Summary
Forkhead Box P3 (Foxp3)-expressing regulatory T (Treg) cells are central to maintaining self-tolerance and immune homeostasis. How Treg cell function and Foxp3 expression are regulated is an important question under intensive investigation. Here, we have demonstrated an essential role for the transcription factor GATA-3, a previously recognized Th2 cell master regulator, in controlling Treg cell function. Treg cell-specific GATA-3 deletion led to a spontaneous inflammatory disorder in mice. GATA-3-null Treg cells were defective in peripheral homeostasis and suppressive function, gained Th17 cell phenotypes and expressed reduced amounts of Foxp3. In addition, GATA-3 controlled Foxp3 expression by binding to and promoting the activity of cis-acting elements of Foxp3. Furthermore, the combined function of GATA-3 and Foxp3 was essential for Foxp3 expression. These findings provide insights into immune regulatory mechanisms and uncover a critical function of GATA-3 in Treg cells and immune tolerance.
Introduction
Regulatory T (Treg) cells are a CD4+ T cell subset possessing potent immune suppressive activities critical for maintaining self-tolerance and immune homeostasis. Defects in Treg cell function invariably result in autoimmunity and inflammatory disease in mammals (Sakaguchi et al., 2008; Shevach, 2000). Foxp3, an X-linked transcription factor, is highly and specifically expressed in Treg cells. Ectopic expression of Foxp3 endows non-Treg cells with immune-suppressive function (Brunkow et al., 2001; Khattri et al., 2003), and Foxp3 deficiency leads to systemic autoimmune syndrome in human IPEX patients and Scurfy mice due to lack of functional Treg cells (Brunkow et al., 2001; Fontenot et al., 2003; Hori et al., 2003; Wildin et al., 2001). Therefore, Foxp3 is recognized as the central regulator of Treg cells. To understand immune tolerance and immune homeostasis, how Treg cell and Foxp3 function are controlled is a critical question yet to be fully addressed. Increasing numbers of transcription factors are shown to be important for Treg cell function (Chaudhry et al., 2009; Kerdiles et al., 2010; Kitoh et al., 2009; Koch et al., 2009; Ouyang et al., 2010; Rudra et al., 2009; Zheng et al., 2009). Curiously, factors controlling the differentiation of Th1 and Th2 cells regulate distinct and specific functions of Treg cells (Kitoh et al., 2009; Koch et al., 2009; Rudra et al., 2009; Szabo et al., 2000; Zheng et al., 2009)}.
GATA-3 is a transcription factor that is highly expressed in Th2 cells and critical for the differentiation of these cells (Zheng and Flavell, 1997; Zhu et al., 2004). GATA-3 is therefore regarded as the “master regulator” for Th2 cells. Nevertheless, GATA-3 expression and function is not limited to Th2 cells. GATA-3 is expressed in multiple tissues and cell types (Yamamoto et al., 1990) and is required for T cell development and natural killer (NK) cell function (Pai et al., 2003; Samson et al., 2003). Thus, GATA-3 plays multi-faceted roles of regulating immune function in a cell-type specific fashion. Importantly, whether and how GATA-3 is involved in controlling Treg cell function in vivo is unknown.
We found that GATA-3 expression was elevated in Treg cells compared to conventional T cells and was suppressed in Treg cells under Th1 cell polarizing condition, where Treg cell function is often found tempered (Caretto et al., 2010; Oldenhove et al., 2009). We therefore hypothesized that GATA-3 expression in Treg cells is important for their optimal function and that defective GATA-3 expression will alter the properties of Treg cells. To test this hypothesis, we deleted GATA-3 specifically in Foxp3-expressing Treg cells and found that GATA-3 deletion in Treg cells led to the development of an inflammatory disorder in mice. GATA-3-deficient Treg cells were intrinsically defective in their homeostasis and showed compromised immune suppressive function in vitro and in vivo. In agreement with these observations, GATA-3-deficient Treg cells expressed decreased amounts of Foxp3 and Treg cell “signature genes” and increased amounts of effector cytokines. In addition, we demonstrated that GATA-3 bound to the regulatory region of Foxp3 locus and promoted the activity of a cis-acting element of Foxp3 gene. Moreover, we showed that the combined function of GATA-3 and Foxp3 was vital for Foxp3 expression, because virtually no Foxp3-expressing cells could be detected when both GATA-3 and Foxp3 were defective. Collectively, this study reveals an essential function of GATA-3 in controlling Treg cell function and Foxp3 expression. It provides further insight into immune regulatory mechanisms and sheds lights on GATA-3 function and how immune responses are controlled.
Results
1. Mice with Treg cell-specific GATA-3 deletion develop an inflammatory disorder
Transcription factors critically involved in directing the differentiation of Th1 and Th2 cells were recently shown to play unique and important roles in controlling Treg cell function (Kitoh et al., 2009; Koch et al., 2009; Rudra et al., 2009; Zheng et al., 2009). However, whether the Th2 cell master regulator GATA-3 is involved in Treg cell function remains unknown. To address this question, we first asked if GATA-3 is expressed by Treg cells. GATA-3 expression was assessed in purified Treg cells and non-Treg CD4+ T (Tn) cells isolated from FIR mice, where Foxp3-expressing cells were marked by the expression of a red fluorescence protein, as described previously (Wan and Flavell, 2005). Treg cells expressed more GATA-3 than Tn cells at both mRNA and protein levels. In addition, GATA-3 expression was down-regulated in Treg cells under Th1 cell polarizing condition associating with reduced Treg cell recovery and Foxp3 expression (Supplementary Fig. S1), agreeing with the finding that Treg cell function is tempered during Th1 response (Caretto et al., 2010; Oldenhove et al., 2009). These observations suggest a potential role of GATA-3 in regulating Treg cell function. We therefore investigated how GATA-3 is involved in Treg cell function. To do this, we generated Treg cell-specific GATA-3 deficient mice by crossing Gata3fl/fl mice (Amsen et al., 2007) with mice bearing a BAC transgene encoding both enhanced green fluorescence protein (EGFP) and Cre recombinase under the control of Foxp3 promoter (Foxp3-EGFP-cre mice (Zhou et al., 2008), hereafter referred to as FGC mice). In FGC mice, EGFP expression faithfully marks Foxp3-expressing Treg cells and Cre-mediated gene deletion occurs specifically in Treg cells (Zhou et al., 2008).
Although Gata-3fl/fl:FGC mice were born normally and matured to adulthood, they spontaneously developed lymphadenopathy and splenomegaly by 16 weeks of age (data now shown). In agreement with this observation, the total numbers of lymphocytes recovered from the peripheral lymph-nodes (PLN) and spleens of Gata-3fl/fl:FGC mice were higher compared to those from Gata-3fl/+:FGC littermates (Fig. 1a), although the distribution of T cell populations in the thymus, spleen and peripheral lymph-nodes was normal and Treg cells were generated in the thymus in Gata-3fl/fl:FGC mice (Supplementary Fig. S2). In addition, histological analysis revealed dramatically increased lymphocytic infiltration in the non-lymphoid organs, such as the lung, pancreas, lacrimal glands, and salivary glands of Gata-3fl/fl:FGC mice (Fig. 1b), suggesting aberrant immune activation. We thus examined the activation and differentiation status of T cells from Gata-3fl/fl:FGC mice. CD4+ Tn and CD8+ T cell populations with an activated phenotype (CD62LlowCD44high) were increased in Gata-3fl/fl:FGC mice compared to those in Gata-3fl/+:FGC littermates (Fig. 1c and supplementary Fig. S2). In addition, interferon-γ (IFN-γ), interleukin-4 (IL-4) and IL-17 production by CD4+ Tn cells were increased in Gata-3fl/fl:FGC mice compared to Gata-3fl/+:FGC littermates (Fig. 1d and 1e). Similarly, CD8+ T cells from Gata-3fl/fl:FGC mice produced increased amounts of IFN-γ (Supplementary S2). Therefore, Treg cell-specific GATA-3 deletion led to an inflammatory disorder in mice, associated with elevated activation and effector function of T cells.
Fig. 1. GATA-3 deletion in Treg cells leads to an inflammatory disorder in mice.
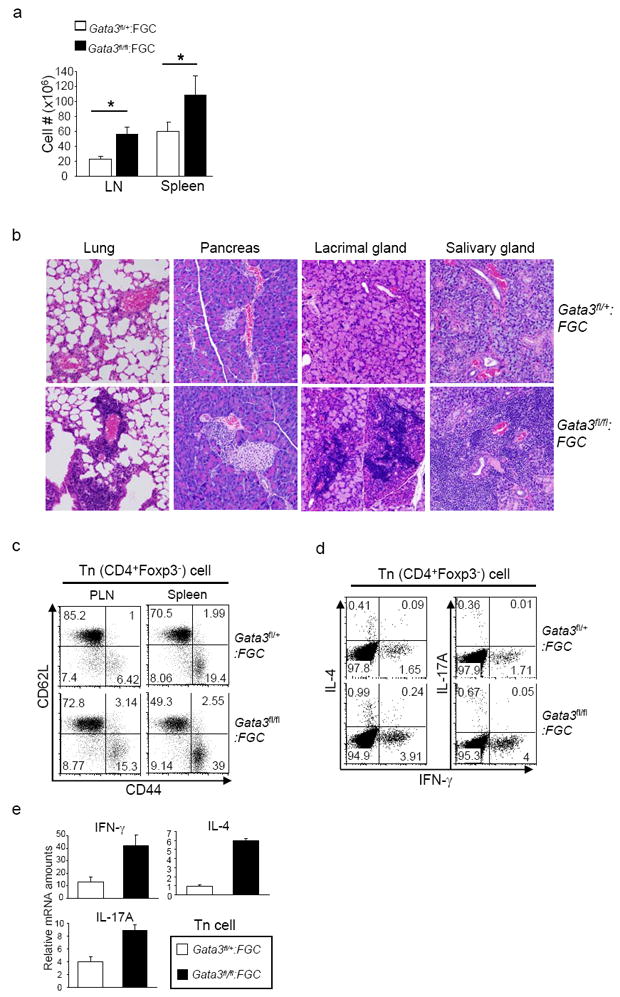
1a, The numbers of lymphocytes in the PLN and spleens from Gata3fl/fl:FGC and Gata3fl/+:FGC littermates. Data are Means ± s.d. of five mice. (*, P<0.05). 1b, Histological analysis of lymphocytic infiltration in the lung, pancreas, lacrimal gland and salivary gland of indicated mice by H&E staining. 1c, Expression of CD44 and CD62L on CD4+Foxp3- (Tn) cells from the lymph nodes and spleens of Gata3fl/fl:FGC mice and Gata3fl/+:FGC littermates. Number in each quadrant showed the percentage of each population. Data represent at least four independent experiments. 1d, IFN-γ, IL-4 and IL-17 expression by CD4+Foxp3- (Tn) cells from the PLNs of Gata3fl/fl:FGC mice and Gata3fl/+:FGC littermates. Number in each quadrant showed the percentage of each population. Results are representative of at least four experiments. 1e, Relative mRNA expression amounts of IFN-γ, IL-4 and IL-17 in sorted CD4+GFP- Tn cells from Gata3fl/fl:FGC mice and Gata3fl/+:FGC littermates. Means ± s.d. of triplicates done in one experiments representative of three are shown.
See also Fig. S2.
2. Treg cells lacking GATA-3 are defective in homeostasis
We then investigated how the Treg cell population was affected by GATA-3 deletion. First, efficient and specific deletion of GATA-3 in Treg cells purified from Gata-3fl/fl:FGC mice was confirmed (Fig. 2a). Initial characterization showed that the numbers of Treg cells were not decreased but moderately increased in Gata-3fl/fl:FGC mice (Fig. 2b). Nevertheless, this observation could be confounded by the inflammatory conditions existed in these mice because Treg cell numbers may be abnormally up-regulated in the hosts with inflammation (Kitoh et al., 2009; Liu et al., 2008; Rudra et al., 2009).
Fig. 2. Intrinsic defects of GATA-3-deficient Tregs in the peripheral homeostasis.
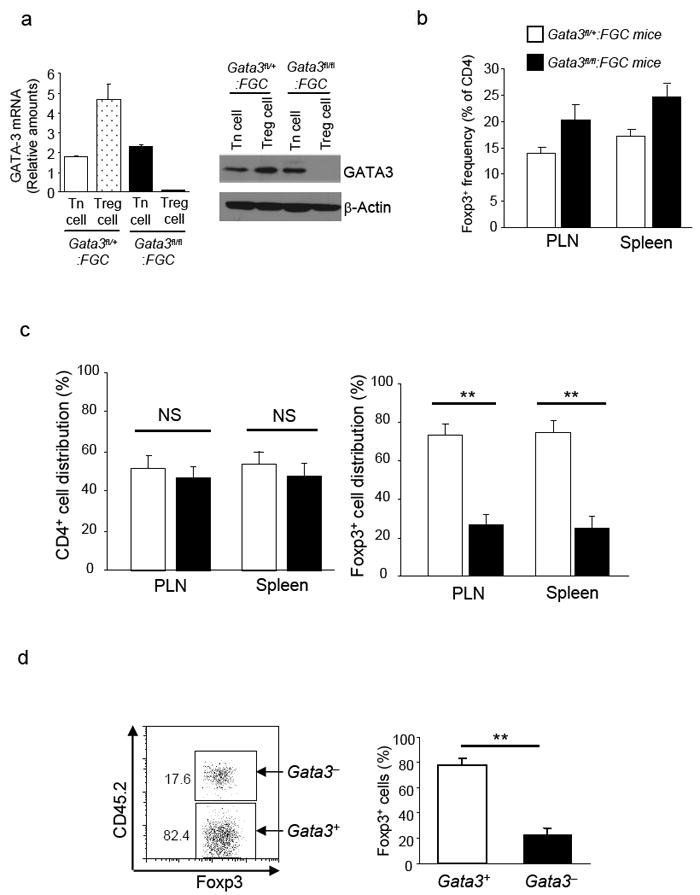
2a, Efficient GATA-3 deletion specifically in Treg cells from Gata3fl/fl:FGC mice. CD4+ Tn cells (CD4+GFP-) and Treg cells (CD4+GFP+) were sorted from Gata3fl/+:FGC and Gata3fl/fl:FGC mice. mRNA and protein expression of GATA-3 in each population was assessed by qRT-PCR and immuno-blotting respectively. Results for qRT-PCR were Means ± s.d. of triplicates done in one experiment representative of three. Results for immuno-blotting were representative of at least three experiments. 2b, The percentages of Foxp3+ Treg cells in CD4 T cells from PLNs and spleens of Gata3fl/+:FGC (open bars) and Gata3fl/fl:FGC (solid bars) mice. Means ± s.d. of four experiments are shown. 2c, Mixed bone marrow chimera were created by transferring equal numbers of bone marrow cells from Gata3fl/+:FGC (CD45.1+) and Gata3fl/fl:FGC (CD45.2+) mice into sub-lethally irradiated Rag1-/- mice. The contributions of cells originated from Gata3fl/+:FGC (open bars) and Gata3fl/fl:FGC (solid bars) bone marrow cells to CD4+ T cell and Foxp3+ Treg cell populations in reconstituted hosts were determined. Means ± s.d. of six mice from one experiment representative of two are shown (NS, Non-significant. **, P<0.01). 2d, GFP+ Treg cells were sorted from Gata3fl/+:FGC (CD45.1+) and Gata3fl/fl:FGC (CD45.2+) mice, mixed at a ratio of 1:1 and then transferred into Rag1-/- mice. Eight weeks after transfer, Foxp3+ Treg cells from different donors were detected by Foxp3 and CD45 co-staining as shown in left plot. The contributions of each donor origin to Treg cell population in the recipients were determined. Means ± s.d. of five mice from one experiment representative of two are shown (**, P<0.01).
See also Fig. S3
We therefore compared the ability of GATA-3-deficient and GATA-3-sufficient Treg cells to repopulate the periphery in the same host. To this end, we generated mixed bone marrow chimera by transferring a mixture of bone marrow cells from Gata-3fl/fl:FGC mice bearing congenic marker CD45.2 and Gata-3fl/+:FGC mice bearing congenic marker CD45.1 into irradiated recipient mice deficient in recombination-activating gene 1 (Rag1-/-). All chimeric mice were grossly normal and showed no signs of inflammatory disorder (data not shown). Accordingly, in the chimeric mice, CD4+ Tn and CD8+ T cells of both donor origins showed naïve phenotype with minimal production of effector cytokines (Supplementary Fig. S3). While total CD4+ T cells were derived equally from both donors, the majority (70-80%) of Foxp3+ Treg cells were derived from GATA-3-sufficient donor. And only approximately 20% of Treg cells were derived from GATA-3-deficient donor (Fig. 2c), suggesting an intrinsic defect in the homeostasis of GATA-3-deficient Treg cells. To confirm that GATA-3-deficient Treg cells were indeed defective in homeostasis in the periphery, we purified Treg cells from Gata-3fl/fl:FGC (CD45.2+) and Gata-3fl/+:FGC (CD45.1+) mice by fluorescence activated cell sorting (FACS). Sorted cells were mixed at 1:1 ratio and then transferred into Rag1-/- mice. Eight weeks after transfer, the contributions of different donor cells to Foxp3+ Treg cell populations in the hosts were assessed. GATA-3-deficient Treg cells were much less abundant than GATA-3-sufficient counterparts (Fig. 2d). Collectively, these findings suggest that GATA-3-deficient Treg cells are intrinsically defective in the homeostasis.
3. Immune suppressive activity is impaired in Treg cells lacking GATA-3
We investigated what functions of Treg cells were affected by GATA-3 deletion. The defining properties of Treg cells are being un-responsive to T cell receptor (TCR) stimulation (anergy) in vitro and being able to suppress CD4+ Tn cell function in vivo and in vitro (Sakaguchi, 2004). We found that GATA-3 deficient Treg cells remained anergic to TCR stimulation, because Treg cells from Gata-3fl/fl:FGC mice did not proliferate in response to TCR stimulation in culture (Fig. 3a). We then studied whether the immune suppressive activity of GATA-3-deficient Treg cells was affected in vivo by using an inflammatory bowel disease (IBD) model induced by transferring naïve CD4+ cells into SCID mice (Powrie et al., 1993). Transferring wild-type naïve (CD25-CD45RBhigh) CD4+ T cells alone elicited IBD in Rag1-/- recipient mice manifested by progressive weight loss (Fig. 3b). Co-transfer with wild-type Treg cells was able to prevent the weight loss (Fig. 3b). In contrast, co-transfer with GATA-3-deficient Treg cells failed to stop naive T cell-elicited weight loss in the recipient mice (Fig. 3b). This observation could be due to reduced numbers of GATA-3-deficient Treg cells in the recipient mice and/or defective suppressive activity of these cells. Further examination revealed that the numbers of Foxp3+ GATA-3-deficient Treg cells recovered from the recipient mice were much lower than those of wild-type Treg cells (Fig. 3c). To directly assess immune suppressive activity of GATA-3-deficient Treg cells, we used an in vitro suppression assay and found that GATA-3-deficient Treg cells showed impaired ability to suppress TCR-stimulated proliferation of co-existing CD4+ Tn cells (Fig. 3d). Therefore, GATA-3 was required for intact immune suppressive activities of Treg cells in vivo and in vitro.
Fig. 3. Immune suppressive activity of Treg cells is impaired in the absence of GATA-3.
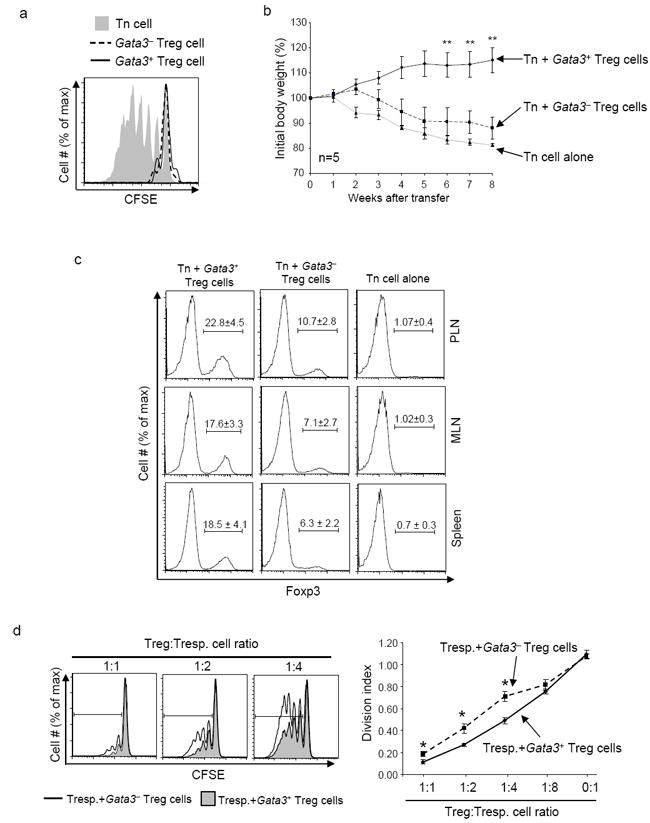
3a, GATA-3-deficient Treg cells remained anergic in vitro. GFP+ Treg cells were sorted from Gata3fl/fl:FGC (Gata3-, dashed line) and Gata3fl/+:FGC (Gata3+, solid line) mice. As a control, GFP- CD4+ Tn (shaded) cells were sorted from Gata3fl/+:FGC mice. Sorted cells were labeled with CFSE and stimulated with soluble anti-CD3 in the presence of irradiated APC. Cell proliferation was assessed by CFSE dilution 72 hours after TCR stimulation in vitro. 3b, GATA-3-deficient Treg cells failed to suppress T cell function in vivo. Rag1-/- mice were transferred with 2×105 CD4+CD25-CD45RBhigh T cells sorted from wild-type C57BL/6 mice alone (dotted line), or together with 1×105 of GFP+ Treg cells sorted from Gata3fl/fl:FGC mice (dashed line) or from Gata3fl/+:FGC mice (solid line). Body weight of recipient mice was monitored weekly for 8 weeks after transfer. The percentage of body weight change was calculated and plotted. Means ± s.e.m of five mice in one experiment representative of two are shown (**, P<0.01). 3c, The percentages (indicated by the numbers above the brackets) of Foxp3+ Treg cells in CD4+ T cells recovered from the peripheral lymph-nodes (PLN), mesenteric lymph-nodes (MLN) and spleens of recipient mice were determined at the end of experiments described in Fig. 3b. Means ± s.d. of five mice in one experiment representative of two are shown. 3d, GATA-3-deficient Treg cells were defective in suppressing T cell function in vitro. CD4+CD25-CD45RBhigh responder T (Tresp.) cells were sorted from wild-type C57BL/6 mice and labeled with CFSE. Labeled responder T cells were then either cultured alone or mixed with varying amounts (as indicated) of GFP+ Treg cells sorted from Gata3fl/fl:FGC or Gata3fl/+:FGC mice. Cell mixtures were stimulated with soluble anti-CD3 in the presence of irradiated APC. The proliferation of responder T cells was assessed by CFSE dilution as shown in left plots. The division-index of responder T cells was determined by FlowJo software and plotted (right panel). Means ± s.d. of triplicate done in one experiments representative of two are shown. (*, P<0.05).
4. GATA-3 deficient Treg cells gain the ability to produce Th17 cell cytokines
Treg cells normally do not produce effector cytokines. However, perturbation of Treg cell function often leads to aberrant cytokine production by these cells (Gavin et al., 2007; Kitoh et al., 2009; Oldenhove et al., 2009; Ouyang et al., 2010; Wan and Flavell, 2007). Our finding that GATA-3 deletion resulted in defective Treg cell function prompted us to ask whether GATA3-deficient Treg cells produced pro-inflammatory cytokines. While freshly isolated GATA-3-deficient Treg cells produced minimal amounts of IFN-γ and IL-4, more than 3% of these cells produced IL-17A, a defining cytokine for Th17 cells (Harrington et al., 2005; Park et al., 2005). At least 3-fold more of IL-17A was produced by GATA-3 -deficient than -sufficient Treg cells at both mRNA and protein levels (Fig. 4a and 4b). This aberrant acquisition of a Th17 cell phenotype from GATA-3-deficient Treg cells was even more pronounced in mice afflicted with IBD (Fig. 4c and 4d). Because the inflammatory cytokine IL-6 promotes IL-17A production in activated Foxp3+ Treg cells in vitro (Xu et al., 2007; Yang et al., 2008), we speculated that IL-6 driven IL-17A expression would be enhanced in Treg cells with GATA-3 deletion. Indeed, upon TCR stimulation in the presence of exogenous IL-6 alone or IL-6 plus transforming growth factor-β (TGF-β), a notably higher percentage of GATA-3-deficient Foxp3+ Treg cells produced IL-17A than that of GATA-3-sufficient Treg cells (Fig. 4e). Therefore, GATA-3-deficient Treg cells acquired enhanced ability to produce Th17 cell cytokines.
Fig. 4. Th17 cytokine production by GATA-3-deficient Treg cells.
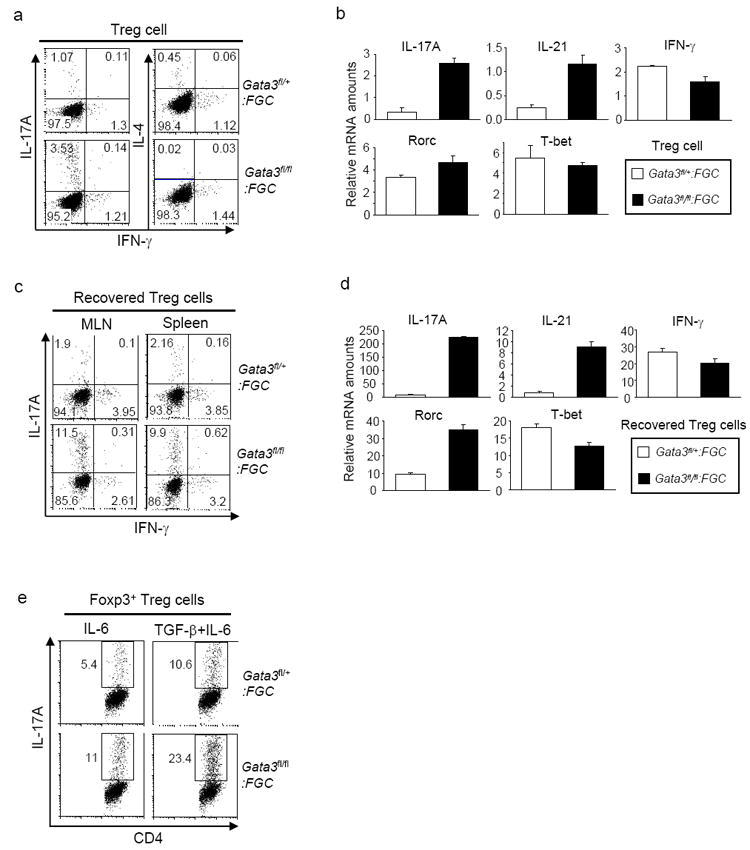
4a and 4b, GFP+ Treg cells were purified from Gata3fl/fl:FGC and Gata3fl/+:FGC mice. Protein expression of IFN-γ, IL-4 and IL-17A in sorted Treg cells was assessed by intracellular staining. The numbers in each quadrant indicate the percentage of relevant population. Representative results of at least five experiments are shown (4a). Relative mRNA amounts of IL-17A, IL-21, IFN-γ, Rorc and T-bet in sorted Treg cells were determined by qRT-PCR. Means ± s.d. of triplicates done in one experiment representative of three are shown (4b). 4c and 4d, At the end of IBD experiment described in Fig. 3b, CD4+ T cells were recovered from mesenteric lymph-nodes (MLN) and spleens of the recipient mice. Protein expression of IL-17A and IFN-γ in recovered Foxp3+ Treg cells was determined by intracellular staining. Representative results of three experiments are shown (4c). Relative mRNA amounts of IL-17A, IL-21, IFN-γ, Rorc and T-bet in sorted GFP+ Treg cells from the spleens were determined by qRT-PCR. Means ± s.d. of triplicates done in one experiment representative of three are shown (4d). 4e. GFP+ Treg cells were sorted from Gata3fl/fl:FGC and Gata3fl/+:FGC mice and then stimulated in vitro with anti-CD3 and anti-CD28 in the presence of IL-6 (40ng/ml) or IL-6 (40ng/ml) + TGF-β (2ng/ml). Four days after stimulation, IL-17A production in Foxp3+ cells was assessed. Results representative of three experiments are shown.
5. The expression of Foxp3 and Treg cell signature genes is decreased in GATA-3 deficient Treg cells
Aforementioned findings suggested that GATA-3 was essential for Treg cell function and prompted us to investigate which factor critical for Treg cell function was affected by GATA-3 deletion. We first noticed that EGFP expression, which reflected Foxp3 expression at the transcription level in FGC mice, was consistently lower in Treg cells from Gata-3fl/fl:FGC mice than from Gata-3fl/+:FGC mice (Fig. 5a). To directly assess Foxp3 mRNA expression, we sorted GATA-3 -deficient and -sufficient Treg cells from Gata-3fl/fl:FGC and Gata-3fl/+:FGC mice respectively and detected Foxp3 expression by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). Indeed, the amount of Foxp3 mRNA was decreased by approximately 50% upon GATA-3 deletion (Fig. 5b). In addition, the amount of Foxp3 protein was reduced to a similar extent in GATA-3-deficient Treg cells (Fig. 5c). Treg cell signature genes, such as CD25, CTLA-4 and GITR, are important for Treg cell function (Sakaguchi, 2004). We then examined whether the expression of these genes was affected by GATA-3 deletion. Compared to Treg cells from Gata-3fl/+:FGC mice, Treg cells from Gata-3fl/fl:FGC mice consistently expressed less CD25, CTLA-4 and GITR at both mRNA and protein levels (Fig. 5d and 5e). Similar observation were made in mixed bone marrow chimera that was reconstituted with bone marrow cells from Gata-3fl/fl:FGC mice (CD45.2+) and Gata-3fl/+:FGC mice (CD45.1+) (Fig. 5f); GATA-3-deficient Treg cells expressed less amounts of Foxp3, CD25, CTLA-4 and GITR than those of GATA-3-suficient counterparts in the same host. Therefore, GATA-3 deletion led to reduced expression of Foxp3 and Treg cell signature genes in Treg cells.
Fig. 5. Decreased expression of Foxp3 and Treg cell signature genes in GATA-3-deficient Treg cells.
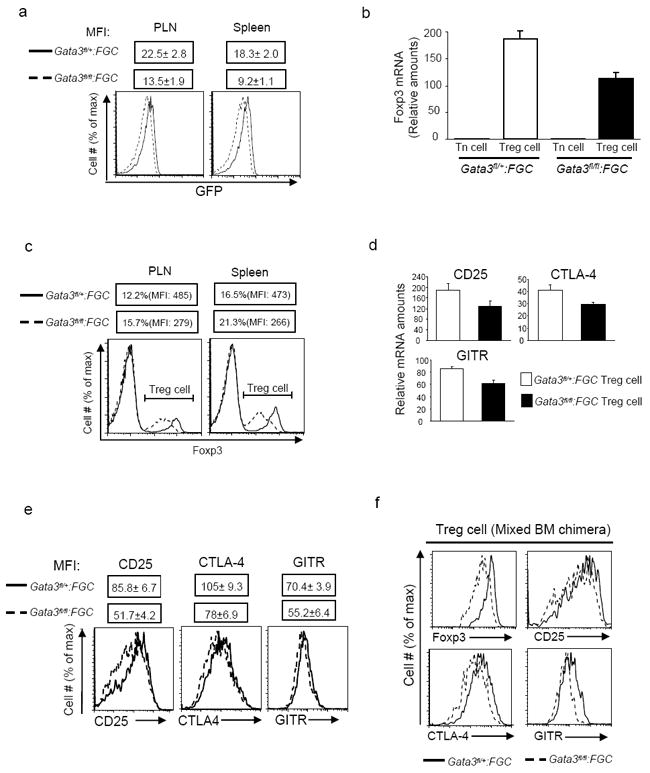
5a, EGFP expression of gated CD4+GFP+ Treg cells from PLNs and spleens of Gata3fl/fl:FGC (dash lines) and Gata3fl/+:FGC (solid lines) mice. Mean fluorescence intensity (MFI) of EGFP is also shown as Means ± s.d. of the results from three mice. 5b, Quantitative RT-PCR analysis of Foxp3 mRNA in CD4+GFP- (Tn) and CD4+GFP+ (Treg) cells sorted from Gata3fl/fl:FGC (solid bars) and Gata3fl/+:FGC (open bars) mice. Means ± s.d. of triplicates done in one experiment representative of at least four experiments are shown. 5c, Flow cytometric analysis of Foxp3 expression in CD4+ T cells in PLNs and spleens of Gata3fl/fl:FGC (dashed lines) and Gata3fl/+:FGC (solid lines) mice. The percentages of Foxp3+ cells and MFI of Foxp3 staining in Foxp3+ cells are also shown. 5d, Relative mRNA amounts of CD25, CTLA-4 and GITR in GFP+ Treg cells sorted from Gata3fl/fl:FGC (solid bars) and Gata3fl/+:FGC (open bars) mice. Means ± s.d. of triplicates done in one experiments representative of four are shown. 5e, The expression of CD25, CTLA-4 and GITR on GFP+ Treg cells from Gata3fl/fl:FGC (dashed lines) and Gata3fl/+:FGC (solid lines) mice were assessed by flow cytometric analysis. MFIs are shown as Means ± s.d. of three mice. 5f, At the end of mixed bone marrow chimera experiments described in Fig. 2c, the expression of Foxp3, CD25, CTLA-4 and GITR in GFP+ Treg cells originated from Gata3fl/fl:FGC (dashed lines) or Gata3fl/+:FGC (solid lines) donors were determined by flow cytometric analysis and compared. Results described in this figure are representative of at least three experiments unless stated otherwise.
6. GATA-3 binds to CNS2 of Foxp3 locus and promotes its activity
Foxp3 is central to controlling various aspects of Treg cell function. The observation that GATA-3 deletion caused a decrease of Foxp3 expression (Fig. 5) prompted us to study the mechanisms by which GATA-3 regulates Foxp3 expression. While reduced CD25 expression in GATA-3-deficient Treg cells can contribute to decreased Foxp3 expression since IL-2 signaling is critical for Foxp3 expression (Fontenot et al., 2005; Rubtsov et al., 2010), we nonetheless hypothesized that GATA-3 may also directly regulate Foxp3 expression. Using PROMO, a transcription factor binding site prediction program, we identified putative GATA-3 binding sites in previously defined Foxp3 regulatory regions (Fig. 6a). Four highly conserved non-coding DNA sequences (CNS) including promoter, CNS1, CNS2 and CNS3 in Foxp3 locus have been identified (Long et al., 2009; Zheng et al., 2010). CNS1 is critical for TGF-β-induced Foxp3 expression, CNS2 is important to maintain Foxp3 expression in thymus-derived Treg cells, and CNS3 acts as a pioneer element for Foxp3 expression. We first investigated whether GATA-3 bound to these putative sites in Treg cells by performing chromatin immuno-precipitation (ChIP) assay. As expected, we found enrichment of GATA-3 binding to the Th2 locus (positive control) (Lee et al., 2006) but not the promoter region of Gmpr gene (negative control). Nuclear lysates prepared from Treg and CD4+ Tn cells sorted from wild-type mice were subjected to ChIP analysis. Enrichment of GATA-3 binding to different sites was determined by quantitative PCR. GATA-3 showed minimal or no binding to the promoter, CNS1 and CNS3 regions of Foxp3 locus in both Treg and CD4+ Tn cells (Fig. 6b). In contrast, a dramatic increase of GATA-3 binding to CNS2 was observed only in Treg cells but not in CD4+ Tn cells, suggesting that GATA-3 binds to and regulates CNS2 activity to control Foxp3 expression specifically in Treg cells (Fig. 6b). To investigate if GATA-3 is able to regulate CNS2 activity, we utilized luciferase reporter assays. Luciferase reporter constructs containing Foxp3 promoter alone or promoter plus CNS2 (Long et al., 2009) were transfected into Jurkat T cells together with either a GATA-3-expressing construct or an empty construct (control). While GATA-3 expression slightly enhanced the activity of Foxp3 promoter in the absence of CNS2, it greatly promoted the activity of Foxp3 promoter in the presence of CNS2 (Fig. 6c). This activity appeared to require direct binding of GATA-3, because GATA-3 promoted activity was virtually abolished when a substitution mutation of the GATA-3 binding site in CNS2 was created (Fig. 6d). Taken together, these findings suggest that GATA-3 controls Foxp3 expression through binding to CNS2 to regulate the activity of cis-acting elements of the Foxp3 gene.
Fig. 6. GATA-3 bound to CNS2 of Foxp3 locus and promoted its activity.
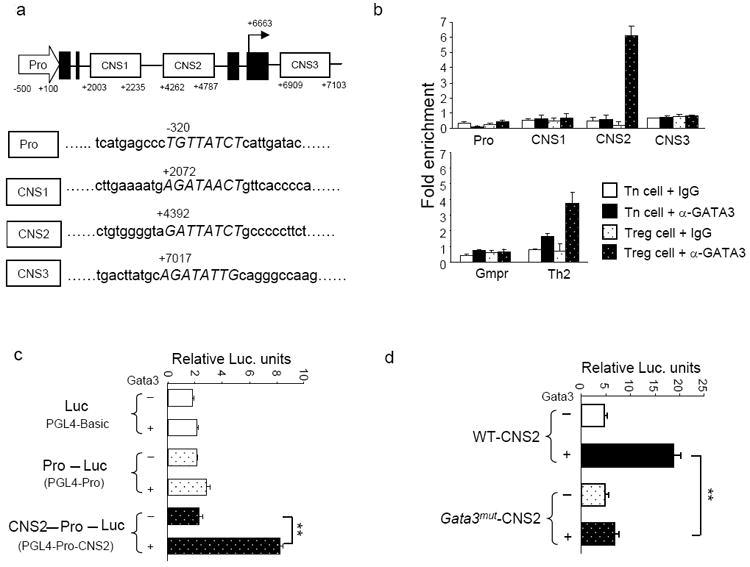
6a, An illustration of putative GATA-3 binding sites in the Foxp3 promoter (Pro), CNS1, CNS2 and CNS3. The numbers denote relative positions to the transcription start site. Exons are marked by filled boxes. 6b, ChIP-coupled quantitative PCR analysis of GATA-3 binding to Foxp3 regulatory regions in sorted GFP-CD4+ Tn cells and GFP+ Treg cells purified from FGC mice. Normal IgG and anti-GATA-3 were used for immuno-precipitation assays. Gmpr locus was used as a negative control and Th2 locus was used as a positive control. Enrichment of GATA-3 binding to each locus was determined. Means ± s.d. of triplicates done in one experiment representative of at least three are shown. 6c and 6d, Luciferase reporter assays. Reporter constructs of PGL4-basic, PGL4 linked with Foxp3 promoter (PGL4-Pro), or PGL4 linked with Foxp3 promoter and CNS2 (PGL4-Pro-CNS2) were transfected into Jurkat T cells together with a GATA-3-expressing plasmid (+) or an empty plasmid (-) (6c). PGL4-Pro-CNS2 reporter constructs containing wild-type CNS2 (WT-CNS2) or containing CNS2 with a substitute mutation of GATA-3 binding site (Gata3mut-CNS2) were transfected into Jurkat T cells together with a GATA-3-expressing plasmid (+) or an empty plasmid (-) (6d). In 6c and 6d, a Renilla luciferase vector (pRL-TK) was co-transfected as internal controls. Transfected cells were cultured for 36 hr before relative luciferase units were determined. Means ± s.d. of triplicates done in one experiment representative of at least three are shown. (**, P<0.01).
See also Table S1.
7. The combined function of GATA-3 and Foxp3 is essential for Foxp3 expression
Multiple transcription factors, including Foxp3 itself, were shown to be important for Foxp3 expression and Treg cell function (Gavin et al., 2007; Kerdiles et al., 2010; Kitoh et al., 2009; Ouyang et al., 2010; Rudra et al., 2009; Zheng et al., 2010). Nevertheless, defects in a single factor rendered incomplete abrogation of Treg cell population and/or function, suggesting that transcription factors may act in combination and redundancy to control Foxp3 expression and Treg cell function. Genome-wide studies to predict transcription factor binding sites showed that Treg cell specific genes and Foxp3 dependent genes tend to have binding sites for both GATA-3 and Foxp3 (Lee et al., 2010), indicating a functional relationship between GATA-3 and Foxp3 in Treg cells. Because Foxp3 is critical for its own expression (Gavin et al., 2007; Zheng et al., 2010) and we found that GATA-3 was important for Foxp3 expression, we hypothesized that the combined function of GATA-3 and Foxp3 is essential to control Foxp3 expression and that defects in both GATA-3 and Foxp3 would lead to drastic reduction of Treg cells.
To test this hypothesis, we first investigated whether GATA-3 remained bound to CNS2 of Foxp3 locus in Treg cells isolated from FILIG mouse, a hypomorphic Foxp3 strain in which Treg cells express greatly reduced Foxp3 and are marked by EGFP (Wan and Flavell, 2007). A strong association of GATA-3 to CNS2 was detected only in Foxp3-expressing Treg cells but not in CD4+ Tn cells purified from FILIG mice (Fig. 7a), suggesting that GATA-3 is involved in Foxp3 expression in FILIG Treg cells. Indeed, by crossing Gata-3fl/fl:FGC mice with FILIG mice, we found that the numbers of Foxp3-expressing Treg cells detected in Gata-3fl/fl:FGC:FILIG mice were lower than in Gata-3fl/+:FGC:FILIG mice (Fig. 7b). A closer examination revealed an incomplete deletion of GATA-3 in Treg cells purified from Gata-3fl/fl:FGC:FILIG mice (Fig. 7c). Thus, Treg cells remained in Gata-3fl/fl:FGC:FILIG mice were “escapees” that expressed GATA-3. Because the Cre expression reflects endogenous Foxp3 expression in FGC mice (Zhou et al., 2008), the great reduction of Foxp3 expression in FILIG mice (Wan and Flavell, 2007) could result in insufficient Cre expression and thus incomplete deletion of GATA-3 in Gata-3fl/fl:FGC:FILIG mice. To circumvent this pitfall and to achieve efficient deletion of GATA-3 in FILIG Treg cells, we bred Gata-3fl/fl:FILIG mice with CD4-Cre transgenic mice (Lee et al., 2001) to generate Gata-3fl/fl:CD4cre:FILIG mice. Sufficient numbers of CD4+ T cells were recovered from Gata-3fl/fl:CD4cre:FILIG mice. In addition, efficient deletion of GATA-3 in these cells was confirmed (Supplementary Fig. S4). While more than 20% of GATA-3-sufficient CD4+ FILIG cells expressed Foxp3, virtually no GATA-3-deficient CD4+ FILIG cells expressed Foxp3 (Fig. 7d). Therefore, defective function of both GATA-3 and Foxp3 led to ablation of Treg cells, suggesting that the combined function of GATA-3 and Foxp3 is essential for Foxp3 expression.
Fig. 7. The combined function of GATA-3 and Foxp3 was essential for Foxp3 expression.
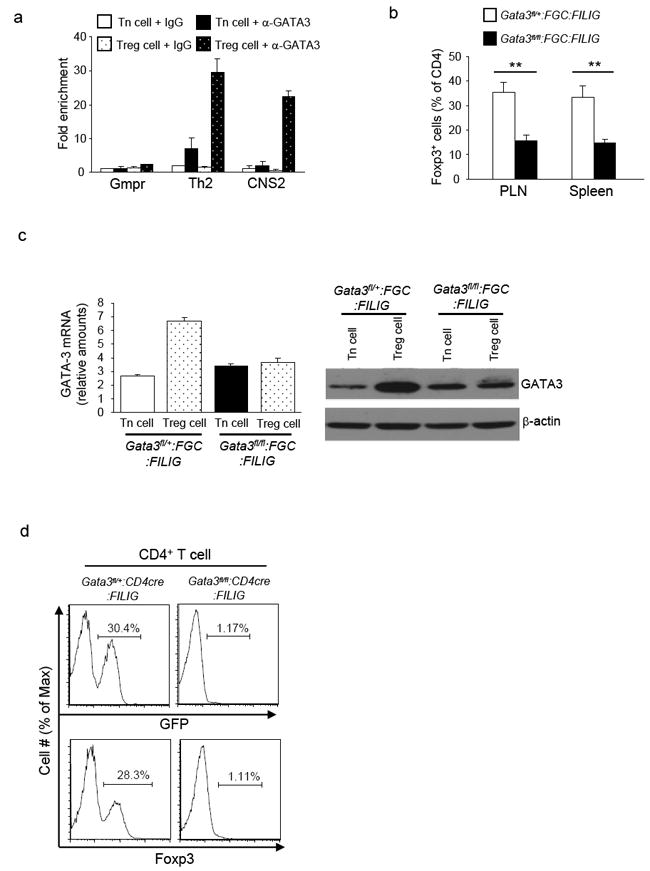
7a, ChIP-coupled quantitative PCR analysis of GATA-3 binding to CNS2 of Foxp3 locus in sorted GFP-CD4+ Tn cells and GFP+ Treg cells from FILIG mice. Normal IgG and anti-GATA-3 were used for immuno-precipitation assays. Gmpr locus was used as a negative control and Th2 locus was used as a positive control. Enrichment of GATA-3 binding to each locus was determined. Means ± s.d. of triplicates done in one experiment representative of three are shown. 7b, The percentages of Foxp3+ Treg cells in the CD4+ T cells isolated from PLN and spleens of Gata3fl/+:FGC:FILIG and Gata3fl/fl:FGC:FILIG mice. Means ± s.d. of four mice are shown. (**, P<0.01). 7c, GATA-3 expression in GFP-CD4+ Tn and GFP+ Treg cells sorted from Gata3fl/+:FGC:FILIG and Gata3fl/fl:FGC:FILIG mice. mRNA expression of GATA-3 in sorted cells were assessed by quantitative RT-PCR (left panel). Means ± s.d. of triplicates done in one experiment representative of three are shown. In addition, protein expression of GATA-3 in sorted cells was assessed by immuno-blotting (right panels). Results are representative of three experiments. 7d, Foxp3-expressing Treg cells in the PLNs of Gata3fl/+:CD4cre:FILIG and Gata3fl/fl:CD4cre:FILIG mice were detected based on GFP and Foxp3 expression. The percentages of Foxp3+ cells were determined (shown above the brackets). Representative results from at least three experiments are shown.
See also Fig. S4
Discussion
This study revealed an indispensible role of GATA-3 in regulating Treg cell function and immune tolerance. Deletion of GATA-3 specifically in Treg cells resulted in an inflammatory syndrome in mice that can be ascribed to defective function of Treg cells. In addition, we found that GATA-3 controlled Foxp3 expression. GATA-3-deficient Treg cells expressed reduced amounts of Foxp3. GATA-3 bound to the regulatory regions of Foxp3 locus and promoted the activity of cis-acting elements of Foxp3 gene. Moreover, we demonstrated that the combined function of GATA-3 and Foxp3 was vital for Foxp3 expression in Treg cells. This study provides insights into the modulation of Treg cell function and sheds lights on how autoimmunity and inflammatory diseases may be controlled.
GATA-3 is a member of GATA-binding protein family consisting of six members (GATA-1-6) that interact with the GATA DNA sequence. GATA-3 regulates an array of biological processes including development, differentiation and tumorigenesis (Pandolfi et al., 1995; Pei et al., 2009). In particular, GATA-3 is critically involved in immune regulation. GATA-3 is highly expressed by Th2 cells and is required for Th2 cell differentiation (Zheng and Flavell, 1997; Zhu et al., 2004). In addition, GATA-3 is essential for T cell development (Pai et al., 2003). Moreover, it is important for the function of NK cells (Samson et al., 2003). Therefore, GATA-3 exerts diverse functions to control immune response in a cell type specific manner. Nevertheless, the understanding of how GATA-3 regulates immune function is far from complete. Indeed, this study unveiled a function of GATA-3 in controlling Treg cell function. Such a function of GATA-3 is vital for self-tolerance and immune homeostasis, because when GATA-3 was deleted in Treg cells, mice spontaneously developed an inflammatory disorder. This finding can have broad implications. Th1 response is important for clearing pathogens and also for causing severe autoimmune diseases, situations where Treg cell function is often tempered (Caretto et al., 2010; Oldenhove et al., 2009). Th1 cell polarizing condition antagonizes GATA-3 expression in non-Treg cells (Szabo et al., 2003). Similarly, we found that Th1 cell polarizing condition also triggers the down-regulation of GATA-3 in Treg cells. It is therefore conceivable that the strong Th1 response during pathogen clearance and inflammation will lead to GATA-3 down-regulation in Treg cells, which may in turn contribute to the reduced function of these cells. In addition, we have demonstrated that GATA-3-deficient Treg cells showed enhanced ability to produce inflammatory cytokines, indicating that GATA-3 down-regulation under inflammatory conditions could allow Treg cells to acquire effector functions that contribute to inflammation. Our study therefore suggests that GATA-3 expression in Treg cells is important for the modulation of Treg cell function and immune response, and thus needs to be considered in order to fully understand how protective (to clear pathogen) and pathogenic (to cause autoimmunity and inflammatory disease) immune responses are controlled.
Treg cells are central to controlling immune tolerance and immune homeostasis. Foxp3 is recognized as a single gene determinant essential for Treg cell function. Perturbations of Foxp3 expression, even slight, often lead to impaired Treg cell function and are associated with various inflammatory diseases and autoimmunity. Therefore, in order to understand immune-tolerance, how Foxp3 transcription is controlled is a critical question under intensive investigation. Many factors including Runx-CBFβ complex, NFκB, Foxp3, FOXO1 and FOXO3, were shown to be important for Foxp3 expression (Gavin et al., 2007; Kerdiles et al., 2010; Kitoh et al., 2009; Long et al., 2009; Ouyang et al., 2010; Rudra et al., 2009; Zheng et al., 2010). Nonetheless, deletion of any one of these genes causes incomplete abrogation of Foxp3 expression. These findings not only underscore the vital importance of maintaining certain amounts of Foxp3 expression but also implicate that multiple factors are involved in promoting Foxp3 expression in a combinatorial and somewhat redundant manner. Indeed, combined deficiency of certain set of genes, such as FOXO1 and FOXO3, results in much more drastic defect in Treg cells than any one gene deficiency achieved (Kerdiles et al., 2010; Ouyang et al., 2010). Similarly, our results demonstrated that, although impaired function of GATA-3 or Foxp3 alone caused decrease of Foxp3 expression, defects in both GATA-3 and Foxp3 led to ablation of Foxp3 expression, suggesting that GATA-3 and Foxp3 control Foxp3 expression in combination. Therefore, while we are investigating how a single factor is involved in controlling Foxp3 expression, we also need to consider how different factors function together to control Foxp3 expression and Treg cell function.
As a transcription factor, GATA-3 exerts its function mainly through DNA binding. It is therefore likely that cell-type specific function of GATA-3 is mediated through its binding to different sets of loci in different cell types. To support this notion, we found that, in non-Treg cells, GATA-3 bound to Th2 locus but not Foxp3 locus. However, in Treg cells, GATA-3 bound to both Th2 locus and CNS2 of Foxp3 locus. Inter-chromosomal interaction has been proposed to regulate activities of cis-acting elements on different chromosomes (Spilianakis et al., 2005). Such inter-chromosomal interactions may be mediated through protein complexes containing specific transcription factors. The observation that GATA-3 bound to both Foxp3 and Th2 loci in Treg cells suggests a potential physical interaction and mutual regulation between Foxp3 and Th2 loci in Treg cells, an issue that warrants further investigation to elucidate the mutual regulation between Th2 and Treg cells. Although we have found that GATA-3 bound to CNS2 but not CNS1 (a site modulating Foxp3 expression in TGF-β-induced Treg cells (Zheng et al., 2010)) of Foxp3 locus in thymic-derived Treg cells, consensus GATA-3 binding sites were identified in CNS1 suggesting that GATA-3 may be also involved in regulating Foxp3 expression in TGF-β-induced Treg cells. Th2 cell polarizing condition was shown to antagonize TGF-β-driven Foxp3 expression and to promote Th9 cell differentiation (Dardalhon et al., 2008; Veldhoen et al., 2008), a process that may involve GATA-3 (Mantel et al., 2007). Further investigation is therefore needed to address if GATA-3 is involved in such a process by regulating Foxp3 expression through CNS1 activities.
Experimental Procedures
1. Mice
Foxp3-GFP-Cre (FGC) mice were purchased from Jackson laboratory and have been backcrossed into C57BL/6 background for more than 5 generations. GATA-3fl/fl, CD4-Cre, FILIG, Rag1-/- and CD45.1 congenic mice were on C57BL/6 background. All mice were housed and bred in specific pathogen-free conditions in the animal facility at the University of North Carolina at Chapel Hill. All mouse experiments were approved by Institution Animal Care and Use Committee of the University of North Carolina.
2. Flow cytometric analysis and cell sorting
Lymphocytes were isolated from the various organs (as described) of age and sex matched mice of 8-16 weeks of age. Fluorescence-conjugated antibodies for CD4, CD8, CD25, CD44, CD62L, GITR, CD45.1, CD45.2, IFN-γ, IL-4, IL-17, Foxp3 (eBioscience) and CTLA-4 (BD Bioscience) were purchased. Surface and Intracellular staining was performed per manufacturer’s protocols. For intracellular cytokine staining, lymphocytes were stimulated with 50 ng/ml of phorobol 12-myristate 13-acetate (PMA) and 1 μM of ionomycin for 3-4 hours in the presence of Brefeldin A. Stained cells were analyzed on a LSRII station (BD Biosciences) or sorted on Moflow cell sorter (Dako cytomation, Beckman Coulter).
3. In vitro cell proliferation and suppression assay
CD4+GFP+ Treg cells (suppressor) and CD4+GFP- non-Treg cells (responder) were sorted. To assess Treg cell proliferation in vitro, 2×104 sorted Treg cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated with soluble CD3 antibody (1μg/ml) in the presence of 1×105 irradiated (3000 cGy) T-cell depleted splenocytes as antigen presenting cells (APC). Cell proliferation was assessed by CFSE dilution detected by flow-cytometric analysis 72 hours post stimulation. To assess the efficacy of Treg cell mediated immune suppression in vitro, 2×104 sorted responder T cells were labeled with CFSE and mixed with varying amounts (as indicated) of Treg suppressor cells. Cell mixtures were stimulated with soluble CD3 antibody (1μg/ml) in the presence of 1×105 irradiated (3000 cGy) T-cell depleted splenocytes as APC. The proliferation of responder cells was assessed by CFSE dilution detected by flow-cytometric analysis 72 hours post stimulation.
4. Luciferase reporter assay
PGL4-Pro and PGL4-Pro-CNS2 luciferase reporter constructs were kindly provided by Dr. S. Ghosh (Columbia University). Substitution mutation of GATA-3-binding site at CNS2 was created with Quickchange Mutagenesis kit (Agilent Technologies). Jurkat T cells were transfected by electroporation with 5μg of reporter plasmid together with 5μg of GATA-3 expressing plasmids. 1μg of pRL-TK plasmid was also co-transfected serving as an internal control. Firefly and Renilla Luciferase activities were determined with a dual-reporter assay kit (Promega) 36 hours post transfection.
5. Adoptive transfer assay, Treg cell-mediated protection of naïve CD4+ T-cell-elicited IBD assay, and bone marrow transplantation assay
To determine the ability of Treg cells to be maintained in the periphery, GATA-3-sufficient and GATA-3-deficient CD4+GFP+ Treg cells bearing different CD45 congenic markers were sorted. 1×105 sorted Treg cells were co-transferred into Rag1-/- recipients via retro-orbital injection. The contribution of each donor to the Treg cell population recovered in the hosts was determined 8 weeks post transfer. To assess Treg cell-mediated protection of naïve T-cell-elicited IBD in vivo, 1×105 sorted Treg cells were mixed with 2×105 naive (CD25-CD45RBhigh) CD4+ T cells sorted from wild-type C57BL/6 mice. Cell mixture was transferred into Rag1-/- via retro-orbital injection. As control, 2×105 naïve CD4+ T cells were also transferred alone into Rag1-/- mice. To monitor IBD development, body weight of the recipient mice was monitored weekly after the transfer. Recipient mice were euthanized 8 weeks after transfer. T cells from these mice were harvested and subjected to immunological analysis. To create mixed bone marrow chimera, bone marrow cells were isolated from the femur bones of Gata3fl/fl:FGC or Gata3fl/+:FGC mice bearing different CD45 congenic markers. 1×106 bone marrow cells from each donor were mixed and transferred into irradiated (500 cGy) Rag1-/- mice. The contribution of each donor to Treg cell population in the recipients was determined 8 weeks after transfer.
6. Chromatin Immuno-precipitation (ChIP) assay
ChIP assay was performed per Upstate Biotechnology’s protocol. Briefly, cells were cross-linked by 1% formaldehyde and lysed in lysis buffer. The lysates were sonicated with a Bioruptor sonicator to shear genomic DNA into 200-500 bp fragments. Chromatin prepared from 2×106 CD4+GFP+ Treg cells and CD4+GFP- Tn cells were subjected to immuno-precipitation overnight at 4°C using goat anti-GATA-3 (D-16, sc-22206, Santa Cruz) or normal goat IgG (sc-2028, Santa Cruz) antibodies. Quantitative real-time PCR was performed to determine the relative abundance of target DNA. Specific primers for analysis of GATA-3 binding to Foxp3 and other target loci are listed in Supplementary Table S1.
7. Statistical analysis
Data from at least three sets of samples were used for statistical analysis. Statistical significance was calculated by Student’s t-test. A P value of less than 0.05 was considered significant.
Supplementary Material
Highlights.
GATA-3 deficiency in Treg cells results in a spontaneous immune disorder.
GATA-3 null Treg cells are defective in homeostasis and display aberrant function.
GATA-3 binds to the Foxp3 locus and regulates its transcription.
The combined function of Foxp3 and GATA-3 is vital for Foxp3 expression.
Acknowledgments
We thank S. Ghosh (Columbia University) for Foxp3 luciferase reporter constructs; J. Engel (University of Michigan) for GATA-3 cDNA constructs; Y. Zheng (Salk Institute) for suggestions on ChIP assays; R. Flavell (Yale University) for FIR and FILIG mice; M. Busslinger and A. Souabni (Research Institute of Molecular Pathology) for Gata3fl/fl mice, N. Fisher and J. Kalnitsky (University of North Carolina Flow-cytometry facility) for cell sorting; J. Ting and G. Matsushima (University of North Carolina) for helpful discussions. This study is supported by the NIH (R00AI072956), the Lupus Research Institute and the University Cancer Research Fund (Y.Y.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H, Kitabayashi I, Tsukada T, Nomura T, Miyachi Y, et al. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31:609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Lee YH, Benary M, Baumgrass R, Herzel H. Prediction of regulatory transcription factors in T helper cell differentiation and maintenance. Genome Inform. 2010;22:84–94. [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, Ho IC, Perou CM, Xiong Y. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15:389–401. doi: 10.1016/j.ccr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F, Buer J, Grosveld F, Godin I, Di Santo JP. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990;4:1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


