Abstract
Respiratory syncytial virus (RSV) is the most common respiratory pathogen in infants and young children. The pathophysiology of this infection in the respiratory system has been studied extensively, but little is known about its consequences in other systems. We studied whether RSV infects human bone marrow stromal cells (BMSCs) in vitro and in vivo, and investigated whether and how this infection affects BMSC structure and hematopoietic support function. Primary human BMSCs were infected in vitro with recombinant RSV expressing green fluorescent protein. In addition, RNA from naive BMSCs was amplified by PCR, and the products were sequenced to confirm homology with the RSV genome. The BMSC cytoskeleton was visualized by immunostaining for actin. Finally, we analyzed infected BMSCs for the expression of multiple cytokines and chemokines, evaluated their hematopoietic support capacity, and measured their chemotactic activity for both lymphoid and myeloid cells. We found that BMSCs support RSV replication in vitro with efficiency that varies among cell lines derived from different donors; furthermore, RNA sequences homologous to the RSV genome were found in naive primary human BMSCs. RSV infection disrupted cytoskeletal actin microfilaments, altered cytokine/chemokine expression patterns, decreased the ability of BMSCs to support B cell maturation, and modulated local chemotaxis. Our data indicate that RSV infects human BMSCs in vitro, and this infection has important structural and functional consequences that might affect hematopoietic and immune functions. Furthermore, we have amplified viral RNA from naive primary BMSCs, suggesting that in vivo these cells provide RSV with an extrapulmonary target.
Keywords: asthma, bronchiolitis, chemotaxis, cytokines, hematopoiesis
CLINICAL RELEVANCE.
This study identifies the bone marrow stroma as a novel target for respiratory syncytial virus (RSV) infection and establishes that this infection causes important structural and functional changes in a cell population that is essential to a functional marrow microenvironment and effective hematopoiesis. These observations imply that, albeit the clinical manifestations of RSV are predominantly circumscribed to the respiratory system, the infection may have systemic implications that can affect its severity during the acute phase, as well as its long-term pathologic sequelae. Consequently, future prophylactic and management strategies should also address the coverage of possible extrapulmonary sites of viral replication.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection in infants and young children (1), and also a frequent cause of morbidity and mortality in the elderly and high-risk adults (2). This virus targets primarily the ciliated bronchiolar epithelium (3), but the ensuing pathology, more than from direct cytopathic effects, results from the host immunoinflammatory response characterized by extensive recruitment of bone marrow–derived cells into the airways and lung parenchyma (4, 5). Furthermore, growing epidemiologic evidence suggests that early-life RSV infections predispose to the development of chronic respiratory dysfunction, possibly related to persistence of the virus itself or to its effects on lung development (6).
Although the tropism of RSV for airway epithelial cells is well documented, it is likely that the infection is not always restricted to the respiratory tract. Viremia and extrapulmonary infections have been shown in experimental RSV infections of rodents (7, 8), and may be a frequent occurrence in infants and young children (9, 10). These observations imply that nonrespiratory cells and tissues may be exposed to RSV, and that previously unrecognized targets for infection exist that contribute to the acute and/or chronic pathological process. Furthermore, if RSV can spread to remote sites, it could also find sanctuary in immunologically privileged extrapulmonary cells and tissues that allow a low-grade, subclinical infection to persist latently and possibly recur.
The bone marrow microenvironment, and particularly bone marrow stromal cells (BMSCs), secrete soluble factors that support survival, differentiation, and proliferation of hematopoietic cells, but also provide a protective niche for both normal and abnormal (e.g., malignant) cells, shielding them from intrinsic (immunologic) and extrinsic (pharmacotherapeutic) host defenses (11, 12). The present study is centered on the hypothesis that BMSCs are a target of human RSV infection, develop structural and functional changes when infected, participate actively in the pathogenesis of the acute disease, and can harbor the virus chronically, allowing persistence of the infection.
To test this hypothesis, we sought to determine whether human primary BMSCs can be infected in vitro by RSV, and whether they can support its complete replicative cycle yielding infectious virions. Select experiments were repeated after inactivation of the virus nucleic acid by ultraviolet (UV) light irradiation to determine whether the observed biological effects were determined by virus gene expression and replication, or by exposure to constituents of the virion. Furthermore, we used RT-PCR with RSV-specific primers to amplify RNA extracted from naive human BMSCs (i.e., not artificially exposed to RSV), and sequenced the PCR products to confirm homology with the viral genome. The structural consequences of the infection on the BMSC cytoskeleton were assessed by immunostaining for actin, which is essential for RSV gene expression, maturation, and budding. Finally, to explore the functional implications of this infection, we analyzed the expression of multiple cytokines, chemokines, and growth factors involved in the regulation of hematopoiesis, evaluated the hematopoietic support capacity of infected BMSCs on a stroma-dependent pro-B cell line, and measured the chemotactic activity for both lymphoid and myeloid cell lines in comparison with RSV-infected lung epithelial cells.
MATERIALS AND METHODS
Complete information on the methods is provided in the online supplement.
Cell lines and Reagents
Primary human BMSCs were derived from adult (P163, P164) and pediatric (P299, P604) consenting donors, as described previously (13), with approval from the West Virginia University institutional review board. Previously published protocols were used for maintenance in culture of murine BMSC line S10 (14), C1.92 pro-B cells (15, 16), and AML14.3D10 myeloid cells (17).
RSV Infection
BMSCs were infected with RSV derived from the RSV-A2 strain and expressing the green fluorescent protein (GFP) gene (rgRSV) (18). Select experiments were controlled using aliquots of rgRSV irradiated with a UV light source for 20 minutes (UV-rgRSV) to inactivate the viral nucleic acid (19).
RSV Detection and Quantification
As in previous studies (20), DNase-treated total RNA extracted from naive primary human BMSCs was amplified by RT-PCR using primers targeting the sequence 1,303–1,712 bp of the RSV strain A2 genome (GenBank accession number M11486), which encodes for the viral nucleocapsid (N) protein (5′-GCGATGTCTAGGTTAGGA-3′ and 5′-GCTATGTCCTTGGGTAGT-3′). PCR products were separated by electrophoresis, purified, sequenced, and analyzed for homology to the RSV-A2 genome using the Genetyx 5.0 software at Eurofins MWG Operon (Huntsville, AL). The number of virus copies yielded from BMSCs infected in vitro with rgRSV was quantitated using a previously published real-time RT-PCR protocol (20).
Actin Immunostaining
P164 and P299 cells were grown on coverslips without gelatin and infected with rgRSV at multiplicity of infection (MOI) of 5. After staining for actin with Alexa Fluor 594 Phalloidin, these preparations were photographed by confocal microscopy at 24, 48, and 72 hours and compared with matched noninfected controls.
Cytokine Expression
Cell-free supernatants from rgRSV-infected BMSCs and matched noninfected controls were collected at 12, 24, 48, and 72 hours and tested for the expression of 30 cytokines and chemokines with a Luminex-based system. Transforming growth factor (TGF)–β1 expression was measured separately by ELISA using etoposide (VP-16)-treated cells as positive control.
Pro-B Cell Expansion
Confluent BMSC layers were infected with rgRSV at MOI of 10. After 6-hour incubation, 1.5 × 105 C1.92 cells were added to each infected and control well. Photographs of these cocultures were taken using inverted fluorescence and bright-field microscopy at 24, 48, and 72 hours to compare qualitatively the number of C1.92 cells attached to infected BMSCs versus noninfected controls. All wells were rinsed at each time point, and the supernatants were collected to count C1.92 cells by the trypan blue method. At the end of 72 hours of pro B-cell:stromal cell coculture, the cells were trypsinized and total pro-B cells were counted with trypan blue.
Chemotaxis Assay
Confluent BMSCs and A549 cell monolayers grown in the bottom chamber of 24-well tissue culture plates were incubated with rgRSV or sterile medium. After 24 hours, transwells with 5-μm pores containing 1.5 × 105 C1.92 pro-B or AML14.3D10 myeloid cells were placed into each well and incubated for 4 hours at 37°C. C1.92 and AML14.3D10 cells migrating to the lower chamber were counted by flow cytometry.
Statistical Analysis
Data are expressed as means (±SEM). Differences among means were analyzed using paired Student's t test or ANOVA (21). Post hoc comparisons were performed using the Holm-Sidak method.
RESULTS
RSV Infects Human BMSCs
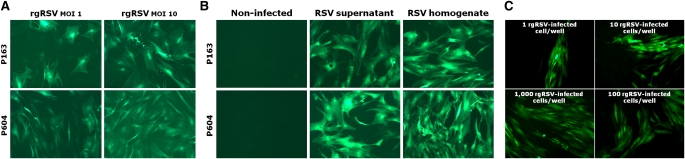
To determine whether the human bone marrow stroma is susceptible to RSV infection, we inoculated primary BMSC cultures with rgRSV at MOI of 1 or 10. After 24 hours of infection, nearly all BMSCs exhibited green fluorescence (Figure 1A), indicating that the virus had infected these cells with high efficiency. After 3 days of infection, cells had elongated processes and diminished adherence. By Day 7, cell damage became manifest by altered morphology. Efficiency varied among BMSCs established from different donors, and was generally greater in BMSCs derived from children compared with adult donors. In addition, the proportion of infected cells was directly proportional to the initial MOI. P164 BMSCs infected with rgRSV at MOI of 10 for 48 hours yielded an average RSV copy number of 1.1 × 107 (±4.6 × 104) (n = 6), as measured by quantitative real-time RT-PCR, whereas no signal was amplified from matched noninfected BMSC controls.
Figure 1.
Respiratory syncytial virus (RSV) infects human bone marrow stromal cells (BMSCs). (A) Fluorescence microscopy shows human primary BMSCs derived from adult (P163) and pediatric (P604) donors expressing recombinant green fluorescent protein (GFP) 24 hours after infection in vitro with an RSV-A2 strain expressing the GFP gene (rgRSV) at multiplicity of infection (MOI) of 1 or 10. Efficiency of infection varies among cell lines derived from different donors, and increases with MOI. (B) Supernatants or homogenates from BMSCs were collected 72 hours after infection with rgRSV at MOI of 10, and then transferred to noninfected BMSC cultures from adult (P163) and pediatric (P604) donors. Fluorescence microscopy after 7 days shows GFP expression in the newly infected BMSCs. Noninfected controls show no detectable autofluorescence. (C) BMSCs infected with rgRSV at MOI of 10 were trypsinized after 72 hours of infection, and their viability was assessed with trypan blue. Serial dilutions ranging from 1 to 1,000 infected cells per well were inoculated on noninfected BMSCs, which were then analyzed for green fluorescence to detect viral propagation. Photomicrographs were taken after 4 days of exposure to rgRSV.
BMSCs Support RSV Replication
To determine if RSV not only infects human BMSCs, but also is able to replicate in these cells, we exposed noninfected BMSCs to the supernatants or homogenates of rgRSV-infected BMSCs. At 1 week after infection, all BMSCs exhibited green fluorescence (Figure 1B). Infection was observed in BMSCs exposed to homogenates earlier than in BMSCs exposed to supernatants from the same cultures. Similarly, cell death was detected earlier in BMSCs exposed to homogenates than in BMSCs exposed to supernatants, suggesting a dose-dependent pattern in both initiation of infection and virus-induced cell death.
In a parallel experiment in which noninfected BMSCs were exposed to varying numbers of rgRSV-infected BMSCs, the same pattern was observed (Figure 1C). Infection was detected after 24 hours in wells exposed to 10,000 or 1,000 infected BMSCs, 48 hours in wells exposed to 100 infected cells, 72 hours in wells exposed to 10 infected cells, and 96 hours in wells exposed to only 1 infected cell.
Interestingly, where infection transfer was undertaken with a single infected cell, there was development of a single focus of fluorescence (Figure 1C, upper left panel), which may be consistent with persistence of endogenous viral RNA in the progeny of the primary infected cells. However, this finding may also reflect transmission of the infection to the naive cells closest to the original infected cell, or a combination of both mechanisms.
RSV Can Be Detected in Naive Primary BMSCs
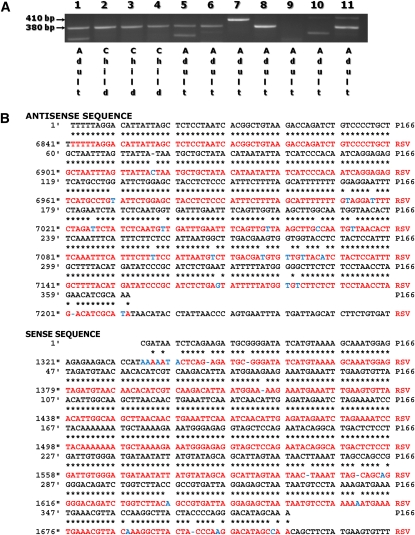
To determine whether human BMSCs can be infected by RSV in vivo and harbor viral genomic RNA, we screened BMSC lines derived from 11 donors (3 children and 8 adults) using primers specific for the nucleocapsid (N) protein of the human RSV-A serotype (Figure 2A). Five of the eight adult-derived BMSC lines (63%) and all children-derived lines (100%) yielded a 380-bp product, the sequence of which shared greater than 95% homology with the RSV-A2 genome (Figure 2B). In contrast, the 410-bp product amplified from subject 7 showed less than 50% sequence homology with RSV-A2. Subjects 9 and 10 were negative for both products.
Figure 2.
RSV can be detected in naive BMSCs. (A) A total of 11 primary human BMSC lines derived from pediatric or adult donors were screened by RT-PCR using primers specific for the nucleocapsid (N) protein of RSV-A. (B) Five of the eight adult-derived BMSC lines (63%) and all three children-derived lines (100%) yielded a 380-bp product, the sequence of which shared greater than 95% homology with the RSV-A genome. Homologous base pairs are marked by asterisks.
RSV Induces Structural and Functional Changes in BMSCs
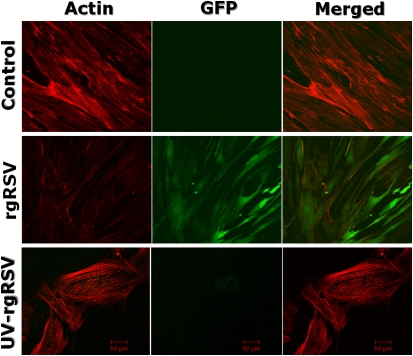
To determine whether RSV interacts with the cytoskeletal network of infected BMSCs, we visualized by immunostaining the cytoskeletal protein, actin (Figure 3). Infection with live rgRSV altered the structure of the actin microfilaments and reduced their immunoreactivity dramatically compared with matched noninfected controls, leading to obvious changes in BMSC morphology (Figure 3, middle panels). Cytoskeletal changes were present at all time points tested (from 24 to 72 h; see Figure E1 in the online supplement), and the extent of rgRSV-induced fragmentation was similar in BMSCs derived from adult and pediatric donors. UV inactivation of the virus nucleic acid abolished the effect of rgRSV on the BMSC cytoskeleton (Figure 3, lower panels), and, as expected, also prevented the expression of GFP, which is critically dependent upon virus gene expression and replication.
Figure 3.
RSV disrupts BMSC cytoskeleton. Cytoskeletal actin microfilaments were visualized by immunostaining. These pictures show noninfected (top) and rgRSV-infected, pediatric-derived BMSCs (P299) at 72 hours after infection. RSV infection altered the structure of the cellular actin filaments and reduced their immunoreactivity dramatically compared with matched noninfected controls, leading to changes in BMSC morphology. Inactivation of the virus with ultraviolet (UV) light abolished its effect on the BMSC cytoskeleton.
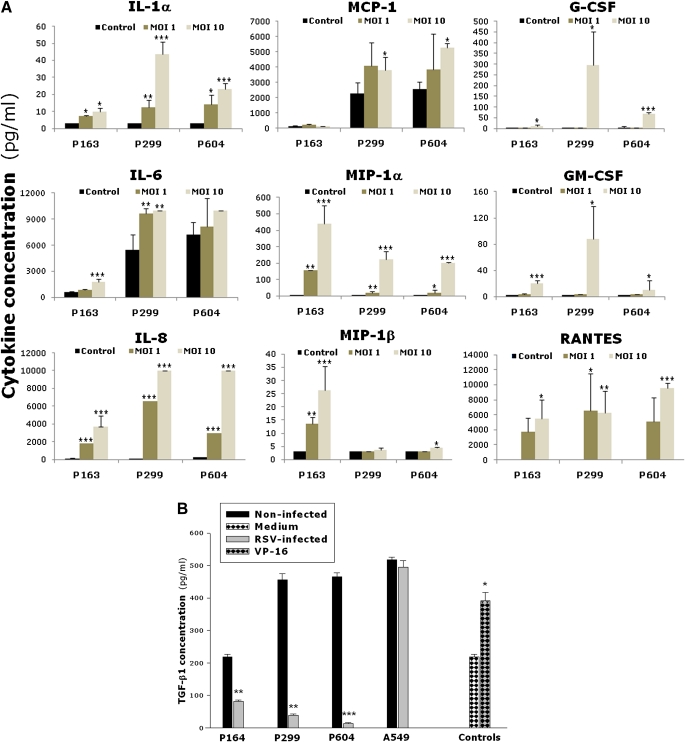
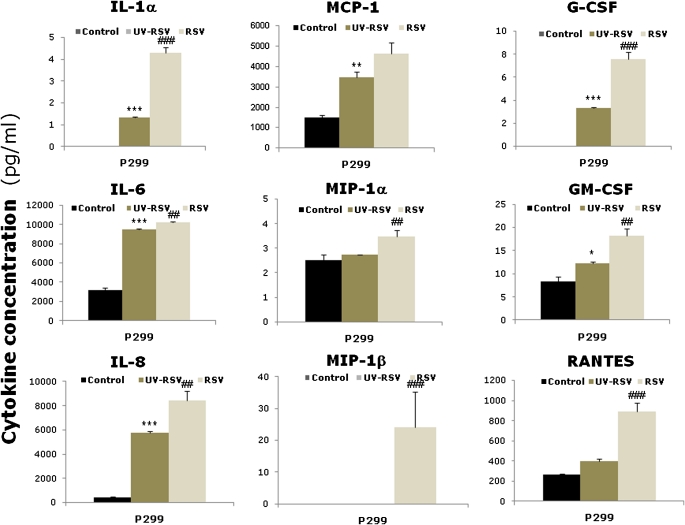
To determine whether RSV infection of BMSCs modifies the expression of soluble factors involved in the regulation of hematopoiesis, we screened the cultures for 30 different cytokines, chemokines, and growth factors using Luminex-based multiplex analysis (Figure 4A). Compared with noninfected controls, rgRSV-infected BMSCs expressed significantly higher levels of IL-1α, IL-6, IL-8, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1a), MIP-1β, granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony–stimulating factor, and RANTES (regulated upon activation, normal T cell expressed and secreted; P < 0.05). In general, BMSCs infected at MOI of 10 had more pronounced elevation of these cytokines compared with BMSCs infected at MOI of 1. Responses also varied among individual bone marrow donors, although the general trends were relatively consistent.
Figure 4.
RSV modifies cytokine expression by BMSCs. (A) Cells were infected with RSV at MOI of 1 or 10. Cell-free supernatants were collected at 72 hours after infection, and cytokine expression was measured by multiplex analysis. The bar graphs show cytokine concentrations of noninfected (closed bars), RSV-infected at MOI of 1 (dark gray bars), and RSV-infected at MOI of 10 (light gray bars). (B) Cells were infected with RSV at MOI of 10. Cell-free supernatants were collected at 72 hours after infection, and TGF-β1 protein expression was measured by ELISA. Etoposide (VP-16, 100 μmol/ml) was added only to P164 cells as positive control, and medium alone served as negative control. TGF-β1 expression decreased significantly in all infected BMSC lines compared with matched noninfected controls, whereas no change was noted between infected and noninfected A549 lung epithelial cells. Data are expressed as the mean (±SEM) (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001 compared with noninfected controls.
The concentrations of IL-1β, IL-1rα, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, CD40L, eotaxin, epidermal growth factor (EGF), fractalkine, IFN-γ, interferon-inducible protein-10 (IP-10), TNF-α, TGF-α, and vascular endothelial growth factor were at the lower limit of detection in both rgRSV-infected BMSCs and noninfected controls. In time-course experiments, ranging from 12 to 72 hours of rgRSV infection (Figure E2), several cytokines and chemokines (e.g., IL-1α, MIP-1α, RANTES) exhibited a general trend of progressive increase in expression, whereas other remained relatively stable across time points (e.g., IL-6 and IL-8). Cytokine levels in noninfected cells remained either undetectable or at low levels of detection throughout the 72-hour culture. Time points beyond 72 hours of infection were not tested because of the extensive cell damage.
In contrast to the generalized overexpression of proinflammatory and mitogenic factors, expression of the inhibitory TGF-β1 protein decreased significantly and consistently in all rgRSV-infected BMSC cell lines (Figure 4B) as compared with matched noninfected controls (P < 0.01). This virtual shutdown of TGF-β1 synthesis seemed specific to BMSCs, as no change was noted between rgRSV-infected and noninfected A549 lung epithelial cells (P = 0.34). VP-16–treated P164 cells used as a positive control exhibited a significant increase in TGF-β1 protein concentration (P < 0.05).
UV inactivation of the viral nucleic acid significantly reduced the potentiating effect of rgRSV on the expression of all proinflammatory cytokines in BMSCs (P < 0.01; Figure 5); this effect did not reach statistical significance only for MCP-1 (P = 0.058), although a strong trend was observed for this cytokine. However, UV-rgRSV still produced significant elevations in these cytokines compared with noninfected BMSC controls (P < 0.05), with the only exception of both MIP isoforms (P = 0.38 and 1.0) and RANTES (P = 0.11). UV-rgRSV also caused only a borderline decrease of TGF-β1 expression in BMSCs compared with noninfected controls (199.0 ± 11.5 pg/ml versus 255.8 ± 25.1 pg/ml; P = 0.05), which was significantly less than the inhibition caused in the same cells by replicating rgRSV (122.4 ± 6.0 ng/ml; P < 0.05).
Figure 5.
UV-inactivation changes RSV-induced cytokines expression. Nucleic acid inactivation reduced significantly virus-induced expression of proinflammatory cytokines in BMSCs. However, the expression of most cytokines was still increased after exposure to inactivated virus compared with noninfected BMSCs, suggesting that this effect of RSV involves expression and replication of viral genes as well as a direct toxic effect of capsid proteins. Data are expressed as the mean (±SEM) (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, compared with noninfected BMSCs; ##P < 0.01, ###P < 0.001, compared with BMSCs exposed to UV-inactivated rgRSV.
RSV Reduces Pro-B Cell Support by BMSCs
rgRSV-infected BMSCs supported pro-B C1.92 cells expansion in coculture less efficiently than matched noninfected controls. Bright-field microscopy at 24, 48, and 72 hours of coculture showed decreased C1.92 cell adherence to rgRSV-infected BMSCs compared with controls (Figure E3A). The average number of C1.92 cells recovered at each time point from the supernatants of noninfected BMSCs was 3.49 (±0.38) × 106, compared with 1.46 (±0.15) × 106 C1.92 cells recovered from rgRSV-infected BMSCs (P < 0.05). At 72 hours, the reduction in total C1.92 pro-B cells recovered from rgRSV-infected BMSC cocultures was roughly 50% (P < 0.01; Figure E3B). The C1.92 pro-B cells recovered from cocultures with infected BMSCs did not exhibit any green fluorescence, and there was no difference in viability between C1.92 pro-B cells collected from rgRSV-infected versus noninfected BMSC cocultures.
RSV Modifies BMSC-Dependent Chemotaxis
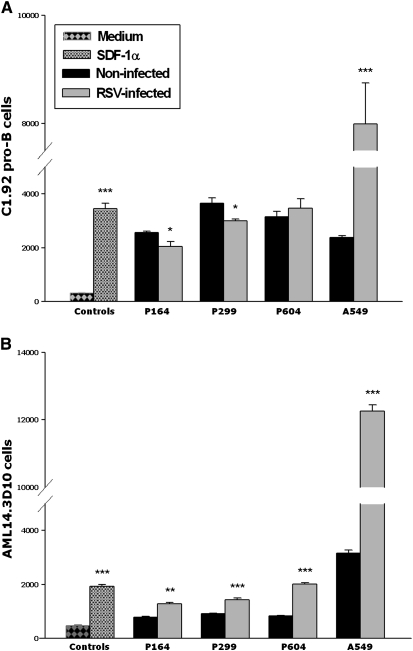
To determine the effect of RSV infection on BMSC-dependent chemotaxis, rgRSV-infected BMSCs, A549 lung epithelial cells, and matched noninfected controls were exposed to lymphoid C1.92 cells or myeloid AML14.3D10 cells. Recombinant stromal cell–derived factor-1α (SDF-1a) and medium served, respectively, as positive and negative control (P < 0.001). rgRSV infection reduced significantly the ability of two out of three BMSC lines to support lymphoid C1.92 cell chemotaxis at 24 hours (P < 0.05; Figure 6A). This inhibitory effect of rgRSV was dependent upon active viral replication, as BMSCs exposed to UV-inactivated virus retained entirely their chemotactic activity on C1.92 pro-B cells compared with matched noninfected controls (4,305 ± 771 C1.92 cells versus 4,264 ± 550 C1.92 cells; n = 3; P = 0.97).
Figure 6.
RSV modifies BMSC-induced chemotaxis. (A) rgRSV infection reduced significantly the ability of two out of three BMSC lines to support lymphoid C1.92 pro-B cells chemotaxis at 24 hours. In contrast, rgRSV infection increased dramatically the chemotactic activity of A549 lung epithelial cells on C1.92 cells. Recombinant stromal cell–derived factor-1 (SCF-1; 100 ng/ml) and medium alone served, respectively, as positive and negative controls. (B) Chemotaxis of myeloid AML14.3D10 cells was significantly increased in all three rgRSV-infected cell lines compared with noninfected controls. rgRSV infection also increased dramatically the chemotactic activity of A549 lung epithelial cells on AML14.3D10 cells. Data are expressed as the mean (±SEM) (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, compared with noninfected controls.
In contrast, chemotaxis of myeloid AML14.3D10 cells was significantly increased in all three cell lines compared with noninfected controls (P < 0.01; Figure 6B). Furthermore, rgRSV infection increased dramatically the chemotactic activity of A549 lung epithelial cells on both C1.92 pro-B and AML14.3D10 cells (P < 0.001).
DISCUSSION
This study shows that primary human BMSCs are infected by RSV in vitro, and that, in these cells, the virus is able to complete its replicative cycle, produce and release progeny virions, and propagate the infection to neighboring cells. To our knowledge, this is the first evidence of RSV infection of human bone marrow. Susceptibility to RSV infection varied among cell lines derived from different donors, and was usually higher with younger donors; this suggests modulation by genetic and epigenetic traits intrinsic to the BMSCs and independent from the host immune function or RSV virulence. In addition, the number of infected cells was proportional to the size of the inoculum, suggesting that, in vivo, the severity and/or duration of the respiratory infection and consequent viremia determines the extent of bone marrow involvement.
The translational significance of our findings is linked to the multiple lines of evidence indicating that RSV can enter the pulmonary microcirculation and then spread hematogenously to extrapulmonary sites. In particular, the virus has been detected in a variety of human tissues, including the central nervous system (22), heart (23, 24), and liver (25), and clinical extrapulmonary manifestations are relatively common in severe RSV infections (9). Interestingly, a previous study using nested PCR detected RSV RNA in the blood of 63% of neonates and 20% of infants whose nasal washes tested positive for RSV antigen (10), suggesting that the probability and/or severity of RSV viremia and extrapulmonary dissemination are inversely proportional to age. It should be noted, however, that presence of viral RNA does not necessarily imply the presence of infectious, replication-competent virus, and, therefore, its biological significance remains to be elucidated.
Another recent report shows that human eosinophils are targets of RSV infection, can support genome replication, and can release infectious virions (26). This observation raises the important question of where the infection of circulating blood cells may occur, either upon recruitment of mature leukocytes to the peripheral site of infection and inflammation (i.e., the lungs), in the bloodstream during a viremic episode, or rather at some stage of the maturation process within the bone marrow. Whatever the answer to this question, the combination of present and previous findings raises the intriguing possibility that RSV may recirculate between lungs and bone marrow using leukocytes as active vectors, which may impact both the severity of the acute infection and its chronic sequelae.
RSV Persistence in BMSCs
Consistent with the evidence outlined previously here, we found PCR-amplifiable RNA sequences with virtually complete homology to the RSV genome in naive primary BMSCs from two-thirds of the adult donors and all of the pediatric donors tested. In contrast, we were unable to find RSV genome in primary human bronchial epithelial cells from four donors (data not shown). In addition, no homologous sequence was identified in the human genome database, suggesting that the message identified in BMSCs was indeed of viral origin, and consequently that the bone marrow may provide an extrapulmonary target if exposed to the virus during viremic episodes (27). Indeed, the bone marrow microenvironment is uniquely suited to act as a sanctuary for virus-infected cells, exactly as it has been extensively shown to do for malignant cells (11, 12).
Notably, these findings in bone marrow aspirates imply that the inoculation of human-derived primary BMSCs with rgRSV in vitro might generate a superinfection on top of a preexisting natural infection. In fact, this was one of the reasons we used rgRSV in our in vitro experiments, to make sure we were detecting only the replication of exogenous tagged virus. However, there still is a possibility that the pre-existence of viral genomic sequences within the cells may interact and/or interfere with the effects of the exogenous virus on BMSC structure and/or function, although this possibility is probably minimal considering that, even at low MOI, the exogenous rgRSV genome would most likely overpower low-level, naturally acquired virus.
The nucleotide sequence of PCR products amplified from human bone marrow differed by less than 5% from the RSV-A2 genomic reference sequence. It is likely that at least some of the few differences derive from the natural genetic variability of the RSV N protein gene. In fact, as with other RNA viruses, RSV has a rather mutable genome by virtue of its dependence on an RNA polymerase that lacks the capacity for RNA proofreading and editing (28). Thus, a clinical isolate will often have a few differences from the A2 strain, because the virus is evolving all the time (29, 30). Although the N protein does not evolve as fast as the G protein of RSV, it is unlikely to be exactly the same as the protein expressed in the reference A2 strain, which was isolated decades ago. In addition, genomic sequences from other coinfecting RSV strains (or other viruses altogether) may have been present in the same bone marrow aspirates, and generated some noise in the sequencing process. Finally, it is possible that some of the mismatch reflects low-level, nonspecific products present in the samples (e.g., residual amplification primers), which may have interfered with some base calls. At any rate, the homology between the two sequences remains highly significant, and provides important evidence for the presence of RSV in primary human BMSCs.
Latent infection has been proposed by several investigators as the mechanism underlying the chronic respiratory sequelae of early-life RSV infection, especially persistent postviral wheezing and asthma in children (31, 32) and lung function abnormalities in adults (33). However, despite multiple attempts, this hypothesis has never been corroborated by objective evidence of viral persistence in human airway cells or lung tissues. RSV can indeed persists in the lungs of some rodent species (34, 35), which are not natural targets of this virus, but can be experimentally infected using large inocula. However, this evidence cannot be simply extrapolated to humans, and studies based on extremely sensitive PCR techniques have concluded that RSV is virtually never detected in the airways of immunocompetent humans after the first 7–10 days of symptoms (36).
In addition, in some studies, low-grade RSV infection has been detected in sputum from 20–30% of patients with stable chronic obstructive pulmonary disease, again leading to the suggestion of RSV latency in diseased lungs (37). Importantly, the mechanism proposed by these investigators involves evasion of antiviral immunity impaired by older age, medications, and chronic tobacco smoking, a concept not easily transferable to childhood asthma. Furthermore, several other large studies have not been able to confirm persistence of RSV in stable chronic obstructive pulmonary disease patients (38).
The present study opens an alternative scenario, suggesting that RSV persistence in a latent state is indeed possible, but primarily in extrapulmonary reservoirs seeded hematogenously: specifically, the bone marrow stroma. From there, the infection may reactivate cyclically, possibly reflecting seasonal fluctuations in systemic antiviral surveillance, or may influence cellular and humoral immunoinflammatory pathways, resulting in persistent or recurrent airway dysfunction. Reactivation of latent RSV from its BMSC reservoir could also be the source of the community outbreaks of this infection in winter after its disappearance in the spring.
RSV Effects on BMSCs
The bone marrow is a complex tissue composed of two distinct but interdependent compartments: the hematopoietic system and the bone marrow stroma. In addition to serving as a mechanical support for differentiating hematopoietic cells, the bone marrow stroma also expresses cell-signaling factors that participate in the differentiation of mature blood cells (39, 40). In respiratory diseases, such as infections, asthma, and fibrosis, the lymphoid and myeloid cells matured in the bone marrow microenvironment are mobilized and migrate to the lungs in response to blood-borne chemotactic stimuli (22–24). Although other viruses, like human cytomegalovirus (41), have been investigated in the context of their bone marrow activity, this is the first study showing a direct pathologic effect of RSV.
In cultures generated from suspensions of marrow cells, colonies derived from adherent precursors of nonhematopoietic origin are defined as bone marrow stromal cells. These are characterized by their capacity to support stroma-dependent hematopoietic cells when supplemented with IL-7, and by constitutive expression of vascular cell adhesion molecule–1, fibronectin, CD44, and a variety of hematopoietic regulatory cytokines, including insulin-like growth factor–1 and the c-kit ligand. In addition to their fibroblast-like morphology, BMSCs share some, but not all, features with fibroblastic cells of other tissues. Importantly, BMSCs contain multipotent progenitors capable of extensive proliferation and differentiation into several phenotypes, and, under appropriate conditions, maintain their multipotential capability during prolonged cultivation and multiple passages in vitro. However, a true “stem cell” character has not been definitively demonstrated (42).
As shown in other cellular systems (43), infection with live virus disrupted the cytoskeletal network of actin microfilaments in human BMSCs, with consequent changes in cellular morphology. These changes are virtually identical to those seen in previous in vitro investigation of the mechanisms of RSV gene expression and characterization of the components of the RSV RNA transcription machinery, and have been correlated with active infection and presence of actin in the virions of most Paramyxoviruses (43). Because the actin microfilaments are essential for the virus to coordinate the expression of its genome, as well as to direct the assembly and budding of new virions, the cytoskeletal changes illustrated in this study constitute a robust piece of evidence confirming that active and productive RSV infection is indeed supported by BMSCs. In vivo, the distorted morphology of infected BMSCs may physically alter surface contact–mediated signaling with hematopoietic progenitors, thereby resulting in significant modifications of steady-state hematopoiesis.
In addition to this direct effect, our data show that RSV has the potential to alter other aspects of hematopoiesis indirectly, as a consequence of the broadly altered expression of multifunctional cytokines, chemokines, and growth factors in the bone marrow microenvironment. In fact, the milieu of proinflammatory and mitogenic agents secreted by RSV-infected BMSCs can regulate, in an autocrine and/or paracrine fashion, various stages of human hematopoiesis. For example, IL-6 promotes B cell differentiation and enhances myeloid and megakaryocyte colony formation (44). IL-8 modulates neutrophil maturation, migration, and activation (45). MIP-1α has been shown to inhibit the proliferation of early progenitor cells in clonogenic assays, but it stimulates the proliferation of more mature progenitors, including granulocyte/macrophage colony–forming cells (46).
As steady-state hematopoiesis results from the balance between negative- and positive-acting cytokines, the most important finding here may be the profound inhibition of the pleiotropic anti-inflammatory cytokine, TGF-β1, in RSV-infected BMSCs. In the bone marrow microenvironment, TGF-β1 plays a central role as a negative controller of hematopoiesis, which prevents progenitor cells from entering the cell cycle by interfering with expression and intracellular signaling of the receptors for growth stimulatory molecules (47). TGF-β1 also modulates negatively the synthesis of proinflammatory cytokines and cytokine receptors (48), and, therefore, its deficiency may indirectly contribute to the changes outlined in the previous paragraph.
Our data indicate that inactivation of the viral nucleic acid by irradiation with UV light reduces significantly the effect of RSV on cytokine expression in BMSCs, implying that this effect is primarily linked to the expression and replication of the viral genome. However, for most cytokines, a significant component of this effect can still be detected after inactivation of the viral nucleic acid, and thus seems to be associated with a direct toxic effect of the capsid proteins. This observation is consistent with a number of previous studies, including a recent report showing minimal differences in direct comparisons between the proteomes from airway epithelial cells exposed to live or UV-inactivated RSV (49). More specifically, in lungs and in circulating leukocytes, UV-inactivated RSV still induces cytokines, such as IL-6 (50) and IL-8 (51), and chemokines, such as CCL6, CCL11, CCL22, and CXCL5 (52), and inhibits lymphocyte responses (53).
RSV Effects on Hematopoiesis
Two interwoven mechanisms seem responsible for the accumulation of myeloid and lymphoid cells in RSV-infected lungs, apparently driven by the same set of proinflammatory cytokines and chemokines. First, the recruitment of circulating cells from the blood stream into the lung tissues and airway lumen by inflammatory mediators released from infected structural cells, primarily the bronchial epithelium acting as primary target of the infection (3). Second, the BMSC-dependent maturation and mobilization of newly formed myeloid and lymphoid cells from the bone marrow. Based on the results of our study, we propose that the latter phase can be driven, at least in part, by active infection of BMSCs, rather than by soluble mediators released into the pulmonary microcirculation.
Despite the overall activation of bone marrow functions in response to the infection, some viruses can selectively disrupt particular hematopoietic lineages by targeting specific differentiation stages of progenitor cells (54). Our study shows that RSV infection markedly decreases BMSC-dependent pro-B cell support. In contrast to the closely related measles virus (55), this effect cannot be explained by direct RSV infection of the lymphoid lineage, as pro-B cells exhibited no green fluorescence or difference in viability. Rather, our study shows reduced BMSC ability to support lymphoid cell chemotaxis when invaded by live virus.
Although these chemotactic changes are relatively small in absolute values, their biological significance might be amplified by the unique geometric factors operating in the bone marrow microenvironment, where BMSC and hematopoietic progenitors at various stages of differentiation are extremely close and involved in tightly regulated cell–cell interactions. Here, minimal disruption of the physical (e.g., cytoskeletal network of actin microfilaments) and/or chemical (expression of cytokines, chemokines, and other soluble factors) milieu can result in significant modifications of steady-state hematopoiesis.
The downstream B cell dysfunction could be exploited by the virus to its advantage, providing an alternative explanation of why the antibody response against RSV is frequently inefficient and transient, making reinfection common even within the same season and across all age groups (56). Furthermore, the same mechanism could generate transient, nonspecific immunosuppression, opening the door to superimposed infections by other viral or bacterial pathogens (54).
In summary, this study identifies BMSCs as a novel target for RSV infection, and establishes that this infection causes important structural and functional changes in a cell population that is essential to a functional marrow microenvironment and effective hematopoiesis. The detection of RSV genome in naive primary human BMSCs indicates that the infection is not merely an artifact of the in vitro model, but can occur in vivo as well. RSV infection has important structural consequences on the BMSC cytoskeleton and functional effects on the synthesis of soluble factors, creating a milieu that favors maturation and mobilization of inflammatory cells. These consequences are primarily a result of the expression and replication of the viral genome, although capsid proteins appear to contribute a direct toxic effect. BMSCs infected with live virus also exhibit a reduced ability to support B cell maturation, which may interfere with the humoral immune response against the virus itself and coinfecting pathogens. Collectively, these observations suggest that, although the clinical manifestations of RSV are predominantly circumscribed to the respiratory system, the infection may have systemic implications that can affect its severity during the acute phase, as well as its long-term pathologic sequelae. Consequently, future prophylactic and management strategies should also address the coverage of possible extrapulmonary sites of viral replication.
Supplementary Material
Acknowledgments
The authors thank James Fortney for the invaluable technical help provided throughout the study, and Dr. Bing-Hua Jiang (West Virginia University, Morgantown, WV) for his help with fluorescence microscopy. The authors are indebted to Dr. Mark Peeples (Nationwide Children's Hospital Research Institute, Columbus, OH) and Dr. Peter Collins (National Institutes of Health, Bethesda, MD) for providing the respiratory syncytial virus (RSV)–green fluorescent protein virus, Dr. Kenneth Dorshkind (University of California, Los Angeles, CA) for providing the murine bone marrow stromal cell line S10, Dr. Kenneth Landreth (West Virginia University, Morgantown, WV) for providing the murine pro-B cell clone C1.92, and Dr. Cassandra Paul (Dayton VA Medical Center, Dayton, OH) for providing the AML14.3D10 subclone. The authors also thank Dr. Peeples and Benjamin Senior (Eurofins MWG Operon, Huntsville, AL) for their input concerning the RSV sequence analysis.
Some of the findings reported in this article were presented at the International Conference of the American Thoracic Society in 2009 (San Diego, CA) and in 2010 (New Orleans, LA).
This work was supported in part by National Institutes of Health grants RO1-HL61007 (G.P.) and RO1-HL56888 (LF.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0121OC on October 22, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005;352:1749–1759. [DOI] [PubMed] [Google Scholar]
- 3.Othumpangat S, Gibson L, Samsell L, Piedimonte G. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PLoS ONE 2009;4:e6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994;71:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007;20:108–119. [DOI] [PubMed] [Google Scholar]
- 6.Piedimonte G. What causes allergy and asthma? Early postnatal influences. Pediatr Pulmonol 2004;26:208–210. [DOI] [PubMed] [Google Scholar]
- 7.Iankevich OD, Dreizin RS, Makhlinovskaia NL, Gorodnitskaia NA. Viremia in respiratory syncytial virus infection. Vopr Virusol 1975;4:455–458. [PubMed] [Google Scholar]
- 8.Liu X-M, Wang Z, Guo Y. Respiratory syncytial virus nephropathy in rats. Kidney Int 2007;71:388–396. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection—a systematic review. Crit Care 2006;10:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohwedder A, Keminer O, Forster J, Schneider K, Schneider E, Werchau H. Detection of respiratory syncytial virus RNA in blood of neonates by polymerase chain reaction. J Med Virol 1998;54:320–327. [DOI] [PubMed] [Google Scholar]
- 11.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 2008;14:2519–2526. [DOI] [PubMed] [Google Scholar]
- 12.Gibson LF. Survival of b lineage leukemic cells: signals from the bone marrow microenvironment. Leuk Lymphoma 2002;43:19–27. [DOI] [PubMed] [Google Scholar]
- 13.Gibson LF, Fortney J, Landreth KS, Piktel D, Ericson SG, Lynch JP. Disruption of bone marrow stromal cell function by etoposide. Biol Blood Marrow Transplant 1997;3:122–132. [PubMed] [Google Scholar]
- 14.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol 1987;138:1082–1087. [PubMed] [Google Scholar]
- 15.Gibson LF, Piktel D, Landreth KS. Insulin-like growth factor–1 potentiates expansion of interleukin-7–dependent pro-B cells. Blood 1993;82:3005–3011. [PubMed] [Google Scholar]
- 16.Clutter SD, Fortney J, Gibson LF. MMP-2 is required for bone marrow stromal cell support of chemotaxis. Exp Hematol 2005;33:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann MA, Paul CC. The AML14 and AML14.3D10 cell lines: a long-overdue model for the study of eosinophils and more. Stem Cells 1998;16:16–24. [DOI] [PubMed] [Google Scholar]
- 18.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 2000;74:10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haeberle HA, Durrstein C, Rosenberger P, Hosakote YM, Kuhlicke J, Kempf VA, Garofalo RP, Eltzschig HK. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE 2008;3:e3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabogal C, Auais A, Napchan G, Mager E, Zhou BG, Suguihara C, Bancalari E, Piedimonte G. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005;57:819–825. [DOI] [PubMed] [Google Scholar]
- 21.Zar JH. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1984.
- 22.Zlateva KT, Van Ranst M. Detection of subgroup B respiratory syncytial virus in the cerebrospinal fluid of a patient with respiratory syncytial virus pneumonia. Pediatr Infect Dis J 2004;23:1065–1066. [DOI] [PubMed] [Google Scholar]
- 23.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr 1980;96:179–186. [DOI] [PubMed] [Google Scholar]
- 24.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003;42:466–472. [DOI] [PubMed] [Google Scholar]
- 25.Nadal D, Wunderli W, Meurmann O, Briner J, Hirsig J. Isolation of respiratory syncytial virus from liver tissue and extrahepatic biliary atresia material. Scand J Infect Dis 1990;22:91–93. [DOI] [PubMed] [Google Scholar]
- 26.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood 2009;114:2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virgin S. Pathogenesis of viral infection. In: Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 329–330.
- 28.Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev 2000;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botosso VF, Zanotto PM, Ueda M, Arruda E, Gilio AE, Vieira SE, Stewien KE, Peret TC, Jamal LF, Pardini MI, et al. Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog 2009;5:e1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiche J, Schweiger B. Genetic variability of group a human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol 2009;47:1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507. [DOI] [PubMed] [Google Scholar]
- 32.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 33.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol 2004;38:155–160. [DOI] [PubMed] [Google Scholar]
- 34.Dakhama A, Vitalis TZ, Hegele RG. Persistence of respiratory syncytial virus (RSV) infection and development of RSV-specific IgG1 response in a guinea-pig model of acute bronchiolitis. Eur Respir J 1997;10:20–26. [DOI] [PubMed] [Google Scholar]
- 35.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJ. Latency and persistence of respiratory syncytial virus despite t cell immunity. Am J Respir Crit Care Med 2004;169:801–805. [DOI] [PubMed] [Google Scholar]
- 36.Falsey AR, Criddle MC, Walsh EE. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol 2006;35:46–50. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJ, Wedzicha JA. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:871–876. [DOI] [PubMed] [Google Scholar]
- 38.Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon MY, Goldman JM, Gordon-Smith EC. Spatial and functional relationships between human hemopoietic and marrow stromal cells in vitro. Int J Cell Cloning 1983;1:429–439. [DOI] [PubMed] [Google Scholar]
- 40.Greenberger JS. The hematopoietic microenvironment. Crit Rev Oncol Hematol 1991;11:65–84. [DOI] [PubMed] [Google Scholar]
- 41.Gilloteaux J, Nassiri MR. Human bone marrow fibroblasts infected by cytomegalovirus: ultrastructural observations. J Submicrosc Cytol Pathol 2000;32:17–45. [PubMed] [Google Scholar]
- 42.Krebsbach PH, Kuznetsov SA, Bianco P, Gehron Robey P. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med 1999;10:165–181. [DOI] [PubMed] [Google Scholar]
- 43.Burke E, Dupuy L, Wall C, Barik S. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 1998;252:137–148. [DOI] [PubMed] [Google Scholar]
- 44.Kishimoto T. The biology of interleukin-6. Blood 1989;74:1–10. [PubMed] [Google Scholar]
- 45.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol 1994;55:97–179. [PubMed] [Google Scholar]
- 46.Broxmeyer HE, Sherry B, Lu L, Cooper S, Oh KO, Tekamp-Olson P, Kwon BS, Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitro by bone marrow myeloid progenitor cells. Blood 1990;76:1110–1116. [PubMed] [Google Scholar]
- 47.Ruscetti FW, Dubois C, Falk LA, Jacobsen SE, Sing G, Longo DL, Wiltrout RH, Keller JR. In vivo and in vitro effects of TGF-beta 1 on normal and neoplastic haemopoiesis. Ciba Found Symp 1991;157:212–227. [DOI] [PubMed] [Google Scholar]
- 48.Robledo MM, Teixidó J. TGF-beta1–binding proteins on human bone marrow stromal cells. Leuk Lymphoma 1997;27:509–515. [DOI] [PubMed] [Google Scholar]
- 49.van Diepen A, Brand HK, Sama I, Lambooy LH, van den Heuvel LP, van der Well L, Huynen M, Osterhaus AD, Andeweg AC, Hermans PW. Quantitative proteome profiling of respiratory virus–infected lung epithelial cells. J Proteomics 201073:1680–1693. [DOI] [PubMed] [Google Scholar]
- 50.Stadnyk AW, Gillan TL, Anderson R. Respiratory syncytial virus triggers synthesis of IL-6 in BALB/c mouse alveolar macrophages in the absence of virus replication. Cell Immunol 1997;176:122–126. [DOI] [PubMed] [Google Scholar]
- 51.König B, Krusat T, Streckert HJ, König W. IL-8 release from human neutrophils by the respiratory syncytial virus is independent of viral replication. J Leukoc Biol 1996;60:253–260. [DOI] [PubMed] [Google Scholar]
- 52.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus–induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis 2004;189:1419–1430. [DOI] [PubMed] [Google Scholar]
- 53.Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J Virol 2002;76:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naniche D, Oldstone MB. Generalized immunosuppression: how viruses undermine the immune response. Cell Mol Life Sci 2000;57:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider-Schaulies S, Niewiesk S, Schneider-Schaulies J, ter Meulen V. Measles virus induced immunosuppression: targets and effector mechanisms. Curr Mol Med 2001;1:163–181. [DOI] [PubMed] [Google Scholar]
- 56.Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2007. p. 1620–1625.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.