Abstract
Cholecystokinin (CCK) is one of the most abundant neuropeptides in the brain, where it interacts with two G protein-coupled receptors (CCK-1 and CCK-2). Activation of both CCK receptors increases the activity of PLC, resulting in increases in intracellular calcium ion (Ca2+) release and activation of PKC. Whereas high density of CCK receptors has been detected in the superficial layers of the entorhinal cortex (EC), the functions of CCK in this brain region have not been determined. Here, we studied the effects of CCK on neuronal excitability of layer III pyramidal neurons in the EC. Our results showed that CCK remarkably increased the firing frequency of action potentials (APs). The effects of CCK on neuronal excitability were mediated via activation of CCK-2 receptors and required the functions of G proteins and PLC. However, CCK-mediated facilitation of neuronal excitability was independent of inositol trisphosphate receptors and PKC. CCK facilitated neuronal excitability by activating a cationic channel to generate membrane depolarization. The effects of CCK were suppressed by the generic, nonselective cationic channel blockers, 2-aminoethyldiphenyl borate and flufenamic acid, but potentiated by gadolinium ion and lanthanum ion at 100 μM. Depletion of extracellular Ca2+ also counteracted CCK-induced increases in AC firing frequency. Moreover, CCK-induced enhancement of neuronal excitability was inhibited significantly by intracellular application of the antibody to transient receptor potential channel 5 (TRPC5), suggesting the involvement of TRPC5 channels. Our results provide a cellular and molecular mechanism to help explain the functions of CCK in vivo.
Keywords: action potential, signal transduction, channel, peptide, depolarization
whereas cholecystokinin (CCK) was discovered originally in the gastrointestinal tract (Mutt and Jorpes 1968), it is one of the most abundant neuropeptides in the brain (Beinfeld et al. 1981). CCK binds to two G protein-coupled receptors (CCK-1 and CCK-2), resulting in increased activity of PLC (Wank 1995). Activation of PLC hydrolyzes phosphatidylinositol 4, 5-bisphosphate (PIP2) into inositol trisphosphate (IP3) to increase intracellular calcium ion (Ca2+) release and diacylglycerol (DAG) to activate PKC. Whereas CCK-1 receptors are present in peripheral tissues and in a few discrete brain regions, including postrema, interpeduncular nucleus, and nucleus tractus solitarius (Hill et al. 1987, 1990; Moran et al. 1986), CCK-2 receptors are the predominant form found in the brain (Van Dijk et al. 1984). CCK is an important neuromodulator that modifies a series of physiological functions, including satiety, analgesia, learning, and memory processes (Beinfeld 2001; Sebret et al. 1999), and pathological disorders such as anxiety (Bradwejn and Koszycki 1994; Crawley and Corwin 1994; Noble and Roques 1999; Rehfeld 2000; Rodgers and Johnson 1995; Wang et al. 2005). However, the underlying cellular and molecular mechanisms of CCK have not been determined completely.
CCK is highly expressed in the limbic structures, including the hippocampus (Greenwood et al. 1981; Hefft and Jonas 2005), cingulate gyrus (Beinfeld et al. 1981), subiculum (Kohler and Chan-Palay 1982), and entorhinal cortex (EC) (Beinfeld et al. 1981; Greenwood et al. 1981; Kohler and Chan-Palay 1982; Lotstra and Vanderhaeghen 1987). In the EC, high density of CCK receptors is detected in the superficial layers (Kohler and Chan-Palay 1988; Kritzer et al. 1988). Whereas an abnormal CCK level has been observed in the EC of schizophrenics (Bachus et al. 1997), and antipsychotic drugs increase CCK expression (Zachrisson et al. 2000), the precise roles of CCK in the EC have not been determined. In the present study, we examined the effects of CCK on neuronal excitability recorded from the pyramidal neurons in layer III of the EC, which contains the highest density of CCK receptors in rats (Kohler and Chan-Palay 1988). Our results demonstrate that CCK increases neuronal excitability via PLC-dependent activation of cationic channels, possibly transient receptor potential channel (TRPC)-like channels.
MATERIALS AND METHODS
Slice preparation.
Horizontal brain slices (400 μm), including the hippocampus, subiculum, and EC, were cut using a vibrating blade microtome (VT1000S, Leica, Wetzlar, Germany) from 15- to 22-day-old Sprague Dawley rats, as described previously (Deng and Lei 2006, 2007; Deng et al. 2009, 2010; Xiao et al. 2009). After being deeply anesthetized with isoflurane, rats were decapitated, and their brains were dissected out in ice-cold saline solution that contained (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2, pH 7.4. Slices were incubated initially in the above solution at 35°C for 40 min for recovery and then kept at room temperature (∼24°C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee (Grand Forks, ND).
Whole-cell recordings from the EC neurons.
Whole-cell recordings using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) in current- or voltage-clamp mode were made from the pyramidal neurons in layer III of the medial EC, visually identified with infrared video microscopy (Olympus BX51WI) and differential interference contrast optics. The recording electrodes were filled with (in mM) 100 potassium (K+)-gluconate, 0.6 EGTA, 2 MgCl2, 8 NaCl, 2 ATP2Na, 0.4 GTPNa, 40 HEPES, and 7 di-tris-phosphocreatine (pH 7.4, 290–300 mOsm/L). For the experiment of buffering intracellular Ca2+, BAPTA (10 mM) and CaCl2 (3.3 mM) were added, and the concentration of HEPES was reduced to 25 mM to maintain the same range of osmolarity. Under these circumstances, the intracellular-free Ca2+ was buffered to 56 nM (calculated using the CABUFFER, Jochen Kleinschmidt, NYU Medical Center, New York, NY). The extracellular solution comprised (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2 (pH 7.4). For the experiments involving gadolinium ion (Gd3+) and lanthanum ion (La3+), the extracellular solution consisted of (in mM) 147 NaCl, 3.5 KCl, 1.5 MgCl2, 2.5 CaCl2, 10 HEPES, and 10 glucose (pH 7.4 adjusted with NaOH). Action potentials (APs) were recorded in the preceding extracellular solution supplemented with bicuculline (10 μM) and CGP55845 (1 μM) to block GABAA and GABAB responses, respectively, and 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and DL-2-amino-5-phosphonopentanoic acid (DL-APV; 50 μM) to block glutamatergic transmission. For most of the cells, a positive current injection was required to bring the resting membrane potential (RMP) to ∼−50 mV to induce AP firing. CCK was applied after the AP firing had been stable for 5∼10 min. To avoid potential desensitization induced by repeated applications of CCK, only one cell was recorded from each slice. Data were filtered at 2 kHz, digitized at 10 kHz, acquired online, and analyzed afterline using pCLAMP 9 software (Molecular Devices). Frequency of APs was calculated by Mini Analysis 6.0.1 (Synaptosoft, Decatur, GA).
Holding currents (HCs) at −60 mV were recorded from layer III pyramidal neurons. The preceding extracellular solution was supplemented with TTX (0.5 μM) to block AP firing. HCs at −60 mV were recorded every 3 s and then averaged per minute. We subtracted the average of the HCs recorded for the last minute prior to the application of CCK from those recorded at different time-points to zero, the basal level of HCs, for better comparison.
Voltage-current relationship was obtained by using a ramp protocol from −100 mV to +60 mV at a speed of 0.1 mV/ms. We compared the voltage-current curves recorded before and during the application of CCK for ∼3–4 min when the effect of CCK was maximal.
Breeding of CCK-2 knockout mice.
Wild-type (WT) and homozygous knockout (KO) mice for CCK-2 receptors were bred in the animal facility of the University of North Dakota. Detailed methods for the generation and genotyping of homozygous KO mice were described previously (Deng et al. 2010; Nagata et al. 1996).
Data analysis.
Data are presented as the means ± SEM. A concentration-response curve of CCK was fit by the Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Student's paired or unpaired t-test or ANOVA was used for statistical analysis as appropriate; P values are reported throughout the text, and significance was set as P < 0.05. Numbers (n) in the text represent the number of cells examined unless stated otherwise.
Chemicals.
CCK was purchased from American Peptide (Sunnyvale, CA). LY225910, thapsigargin, heparin, 2-aminoethoxydiphenyl borate (2-APB), xestospongin C, DNQX, bicuculline, and DL-APV were purchased from Tocris Cookson (Ellisville, MO). GDP-β-S, GTP-γ-S, U73122, U73343, calphostin C, and Ro318220 were products of ENZ Life Sciences (Plymouth Meeting, PA). 1-O-Octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (edelfosine) was purchased from Calbiochem (Darmstadt, Germany). Anti-TRPC1 (catalog number ACC-010), anti-TRPC4 (catalog number ACC-018), and anti-TRPC5 (catalog number ACC-020) were purchased from Alomone Labs (Jerusalem, Israel). Other chemicals were products of Sigma-Aldrich (St. Louis, MO).
RESULTS
CCK increases AP firing frequency in layer III pyramidal neurons of the EC.
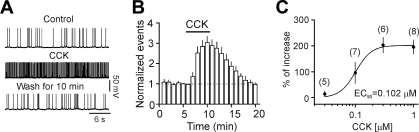
Because the highest density of CCK receptors has been detected in layer III of the rat EC (Kohler and Chan-Palay 1988), we initially examined the effects of CCK on neuronal excitability by recording AP firing from these neurons. Bath application of CCK (0.3 μM) for 5 min significantly increased the firing frequency of APs (control: 1.26 ± 0.16 Hz; CCK: 3.89 ± 0.66 Hz; n = 6; P = 0.001; Fig. 1, A and B). The maximal effect could be observed in 3–5 min after the beginning of the CCK application and returned to the control level after wash for 10 min in CCK-free extracellular solution (Fig. 1, A and B). The EC50 value of CCK was 0.102 μM (Fig. 1C). Because the maximal effect of CCK could be observed at 0.3 μM, we used this concentration for the rest of the experiments.
Fig. 1.
Bath application of cholecystokinin (CCK) concentration dependently increased the firing frequency of action potentials (APs) recorded from layer III pyramidal neurons in the entorhinal cortex (EC). A: APs recorded prior to, during, and after application of CCK (0.3 μM). B: time course of CCK-induced increases in AP firing frequency (n = 6). C: concentration-response curve of CCK-induced increases in AP firing frequency. Numbers in the parenthesis were numbers of cells recorded for each concentration.
CCK increases AP firing frequency via activation of CCK-2 receptors.
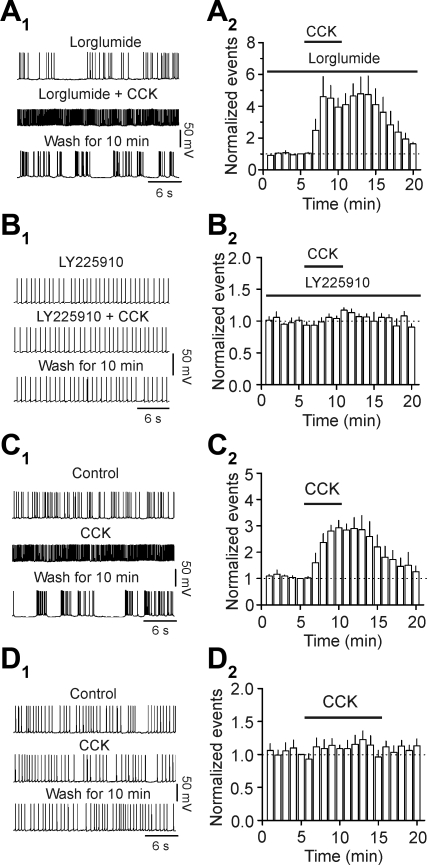
We then tested the involvement of CCK receptors in CCK-mediated augmentation of AP firing frequency. Slices were pretreated with the CCK-1 receptor antagonist lorglumide (Ki = 0.049 μM) (van der Bent et al. 1992) at the concentration of 1 μM for ∼1 h, and the bath was perfused continuously with the same concentration of lorglumide. At this concentration, lorglumide was effective at blocking the effects of CCK via CCK-1 receptors (Tsujino et al. 2005; Wan et al. 2007). Under this condition, application of CCK still significantly increased the firing frequency of APs (control: 1.25 ± 0.18 Hz; CCK: 4.81 ± 0.82; n = 6; P = 0.04; Fig. 2, A 1 and A2), suggesting that CCK-1 receptors are not required for CCK-induced facilitation of AP firing. We then tested the roles of CCK-2 receptors by using the selective antagonist, LY225910 (Ki = 470–760 nM in guinea pig cortex) (Suman-Chauhan et al. 1996). Pretreatment of slices with and continuous bath application of LY225910 (5 μM) blocked CCK-induced increases in AP firing frequency (control: 1.50 ± 0.21 Hz; CCK: 1.58 ± 0.24 Hz; n = 8; P = 0.21; Fig. 2, B1 and B2). We further confirmed the role CCK-2 receptors by using CCK-2 KO mice. Application of CCK increased AP firing frequency in six cells from three WT mice (control: 1.59 ± 0.28 Hz; CCK: 4.42 ± 0.69 Hz;P < 0.001; Fig. 2, C1 and C2), whereas application of the same concentration of CCK failed to increase AP firing frequency significantly in nine cells from three CCK-2 KO mice (control: 1.81 ± 0.10 Hz; CCK: 2.10 ± 0.28 Hz; P = 0.48; Fig. 2, D1 and D2). These results together demonstrate that CCK increases AP firing frequency in layer III pyramidal neurons of the EC via activation of CCK-2 receptors.
Fig. 2.
CCK increases the firing frequency of APs via activation of CCK interacting with G protein-coupled receptor (CCK-2). A1 and A2: pretreatment of slices with and continuous bath application of the selective CCK-1 antagonist lorglumide (1 μM) did not block CCK-induced facilitation of AP firing frequency. A1: APs recorded before, during, and after the application of CCK (0.3 μM) in the presence of lorglumide. A2: summarized time course (n = 6). B1 and B2: pretreatment of slices with and continuous bath application of the selective CCK-2 antagonist LY225910 (5 μM) blocked CCK-induced increases in AP firing frequency. B1: APs recorded before, during, and after the application of CCK (0.3 μM) in the presence of LY225910. B2: summarized time course (n = 8). C1 and C2: bath application of CCK (0.3 μM) significantly increased the firing frequency of APs recorded from wild-type (WT) mice. C1: APs recorded before, during, and after the application of CCK (0.3 μM). C2: summarized time course (n = 6 cells from 3 WT mice). D1 and D2: bath application of CCK (0.3 μM) failed to change significantly the firing frequency of APs recorded from CCK-2 knockout (KO) mice. D1: APs recorded before, during, and after the application of CCK (0.3 μM). D2: summarized time course (n = 9 cells from 3 CCK-2 KO mice).
CCK-induced increases in AP firing frequency require the functions of G proteins and PLC but are independent of IP3 receptors and PKC activity.
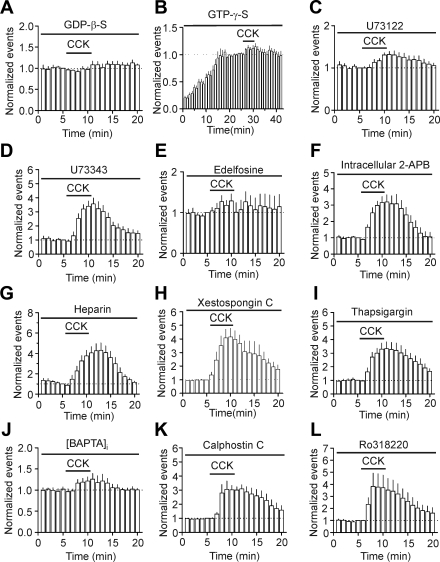
Because CCK-2 receptors are G protein coupled, we tested the roles of G proteins in CCK-mediated facilitation of AP firing. We included the G protein inactivator, GDP-β-S (4 mM), in the recording pipettes and waited for >20 min after the formation of whole-cell configuration to allow the dialysis of GDP-β-S into cells. Intracellular application of GDP-β-S via the recording pipettes completely blocked CCK-induced increases in AP firing frequency (control: 1.34 ± 0.21 Hz; CCK: 1.22 ± 0.17 Hz; n = 6; P = 0.15; Fig. 3A). We also used the nonhydrolyzable GTP analog GTP-γ-S to further examine the roles of G proteins. Inclusion of GTP-γ-S (0.5 mM) in the recording pipettes for 25 min after the formation of whole-cell configuration resulted in significant increases in AP firing frequency (control: 0.93 ± 0.19 Hz; GTP-γ-S: 4.71 ± 0.75 Hz; n = 6; P = 0.002; Fig. 3B). In the presence of GTP-γ-S, application of CCK did not significantly increase AP firing frequency further (GTP-γ-S: 4.71 ± 0.75 Hz; CCK: 5.15 ± 0.85 Hz; n = 6; P = 0.18; Fig. 3B). These data together demonstrate the requirement of G proteins. We then tested the roles of PLC by using the selective PLC inhibitor, U73122. The inactive compound U73343 was used as a control. Slices were pretreated with U73122 or U73343 at 5 μM for ∼1 h, and the extracellular solution contained the same concentration of these drugs. Under this condition, CCK-induced increases in AP firing frequency were reduced significantly (P < 0.001; control: 1.33 ± 0.12 Hz; CCK: 1.73 ± 0.15 Hz; n = 8; Fig. 3C) compared with those in the presence of U73343 (control: 1.41 ± 0.21 Hz; CCK: 4.99 ± 0.87 Hz; n = 5; Fig. 3D). Likewise, pretreatment of slices with and continuous bath application of another PLC inhibitor, edelfosine (10 μM), blocked CCK-induced facilitation of AP firing frequency (control: 1.46 ± 0.25 Hz; CCK: 1.75 ± 0.28 Hz; n = 8; P = 0.12; Fig. 3E). These results together demonstrate that the function of PLC is required for CCK-induced increases in AP firing frequency. We then tested the roles of the two downstream targets, IP3 receptors and PKC, in CCK-induced facilitation of AP firing frequency. We included separately three selective IP3 receptor inhibitors—2-APB, heparin, and xestospongin C—in the recording pipettes and waited for >20 min after the formation of a whole-cell configuration to permit diffusion of these inhibitors into the cells. Intracellular application of 2-APB (100 μM) failed to change significantly CCK-induced facilitation of AP firing frequency (control: 1.52 ± 0.09 Hz; CCK: 5.07 ± 0.85 Hz; n = 8; P = 0.003; Fig. 3F). Similarly, intracellular application of heparin (2 mg/ml) did not significantly alter CCK-induced augmentation of AP firing frequency (control: 1.16 ± 0.23 Hz; CCK: 5.04 ± 1.06 Hz; n = 5; P = 0.01; Fig. 3G). Furthermore, intracellular dialysis of xestospongin C (5 μM), via the recording pipettes, failed to change significantly CCK-induced increases in AP firing frequency (control: 1.34 ± 0.16 Hz; CCK: 5.55 ± 1.01 Hz; n = 5; P = 0.01; Fig. 3H). These results together suggest that the function of IP3 receptors is not necessary for CCK-induced facilitation of AP firing frequency. We further tested whether intracellular Ca2+, released from other stores, is required for the effects of CCK on AP firing. Intracellular application of thapsigargin (10 μM) via the recording pipettes to deplete the intracellular Ca2+ store did not significantly change CCK-induced facilitation of AP firing frequency (control: 1.70 ± 0.17 Hz; CCK: 5.44 ± 0.55 Hz; n = 5; P = 0.003; Fig. 3I), confirming further that intracellular Ca2+ release is unnecessary for CCK-mediated augmentation of AP firing. We finally clamped the intracellular Ca2+ close to the basal level (∼56 nM) by adding BAPTA (10 mM) and CaCl2 (3.3 mM) to the recording pipettes (see materials and methods, Whole-cell recordings from the EC neurons). Under this condition, bath application of CCK failed to increase AP firing frequency significantly (control: 1.34 ± 0.12 Hz; CCK: 1.68 ± 0.23; n = 7; P = 0.12; Fig. 3J), suggesting that increases in intracellular Ca2+ concentration, possibly from the extracellular side, are necessary for CCK-induced facilitation of AP firing frequency. To test the roles of PKC in CCK-mediated facilitation of AP firing, we pretreated slices separately with two selective PKC inhibitors—calphostin C (1 μM) and Ro318220 (1 μM). The same concentrations of the PKC inhibitors were bath applied. Under these circumstances, application of CCK in the presence of calphotin C still significantly increased the firing frequency of APs (control: 1.11 ± 0.14 Hz; CCK: 3.43 ± 0.75 Hz; n = 6; P = 0.019; Fig. 3K). Likewise, application of Ro318220 (1 μM) failed to change significantly CCK-mediated facilitation of AP firing frequency (control: 1.61 ± 0.26 Hz; CCK: 6.39 ± 2.0 Hz; n = 7; P = 0.04; Fig. 3L). These results together demonstrate that PKC activity is not required for CCK-induced facilitation of AP firing frequency.
Fig. 3.
CCK-mediated facilitation of AP firing requires the functions of G proteins and PLC but is independent of intracellular calcium ion (Ca2+) release and PKC activity. A: intracellular application of GDP-β-S via the recording pipettes blocked CCK-induced increases in AP firing frequency. B: intracellular perfusion of GTP-γ-S resulted in facilitation of AP firing frequency by itself and blocked CCK-induced increases in AP firing frequency. C: pretreatment of slices with and continuous bath application of U73122 significantly reduced CCK-induced facilitation of AP firing frequency. D: pretreatment of slices with and continuous bath application of U73343 failed to change significantly CCK-induced facilitation of AP firing frequency. E: pretreatment of slices with and continuous bath application of 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (edelfosine) blocked CCK-induced facilitation of AP firing frequency. F: intracellular application of 2-aminoethoxydiphenyl borate (2-APB) did not block CCK-induced increases in AP firing frequency. G: intracellular application of heparin failed to alter the effect of CCK on AP firing frequency. H: intracellular dialysis of xestospongin C did not significantly change CCK-induced facilitation of AP firing frequency. I: intracellular application of thapsigargin via the recording pipettes did not change significantly CCK-mediated facilitation of AP firing frequency. J: buffering intracellular Ca2+ to the basal level (∼56 nM) by adding BAPAT (10 mM) and CaCl2 (3.3 mM) via the recording pipittes prevented CCK-induced increases in AP firing frequency. K and L: pretreatment of slices with and continuous bath application of 2 PKC inhibitors, calphostin C (K) and Ro318220 (L), had no effects on CCK-mediated facilitation of AP firing frequency.
One possibility for the negative results of using the above inhibitors for intracellular Ca2+ release and PKC was that these inhibitors failed to block intracellular Ca2+ release and did not inhibit PKC. We therefore performed positive control experiments to test this possibility. Because intracellular Ca2+ release and PKC are required for CCK-induced increases in glutamate release at the perforant path-granule cell synapses in the hippocampus (Deng et al. 2010), we assessed the biological efficacy of these inhibitors by testing their roles in CCK-mediated facilitation of evoked α-amino-3-hydroxy-5methylisoxazole-4-proprionate excitatory postsynaptic currents (AMPA EPSCs) at this synapse. Bath application of CCK increased the amplitude of AMPA EPSCs evoked by stimulation of the medial perforant path to 189 ± 12% of control (n = 8; P < 0.001; Supplemental Fig. 1). CCK-induced increases in AMPA EPSCs were reduced significantly when slices were pretreated with 2-APB (100 μM; 130 ± 6% of control, n = 8, P = 0.002 vs. baseline; Supplemental Fig. 1) or xestospongin C (1 μM; 125 ± 5% of control, n = 7, P = 0.002 vs. baseline; Supplemental Fig. 1). CCK-mediated facilitation of AMPA EPSCs was blocked completely by pretreatment of slices with thapsigargin (10 μM; 122 ± 9% of control, n = 7, P = 0.06 vs. baseline; Supplemental Fig. 1), calphostin C (1 μM; 96 ± 6% of control, n = 8, P = 0.54 vs. baseline; Supplemental Fig. 1), or Ro318220 (1 μM; 105 ± 10% of control, n = 9, P = 0.66 vs. baseline; Supplemental Fig. 1). These data together demonstrate that the incapacity of these inhibitors to block the effects of CCK on AP firing frequency in the EC is not due to their biological inefficacy.
CCK generates membrane depolarization via activation of a cationic conductance.
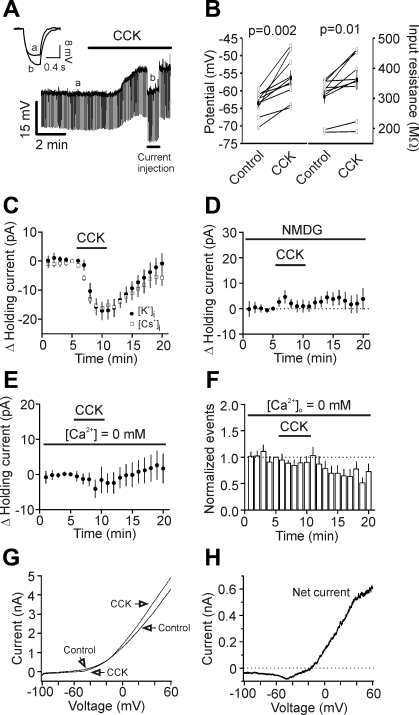
We next examined the effects of CCK on the RMP and input resistance. Bath application of CCK generated membrane depolarization (control: −63.6 ± 1.2 mV; CCK: −56.4 ± 2.1 mV; n = 9; P = 0.002; Fig. 4, A and B) and slightly but significantly increased the input resistance (control: 306 ± 22 MΩ; CCK: 361 ± 33 MΩ; n = 9; P = 0.01; Fig. 4, A and B). In voltage-clamp mode, CCK induced an inward shift of the HCs recorded at −60 mV (−17.1 ± 3.1 pA; n = 9; P < 0.001; Fig. 4C), further confirming the depolarizing effect of CCK. We then tested whether CCK-induced increases in inward HCs are responsible for its effects on AP firing by injecting a comparable positive current into cells and measuring the changes of the AP firing frequency. Injection of the averaged HC (+17 pA) into the cells increased AP firing frequency from 1.08 ± 0.08 Hz to 3.62 ± 0.26 Hz (n = 7; P < 0.001; data not shown), an increase (347 ± 35% of control; n = 7) statistically indistinguishable from a CCK-induced increase of AP firing frequency (303 ± 30% of control; n = 6; P = 0.33, unpaired t-test). We then probed the ionic mechanisms underlying CCK-induced depolarization. CCK could generate membrane depolarization by activating a cationic conductance or inhibiting a background K+ channel or both. The following results indicate that CCK-induced depolarization is due to the opening of a cationic conductance. First, application of CCK still induced a comparable, inward HC when the intracellular K+-gluconate was replaced with the same concentration of Cs-gluconate (−16.2 ± 1.6 pA; n = 6; P < 0.001; Fig. 4C), suggesting that CCK-induced depolarization is not mediated by inhibition of background K+ channels. Second, if CCK-induced depolarization were due to the activation of a cationic conductance, influx of extracellular sodium (Na+) ions should be the major ions responsible for membrane depolarization. Replacement of extracellular NaCl with the same concentration of N-methyl-d-glucamine chloride (NMDG-Cl) completely blocked CCK-induced increases in inward HCs (1.0 ± 2.5 pA; n = 5; P = 0.72; Fig. 4D), supporting the involvement of cationic channels. Third, substitution of the extracellular Ca2+ with the same concentration of Mg2+ and inclusion of 1 mM EGTA in the extracellular solution to chelate, the ambient Ca2+, also blocked CCK-induced increases in inward HCs (−4.1 ± 2.5 pA; n = 7; P = 0.16; Fig. 4E). We also tested the effects of zero extracellular Ca2+ on CCK-induced facilitation of AP firing frequency. Depletion of extracellular Ca2+ blocked CCK-induced increases in AP firing frequency (control: 1.59 ± 0.12 Hz; CCK: 1.37 ± 0.16 Hz; n = 14; P = 0.4; Fig. 4F), suggesting that the effects of CCK are dependent on extracellular Ca2+. Finally, we measured the reversal potential of CCK-induced net currents by applying a ramp protocol before and during the application of CCK. CCK-induced net currents had an initial negative slope and then reversed at −25.4 ± 1.1 mV (n = 7; Fig. 4, G and H). This type of voltage-current relationship (initial negative slope at negative potentials) has also been observed in numerous types of cells (Brown et al. 2002; Faber et al. 2006; Haj-Dahmane and Andrade 1996; Meis et al. 2007; Zhang et al. 2011; Zhang and Seguela 2010), suggesting the involvement of TRPCs.
Fig. 4.
CCK-induced depolarization is mediated by activation of cation channels. A: application of CCK (0.3 μM) generated membrane depolarization and increased input resistance in layer III pyramidal neurons of the EC. Resting membrane potential (RMP) was recorded in current-clamp mode, and a hyperpolarizing current (−50 pA; 500 ms) was injected every 5 s to measure the input resistance. Note that CCK generated membrane depolarization and increased input resistance. The inset is the voltage traces taken before (a) and during (b) the application of CCK. B: summarized data for CCK-induced changes in RMPs and input resistance in layer III pyramidal neurons. C: bath application of CCK induced comparable inward holding currents (HCs) in intracellular solution containing potassium-gluconate ([K+]i) or cesium-gluconate ([Cs+]i). D: replacement of extracellular sodium with N-methyl-d-glucamine (NMDG) blocked CCK-induced increases in inward HCs. E: depletion of extracellular Ca2+ blocked CCK-induced increases in inward HCs. F: depletion of extracellular Ca2+ blocked CCK-induced increases in AP firing frequency. G: voltage-current relationship obtained by a ramp protocol before and during the application of CCK. H: CCK-induced net voltage-current relationship obtained by subtracting the control curve from that in the presence of CCK (in G). Note that there was a negative slope at the negative potentials, and the curve reversed polarity at ∼−20 mV.
Involvement of TRPC-like channels.
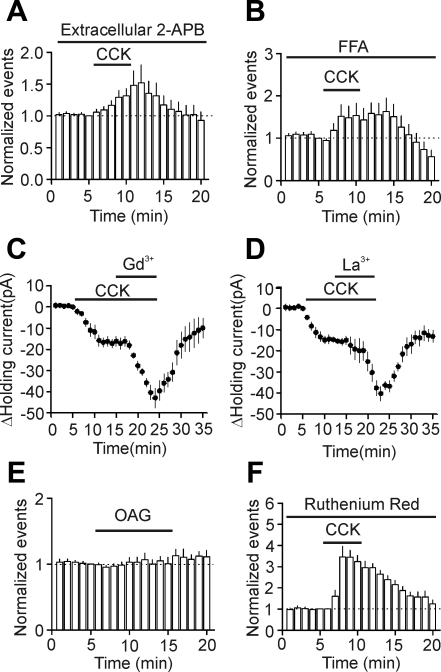
The voltage-current relationship of the CCK-induced net currents resembles that of the heteromers formed by TRPC1/TRPC4 or TRPC1/TRPC5 (Strubing et al. 2001). Interestingly, the EC expresses TRPC1, TRPC4, and TRPC5 (Fowler et al. 2007; von Bohlen Und Halbach et al. 2005). We accordingly tested the roles of TRPC-like channels in CCK-induced increases in AP firing frequency by applying TRPC channel blockers. Extracellular application of 2-APB has been shown to inhibit several TRPC channel subtypes including TRPC1, TRPC3, TRPC5, TRPC6, and TRPC7 (Lievremont et al. 2005; Trebak et al. 2002; Xu et al. 2005). The action site of 2-APB is on the extracellular side (Xu et al. 2005). Bath application of 2-APB (100 μM) inhibited CCK-mediated increases in AP firing frequency (control: 1.52 ± 0.25 Hz; CCK: 2.10 ± 0.33 Hz; n = 8; P = 0.06; Fig. 5A), suggesting the involvement of TRPC-like channels. Similarly, bath application of flufenamic acid (FFA; 100 μM), an inhibitor for TRPC3, TRPC5, and TRPC7 (Lee et al. 2003), also suppressed the effect of CCK on AP firing (control: 1.58 ± 0.19 Hz; CCK: 2.52 ± 0.64 Hz; n = 10; P = 0.12; Fig. 5B), further confirming the involvement of TRPC-like channels. Micromolar concentrations of two trivalent ions (Gd3+ and La3+) have been shown to inhibit TRPC3, TRPC6, and TRPC7 but potentiate TRPC4 and TRPC5 (Jung et al. 2003; Strubing et al. 2001). We used the HEPES-based extracellular solution (see materials and methods, Whole-cell recordings from the EC neurons) to prevent Gd3+- and La3+-induced precipitation. We initially recorded APs and applied CCK to induce increases in AP firing. When the maximal effect of CCK on AP firing frequency was observed, we applied 100 μM Gd3+ or La3+ to test whether they still further increase AP firing frequency. This application method of Gd3+ and La3+ was used previously by Strubing et al. (2001) in transfected cells. However, when CCK-induced maximal firing frequency was observed, coapplication of Gd3+ or La3+ induced further depolarization without repolarization to keep continuous firing of APs. We therefore instead tested the effects of Gd3+ and La3+ by recording the HCs at −60 mV. Initial application of CCK induced an inward HC and subsequent application of 100 μM Gd3+ (n = 7; P = 0.002; Fig. 5C), and La3+ (n = 8; P < 0.001; Fig. 5D) further increased the inward HCs, suggesting that Gd3+ and La3+ facilitate the effects of CCK. Furthermore, bath application of 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM), a recognized membrane-permeable activator of TRPC3, TRPC6, and TRPC7 (Ramsey et al. 2006), failed to increase significantly the firing frequency of APs in layer III pyramidal neurons (control: 1.41 ± 0.17 Hz; CCK: 1.46 ± 0.23 Hz; n = 5; P = 0.7; Fig. 5E). Finally, bath application of the transient receptor potential cation channel subfamily V (TRPV) channel blocker, ruthenium red (50 μM) (Ramsey et al. 2006), did not significantly change CCK-induced facilitation of AP firing frequency (control: 1.33 ± 0.32 Hz; CCK: 4.27 ± 0.75 Hz; n = 7; P = 0.002; Fig. 5F), arguing against the involvement of TRPV. Collectively, these data suggest that activation of CCK-2 receptors increases the firing frequency of APs in layer III pyramidal neurons via activation of TRPC-like channels.
Fig. 5.
Effects of some cationic channel blockers on CCK-induced increases in neuronal excitability. A: bath application of 2-APB (100 μM) inhibited CCK-induced enhancement of AP firing frequency. B: bath application of flufenamic acid (FFA; 100 μM) suppressed CCK-mediated increases in AP firing frequency. C: bath application of CCK induced an inward HC. When the effect of CCK reached maximal, bath application of gadolinium ion (Gd3+; 100 μM) in the presence of CCK further increased the inward HCs. D: bath application of CCK induced an inward HC. When the effect of CCK reached maximal, bath application of lanthanum ion (La3+; 100 μM) in the presence of CCK further increased the inward HCs. E: bath application of 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM) failed to change AP firing frequency significantly. F: bath application of ruthenium red (50 μM) did not alter CCK-mediated increases in AP firing frequency.
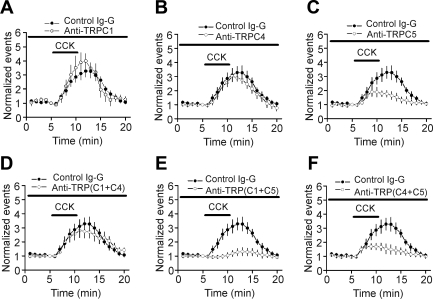
Because there are no specific antagonists for TRPC-like channels and as numerous studies demonstrate that application of the TRPC antibodies—which are against their intracellular epitopes to the cytoplasmic site in either a whole-cell or inside-out, patch-clamp configuration—is effective on inhibiting the functions of the corresponding TRPC channels (Albert et al. 2006; Alvarez et al. 2008; Chen et al. 2009; Faber et al. 2006; Fauconnier et al. 2007; Ma et al. 2003; Saleh et al. 2006, 2008; Xiao et al. 2010), we tested the roles of TRPC1, TRPC4, and TRPC5 by applying their specific antibodies via the recording pipettes. To ensure complete intracellular dialysis of these antibodies, we waited for >40 min after the formation of whole-cell configurations. As a control, application of control IgG (4 μg/ml), via the recording pipettes, did not significantly change CCK-induced increases in AP firing frequency (n = 7; P = 0.48 vs. CCK alone, two-way ANOVA; Fig. 6), suggesting that intracellular infusion of IgG had no nonspecific effects on CCK-induced facilitation of AP firing frequency. Intracellular application of antibodies to TRPC1 (4 μg/ml; n = 6; P = 0.87; Fig. 6A) and TRPC4 (4 μg/ml; n = 11; P = 0.26; Fig. 6B) did not significantly alter CCK-mediated augmentation of AP firing frequency compared with the effect of CCK in the presence of control IgG (two-way ANOVA). However, intracellular perfusion of anti-TRPC5 (4 μg/ml) significantly reduced the effect of CCK on AP firing (n = 5; P = 0.02 vs. control IgG, two-way ANOVA; Fig. 6C), suggesting the involvement of TRPC5. Because TRPC heteromers, such as TRPC1/TRPC4 and TRPC1/TRPC5, have been detected in neurons (Strubing et al. 2001), we alternately applied the antibodies to two different types of channels via the recording pipettes. Intracellular application of anti-TRPC1 with anti-TRPC4 failed to change significantly CCK-mediated increases in AP firing frequency (n = 9; P = 0.78 vs. control IgG, two-way ANOVA; Fig. 6D), whereas intracellular perfusion of anti-TRPC1 with anti-TRPC5 (n = 6; P = 0.003; Fig. 6E) or anti-TRPC5 together with anti-TRPC4 (n = 11; P = 0.015; Fig. 6F) significantly reduced the effect of CCK on AP firing (two-way ANOVA vs. control IgG). Statistical comparison of the extent of the inhibition mediated by anti-TRPC5 alone with the inhibitions in the presence of anti-TRPC1 and anti-TRPC5 (P = 0.18, two-way ANOVA) or anti-TRPC4 and anti-TRPC5 (P = 0.96, two-way ANOVA) showed no significant differences, suggesting that TRPC5 is the principal target of CCK.
Fig. 6.
CCK-induced facilitation of AP firing frequency is sensitive to intracellular application of transient receptor potential channel 5 (TRPC5) antibody via the recording pipettes. A: intracellular application of anti-TRPC1 did not significantly change CCK-induced increases in AP firing frequency. B: intracellular application of anti-TRPC4 did not significantly change CCK-induced increases in AP firing frequency. C: intracellular application of anti-TRPC5 significantly reduced CCK-induced increases in AP firing frequency. D: coapplication of anti-TRPC1 and anti-TRPC4 via the recording pipettes did not significantly alter CCK-mediated increases in AP firing frequency. E: coapplication of anti-TRPC1 and anti-TRPC5 via the recording pipettes significantly decreased CCK-mediated augmentation of AP firing frequency. F: coapplication of anti-TRPC4 and anti-TRPC5 via the recording pipettes significantly inhibited CCK-mediated facilitation of AP firing frequency.
DISCUSSION
Our results demonstrate that activation of CCK-2 receptors facilitates neuronal excitability of layer III pyramidal neurons in the EC via activation of TRPC-like channels. CCK-mediated excitation requires the functions of G proteins and PLC but is independent of IP3 receptors and PKC activity. CCK-induced facilitation of AP firing frequency was suppressed by extracellular application of 2-APB and FFA, whereas CCK-induced increases in inward HCs were potentiated by Gd3+ and La3+. Furthermore, intracellular application of the antibody to TRPC5, not the antibodies to TRPC1 and TRPC4, via the recording pipettes, significantly reduced CCK-induced facilitation of AP firing frequency. These data collectively suggest that CCK enhances neuronal excitability in layer III pyramidal neurons via activation of TRPC-like channels.
Whereas an autoradiographic study indicates that layer III pyramidal neurons in a rat EC express the highest density of CCK receptors (Kohler and Chan-Palay 1988), the identities of CCK receptors have not been determined. With both pharmacological and transgenic approaches, we demonstrate that these CCK receptors detected in layer III are CCK-2 receptors, because CCK-induced increases in AP firing frequency are insensitive to CCK-1 receptor antagonist but sensitive to CCK-2 receptor blockers. Furthermore, application of CCK failed to increase the AP firing frequency in CCK-2 KO mice but still induced robust facilitation of AP firing frequency in WT mice. Because CCK-2 receptors are coupled to Gq/11, resulting in activation of PLC, which further hydrolyzes PIP2 to generate IP3 to increase intracellular Ca2+ release and DAG to activate PKC (Wank 1995), we probed the roles of these intracellular signaling molecules in CCK-induced facilitation of AP firing. Our results demonstrate that G proteins and PLC are required, whereas IP3 receptors and PKC are unnecessary for CCK-mediated increases in AP firing frequency.
Dependent on the neuronal types, CCK has been shown to facilitate neuronal excitability by inhibiting K+ channels (Branchereau et al. 1993; Chung and Moore 2009; Chung et al. 2009; Cox et al. 1995; Deng and Lei 2006; Deng et al. 2010; Meis et al. 2007; Miller et al. 1997) and/or by activating cationic channels (Chung and Moore 2009; Meis et al. 2007; Thorn and Petersen 1992; Tsujino et al. 2005; Wang et al. 2005; Wu and Wang 1996a, b). Our results show that CCK excites entorhinal neurons in the EC by activating cationic channels based on the following lines of evidence. First, replacement of extracellular NaCl with the same concentration of NMDG-Cl blocked CCK-mediated inward shifting of HCs, suggesting that the influx of extracellular Na+ ions is the major mediator to generate membrane depolarization. Second, CCK-induced membrane depolarization was not affected when intracellular K+ was substituted with the same concentration of cesium, suggesting that K+ channels are not the targets for CCK in layer III pyramidal neurons of the EC. Third, the reversal potential of CCK-induced net currents is close to −25 mV, which is different from the K+ reversal potential. However, our results do demonstrate that CCK slightly but significantly increased the input resistance, seemingly suggesting the involvement of K+ channels. CCK-induced, slight increases in input resistance could be due to the negative slope of the net current induced by CCK at the negative potentials (Fig. 4H). This phenomenon—i.e., G protein-coupled receptors activate cationic channels but increase the input resistance—has also been observed for muscarinic receptors in the pyramidal neurons of the association cortex (Haj-Dahmane and Andrade 1996).
Our results suggest that TRPC-like channels are the targets for CCK in the entorhinal neurons based on the following bodies of evidence. First, TRPC1, TRPC4, and TRPC5 have been detected in the EC (Fowler et al. 2007; von Bohlen Und Halbach et al. 2005). Second, CCK-mediated facilitation of neuronal excitability was inhibited by applications of the TRPC channel blockers such as 2-APB and FFA but potentiated by Gd3+ (100 μM) and La3+ (100 μM). Both Gd3+ and La3+ at this concentration have been shown to potentiate TRPC4 and TRPC5 channels (Jung et al. 2003; Strubing et al. 2001). Third, intracellular perfusion of the antibody to TRPC5 significantly reduced CCK-mediated increases in AP firing frequency. Finally, the voltage-current relationship of the net current induced by CCK in layer III neurons resembles that observed for the heteromers formed by TRPC1/TRPC4 and TRPC1/TRPC5 (Strubing et al. 2001). However, we acknowledge that our conclusion is limited by the specificities of the inhibitors.
Whereas CCK has been shown to increase neuronal excitability in a variety of neurons, there are inconsistent results as to whether extracellular Ca2+ is required for the effects of CCK. In the projection neurons of mouse basolateral amygdala, extracellular Ca2+ is not required for CCK-induced depolarization (Meis et al. 2007). However, omission of extracellular Ca2+ has been shown to enhance CCK-induced membrane depolarization in the interneurons of the basolateral amygdala (Chung and Moore 2009) and orexin neurons (Tsujino et al. 2005). Our results demonstrated that extracellular Ca2+ is required for CCK-mediated facilitation of neuronal excitability in the EC. There are several plausible mechanisms to explain the observed discrepancies. First, different CCK receptors may generate distinct effects. The effects of CCK in orexin neurons are mediated via activation of CCK-1 receptors (Tsujino et al. 2005), whereas those in the projection neurons (Meis et al. 2007) and interneurons (Chung and Moore 2009) in the basolateral amygdala and layer III pyramidal neurons of the EC were mediated via CCK-2 receptors. Second, the ion channels activated by CCK likely have different sensitivity to extracellular Ca2+. Whereas TRPCs are involved in CCK-mediated depolarization in the projection neurons in the basolateral amygdala (Meis et al. 2007), orexin neurons (Tsujino et al. 2005), and layer III pyramidal neurons of the EC, both K+ and cation channels are required for the effects of CCK on the interneurons in the basolateral amygdala (Chung and Moore 2009). Among the TRPC channels, TRPC5 (Blair et al. 2009; Nishida et al. 2006) and TRPC7 (Nishida et al. 2006) are gated by extracellular Ca2+, and TRPC7 is also activated by DAG (Nishida et al. 2006). Our result that bath application of OAG, the membrane-permeable analog of DAG, failed to induce an increase in AP firing frequency suggests that TRPC7 is unlikely to be involved. However, we cannot exclude the possibility that TRPC7 combines with other TRPC subtypes, such as TRPC1, TRPC4, or TRPC5, in layer III pyramidal neurons, because the sensitivity to OAG of TRPC channels is likely changed in heteromers (Qiu et al. 2010; Strubing et al. 2003; Zhang et al. 2008). Therefore, based on the sensitivities to extracellular Ca2+ and intracellular application of anti-TRPC5, the most likely target of CCK in the EC is TRPC5.
Our results indicate that CCK-mediated facilitation of neuronal excitability does not require the functions of IP3 receptors DAG and PKC. The mechanism underlying the activation of TRPC4/TRPC5 channels by G protein-coupled receptors is still an unsolved mystery. It is generally accepted that PLC is required for the opening of TRPC4/TRPC5 channels activated by Gq/11-coupled receptors, and the downstream targets of PLC, IP3, and DAG do not activate TRPC4 and TRPC5 (Clapham et al. 2001; Nishida et al. 2006). Activation of PLC hydrolyzes PIP2 to generate IP3 and DAG. PIP2 has been shown to modulate a variety of ion channels (Suh and Hille 2008), including TRPC5 channels (Kim et al. 2008; Trebak et al. 2009). Application of exogenous PIP2 via the recording pipettes has been shown to slow the desensitization of TRPC5 channels activated by muscarinic receptors (Kim et al. 2008). Depletion of endogenous PIP2 through inhibition of phosphatidylinositol 4-kinase results in activation of TRPC5 channels, whereas direct application of PIP2 to the cytoplasmic side in inside-out patches results in activation of TRPC5 channels (Trebak et al. 2009). These results together suggest that PIP2 may have complex functions on TRPC5 channels.
CCK has been implicated in a variety of behavioral functions including satiety, analgesia, anxiety, learning, and memory processes (Beinfeld 2001; Rehfeld 2000; Sebret et al. 1999). For example, CCK has been shown to facilitate learning and memory processes (Gulpinar and Yegen 2004; Sebret et al. 1999; Voits et al. 2001). Because the EC is a critical structure involved in cognitive activity, CCK-induced increases in neuronal excitability in the EC likely contribute to its facilitation of learning and memory processes. Furthermore, it has been known for decades that CCK induces anxiety (Beinfeld 2001; Rehfeld 2000). Because the EC is an important limbic structure involved in emotional control, up-regulation of neuronal excitability mediated by CCK in the EC may also be related to its anxiogenic effect. Therefore, our results may provide a novel cellular and molecular mechanism to explain the roles of CCK in anxiety and learning and memory processes.
GRANTS
This work was supported by National Institute of Mental Health Grant R01MH082881 (S. Lei).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Albert AP, Pucovsky V, Prestwich SA, Large WA. TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol 571: 361–369, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Coulombe A, Cazorla O, Ugur M, Rauzier JM, Magyar J, Mathieu EL, Boulay G, Souto R, Bideaux P, Salazar G, Rassendren F, Lacampagne A, Fauconnier J, Vassort G. ATP/UTP activate cation-permeable channels with TRPC3/7 properties in rat cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H21–H28, 2008 [DOI] [PubMed] [Google Scholar]
- Bachus SE, Hyde TM, Herman MM, Egan MF, Kleinman JE. Abnormal cholecystokinin mRNA levels in entorhinal cortex of schizophrenics. J Psychiatr Res 31: 233–256, 1997 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. An introduction to neuronal cholecystokinin. Peptides 22: 1197–1200, 2001 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212: 51–57, 1981 [DOI] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133: 525–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D. The cholecystokinin hypothesis of anxiety and panic disorder. Ann N Y Acad Sci 713: 273–282, 1994 [DOI] [PubMed] [Google Scholar]
- Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokinin-gated currents in neurons of the rat solitary complex in vitro. J Neurophysiol 70: 2584–2595, 1993 [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 22: 8850–8859, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Crossland RF, Noorani MM, Marrelli SP. Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am J Physiol Heart Circ Physiol 297: H417–H424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L, Moore SD. Cholecystokinin excites interneurons in rat basolateral amygdala. J Neurophysiol 102: 272–284, 2009 [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD, Cox CL. Cholecystokinin action on layer 6b neurons in somatosensory cortex. Brain Res 1282: 10–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci 2: 387–396, 2001 [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol 74: 990–1000, 1995 [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides 15: 731–755, 1994 [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol 572: 425–442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol 97: 727–737, 2007 [DOI] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin HS, Porter JE, Geiger JD, Lei S. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci 30: 5136–5148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron 63: 230–243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience 137: 781–794, 2006 [DOI] [PubMed] [Google Scholar]
- Fauconnier J, Lanner JT, Sultan A, Zhang SJ, Katz A, Bruton JD, Westerblad H. Insulin potentiates TRPC3-mediated cation currents in normal but not in insulin-resistant mouse cardiomyocytes. Cardiovasc Res 73: 376–385, 2007 [DOI] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One 2: e573, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood RS, Godar SE, Reaves TA, Jr, Hayward JN. Cholecystokinin in hippocampal pathways. J Comp Neurol 203: 335–350, 1981 [DOI] [PubMed] [Google Scholar]
- Gulpinar MA, Yegen BC. The physiology of learning and memory: role of peptides and stress. Curr Protein Pept Sci 5: 457–473, 2004 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci 16: 3848–3861, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8: 1319–1328, 2005 [DOI] [PubMed] [Google Scholar]
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci 7: 2967–2976, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Shaw TM, Graham W, Woodruff GN. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci 10: 1070–1081, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278: 3562–3571, 2003 [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim MT, Jeon JH, Kim SJ, So I. Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol Pharm Bull 31: 1733–1738, 2008 [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V. Cholecystokinin-octapeptide (CCK-8) receptors in the hippocampal region: a comparative in vitro autoradiographic study in the rat, monkey and the postmortem human brain. Neurosci Lett 90: 51–56, 1988 [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V. The distribution of cholecystokinin-like immunoreactive neurons and nerve terminals in the retrohippocampal region in the rat and guinea pig. J Comp Neurol 210: 136–146, 1982 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Innis RB, Goldman-Rakic PS. Regional distribution of cholecystokinin receptors in macaque medial temporal lobe determined by in vitro receptor autoradiography. J Comp Neurol 276: 219–230, 1988 [DOI] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol 284: G604–G616, 2003 [DOI] [PubMed] [Google Scholar]
- Lievremont JP, Bird GS, Putney JW., Jr Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Mol Pharmacol 68: 758–762, 2005 [DOI] [PubMed] [Google Scholar]
- Lotstra F, Vanderhaeghen JJ. High concentration of cholecystokinin neurons in the newborn human entorhinal cortex. Neurosci Lett 80: 191–196, 1987 [DOI] [PubMed] [Google Scholar]
- Ma R, Rundle D, Jacks J, Koch M, Downs T, Tsiokas L. Inhibitor of myogenic family, a novel suppressor of store-operated currents through an interaction with TRPC1. J Biol Chem 278: 52763–52772, 2003 [DOI] [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci 35: 356–367, 2007 [DOI] [PubMed] [Google Scholar]
- Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci 17: 4994–5003, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res 362: 175–179, 1986 [DOI] [PubMed] [Google Scholar]
- Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem 6: 156–162, 1968 [DOI] [PubMed] [Google Scholar]
- Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci USA 93: 11825–11830, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Hara Y, Yoshida T, Inoue R, Mori Y. TRP channels: molecular diversity and physiological function. Microcirculation 13: 535–550, 2006 [DOI] [PubMed] [Google Scholar]
- Noble F, Roques BP. CCK-B receptor: chemistry, molecular biology, biochemistry and pharmacology. Prog Neurobiol 58: 349–379, 1999 [DOI] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30: 1560–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Cholecystokinin and panic disorder—three unsettled questions. Regul Pept 93: 79–83, 2000 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Cholecystokinin and anxiety: promises and pitfalls. Crit Rev Neurobiol 9: 345–369, 1995 [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol 586: 2463–2476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebret A, Lena I, Crete D, Matsui T, Roques BP, Dauge V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci 19: 7230–7237, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278: 39014–39019, 2003 [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001 [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman-Chauhan N, Meecham KG, Webdale L, Hunter JC, Pritchard MC, Woodruff GN, Hill DR. The influence of guanyl nucleotide on agonist and antagonist affinity at guinea-pig CCK-B/gastrin receptors: binding studies using [3H]PD140376. Regul Pept 65: 37–43, 1996 [DOI] [PubMed] [Google Scholar]
- Thorn P, Petersen OH. Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J Gen Physiol 100: 11–25, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Bird GS, McKay RR, Putney JW., Jr Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem 277: 21617–21623, 2002 [DOI] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch 457: 757–769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci 25: 7459–7469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bent Blommaert A, Melman AG, IJ AP, CT, van Wijngaarden I, Soudijn W. Hybrid cholecystokinin-A antagonists based on molecular modeling of lorglumide and L-364,718. J Med Chem 35: 1042–1049, 1992 [DOI] [PubMed] [Google Scholar]
- Van Dijk A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin8 as radioligand. J Neurosci 4: 1021–1033, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voits M, Hasenohrl RU, Huston JP, Fink H. Repeated treatment with cholecystokinin octapeptide improves maze performance in aged Fischer 344 rats. Peptides 22: 1325–1330, 2001 [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O, Hinz U, Unsicker K, Egorov AV. Distribution of TRPC1 and TRPC5 in medial temporal lobe structures of mice. Cell Tissue Res 322: 201–206, 2005 [DOI] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 293: G484–G492, 2007 [DOI] [PubMed] [Google Scholar]
- Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev 29: 1361–1373, 2005 [DOI] [PubMed] [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol 269: G628–G646, 1995 [DOI] [PubMed] [Google Scholar]
- Wu T, Wang HL. The excitatory effect of cholecystokinin on rat neostriatal neurons: ionic and molecular mechanisms. Eur J Pharmacol 307: 125–132, 1996a [DOI] [PubMed] [Google Scholar]
- Wu T, Wang HL. G alpha q/11 mediates cholecystokinin activation of the cationic conductance in rat substantia nigra dopaminergic neurons. J Neurochem 66: 1060–1066, 1996b [DOI] [PubMed] [Google Scholar]
- Xiao JH, Zheng YM, Liao B, Wang YX. Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol 43: 17–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE, Lei S. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J Biol Chem 284: 10980–10991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145: 405–414, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachrisson O, Nomikos GG, Marcus MM, Svensson TH, Lindefors N. Effects of antipsychotic drugs on cholecystokinin and preprotachykinin (substance P) mRNA expression in the rat hippocampal formation. Eur Neuropsychopharmacol 10: 355–363, 2000 [DOI] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28: 4423–4434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Reboreda A, Alonso A, Barker PA, Seguela P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus 20: 386–397, 2011 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Seguela P. Metabotropic induction of persistent activity in layers II/III of anterior cingulate cortex. Cereb Cortex 20: 2948–2957, 2010 [DOI] [PubMed] [Google Scholar]