Abstract
Cell growth is influenced by environmental stress. Mammalian target of rapamycin (mTOR), the central regulator of cell growth, can be positively or negatively regulated by various stresses through different mechanisms. The p38 MAP kinase pathway is essential in cellular stress responses. Activation of MK2, a downstream kinase of p38α, enhances mTOR complex 1 (mTORC1) activity by preventing TSC2 from inhibiting mTOR activation. The p38β-PRAK cascade targets Rheb to inhibit mTORC1 activity upon glucose depletion. Here we show the activation of p38β participates in activation of mTOR complex 1 (mTORC1) induced by arsenite but not insulin, nutrients, anisomycin, or H2O2. Arsenite treatment of cells activates p38β and induces interaction between p38β and Raptor, a regulatory component of mTORC1, resulting in phosphorylation of Raptor on Ser863 and Ser771. The phosphorylation of Raptor on these sites enhances mTORC1 activity, and contributes largely to arsenite-induced mTORC1 activation. Our results shown here and in previous work demonstrate that the p38 pathway can regulate different components of the mTORC1 pathway, and that p38β can target different substrates to either positively or negatively regulate mTORC1 activation when a cell encounters different environmental stresses.
Keywords: MAP Kinases (MAPKs), mTOR, mTOR Complex (mTORC), p38, p38 MAPK
Introduction
The p38 mitogen-activated protein kinase (MAPK) signal pathway plays an important role in a variety of biological processes, including inflammation, cell differentiation, and cell death (1–3). The p38 group of MAPK has four members: p38α, p38β, p38γ, and p38δ (4–7). Although similarities in activation and function have been observed, each p38 isoform also has distinct functions (8). Although activation of p38 MAPKs by different stimuli is cell type-dependent, various stress stimuli appear to activate the p38 pathway in all types of cells, and thus the p38 pathway is considered to be a major stress-activated signaling pathway (9). The activation of transcription factors and subsequent gene expression is a major mechanism by which the p38 pathway mediates biological responses (10–12). The activation of other types of cellular proteins is also essential for the p38 pathway to execute its function (13).
The mammalian target of rapamycin (mTOR)3 is a serine/threonine kinase that acts as an environmental sensor to regulate a plethora of cellular biosynthetic processes (14). mTOR activation promotes cell growth and proliferation, whereas mTOR inhibition stops cell growth and initiates catabolic processes (15). The p70 S6 kinase (S6K) and eIF4E-binding protein 1 (4EBP1) are key regulators of mRNA translation, and are the most well characterized targets of mTOR (16, 17). Phosphorylation of S6K and 4EBP1 by mTOR leads to increased levels of translation of specific mRNAs (18, 19). mTOR exists in two distinct functional complexes, mTOR complex 1 (mTORC1) and mTORC2. mTORC1 is potently and specifically inhibited by rapamycin, and it regulates cell size, autophagy, ribosome biogenesis, protein translation, transcription, and cellular viability (15). mTORC2, which is much less sensitive to rapamycin, is important for cytoskeletal regulation and AKT activation (20).
mTORC1 is a central signal integrator that receives signals arising from growth factors, nutrients, cellular energy metabolism, and environmental stresses (21). Growth factors, insulin, and energy depletion work via different pathways to activate or inhibit the TSC1/TSC2 complex (22–26). The tumor suppressors TSC1 and TSC2 negatively regulate mTOR by acting as the GTPase-activating protein for the small GTPase Ras-homology enriched in brain (Rheb), and thus they decrease Rheb-GTP and prevent Rheb from stimulating mTOR activity (27, 28). TSC1/TSC2 is essential for growth factor-, nutrient-, and energy-mediated regulation of mTORC1; however, recent studies have shown that other proteins can also play an essential role. The small G protein RAG can sense nutrients and directly mediate mTORC1 relocalization and activation (29, 30). mTOR can self-enhance its activity by phosphorylation of Raptor (regulatory associated protein of TOR), a component of the mTORC1 complex (31). It was suggested that the mTOR-mediated Raptor phosphorylation is a biochemical rheostat that modulates mTORC1 activity in accordance with environmental cues (32).
Cross-talk between the p38 and mTOR pathways has been reported. Phosphorylation of serine 1210 on TSC2 by MK2, a downstream kinase of p38α, creates a 14-3-3 binding site, which has been implicated to play a role in inhibiting TSC2 and thus promote anisomycin-induced mTOR activation (33). A recent paper suggested that p38α or p38β and Drosophila p38b promote mTOR activation under various conditions (34). Our recent study shows that the p38β-PRAK (p38-regulated/activated protein kinase, or MK5) cascade selectively participates in glucose depletion-induced inactivation of mTORC1. PRAK directly phosphorylates Rheb on serine 130, which in turn impairs the GTP-binding ability of Rheb and prevents Rheb from stimulating mTOR activity (35). It is clear that there are multiple different interplays between the p38 pathway and mTOR pathway.
Although energy stress leads to inactivation of mTORC1, other cell stresses can either inhibit or activate mTORC1 (14). Arsenite is a contaminant toxicant and is also used as a medicinal compound. It activates mTORC1, but the mechanism of this activation is unknown. Here we report that p38β selectively participates in arsenite-induced mTORC1 activation, but not in other stress-induced activation of mTOR. Arsenite-induced activation of mTOR is independent from AMPK and TSC2, and occurs at least partially via direct phosphorylation of Raptor by p38β to activate mTORC1.
EXPERIMENTAL PROCEDURES
Materials
Sodium arsenite, rapamycin, insulin, and anisomycin were obtained from Sigma. PD98059 and SB203580 were obtained from Calbiochem. Immobilon-P PVDF membrane and Immobilon Western Chemiluminescent HRP Substrate were obtained from Millipore. Autoradiography film was obtained from Kodak.
Generation and Validation of p38α/β/γ/δ Conditional Knock-out Mouse Embryonic Fibroblasts (MEF)
p38αlox/lox, p38βlox/lox, p38γlox/lox, and p38δlox/lox primary MEFs were prepared from E13.5 embryos of respective mice with a locus flanked by LoxP sites and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and 10 units/ml of penicillin/streptomycin. Cells were validated by genomic PCR and then immortalized by SV40 large T antigen. To generate gene knock-out MEF cells, the p38αlox/lox, p38βlox/lox, p38γlox/lox, and p38δlox/lox MEF cells were infected with Cre to excise the loxed gene. The gene knock-out was confirmed by genomic PCR.
DNA Constructs
Expression constructs of MKK6b(E), mTOR(KD), murine p38α, human p38β, human p38γ, and human p38δ were described previously (7). Full-length cDNAs of p38α, p38β, p38γ, and p38δ were subcloned into pBOBI vector for lentiviral gene delivery. p38β RNAi constructs were as described previously (35). Full-length or truncated cDNAs of Raptor, Deptor, mLST8/GβL, Rheb, S6K1, and p38β were cloned into mammalian expression vectors pcDNA6 or pCMV5, or a lentiviral vector pBOBI, or the bacterial expression vector pGEX-4T-1. The Raptor-Rheb(C20) expression plasmid was kind gift of Dr. Kunliang Guan (University of California, San Diego, CA). Point mutations of Raptor were generated by a PCR-based site-directed mutagenesis method using Pfu polymerase (Stratagene). The following oligonucleotides were used to create the point mutations; the underlined nucleotides indicate the mismatch (5′ → 3′): S771A-F, AGCAGCACCCTGGGCGCCCCCGAGAATGAGGA; S771A-R, TCCTCATTCTCGGGGGCGCCCAGGGTGCTGCT; S792A-F, ATGCGCCGCGCCAGCGCCTACTCCTCCCTCA; S792A-R, TGAGGGAGGAGTAGGCGCTGGCGCGGCGCAT; S795A-F, GCCAGCTCCTACTCCGCCCTCAACTCCCTCA; S795A-R, TGAGGGAGTTGAGGGCGGAGTAGGAGCTGGC;S863A-F, CAGTCGGCCCCCGCCGCCCCCACCAACAAGGG; S863A-R, CCCTTGTTGGTGGGGGCGGCGGGGGCCGACTG; T865A-F, GCCCCCGCCAGCCCCGCCAACAAGGGCGTGC; T865A-R, GCACGCCCTTGTTGGCGGGGCTGGCGGGGGC; S877A-F, CACCAGGCGGGGGGCGCCCCTCCGGCGTCCA; S877A-R, TGGACGCCGGAGGGGCGCCCCCCGCCTGGTG; S863A on T865A-F, CAGTCGGCCCCCGCCGCCCCCGCCAACAAGGG; S863A on T865A-R, CCCTTGTTGGCGGGGGCGGCGGGGGCCGACTG. shRNA targeted the 3′ UTR of human Raptor and were cloned into pll3.7 vectors. The target sequences were: 1) GGAAACCAAGAGAGAGGAA; 2) GCGAATTGAGACGGACTTT; 3)GCCTGCTGTACATAGTGAA; 4) GCAAGCAGGGACATTTCCT; 5) GGACATTTCCTAGCCAGCT; 6) GCTGTCGTGTCTGGAATGT; 7) GCCTAGAGGCTCATTATCT; 8) CCTAGAGGCTCATTATCTA; 9) CCATGTAATCAGAGCATTA; 10) GAGAGTGACCAACAGTAAA. The following oligonucleotides were used for qPCR (5′ → 3′): (m)p38α_qF, ACCGAGCCCCAGAGATCAT; (m)p38α_qR, CACGGACCAAATATCCACTGTCT; (m)p38β_qF, AGCACGAGAACGTCATAGGACTT; (m)p38β_qR, CATCAGGGTCGTCACGAGGTA; (m)p38γ_qF, TGCGCCTCCTCAAACACAT; (m)p38γ_qR, GAGACTCATCAGGTGTGAACACATC; (m)p38δ_qF, CAAGGCGGCCAAATCCTATA; (m)p38δ_qR, TGGCGCGTGGGAAGAG; (h)p38α_qF, GGTTACGTGTGGCAGTGAAG; (h)p38α_qR, AACGTCCAACAGACCAATCA; (h)p38β_qF, GCCATATGATGAGAGCGTTG; (h)p38β_qR, GTGAGCTCCTTCCACTCCTC; (h)p38γ_qF, AGATGATCACAGGCAAGACG; (h)p38γ_qR, TGTAGTTCTTGGCCTCATCG; (h)p38δ_qF, TCCTCAGCTGGATGCACTAC; (h)p38δ_qR, TGGTCCAGGTAATCTTTCCC. All constructs were verified by DNA sequencing.
Cell Culture, Stimulation, Transfection, and Lentivirus Infection
Primary p38β+/+ and p38β−/− MEFs were obtained from E13.5 embryos of the respective mice. PRAK+/+ and PRAK−/− MEFs were described previously (35). TSC2+/+ and TSC2−/− cells were described previously (37). AMPKα1/α2 double knock-out cells were kindly provided by Drs. Benoit Viollet (Universite Paris Descartes) and Keith R. Laderoute (Stanford University School of Medicine). MK2−/− cells were kindly provided by Dr. Matthias Gaestel (Hannover Medical School). The HEK293T cell line was obtained from ATCC. All cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen) and 10 units/ml of penicillin/streptomycin at 37 °C in a humidified incubator containing 5% CO2.
For insulin or H2O2 stimulation, cells were serum-starved for 24 h followed by insulin or H2O2 stimulation, as indicated. For glucose starvation, cells were cultured with d-glucose-free DMEM (Invitrogen) containing 25 mm HEPES and 10% dialyzed FBS. Cells were stimulated with 25 mm glucose for 15–60 min as indicated. For amino acid starvation, cells were serum-starved for 24 h and incubated in DPBS (PBS containing 25 mm glucose) for 30 min. DMEM containing all amino acids was then added for 10–30 min as indicated.
293T cells were transfected with Lipofectamine 2000 and the corresponding empty vector was used for adjustment to ensure an equal amount of total DNA. 36 h after transfection, cells were collected using lysis buffer as indicated.
For lentivirus production, 293T cells were transfected with pLV-EF1α-Cre-IRES-Bsd, pBOBI-HA-Raptor, and pBOBI-MKK6b(E) with lentivirus-packaging plasmids (VSVG/PMDL/REV) using Lipofectamine 2000. The transfected cells were cultured at 37 °C for 48 h and the virus generated was used for infection.
Antibodies
Antibodies to phospho-S6K(T389), S6K, phospho-S6(S235/236), phospho-4EBP1(T37/46), 4EBP1, phospho-p38(T180/Y182), p38β, Raptor, and mTOR were obtained from Cell Signaling Technology. Anti-phospho-Raptor(Ser863) and anti-HA(F-7) antibodies were obtained from Santa Cruz Biotechnology. Anti-FLAG M2 antibodies, anti-FLAG M2 beads, and anti-HA beads were obtained from Sigma. Polyclonal antibodies to phospho-Raptor (Ser771) were generated in rabbits against keyhole limpet hemocyanin-coupled peptides (amino acids 767–775: STLG(pS)PENE) using Abmart's custom antibody service. Goat anti-mouse HRP and goat anti-rabbit HRP antibodies were from Thermo Scientific.
Cell Lysis, Immunoprecipitation, Western Blotting
Cells rinsed once with ice-cold PBS were lysed in ice-cold CHAPS lysis buffer (40 mm HEPES, pH 7.5, 120 mm NaCl, 1 mm EDTA, 10 mm pyrophosphate, 10 mm β-glycerophosphate, 50 mm NaF, 1.5 mm Na3VO4, 0.3% CHAPS, 1 μg/ml of leupeptin, and 1 mm PMSF) as described (38). The soluble fractions of cell lysates were isolated by centrifugation at 19,000 × g for 20 min at 4 °C in a microcentrifuge.
For immunoprecipitations, anti-FLAG M2 beads or anti-HA beads were added to the lysates and incubated with rotation for 3 h or overnight at 4 °C. The immunoprecipitates were then washed three times each with lysis buffer followed by addition of 1× SDS sample buffer, boiling for 10 min, resolved by SDS-PAGE, and analyzed by immunoblotting.
Calf Intestinal Alkaline Phosphatase (CIAP) Treatment
As described (24), anti-HA beads used for immunoprecipitating HA-Raptor deletions were washed three times in RIPA buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Nonidet P-40, 1% sodium deoxycholate, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml of leupeptin, 0.1% SDS, and 1 mm PMSF) followed by two additional washes in CIAP buffer (100 mm NaCl, 50 mm Tris, pH 7.9, 10 mm MgCl2, 1 mm dithiothreitol). Beads were then incubated with 1 μl of 30 units/μl of CIAP (Takara Bio, Inc.) in 50 μl of CIAP buffer for 1 h at 37 °C. Reactions were terminated by washing twice in CIAP buffer and boiling in 1× SDS sample buffer.
In Vitro Kinase Assay
p38β kinase assays were performed in a final volume of 20 μl consisting of p38 kinase buffer with 250 μm ATP and bacterial-derived GST-Raptor-(741–887) fragments as substrates for 30 min at 30 °C. The reactions were stopped by 2× SDS sample buffer, and subjected to SDS-PAGE and immunoblotting.
For the mTORC1 kinase assay, 293T cells were transfected with HA-Raptor WT or S771A, S863A, and S771A/S863A mutants. Transfected cells were treated with 1 mm arsenite for 30 min as indicated in the figures and then lysed in CHAPS lysis buffer followed by immunoprecipitation by HA antibodies. As described (39), the immunoprecipitates were washed three times in CHAPS lysis buffer and then twice in 25 mm HEPES, pH 7.4, 20 mm potassium chloride. Kinase assays were carried out in a volume of 15 μl consisting of mTORC1 kinase buffer (25 mm HEPES, pH 7.4, 50 mm KCl, 10 mm MgCl2, 250 μm ATP) and bacterial-derived GST-S6K as a substrate for 30 min at 30 °C. The reactions were terminated by 2× SDS sample buffer, and subjected to SDS-PAGE and immunoblotting.
RESULTS
p38β Participates in Arsenite-induced Activation of mTORC1
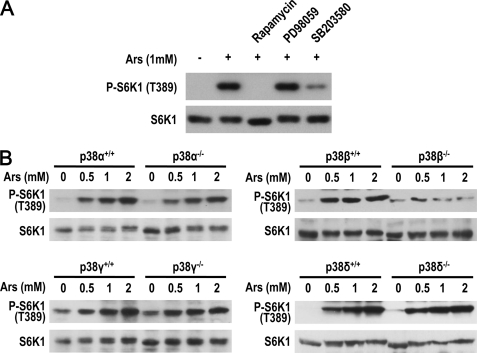
To study the effect of arsenite on mTOR activity, we measured phosphorylation levels of S6K1 and 4EBP1, substrates of mTOR, in arsenite-treated MEF cells. Arsenite-induced Thr389 phosphorylation of S6K1 and Thr37/46 phosphorylation of 4EBP1 (Fig. 1A and supplemental Fig. S1A). The induction is rapamycin sensitive, indicating arsenite-activated mTORC1 (Fig. 1A). Arsenite can activate the ERK pathway, a pathway that plays a role in EGF-stimulated mTORC1 activation (24). To determine whether arsenite works via ERK to activate mTORC1, we used the MEK inhibitor PD98059 and found that inhibition of the ERK pathway had no effect on arsenite-induced mTORC1 activation (Fig. 1A). In contrast, the p38α/β inhibitor SB203580 effectively inhibited arsenite-induced mTORC1 activation (Fig. 1A), suggesting involvement of the p38 pathway. To unambiguously determine the participation of the p38 pathway in arsenite-induced mTORC1 activation, and to determine which p38 group member plays the role, we analyzed arsenite-induced mTORC1 activation in p38α−/−, p38β−/−, p38γ−/−, p38δ−/−, and their corresponding p38α+/+, p38β+/+, p38γ+/+, and p38δ+/+ MEF cells. These cells were generated by in vitro treatment of the large T immortalized p38αlox/lox, p38βlox/lox, p38γlox/lox, and p38δlox/lox MEF cells with a lentivirus-expressing Cre recombinase or a control virus as described previously (35). We treated these cells with different doses of arsenite and found that only p38β deletion impaired arsenite-induced S6K1 phosphorylation (Fig. 1B). To confirm this phenotype of p38β−/− MEF, we tested two pairs of primary wild-type and p38β−/− MEF cells and observed a similar deficiency in arsenite-induced mTORC1 activation (supplemental Fig. S1B). siRNA knockdown of p38β in 293T cells also reduced arsenite-induced mTORC1 activation (supplemental Fig. S1C). The importance of p38β is not due to the abundance of p38β because its expression is not higher than other p38 isoforms in MEF and 293T cells (supplemental Fig. S1, D and E). Thus, p38β is involved in arsenite-induced mTORC1 activation.
FIGURE 1.
p38β is involved in arsenite-induced activation of mTORC1. A, MEF cells were preincubated for 30 min with rapamycin (20 nm), PD98059 (20 μm), SB203580 (20 μm), or a vehicle, followed by 1 mm arsenite stimulation for 30 min. Phosphorylation of endogenous S6K1 was detected by immunoblotting. B, p38α−/−, p38β−/−, p38γ−/−, or p38δ−/− and their corresponding wild-type MEFs were treated with arsenite as indicated for 30 min. Phosphorylation of endogenous S6K1 was detected by immunoblotting.
It was a surprise to us that p38β plays a promoting role in arsenite-induced mTORC1 activation, because our recent study showed that the p38β-PRAK cascade negatively regulates mTORC1 in 2-deoxyglucose-treated cells (35). We did a side by side comparison and confirmed that p38β is required for 2-deoxyglucose-induced inactivation of mTORC1 and arsenite-induced activation of mTORC1 (supplemental Fig. S2). In contrast to p38β, PRAK is required for 2-deoxyglucose-induced inactivation of mTORC1 but has no role in arsenite-induced mTORC1 activation (supplemental Fig. S3, A and B). It appears that p38β targets different downstream proteins to execute different functions in 2-deoxyglucose- and arsenite-treated cells.
p38β Activation Is Selectively Involved in Arsenite-induced Activation of mTORC1
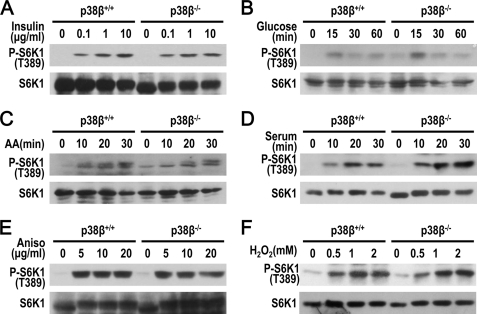
Activation of mTORC1 has been reported in a number of conditions. Replenishment of insulin, glucose, amino acid, or serum all activates mTORC1. We found that p38β was not required for mTORC1 activation under these conditions (Fig. 2, A–D). The protein synthesis inhibitor anisomycin can activate a variety of intracellular signaling pathways, including the p38 and mTORC1 pathways. It was shown that serum refurnishing or anisomycin treatment both activated MK2, which in turn phosphorylates TSC2 on Ser1210 and enables TSC2 binding to 14-3-3, and thus promotes mTORC1 activation (33). We found that, as in serum refurbishment, p38β has no role in anisomycin-induced S6K1 phosphorylation, excluding the possibility that p38β functions via MK2 to activate mTORC1 (Fig. 2E). Under serum-starved conditions, H2O2 can activate mTOR, and we found that p38β has no role in H2O2-induced mTOR activation (Fig. 2F). Collectively, of all the activation of mTORC1 tested, arsenite-induced mTORC1 activation is the only one that requires p38β.
FIGURE 2.
p38β has no role in insulin, anisomycin, H2O2, and refurnishing of glucose, amino acid, or serum-induced mTORC1 activation. Phosphorylation of endogenous S6K1 was detected by immunoblotting after the cells were treated as described below. A, p38β+/+ and p38β−/− MEFs were serum-starved for 24 h and stimulated by increasing concentrations of insulin (0, 0.1, 1, and 10 μg/ml) for 30 min. B, p38β+/+ and p38β−/− MEFs were starved with glucose-free DMEM containing 25 mm HEPES and 10% dialyzed FBS and stimulated with 25 mm glucose for the indicated times. C, p38β+/+ and p38β−/− MEFs were serum-starved for 24 h and incubated in DPBS (PBS containing 25 mm glucose) for 30 min. DMEM containing all amino acids was then added for 10–30 min as indicated. D, p38β+/+ and p38β−/− MEFs were serum-starved for 24 h and stimulated by 10% serum for the indicated time. E, p38β+/+ and p38β−/− MEFs were treated with increasing concentrations of anisomycin (0, 5, 10, and 20 μg/ml) for 30 min. F, p38β+/+ and p38β−/− MEFs were serum-starved for 24 h and treated with increasing concentrations of H2O2 (0, 0.5, 1, and 2 mm) for 30 min.
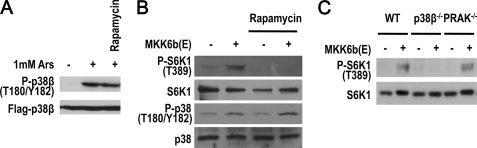
It is known that arsenite activates all p38 group members, including p38β (7). Because of the similar size of p38 family members, there is currently no method available to specifically detect p38β activation. To determine whether p38β is activated in our experimental setting, we used transiently expressed FLAG-p38β to determine whether arsenite activates p38β. 293T cells expressing FLAG-p38β were treated with arsenite and the phosphorylation of FLAG-p38β was detected by Western blotting with anti-phospho-p38 antibodies (Fig. 3A). Arsenite-induced p38β phosphorylation and rapamycin had no effect on arsenite-induced p38β activation, suggesting that p38β activation is upstream of mTORC1. Ectopically expressing constitutively active MKK6 (MKK6b(E)) in cells can also lead to activation of all p38 group members (40). We tested whether expression of MKK6b(E) in MEF cells can mimic arsenite treatment and found a low level increase in rapamycin-sensitive S6K1 phosphorylation (Fig. 3B), and found this phosphorylation to be p38β- but not PRAK-dependent (Fig. 3C). It appears that overactivation of p38β alone is sufficient to increase mTORC1 activity, but the increase of mTORC1 activity is limited. This data are consistent with the results showing that p38β deletion or SB203580 treatment did not completely inhibit arsenite-induced mTORC1 activation (Fig. 1).
FIGURE 3.
Expression of constitutively active MKK6 (MKK6b(E)) mutant partially mimics arsenite-induced mTORC1 activation. A, arsenite induces p38β phosphorylation. 293T cells transfected with FLAG-p38β expression vector were treated with 1 mm arsenite for 30 min. Phosphorylation of FLAG-p38β was detected by immunoblotting. B, expression of MKK6b(E) induces S6K1 phosphorylation. HEK293 cells were infected by MKK6b(E)-expressing lentivirus. After 36 h, infected cells were treated with 20 nm rapamycin for 30 min and subjected to immunoblotting with the indicated antibodies. C, p38β but not PRAK is required for MKK6b(E)-induced S6K1 phosphorylation. WT, p38β−/−, and PRAK−/− MEF cells were infected by MKK6b(E)-expressing lentivirus. After 48 h, the cells were subjected to immunoblotting with the indicated antibodies.
Arsenite-induced Activation of mTORC1 Is Independent from TSC2 and AMPK
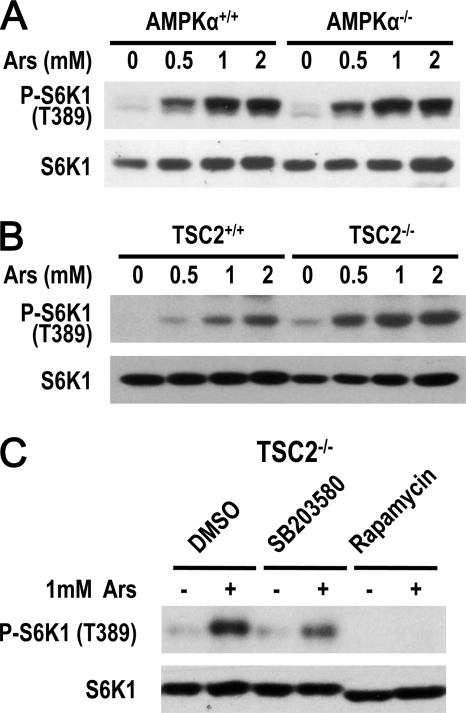
Because AMPK and TSC2 are important regulators of mTORC1, we determined their role in arsenite-induced mTORC1 activation. To examine whether AMPK is involved in arsenite-induced activation of mTORC1, we used AMPKα1/α2 double knock-out MEFs, and found that arsenite-induced mTORC1 activation was not affected by AMPK deletion (Fig. 4A). We then used TSC2−/− cells to determine the involvement of TSC2 and found that arsenite-induced mTORC1 activation was not affected by TSC2 deletion (Fig. 4B). As anticipated, arsenite-induced mTORC1 activity can be inhibited in TSC2−/− cells by SB203580 and rapamycin (Fig. 4C). Thus, arsenite-induced mTORC1 activation is independent from AMPK and TSC2.
FIGURE 4.
AMPK and TSC2 are not involved in arsenite-induced mTORC1 activation. A, AMPKα1/α2 double deletion and control wild-type cells were treated with different concentrations of arsenite for 30 min. Cell lysates were immunoblotted with anti-phospho-S6K1(T389) and S6K1 antibodies. B, the same as in A, except TSC2+/+ and TSC2−/− MEF cells were used. C, TSC2−/− cells were preincubated with SB203580, rapamycin, or dimethyl sulfoxide (DMSO) for 30 min and then treated with 1 mm arsenite for 30 min. Cell lysates were immunoblotted with anti-phospho-S6K1(T389) and S6K1 antibodies.
Arsenite Induces Interaction between p38β and Raptor
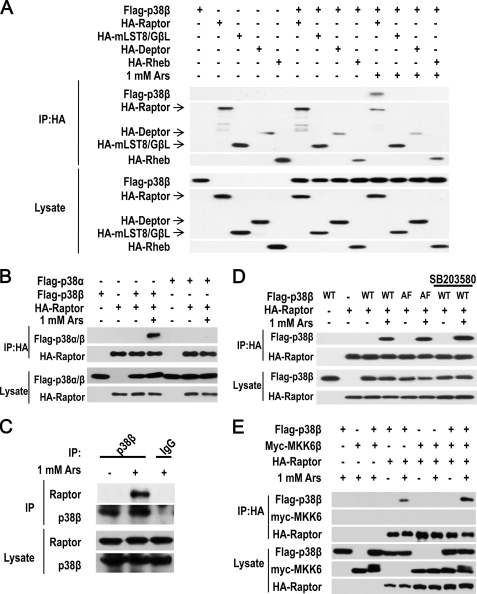
To elucidate the pathway of p38β-mediated mTORC1 activation, we coexpressed p38β with different components of the mTORC1 complex in 293T cells and treated the cells with or without arsenite. We immunoprecipitated HA-tagged Raptor, mLST8/GβL, Deptor, and Rheb, finding that p38β did not interact with any of these proteins in the absence of arsenite treatment, but interacted with Raptor but not mLST8/GβL, Deptor, or Rheb in the presence of arsenite stimulation (Fig. 5A). To test whether the interaction between p38β and Raptor is p38β-specific, we compared p38β with p38α in their interaction with Raptor and found that only p38β can interact with Raptor in arsenite-treated cells (Fig. 5B). We then checked whether arsenite treatment induces interaction between endogenous p38β and Raptor. 293T cells were treated with or without 1 mm arsenite for 30 min and p38β was immunoprecipitated with anti-p38β antibodies. We detected Raptor in the immunoprecipitates from arsenite-treated cells, but not in the immunoprecipitates from control non-treated cells (Fig. 5C). The specificity of p38β immunoprecipitation was demonstrated by the absence p38β and Raptor in the immunoprecipitates using control IgG (Fig. 5C). Because arsenite induces p38β activation, we examined whether the activity of p38β is required for its interaction with Raptor. We coexpressed Raptor with wild-type p38β or p38β inactive mutant (p38β(AF)) in which the regulatory phosphorylation sites TGY were mutated to AGF, and treated the cells with or without arsenite. Both p38β and p38β(AF) could co-immunoprecipitate with Raptor after the cells were treated with arsenite (Fig. 5D). Consistently, treatment of the cells with SB203580 also did not affect arsenite-induced interaction between Raptor and p38β (Fig. 5D). We also examined whether the MKK6-p38β module has any role in arsenite-induced p38β-Raptor interaction and found that MKK6 was not associated with the p38β-Raptor complex (Fig. 5E). Our data indicated that arsenite-induced interaction between p38β and Raptor does not require p38β activating phosphorylation and p38β activity.
FIGURE 5.
Arsenite induces interaction between p38β and Raptor. A, FLAG-p38β expression vector was cotransfected with the expression vector of HA-Raptor, HA-mLST8/GβL, HA-Deptor, or HA-Rheb as indicated. 36 h later, the cells were treated with or without 1 mm arsenite for 30 min. The cells were harvested in 0.3% CHAPS lysis buffer and then immunoprecipitated (IP) with anti-HA antibodies. The immunoprecipitates and lysates were immunoblotted with anti-HA or anti-FLAG antibodies. B, p38β but not p38α can interact with Raptor upon arsenite treatment. 293T cells cotransfected with expressing vectors of FLAG-p38β, FLAG-p38α, and HA-Raptor in different combinations as indicated. The immunoprecipitation (IP) and immunoblotting were performed as described in A. C, endogenous p38β interacts with Raptor. 293T cells were treated with arsenite as indicated and harvested in 0.3% CHAPS lysis buffer. Cell lysates were immunoprecipitated with anti-p38β antibodies or control IgG. The immunoprecipitates and lysates were immunoblotted with anti-Raptor and anti-p38β antibodies. D, the interaction between p38β and Raptor is not dependent on p38β kinase activity. 293T cells were cotransfected with expression vectors of FLAG-p38β(WT), FLAG-p38β(AF), and HA-Raptor as indicated. 12 h later, 20 μm SB203580 was added into the culture medium as indicated. At 24 h after transfection, the cells were harvested and analyzed as described in A. E, MKK6 is not in p38β-Raptor complex. 293T cells were cotransfected with expression vectors of FLAG-p38β, Myc-MKK6b, and HA-Raptor as indicated. At 36 h after transfection, the cells were harvested and analyzed as described in A.
p38β Phosphorylates Raptor
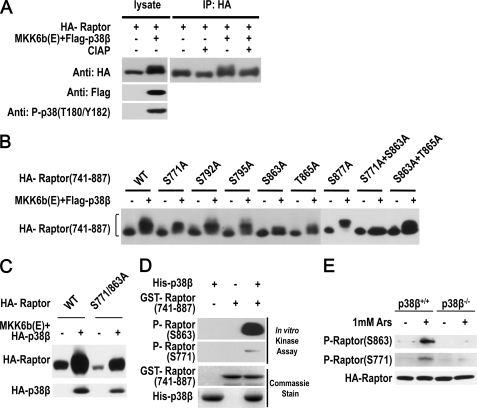
The inducible interaction between p38β and Raptor suggests that Raptor is a target of p38β in arsenite-treated cells. To explore the effect of p38β on Raptor, we coexpressed Raptor with p38β and included MKK6b(E) in some samples to activate p38β in 293T cells. We found that activation of p38β by coexpressed MKK6b(E) led to a band shift of Raptor, and this shift is at least partially due to phosphorylation, because treatment of the sample with CIAP reduced the band shift (Fig. 6A). To identify the phosphorylation site(s) we created a number of deletion mutants of Raptor and determined which fragment could be modified by MKK6b(E)-activated p38β. Western blotting analysis of Raptor mutants that coexpressed with MKK6b(E) and p38β in 293T cells revealed that the band shift of Raptor is primarily caused by a fragment of 741–887 amino acids (supplemental Fig. S4, A–C). The band shift of this fragment is due to phosphorylation, because treatment of immunoprecipitated Raptor with CIAP eliminated the band shift (supplemental Fig. S4D).
FIGURE 6.
p38β phosphorylates Raptor on Ser863 and Ser771. A, p38β causes a mobility shift of Raptor. 293T cells were cotransfected with expressing vector of FLAG-p38β, HA-Raptor, and MKK6b(E) as indicated. After 36 h, the cells were harvested in RIPA lysis buffer, immunoprecipitated with anti-HA beads, and incubated with or without CIAP before being subjected to immunoblotting with anti-HA antibodies. To show the shift, an 8% SDS-PAGE was used. B, Ser863 and Ser771 are required for p38β-induced mobility shift of the 741–887 Raptor fragment. 293T cells were cotransfected with expressing vector of FLAG-p38β, MKK6b(E), and HA-Raptor-(741–887) fragment with point mutations as indicated. Cell lysates were immunoblotted with anti-HA antibodies. C, Ser863 and Ser771 constitute the major phosphorylation sites that are responsible for p38β-induced mobility shift of full-length Raptor. 293T cells were cotransfected with expressing vector of FLAG-p38β plus MKK6b(E) with wild-type or S771A/S863A Raptor as indicated. After 36 h, the cells were harvested and subjected to immunoblotting with anti-HA antibodies. D, p38β phosphorylates Raptor on Ser863 and Ser771 in vitro. Bacterial expressed His-p38β and GST-Raptor-(741–887) were incubated as indicated and immunoblotted with antibodies for phospho-Raptor(Ser863) and phospho-Raptor(Ser771). E, arsenite-induced Ser771 and Ser863 phosphorylation of Raptor requires p38β. p38β+/+ and p38β−/− MEF cells were infected with lentiviral vector expressing HA-Raptor. The cells were treated with arsenite for 30 min. Cell lysates were immunoblotted with antibodies for phospho-Raptor(Ser863), phosphor-Raptor(Ser771), and HA.
We mutated each of the serine and threonine sites (41) to alanine in the 741–887 region of Raptor and determined which sites are involved in the phosphorylation-mediated band shift. Converting Ser863 and Ser771 to Ala both slightly reduced the band shift, and simultaneous mutation of Ser863 and Ser771 completely abolished the shift (Fig. 6B). We then checked whether mutation of Ser771 and Ser863 to Ala affected the MKK6b(E)/p38β-mediated shift of full-length Raptor and found that the S771A/S863A mutant has much less band shift in comparison with wild-type Raptor (Fig. 6C). Thus, Ser863 and Ser771 constitute the major phosphorylation sites that are responsible for the p38β-induced band shift of Raptor.
We obtained anti-Ser863 phospho-Raptor and anti-Ser771 phospho-Raptor antibodies. An in vitro kinase reaction using recombinant His-p38β and GST-Raptor-(741–887) was performed and the reaction mixtures were analyzed by immunoblotting using the anti-phospho-Raptor antibodies. Phosphorylation of Ser863 and Ser771 were both detected in the products of the kinase reaction (Fig. 6D). The weak signal of Ser771 phosphorylation is most likely due to the titer of the anti-Ser771 phospho-Raptor being low, and we concentrated anti-Ser771 antibody 40-fold by using an Amicon ultra filter for further studies. We determined that the concentrated antibody can specifically detect Ser771 phospho-Raptor and the anti-Ser863 phospho-Raptor antibody can specifically detect Ser863 phospho-Raptor in arsenite-treated cells expressing HA-Raptor (supplemental Fig. S5A), although the titers of these antibodies are still not high enough to detect endogenous protein. By using these antibodies, we analyzed wild-type and p38β−/− MEF cells that had infected HA-Raptor expressing lentivirus, and found that arsenite treatment induced Ser771 and Ser863 phosphorylation of Raptor in wild-type but not p38β−/− cells (Fig. 6E). The requirement of p38β in arsenite-induced Raptor phosphorylation was also observed when different doses of arsenite were used (supplemental Fig. S5B). Thus, arsenite induces p38β-dependent phosphorylation of Raptor on Ser771 and Ser863.
Arsenite-induced Raptor Ser863 Phosphorylation Is mTORC1 Independent
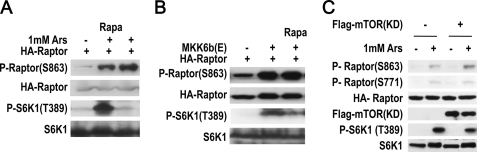
Ser863 phosphorylation of Raptor was previously reported to be executed by mTORC1 during insulin stimulation (31). Here we need to determine whether mTORC1 has any role in arsenite-induced Ser863 phosphorylation of Raptor. To determine whether arsenite-induced phosphorylation of Raptor is mTORC1-dependent, we treated the cells with the mTORC1 inhibitor rapamycin. Rapamycin inhibited arsenite-induced S6K1 phosphorylation, but it did not block arsenite-induced Raptor Ser863 phosphorylation (Fig. 7A). Similar results were obtained in MKK6b(E)-expressing cells (Fig. 7B). We also evaluated whether inhibition of mTOR by overexpressing inactive mutant of mTOR (mTOR(KD)) in 293T cells can affect arsenite-induced phosphorylation of Raptor, and found that overexpression of FLAG-mTOR(KD) inhibited arsenite-induced S6K1 phosphorylation but not Ser863 and Ser771 phosphorylation on Raptor (Fig. 7C). Collectively, these data demonstrated that arsenite-induced Raptor phosphorylation is independent from mTORC1 activity.
FIGURE 7.
Arsenite-induced Raptor Ser863 phosphorylation is mTORC1-independent. A, rapamycin does not inhibit arsenite-induced Raptor Ser863 phosphorylation. 293T cells were transfected with HA-Raptor and treated with 1 mm arsenite for 30 min in the presence or absence of rapamycin (20 nm, for 30 min). Cell lysates were immunoblotted with antibodies as indicated. B, rapamycin does not inhibit MKK6b(E)-induced Raptor Ser863 phosphorylation. 293T cells were cotransfected with an expression vector of HA-Raptor and MKK6b(E) and treated with rapamycin as in A. Cells were harvested and immunoblotted with antibodies as indicated. C, kinase-dead (KD) mutant of mTOR has no effect on arsenite-induced Ser863 and Ser771 phosphorylation of Raptor. 293T cells were transfected with or without expression vectors of FLAG-mTOR(KD) and HA-Raptor as indicated. The cells were treated with or without arsenite for 30 min and the cell lysates were immunoblotted with antibodies for phospho-Raptor(Ser863), phospho-Raptor(Ser771), HA, FLAG, phospho-S6K1(Thr389), and S6K1.
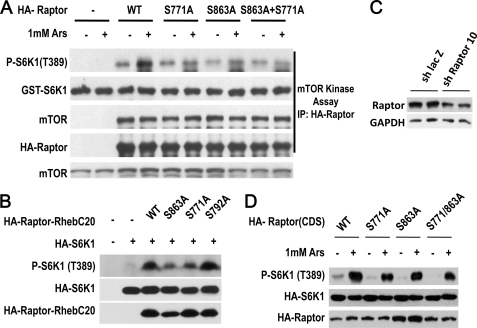
mTOR-mediated Ser863 phosphorylation was shown to enhance mTORC1 activity (31, 32). We examined whether S863A, S771A, and S863A/S771A mutations affect on the function of Raptor in cells treated with arsenite. We expressed wild-type and mutants of HA-Raptor in 293T cells and stimulated the cells with or without arsenite. The cells were lysed with CHAPS buffer, because in this buffer mTOR is still associated with Raptor (38). Raptor was immunoprecipitated with anti-HA antibodies and the Raptor-mTOR complexes were subjected to a kinase assay using recombinant GST-S6K1 as a substrate as described (39). Wild type-Raptor complex isolated from arsenite-treated cells had increased activity in the phosphorylation of S6K1 (Fig. 8A), which reflects the activation of mTOR by arsenite treatment. S771A, S863A, and S863A/S771A mutations all reduced arsenite-induced mTOR activity in the immunocomplex (Fig. 8A).
FIGURE 8.
Phosphorylation of Ser863 and Ser771 on Raptor enhances mTORC1 activation. A, 293T cells were transfected, treated with 1 mm arsenite for 30 min as indicated, and harvested in 0.3% CHAPS lysis buffer. Wild-type (WT), or S863A, S771A, and S863A/S771A mutants of HA-Raptor were immunoprecipitated by anti-HA antibodies and then incubated with bacterial derived recombinant GST-S6K1 in a kinase buffer. Proteins from this assay were immunoblotted with antibodies as indicated. B, mutations of Ser863 or Ser771, but not Ser792, to Ala in a constitutively active Raptor mutant reduce its activity. 293T cells were cotransfected with expression vectors of HA-Raptor-Rheb(C20) WT, S863A, S771A, S792A, and HA-S6K1 as indicated. Cell lysates were immunoblotted with antibodies as indicated. C, 293T cells were transfected with expression vector of short hairpin LacZ or short hairpin Raptor 10 that targets the 3′ UTR of Raptor. The protein level of Raptor at 36 h after transfection were measured by immunoblotting with anti-Raptor antibody. GAPDH was used as control. D, the Raptor knockdown cells shown in C were transfected with expression plasmids containing coding region of wild-type (WT) Raptor, S771A Raptor, Ser863 Raptor, S771A/S863A Raptor. 36 h later, the cells were treated with arsenite for 30 min and the cell lysates were subjected to immunoblotting with antibodies for phospho-S6K1(Thr389) and HA.
Because fusion of Raptor with the C-terminal 20-amino acid portion of Rheb creates a dominant-active Raptor (Raptor-Rheb(C20)), we mutated Ser863 and Ser771 to Ala in this dominant-active Raptor and analyzed the effect of these mutations on the activity of Raptor-Rheb(C20). The S792A mutant was included as a control. Mutation of Ser863 or Ser771, but not Ser792, reduced Raptor-Rheb(C20)-induced increase of S6K1 phosphorylation (Fig. 8B).
Knockdown of Raptor and then reconstituting the cells with wild-type or phosphorylation site mutants of Raptor is an approach to evaluate the effect of the phosphorylation site mutations, and was used in our study. We designed a number of shRNA to target 3′ untranslated region (3-UTR) of Raptor and identified effective shRNAs. As shown in Fig. 8C, short hairpin Raptor 10 effectively reduced Raptor protein in 293T cells. We transfected expression plasmid containing the coding region of wild-type Raptor, S771A Raptor, S863A Raptor, or S771A/S863A Raptor into Raptor knockdown cells, and examined arsenite-induced phosphorylation of S6K1. The cells with the reconstitution of S771A Raptor, S863A Raptor, or S771A/S863A Raptor had a lower level of arsenite-induced phospho-S6K1 in comparison with the cells that were reconstituted with wild-type Raptor (Fig. 8D). Collectively, the data shown in Fig. 8 suggest the role of phosphorylation on Ser863 and Ser771 of Raptor in arsenite-induced activation of mTORC1.
DISCUSSION
Cellular stresses have profound effects on cell growth. One of the mechanisms by which stresses influence cell growth is modulation of mTOR activity. In this report, we demonstrate that a specific p38 group member, p38β, plays an important role in arsenite-induced activation of mTORC1. We show that p38β is activated by arsenite treatment and can directly phosphorylate Raptor on Ser863 and Ser771. Phosphorylation of Raptor enhances activity of mTORC1. The data presented here and in previous publications (33, 35) demonstrate that the p38 pathway can regulate the mTOR pathway at multiple sites to enhance or inhibit the activity of mTORC1 (supplemental Fig. S6). Although not being specifically addressed, p38αα may be able to regulate mTOR (33), for example, through activation of MK2. MK2 can phosphorylate TSC2 to sequester TSC2 at the 14-3-3 site and thus release the inhibition of mTORC1 (33). It was known that MK2 plays a role in anisomycin-induced mTORC1 activation (33), whereas it has no role in arsenite-induced mTORC1 activation (supplemental Fig. S7, A and B). Consistently, arsenite-induced Ser863 phosphorylation of Raptor was not affected by MK2 deletion (supplemental Fig. S7C). We recently showed that p38β plays an essential role in the energy depletion-induced inactivation of mTORC1 (35). The specificity of this regulation is conferred by the downstream kinase PRAK (supplemental Fig. S3A). PRAK is essential for p38β-mediated inactivation of mTORC1 in response to energy depletion, but has no role in arsenite-induced mTORC1 activation and p38β-dependent phosphorylation of Raptor (supplemental Fig. S3, B and C). Distinct from MK2, deletion of PRAK had no effect on anisomycin-induced mTORC1 activation (supplemental Fig. S3D). It is clear that the p38 pathway can regulate mTOR in many different ways, and the mechanisms used are stimuli-dependent (supplemental Fig. S6).
It is very challenging to address how p38β has different roles in regulating mTORC1 in response to different cellular stresses. We showed that PRAK and Raptor are substrates of p38β in response to energy starvation and arsenite, respectively. It is unknown how p38ββ selects different substrates in response to different stimuli. It is clear that formation of different signaling complexes plays a role in determining the divergent functions of p38β in regulating mTOR upon different stresses, as we observed that interaction between p38β and Raptor only can be induced by arsenite treatment but not 2-deoxyglucose or anisomycin treatments (supplemental Fig. S8). It is possible that arsenite and energy starvation activate different populations of p38β located in either different protein complexes or subcellular locations and thus can access different substrates. It is also possible that the time and levels of p38β activation play a role in determining the different complex formations of p38β. It is well accepted that a cell can achieve temporal, spatial, and stress-specific regulation in response to different stresses, and the paradoxical role of p38β in regulating mTOR in response to different stresses could be the result from these complicated activation mechanisms.
Raptor is believed to be a key mTORC1 scaffolding protein that binds mTOR substrates via the TOR signaling (TOS) motif, facilitating their phosphorylation by mTOR. A number of recent studies have shown phosphorylation of Raptor on various sites in the up- or down-regulation of mTORC1 activity. Phosphorylation at Ser722 and Ser792 by AMPK create 14-3-3 binding sites and inhibit mTORC1 activity in response to energy stress (41); phosphorylation of Raptor by Rsk at Ser721 and by mTOR at Ser863 have been shown to enhance mTORC1 activity (31, 32, 42); phosphorylation of Raptor on Ser696 and Thr706 by Cdc2 was observed during mitosis (36). We show in this report that arsenite induces a p38β-dependent Raptor phosphorylation on Ser863 and Ser771, and phosphorylation of Raptor on these sites enhances mTORC1 activity. Our data and others suggested that Ser863 on Raptor can be phosphorylated by more than one kinase, and we observed that arsenite induces phosphorylation of Ser863 and Ser771 simultaneously, whereas anisomycin induces only Ser863 phosphorylation (supplemental Fig. S8). The mechanism of augmentation or inhibition of mTOR activity by Raptor phosphorylation remains elusive at present; however, we can speculate that phosphorylation may change the conformation of Raptor, which affects its interaction with mTOR substrates and thus positively or negatively influences the phosphorylation of mTOR substrates by mTOR.
The complexity of mTOR regulation in response to stresses provides an example of different stress pathways being integrated within a cell, and cells engaging in different strategies to use the same protein to differentially respond to various stresses. It is clear that new technologies and strategies need to be developed to study the spatial dependence of signaling transduction.
Supplementary Material
Acknowledgments
We thank Dr. Huiping Jiang (Boehringer Ingelheim Pharmaceuticals, Inc.) for providing p38 conditional knock-out mice, Drs. Benoit Viollet and Keith R. Laderoute for AMPKα1/α2 double knock-out cells, and Dr. Matthias Gaestel for MK2−/− cells.
This work was supported, in whole or in part, by National Institutes of Health Grants National Institutes of Health AI41637 and AI68896, and NSF China Grant 30830092, 30921005, 30828219, and 31070688, Fujian Provincial Department of Science & Technology Grant 2010J01228, and Science Planning Program of Fujian Province Grant 2009J1010, 973 program 2009CB522200.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- mTOR
- mammalian target of rapamycin
- S6K
- p70 S6 kinase
- 4EBP1
- eIF4E-binding protein 1
- MEF
- mouse embryonic fibroblast
- AMPK
- AMP-activated protein kinase
- CIAP
- calf intestinal alkaline phosphatase.
REFERENCES
- 1. Ono K., Han J. (2000) Cell. Signal. 12, 1–13 [DOI] [PubMed] [Google Scholar]
- 2. Nebreda A. R., Porras A. (2000) Trends Biochem. Sci. 25, 257–260 [DOI] [PubMed] [Google Scholar]
- 3. Cuenda A., Rousseau S. (2007) Biochim. Biophys. Acta 1773, 1358–1375 [DOI] [PubMed] [Google Scholar]
- 4. Han J., Lee J. D., Bibbs L., Ulevitch R. J. (1994) Science 265, 808–811 [DOI] [PubMed] [Google Scholar]
- 5. Li Z., Jiang Y., Ulevitch R. J., Han J. (1996) Biochem. Biophys. Res. Commun. 228, 334–340 [DOI] [PubMed] [Google Scholar]
- 6. Jiang Y., Gram H., Zhao M., New L., Gu J., Feng L., Di Padova F., Ulevitch R. J., Han J. (1997) J. Biol. Chem. 272, 30122–30128 [DOI] [PubMed] [Google Scholar]
- 7. Jiang Y., Chen C., Li Z., Guo W., Gegner J. A., Lin S., Han J. (1996) J. Biol. Chem. 271, 17920–17926 [DOI] [PubMed] [Google Scholar]
- 8. Zarubin T., Han J. (2005) Cell Res. 15, 11–18 [DOI] [PubMed] [Google Scholar]
- 9. Rincón M., Davis R. J. (2009) Immunol. Rev. 228, 212–224 [DOI] [PubMed] [Google Scholar]
- 10. Dong C., Davis R. J., Flavell R. A. (2002) Annu. Rev. Immunol. 20, 55–72 [DOI] [PubMed] [Google Scholar]
- 11. English J. M., Cobb M. H. (2002) Trends Pharmacol. Sci. 23, 40–45 [DOI] [PubMed] [Google Scholar]
- 12. Roux P. P., Blenis J. (2004) Microbiol. Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaestel M. (2008) Front. Biosci. 13, 6050–6059 [DOI] [PubMed] [Google Scholar]
- 14. Sengupta S., Peterson T. R., Sabatini D. M. (2010) Mol. Cell 40, 310–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 16. Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. (1995) Nature 377, 441–446 [DOI] [PubMed] [Google Scholar]
- 17. Fingar D. C., Salama S., Tsou C., Harlow E., Blenis J. (2002) Genes Dev. 16, 1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 19. Ma X. M., Blenis J. (2009) Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 20. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 21. Reiling J. H., Sabatini D. M. (2006) Oncogene 25, 6373–6383 [DOI] [PubMed] [Google Scholar]
- 22. Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 23. Potter C. J., Pedraza L. G., Xu T. (2002) Nat. Cell Biol. 4, 658–665 [DOI] [PubMed] [Google Scholar]
- 24. Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005) Cell 121, 179–193 [DOI] [PubMed] [Google Scholar]
- 25. Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13489–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoki K., Zhu T., Guan K. L. (2003) Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 27. Inoki K., Li Y., Xu T., Guan K. L. (2003) Genes Dev. 17, 1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garami A., Zwartkruis F. J., Nobukuni T., Joaquin M., Roccio M., Stocker H., Kozma S. C., Hafen E., Bos J. L., Thomas G. (2003) Mol. Cell 11, 1457–1466 [DOI] [PubMed] [Google Scholar]
- 29. Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008) Nat. Cell Biol. 10, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008) Science 320, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L., Lawrence J. C., Jr., Sturgill T. W., Harris T. E. (2009) J. Biol. Chem. 284, 14693–14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foster K. G., Acosta-Jaquez H. A., Romeo Y., Ekim B., Soliman G. A., Carriere A., Roux P. P., Ballif B. A., Fingar D. C. (2010) J. Biol. Chem. 285, 80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y., Inoki K., Vacratsis P., Guan K. L. (2003) J. Biol. Chem. 278, 13663–13671 [DOI] [PubMed] [Google Scholar]
- 34. Cully M., Genevet A., Warne P., Treins C., Liu T., Bastien J., Baum B., Tapon N., Leevers S. J., Downward J. (2010) Mol. Cell Biol. 30, 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng M., Wang Y. H., Wu X. N., Wu S. Q., Lu B. J., Dong M. Q., Zhang H., Sun P., Lin S. C., Guan K. L., Han J. (2011) Nat. Cell Biol. 13, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gwinn D. M., Asara J. M., Shaw R. J. (2010) PLoS One 5, e9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang H., Cicchetti G., Onda H., Koon H. B., Asrican K., Bajraszewski N., Vazquez F., Carpenter C. L., Kwiatkowski D. J. (2003) J. Clin. Invest. 112, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 39. Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007) Mol. Cell 25, 903–915 [DOI] [PubMed] [Google Scholar]
- 40. Huang S., Jiang Y., Li Z., Nishida E., Mathias P., Lin S., Ulevitch R. J., Nemerow G. R., Han J. (1997) Immunity 6, 739–749 [DOI] [PubMed] [Google Scholar]
- 41. Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carrière A., Cargnello M., Julien L. A., Gao H., Bonneil E., Thibault P., Roux P. P. (2008) Curr. Biol. 18, 1269–1277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.