-2-transduced DCs are irradiated and used to initiate the LMP-specific CTL.
Step 3. Generation of LMP-specific CTL. Patient PBMCs are co-cultured with autologous, irradiated transduced DCs at a ratio of 20 PBMCs to 1 DC in the presence of IL-15. Cultures are then stimulated on day 10 with irradiated Ad5F35-ΔLMP1-I-LMP2-transduced LCL at a ratio of 4:1. IL-2 is first added on day 13 and then used twice weekly thereafter. Weekly stimulations with transduced LCL in the presence of IL-2 are continued until sufficient numbers of LMP-specific CTL are generated.
A maximum of 60 ml of peripheral blood × 2 for a total maximum amount of blood of 120 ml, is collected from the patient or stem cell donor (subjects must be at least 12 kg or 24 pounds). For donors or patients <18 years a maximum of 3mls/kg blood is taken in an 8 week period. PBMCs are isolated on ficoll (Lymphoprep, Cosmo Bio USA, Carlsbad, CA) gradients. Each component, T cells, LCLs, monocytes and DCs can be prepared from fresh or cryopreserved PBMC.
CTL Initiation
CTL specific for the EBV tumor-associated antigens LMP 1 and 2 are prepared according to GMP SOP D03.16.[4] 1. For patient-derived LMP-specific CTL, autologous DCs are used as APCs for the first stimulation. DC are manufactured according to SOP D03.15 by culture of adherent PBMC-derived monocytes with cytokines (GM-CSF, IL-4) followed by transduction on day 5 or 6 with a replication-incompetent adenovirus vector expressing inactive LMP1 and LMP2 separated by an internal ribosomal entry site (Ad5f35-ΔLMP1-I-LMP2; produced by the Gene Vector Laboratory of the Center for Cell and Gene Therapy, BCM)[5], [6] at a vp:cell ratio of 30,000:1 and maturation with a cytokine cocktail containing IL-1β, IL-6, TNF-αand PGE1. After 48 hours, mature, transduced DC are used to stimulate LMP-CTL according to SOP D03.15. Transduced DCs are harvested, washed and irradiated (30Gy) and then cocultured with autologous PBMC at an E:T ratio of 20:1 in the presence of IL-15. In this case, dendritic cells are prepared from about 40 mL of blood and the T cells are derived from approximately 20mL of blood. If the DCs are established from fresh blood, then non-adherent PBMCs can be cryopreserved as the source of CTLs.
2. For donor-derived LMP-specific CTL, donor PBMC are adhered overnight in 24 well plates in X-Vivo 15 media (BioWhittaker; Walkersville, Maryland) at a concentration of 2×106 cells per well. The following day, the activated monocytes are scraped from the wells, transduced with the adenovirus vector (Ad5f35-LMP1/2), at a viral particle (vp) to cell ratio of 30,000:1 for 2 hours. This procedure is critical since adenovirus infection of fresh monocytes results in monocyte death.[7]After this time the cells are resuspended at a concentration of 1 × 106/ml in CTL media containing Advanced RPMI (Invitrogen, Carlsbad, CA), Clicks EHAA (Irvine Scientific, Santa Ana, CA), 10% FCS (Hyclone, Logan, UT) and glutamine (Invitrogen). In this strategy the transduced monocyte fraction of PBMC will express and present LMP1 and LMP2 peptide epitopes to the LMP-specific T cell fraction of the PBMC. This step requires 20 to 40 × 106 PBMC from about 40 mL of blood. Monocytes are effective for the reactivation of LMP-specific T cells from healthy donors, but not from lymphoma patients, likely because their T cells are anergized, low frequency or fragile.
Expansion of LMP-specific CTL
For the second and subsequent stimulations autologous LCLs transduced with Ad5f35-LMP1-2 are used as APCs.[8], [9] Although LCLs express endogenous LMPs, overexpression of LMPs from the adenovirus vector ensures that LMP peptides are over-represented as HLA:peptide complexes at the cell surface to ensure that LMP-specific T cells are preferentially expanded. This stimulation can be performed either in 24 well plates or in the G-Rex gas permeable cell expansion device according to SOP D03.16.[10]
Manufacture of APCs
LCLs are manufactured according to SOP D03.01. EBV-LCLs are derived from PBMC by infection with a clinical grade, laboratory strain of EBV(B95-8).[11], [12] About 5 × 106 PBMC, or 5 to 10 mLs of blood is required to generate the EBV-LCL. Establishment of LCLs takes 4 to 6 weeks and in patients with disease, this process may be prolonged even further. To expand the LMP-specific T cells EBV-LCLs are transduced with the Ad5f35-LMP1-I-2 vector at a vp:LCL ratio of 100,000:1 according to SOPs D03.14 (however the multiplicity of infection is established for every new lot of vector). This transduction allows the EBV-LCLs to present excess LMP1 and LMP2 peptides to the T cells. The second CTL stimulation using transduced LCLs is performed approximately 10 days after the first stimulation with DCs or monocytes. Transduced LCL are harvested 48 hours after transduction and used fresh or can be cryopreserved for future stimulations. Transduced LCLs are irradiated (40Gy), washed and then cocultured with the LMP-activated T cells at a responder to stimulator ratio of 4:1 if culturing cells in 24 well plates or at a ratio of 1:5 if culturing CTL in a G-Rex. The first IL-2 feed is performed 3-4 days after the first LCL stimulation and continues twice weekly thereafter. Earlier addition of IL-2 may result in the expansion of non-antigen-specific T cells or even T regulatory cells. Additional LCL stimulations are usually required to expand CTL to the numbers required for clinical use.
If CTLs are growing poorly, they receive stimulation with a mitogenic cocktail of irradiated allogenic PBMCs, autologous LCLs, submitogenic doses of OKT3 (50 ng per mL) and IL-2 (100 units per mL).[13] This regimen consistently rescues cultures, so that they can be transduced after subsequent stimulation with LCL alone. The addition of 45% EHAA to our initial RPMI medium, and the replacement of RPMI with Advanced RPMI has largely overcome the previously common requirement for superexpansion, but superexpansion may still be required and more than one superexpansion may be needed in cases of extreme lymphocyte fragility. CTLs are cryopreserved when sufficient cells for the dose level and QC have been produced.
QA/QC and Release Criteria
Cropreservation of the CTL is performed according to SOP D03.05 using a Cryomed (Thermo Fisher Scientific Inc, Waltham, MA) and includes 4 washes to remove FCS and phenol. At the time of freezing, aliquots of the CTL culture; cells, cell culture supernatant, final wash and final product are collected for sterility testing. Aliquots of cells are HLA typed for identity, and phenotyped to assess T cell subsets and confirm the absence of genetically modified B cells and monocytes or DCs. The CTL line is also tested for cytotoxic specificity. Lack of killing of patient lymphoblasts is a release criterion to exclude autoreactivity or alloreactivity. Samples are archived for immunological characterization and for possible additional testing. The frequency of LMP-specific CTL is determined using ELISpot analysis and multimer reagents if available. Release criteria for administering the CTL to patients include viability >70%, negative culture for bacteria and fungi after 14 days, endotoxin testing ≤ 5EU/ml, negative result for mycoplasma, <10% killing of patient PHA blasts or skin fibroblasts at 20:1 ratio, <2% CD19 positive B cells, <2% CD14 positive monocytes (or <2% CD83 positive/CD3 negative cells if DCs were used as stimulators) and HLA identity. After quality assurance testing is complete a certificate of analysis is issued.
Expected results
Before the CTL line is released for infusion, all testing must have produced appropriate results or the line will be ineligible. Patient-derived lymphoblasts or fibroblasts should be killed only if expressing tumor antigens. It is expected that the adenovirus vector itself induces some adenovirus-specific killing, so the presence of adenovirus-specific T cells is acceptable and it is the expectation that these constitute only a minor fraction of the CTL line. It is usually possible to inhibit killing with antibodies to HLA class I and/or class II molecules, although this will not be a release criterion, since the lines sometimes contain a component of NK-like activity from CD56+ CD3+ T cells, whose activity may be enhanced in the presence of antibody.
Potential Problems and Troubleshooting
A major problem with this protocol is the quality of blood from patients with lymphoma who have immunosuppressive tumors and have received multiple rounds of aggressive therapy. LCL generation is frequently prolonged and fails in about 10% of the cases. Hence, if the LCL is not expanding as expected after 2 weeks, we routinely request additional blood and repeat the attempt using greater lymphocyte numbers and reduced amounts of cyclosporin A. This strategy is often successful, unless patients have received B cell depletion with rituximab as a part of therapy, in which case we wait until B cells recover before LCL initiation. Many Hodgkin Lymphoma patients have very low lymphocyte counts. In such cases, we need to collect and freeze multiple blood samples before beginning the DC/CTL cultures. We have in the majority of cases, been able to generate CTLs from less than 120mls of peripheral blood. Furthermore, we have shown that the ability of these patient-derived CTL to expand in vitro was also optimized by finding the right medium and serum and from the right source, highlighting the fact that it is critically important to test these critical reagents obtained from different companies and different lots. Finally, in a proportion of patients we were unable to elicit a response to LMP1 and/or LMP2 especially when the patients or donors were HLA A0201 negative. However, since modifying our manufacturing protocol to add IL-15 at the time of CTL initiation we have routinely been able to generate CTL that are specific for these LMP antigens irrespective of HLA type.
Acknowledgements
This work was supported by NIH grants PO1 CA94237 (CMB, SG, APG and CMR), P50CA126752 (CMB, SG, AML, APG, CMR), the Leukemia Lymphoma Society (CMB, CMR, SG) and a Production Assistance for Cellular Therapies grant (N01-HB-37163) (CMR, APG). CMB was also supported by an award from the Gillson Longenbaugh Foundation. The LMP1-I-2 vector was provided by a grant from the National Gene Vector Laboratories (NIH-NCRR U42 RR16578).
Footnotes
The authors declare no competing financial interests.
DISCLAIMER - The information in this article is intended for educational purposes only and should not be considered as a standard of care or recommended treatment for any particular patient.
Reference List
- 1.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollard CM, et al. Administration of tumor-specific Cytotoxic T Lymphocytes engineered to resist TGF-beta to patients with EBV-associated lymphomas. Blood. 2010;116:520. Ref Type: Abstract. [Google Scholar]
- 3.Roskrow MA, Suzuki N, Gan Y-J, Sixbey JW, Ng CYC, Kimbrough S, et al. EBV-specific cytotoxic T lymphocytes for the treatment of patients with EBV positive relapsed Hodgkin’s disease. Blood. 1998;91:2925–34. [PubMed] [Google Scholar]
- 4.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T Lymphocyte Therapy for Epstein-Barr Virus+ Hodgkin’s Disease. J. Exp. Med. 2004;200:1623–33. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTL against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive Immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–12. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 6.Ratnayake M, Leen AM, Rooney CM, Brenner MK, Gottschalk S. Activation of LMP1- and LMP2-specific T-cells with an adenoviral vector encoding full length LMP1 and LMP2: towards a vaccine for NPC. East-West Symposium on Nasopharyngeal Cancer. 2005 Abstract 29. [Google Scholar]
- 7.Leen AM, Ratnayake M, Foster AE, Heym K, Ahmed N, Rooney CM, et al. Contact activated Monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J Immunother. 2007:96–107. doi: 10.1097/01.cji.0000211325.30525.84. [DOI] [PubMed] [Google Scholar]
- 8.Sili U, Huls MH, Davis AR, Gottschalk S, Brenner MK, Heslop HE, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocytes using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–56. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bollard CM, Straathof KC, Huls MH, Leen A, Lacuesta K, Davis A, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27:317–27. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller G, Lipman M. Release of Infectious Epstein-Barr Virus by Transformed Marmoset Leukocytes. Proc Natl Acad Sci USA. 1973;70:190–4. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CA, Ng CYC, Heslop HE, Holladay MS, Richardson S, Turner EV, et al. Production of genetically modified EBV-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995;4:73–9. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 13.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand antigen specific T cells. J. Immunol. Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]