Abstract
Background
Anal cancer remains rare (incidence of ∼1.5 per 100,000 women annually) but rates are increasing in many countries. Human papillomavirus-16 (HPV16) infection causes most cases. We evaluated vaccine efficacy (VE) of an ASO4-adjuvanted HPV16/18 vaccine against anal HPV16/18 infection.
Methods
In a randomized double-blind controlled trial designed to evaluate VE against persistent cervical HPV16/18 infections and associated precancerous lesions in Costa Rica, 4210 healthy women underwent anal specimen collection (4224 of 5968= 70.8% of eligible women) at the final blinded study visit 4 years after vaccination to evaluate anal HPV16/18 VE. Cervical HPV16/18 VE among the same women at the same visit was calculated as a comparator. For this ancillary work, analyses were conducted in a restricted cohort of women both cervical HPV16/18 DNA negative and HPV 16/18 seronegative prior at enrollment (N=1989), and in the full cohort (all women with an anal specimen).
Findings
In the restricted cohort, VE against prevalent HPV16/18 anal infection measured one-time, four-years post-vaccination was 83.6% (95%CI 66.7% to 92.8%), which was comparable to cervical HPV16/18 VE (87.9%, 95%CI 77.4% to 94.0%). In the full cohort, HPV16/18 VE was statistically lower at the anus (62.0%, 95%CI 47.1% to 73.1%) compared to the cervix (76.4%, 95%CI 67.0% to 83.5%) (p for anatomic-site interaction =0.03). Significant and comparable VE estimates against a composite endpoint of HPV31/33/45 (i.e.: cross-protection) was observed at the anus and cervix.
Interpretation
The ASO4-adjuvanted vaccine affords strong protection against anal HPV, particularly among women more likely to be HPV naïve at vaccination. Funding. The Costa Rica HPV Vaccine Trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health, and conducted with support from the Ministry of Health of Costa Rica. Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK), under a Clinical Trials Agreement with the NCI.
Keywords: Human papillomavirus vaccine, HPV, anal cancer
Introduction
Anal cancer remains rare, with annual age-standardized incidence rates in the general population of ∼1.5/100,000 for women (1); but, rates have been approximately doubled over the past several decades in many countries, including the US and several European nations (2-5). The absolute burden of anal cancer is higher for women than men (3-5), yet, anal cancer disproportionately affects HIV-positive individuals and men who have sex with men (MSM), even if they are HIV-negative (6, 7).
Human papillomavirus (HPV) causes the majority of anal cancers, with an estimated 75 to 80% of HPV-associated anal cancers caused by HPV type 16 or 18 (8; 9). For other HPV-associated extra-cervical cancers, including cancers of the oropharynx, vagina, vulva, and penis (9), variable proportions are caused by HPV infection but, where HPV is involved, HPV16 is the vastly predominant type implicated in the cancer (range 75% to 95% of the HPV-associated cancers) (9).
Two vaccines prevent HPV types 16 and 18 infections: the bivalent HPV16/18 vaccine (Cervarix®*, GlaxoSmithKline Biologicals)(10) and the quadrivalent HPV 6/11/16/18 vaccine (Gardasil™, Merck and Co, Inc,) (11). Among women, vaccine efficacy (VE) against cervical (both vaccines) (10, 11) and vaginal and vulvar (quadrivalent only) (12) HPV infections and related diseases has been demonstrated. For men, VE against penile, perianal, perineal HPV infections as a combined endpoint has been demonstrated (quadrivalent only) (13). Direct evidence for efficacy against anal HPV infection and disease is lacking with the exception of one unpublished trial of the quadrivalent vaccine among ∼600 MSM (14). The bivalent vaccine has not been evaluated at extra-cervical sites.
We conducted a study to evaluate efficacy of the bivalent HPV vaccine to reduce anal HPV infection, nested in a community-based randomized cervical vaccine efficacy trial of adult young women.
Methods
Women included in the present evaluation are participants in a randomized clinical trial initially designed to evaluate the efficacy of a bivalent HPV vaccine against persistent type-specific HPV16/18 infection and associated precancerous lesions at the cervix (15, 16). The study enrolled women residing in Guanacaste and selected areas of Puntarenas, Costa Rica, identified via a census, between 2004-2005. Main eligibility requirements were: age 18-25 years, planned residence in the area for the 6 months following enrollment, in good general health, neither pregnant nor breastfeeding, and willing to provide written informed consent. Women were excluded if they had preexisting medical conditions that required chronic treatment or caused immunosuppression, had a history of hepatitis A or previous vaccination against it, or were unwilling to use contraception during the vaccination period. 7,466 women were enrolled; they represented 59.1% of eligible and 30.5% of all (eligible plus ineligible) women from the census (15). The trial was approved by the human subjects review committees of the United States (US) National Cancer Institute and INCIENSA (Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud) in Costa Rica.
At the enrollment visit (following informed consent and risk-factor interview), a pelvic examination was performed on sexually experienced women, exfoliated cervical cells were collected in PreservCyt medium (Cytyc Corp, now Hologic, Marlborough, Massachusetts) for Thinprep (Cytyc Corp) cytologic evaluation and HPV DNA testing, and blood was collected for HPV16/18 serology. Next, women were randomized in a double-blinded fashion to receive the HPV vaccine (Cervarix, GlaxoSmithKline Biologicals) or a “Control” hepatitis A vaccine (a modified preparation of Havrix, GSK Biologicals). Both vaccines were formulated in three 0.5 ml doses with identical packaging. HPV and control vaccines were assigned random vaccine identification numbers at the time of labeling by the manufacturer. Study personnel at the Costa Rican study site randomized participants by assigning each eligible participant to the next available sequential vaccine identification number. The protocol called for a dose of vaccine at each of 3 study visits: at enrollment, 1 month following the initial dose (allowable range, 21-120 days), and 6 months following the initial dose (allowable range, 121-300 days). Women not attending their visits in the allowable range for the 2nd dose (21-120 days after enrollment) remained eligible for the final dose; women who missed the window for the final dose (121-300 days after enrollment) did not receive that dose (15).
At annual follow-up visits, clinicians collected from sexually active women exfoliated cervical cells (same method as above) for cytologic evaluation and HPV DNA testing Women with low-grade cytologic abnormalities were evaluated every six-months until 3 consecutive normal cytologic results returned them to yearly follow-up. Women with cervical high-grade disease or persistent low-grade abnormalities were referred to cervical colposcopy for evaluation and treatment if needed.
Sampling of the anus was introduced at the four-year study visit, the final blinded study visit of the trial. At this study visit, a questionnaire was administered that included questions on anal sexual behaviors; while some under-reporting of stigmatized behaviors such as anal sex is expected, we don't anticipate differential reporting by vaccination status that would bias our efficacy estimates.
The anal specimen was collected prior to the pelvic exam among sexually active women (defined by vaginal intercourse) by inserting a dry swab 3-4 cm into the anal canal, rotating one time, and then removing the swab while rotation continued using gentle pressure against the wall of the anal canal. The swab was placed in 1ml of PreservCyt and frozen immediately in liquid nitrogen. While the predictive value of one-time detection of anal HPV16 or 18 likely for anal precancer or cancer is likely very low, and there are not standard clinical recommendations for need of follow-up in such cases, a subset of women with anal HPV16/18 infection will be monitored during the long-term follow-up phase of the trial. Most women will be followed up to 10 years since initial vaccination; as part of this effort, long-term type-specific anal HPV persistence and related disease will be monitored, and clinical management will be provided if necessary.
Anal and cervical samples were tested for HPV DNA using the SPF10 PCR primer system and a DNA enzyme immunoassay detection of amplimers (DEIA; DDL Diagnostic Laboratory [Voorburg, the Netherlands]); if positive, genotyping was conducted using the line probe assay (LiPA25) (SPF10PCR/LiPA25 HPV genotyping assay system, version 1, Labo Bio-medical Products, Rijswijk, the Netherlands) (17). LiPA25 detects 25 HPV genotypes, including carcinogenic (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68/73) and non-carcinogenic (6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74) types. To ensure that HPV16 and HPV18 infections were not missed, all positive specimens on SPF10 PCR/DEIA that were negative for HPV16 or HPV18 by LiPA25 were also tested using HPV16 or 18 type-specific primers (18, 19).
Serum collected at enrollment was used to determine HPV16 and HPV18 serological status using a VLP-based direct ELISA, a standard measure that measures polyclonal antibodies (GlaxoSmithKline Biologicals, Rixensart, Belgium), as described previously (20, 21). Antibody results were dichotomized using standard cutoff points calculated from antibody titer values 3 SDs above the geometric mean titers taken from a group of HPV-negative individuals (20). Cut points were optical density of at least 8 EU/mL for anti-HPV16 and at least 7 EU/mL for anti-HPV18 (20, 21).
Statistical Analysis
Characteristics between women who accepted and declined the anal specimen collection were compared using the chi-squared test for categorical variables. Among women who accepted anal specimen collection, general characteristics from both the enrollment and 4-year post-vaccination visits were compared between women in the HPV and control arms; this test shows that despite attrition over time, the two analysis groups remain roughly similar as per the randomization. Median follow-up time from enrollment was calculated in months overall and compared by arm using the Kruskal-Wallis test.
Prevalence of anal HPV 16 or 18 infections approximately four years after vaccination was the primary endpoint (defined as detection of either HPV16 or HPV18 or both at the four year study visit); cervical HPV 16 or 18 infections among the same women at the same time point were evaluated for comparison purposes.
The full analysis cohort (presented in the CONSORT Figure) included all women who had anal specimens collected and had HPV results available (“full cohort”); thus, no exclusions were based on HPV DNA positivity, HPV serostatus, or number of vaccine doses received, in this cohort. The “restricted cohort” included women from the full cohort with no evidence of prevalent cervical HPV16/18 infection or HPV16/18 antibodies prior to vaccination, who received three doses of the HPV or control vaccine. By restricting based on cervical infections which are correlated with concomitant anal infections (e.g.: in our population, type-specific HPV16 agreement at the anus and the cervix measured at the four-year study visit was 30.5% (47 out of 154) percent positive agreement and kappa of 0.44), we intended to remove women from this secondary analytic cohort who may have had past or prevalent anal HPV infection (22), since a pre-vaccination anal specimen was not collected to allow for this exclusion based directly on anal HPV detection. Specifically, to compose the restricted cohort, women were excluded from the full cohort if they met any of the following criteria: 1) cervical HPV 16 or 18 DNA positive at enrollment (or missing results), 2) HPV 16 or 18 seropositive at enrollment (or missing results), 3) biopsied for possible cervical intraepithelial neoplasia (CIN) or treated by LEEP, following a positive screening test during the vaccination phase (e.g.: until the 6-month study visit, which could functionally occur 4-10 months after enrollment), or 4) recipients of fewer than three doses of either vaccine.
figure. CIN2+: cervical intraepithelial neoplasia grade 2 or higher.
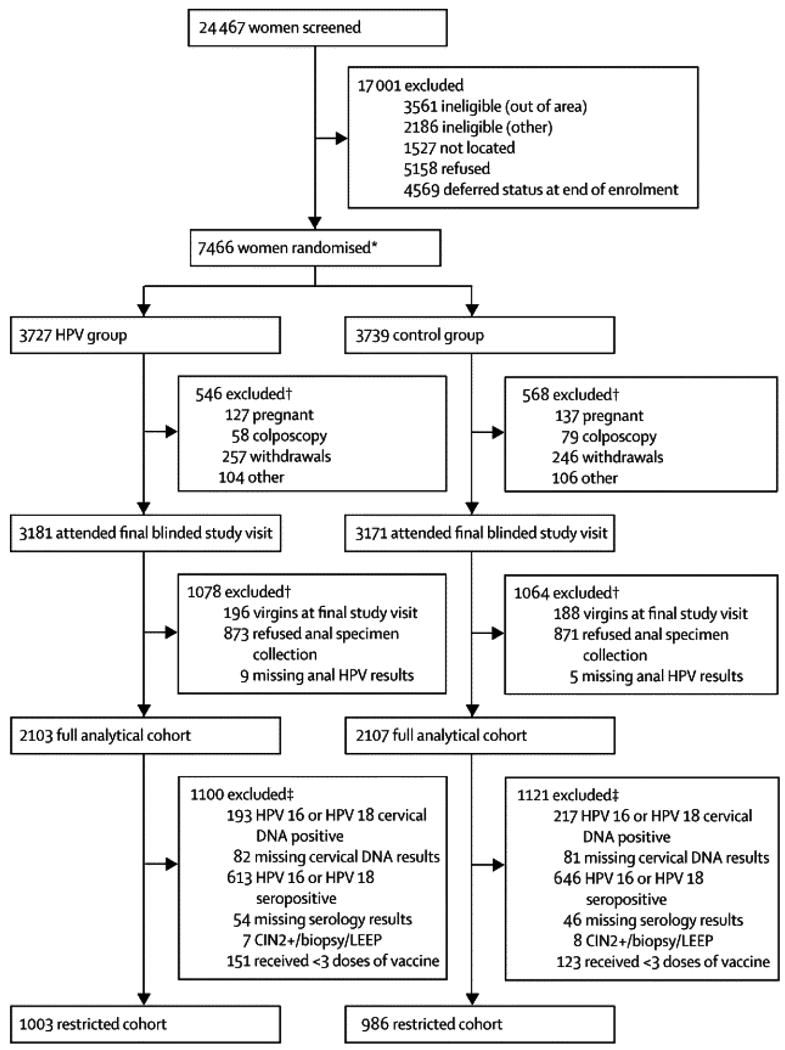
1Four women received discordant vaccines (one woman was enrolled twice and received three doses of each vaccine and three women received two doses of one vaccine and one dose of the other vaccine). For the purpose of this analysis, the women were assigned to the arm for which the first dose was administered.
2 Used data collected at the four-year study visit when anal specimen collection occurred to create the full analytic cohort.
3 Used data collected at enrollment (cervical HPV DNA and serology status) and throughout the vaccination phase (CIN2+/biopsy/LEEP and fewer than three doses) to exclude women from the restricted cohort.
For each arm, the prevalences of anal and cervical HPV16/18 combined and separately measured four-years post-vaccination were expressed as the number of infected women per 100 women vaccinated (stratified by HPV vs. control vaccine); asymptotic confidence intervals (95%CI) were estimated except when cells had less than five events, in which case exact confidence limits were reported. The complement of the ratios of the prevalence for the HPV and control arms comprised the VE estimates. In this woman-level analysis, each woman could only contribute once to the numerator and denominator, even if multiple HPV types were detected. Exact confidence intervals (23) for vaccine efficacy were calculated based on the binomial distribution of the number of events in the HPV arm among the total number of events in the HPV and control arms (24). Anal and cervical VE estimates were calculated and compared by including an arm*anatomical site interaction variable in a GEE model (25) and evaluating whether the beta coefficient for the interaction variable varied significantly from 0.
In our pre-specified plan, the main objective of our analysis was to evaluate VE against anal HPV16/18 infection four years following enrollment and administration of the first vaccine dose (regardless of vaccine type); cervical VE in the exact same cohorts was estimated as a comparator. Because of evidence for cross-protection in cervical VE studies (10, 11), anal (and cervical) VE against a pre-specified composite endpoint of HPV31/33/45, and then individually by type, was evaluated. In the restricted cohort, women who were HPV31, 33, or 45 DNA positive at enrollment were excluded from each respective analysis; no serologic restrictions were made because serologic testing for these HPV types was not conducted. Anal and cervical VE against all other carcinogenic types (after removing HPV16/18/31/33/45) was evaluated in the full cohort.
HPV16/18 VE was estimated by self-reported anal sex (yes/no) assessed by questionnaire at the study visit when anal specimen collection occurred, in the full and restricted cohorts.
This trial is registered at clinicaltrials.gov, identifier: NCT00128661.
Role of the Funding Source
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health, and conducted with support from the Ministry of Health of Costa Rica. Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK), under a Clinical Trials Agreement with the NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. Drs Schiller and Lowy report that they are named inventors on US government–owned HPV vaccine patents that are licensed to GSK and Merck and for which the NCI receives licensing fees. Drs Schiller and Lowy are entitled to limited royalties as specified by federal law. No other financial disclosures were reported. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The NCI and Costa Rica investigators make final editorial decisions on this and subsequent publications; GSK has the right to review and comment.
At the time of this analysis, fieldwork was on-going and individual information remained blinded. Thus, analyses were conducted by an external group, Information Management Systems (by Sabrina Chen; Rockville, MD), under the direction of the investigators who remained masked to individual random assignments. SAS 9.2 TS2M3 was used for analysis.
Results
Of the 7466 women randomized (CONSORT), 6352 attended the four-year study visit (3181 HPV; 3171 Control). 1744 (873 HPV; 871 Control) women refused anal specimen collection and virgins (n=384) were not eligible; the acceptance rate of anal specimen collection among eligible women was 70.8% (4224 out of 5968). After excluding 14 inadequate anal specimens, the full analytic cohort comprised 4210 women (2103 HPV; 2107 Control). Median follow-up time was 48.8 months (4.1 years) and was similar between arms (HPV 48.9 months [range 39.5–74.5 months]; Control 48.8 months [range 38.7-74.5 months]; p=0.9). The restricted cohort of women who were negative for cervical HPV16/18 DNA and serology at enrollment included 1989 women (1003 HPV; 986 Control).
Percentages of women who accepted specimen collection were the same in both arms (70.8% for both HPV [2112 out of 2985] and Control [2112 out of 2983]). Women who provided an anal specimen were older (46.6% [1970/4224] vs. 39.9% [695/1744] in the older age category at enrollment [22 to 25 yrs], p<0.001), more likely to have cervical HPV16 positivity at enrollment (7.2% [305/4224] vs. 5.2% [90/1744], p<0.001), more likely to have 4+ lifetime sexual partners (38.0% [1604/4224] vs. 31.3% [546/1744], p<0.001), and more likely to report anal sex (22.3% [944/4224] vs. 8.2% [143/1744], p<0.001) than women who declined.
Distributions of pre- and post-randomization characteristics were similar in the HPV and control arms, including age at entry, enrollment cervical cytology, cervical HPV DNA positivity, and HPV seropositivity and self-reported anal sex (Table 1).
Table 1. Balance by vaccine arm on selected characteristics for the full analytic cohort.
| HPV (N=2103) N (%) | Control (N=2107) N (%) | p value | |
|---|---|---|---|
| Age at entry1,y | |||
|
| |||
| 18-19 | 595 (28.3%) | 639 (30.3%) | 0.3 |
| 20-21 | 516 (24.5%) | 496 (23.5%) | |
| 22-23 | 511 (24.3%) | 524 (24.9%) | |
| 24-25 | 481 (22.9%) | 448 (21.3%) | |
|
| |||
| Cervical HPV16/18 DNA status at enrollment2,3 | |||
|
| |||
| Negative | 1646 (89.5%) | 1615 (88.2%) | 0.2 |
| Positive2 | 193 (10.5%) | 217 (11.8%) | |
|
| |||
| Serologic HPV16/18 status at enrollment3 | |||
|
| |||
| Negative | 1246 (61.2%) | 1210 (59.1%) | 0.1 |
| Positive | 791 (38.8%) | 837 (40.9%) | |
|
| |||
| Enrollment Cervical Cytology2 | |||
|
| |||
| Inadequate | 8 (0.4%) | 10 (0.55%) | 0.6 |
| Normal | 1544 (84.0%) | 1557 (85.0%) | |
| HPV+ ASCUS or LSIL | 246 (13.4%) | 233 (12.7%) | |
| HSIL+ | 41 (2.2%) | 32 (1.75%) | |
|
| |||
| Time Since Sexual Debut (measured at enrollment visit)4 | |||
|
| |||
| Virgin at Enrollment | 264 (12.6%) | 275 (13.1%) | 0.95 |
| <2 years | 206 (9.8%) | 211 (10.0%) | |
| 2 to 3 years | 435 (20.7%) | 430 (20.4%) | |
| 4 or more years | 1198 (57.0%) | 1191 (56.5%) | |
|
| |||
| Anal sex5 | |||
|
| |||
| No | 1613 (76.7%) | 1655 (78.5%) | 0.2 |
| Yes | 490 (23.3%) | 452 (21.5%) | |
Full analysis cohort included all women who accepted anal specimen collection.
Two women enrolled at age 17 are included in the 18-19 age group
No cervical specimen was collected for HPV testing or cervical cytology among virgins.
Indicates positive for either or both HPV16 and 18 at enrollment.
The few (N<5) “Unknown” responses were collapsed into the “4 or more years” category.
The few (N<5) “Refused” and “Don't know” responses were collapsed into the “No” category.
In the restricted cohort, VE against HPV16/18 infection detected at the anus four-years following administration of the first vaccine dose was 83.6% (95%CI 66.7% to 92.8%; 8 and 48 events in the HPV and control arms, respectively), which was comparable to the cervical HPV16/18 VE among the same women from specimens collected at the same time point (87.9%, 95%CI 77.4% to 94.0%; 10 and 81 events in the HPV and control arms, respectively).
In the full analytic cohort, VE against HPV16/18 infection detected at the anus four-years post-vaccination was 62.0% (95%CI 47.1% to 73.1%; 47 and 124 events in the HPV and control arms, respectively); the corresponding cervical VE was 76.4% (95%CI 67.0% to 83.5%; 40 and 170 events in the HPV and control arms, respectively) (p for anatomic-site interaction =0.031) (Table 2). VE against anal HPV16 was 68.2% (95%CI 51.4% to 79.7%; 27 and 85 events in the HPV and control arms, respectively) and against anal HPV18 was 55.5% (95%CI 25.2% to 74.2%; 20 and 45 events in the HPV and control arms, respectively); VE against cervical HPV16 was 75.8% (95%CI 63.8% to 84.2%; 28 and 116 events in the HPV and control arms, respectively) and cervical HPV18 was 78.6% (95%CI 62.0% to 88.7%; 13 and 61 events in the HPV and control arms, respectively) (p for anatomic-site interaction: HPV16=0.3; HPV18=0.05).
Table 2. Estimated vaccine efficacy against anal and cervical HPV16 or 18 infections.
| Cohort | Anatomic Site | Arm | # Women | # HPV16/18 Infections | HPV16/18 Prevalence (95%CI) | HPV Vaccine Efficacy (95%CI) | p value for difference in VE by anatomic site |
|---|---|---|---|---|---|---|---|
| Full1 | Anus | HPV | 2103 | 47 | 2.2% (1.7% to 2.9%) | 62.0% (47.1% to 73.1%) | 0.031 |
|
| |||||||
| Control | 2107 | 124 | 5.9% (4.9% to 7.0%) | ||||
|
| |||||||
| Cervix | HPV | 2103 | 40 | 1.9% (1.4% to 2.6%) | 76.4% (67.0% to 83.5%) | ||
|
| |||||||
| Control | 2107 | 170 | 8.1% (7.0% to 9.3%) | ||||
|
| |||||||
| Restricted2 | Anus | HPV | 1003 | 8 | 0.8% (0.4% to 1.5%) | 83.6% (66.7% to 92.8%) | 0.55 |
|
| |||||||
| Control | 986 | 48 | 4.9% (3.7% to 6.3%) | ||||
|
| |||||||
| Cervix | HPV | 1003 | 10 | 1.0% (0.5% to 1.8%) | 87.9% (77.4% to 94.0 %) | ||
|
| |||||||
| Control | 986 | 81 | 8.2% (6.6% to 10.1%) | ||||
Full analysis cohort included all women who accepted anal specimen collection.
Restricted cohort included women from the full cohort with no evidence of prevalent cervical HPV16/18 infection or HPV16/18 antibodies prior to vaccination, who received three doses of the HPV or control vaccine.
In the full cohort, significant and comparable cross-protection against a composite endpoint of infection with HPV31/33/45 was observed at the anus (VE 49.4%, 95%CI 30.3% to 63.6%; 55 and 109 anal events in the HPV and control arms, respectively) and the cervix (VE 45.2%, 95%CI 27.7% to 58.7%; 76 and 139 cervical events in the HPV and control arms, respectively) (p for interaction = 0.65) (Table 3). Individually, significant VE was present against HPV 31 and 45 but not 33 (Supplementary table 1). The results for VE against heterologous HPV types observed in the full cohort were similar to the results in the restricted cohort (Table 2 and Supplementary table 1). No VE was observed for all other carcinogenic HPV types (after excluding 16/18/31/33/45) (full cohort: anal VE -3.2% [95%CI -18.6% to 10.1%], 405 and 393 anal events in the HPV and control arms, respectively; cervical VE 3.9% [95%CI -9.4% to 15.6%], 445 and 464 cervical events in the HPV and control arms, respectively).
Table 3. Estimated vaccine efficacy against anal and cervical HPV 31, 33, or 45 infection (i.e.: cross-protection).
| Cohort | Anatomic Site | Arm | # Women | # HPV 31, 33, or 45 Infection | HPV 31, 33, 45 Prevalence (95%CI) | HPV 31, 33, 45 Vaccine Efficacy (95%CI) | p value for difference in VE by anatomic site |
|---|---|---|---|---|---|---|---|
| Full1 | Anus | HPV | 2103 | 55 | 2.6% (2.0% to 3.4%) | 49.4% (30.3% to 63.6%) | 0.65 |
|
| |||||||
| Control | 2107 | 109 | 5.2% (4.3% to 6.2%) | ||||
|
| |||||||
| Cervix | HPV | 2103 | 76 | 3.6% (2.9% to 4.5%) | 45.2% (27.7% to 58.7%) | ||
|
| |||||||
| Control | 2107 | 139 | 6.6% (5.6% to 7.7%) | ||||
|
| |||||||
| Restricted2 | Anus | HPV | 1629 | 31 | 1.9% (1.3% to 2.7%) | 61.8% (42.8% to 75.0%) | 0.28 |
|
| |||||||
| Control | 1684 | 84 | 5.0% (4.0% to 6.1%) | ||||
|
| |||||||
| Cervix | HPV | 1629 | 49 | 3.0% (2.3% to 3.9%) | 51.3% (31.9% to 65.5%) | ||
|
| |||||||
| Control | 1684 | 104 | 6.2% (5.1% to 7.4%) | ||||
Full analysis cohort included all women who accepted anal specimen collection.
Restricted cohort included women from the full cohort with no evidence of prevalent cervical HPV 31, 33, or 45 infections prior to vaccination, and who received three doses of the HPV or control vaccine.
At the final study visit when anal sex was queried, women in the full cohort who reported anal sex had an anal VE of 73.9% (95%CI 52.7% to 86.4%) while the anal VE among women who did not was 55.3% (95%CI 33.5% to 70.4%; p for interaction by anal sex status =0.13); cervical VE was comparable by reported anal sex status (77.5% [57.4% to 88.8%] and 76.2% [64.7% to 84.3%], respectively, p for interaction by anal sex status =0.89) (Table 4). In the restricted cohort, anal and cervical VE estimates were comparably high, regardless of anal sex (Supplementary table 2).
Table 4. Estimated vaccine efficacy against anal and cervical HPV16 or 18 infections by self-reported anal-sex status in the full cohort1.
| Anatomic site | Anal Sex | Arm | # Women | # HPV16/18 Infections | HPV16/18 Prevalence (95%CI) | HPV16/18 Vaccine Efficacy (95%CI) | p value for difference in VE by anal sex status |
|---|---|---|---|---|---|---|---|
| Anus | No | HPV | 1613 | 34 | 2.1% (1.5% to 2.9%) | 55.3% (33.5% to 70.4%) | 0.13 |
|
| |||||||
| Control | 1655 | 78 | 4.7% (3.8% to 5.8%) | ||||
|
| |||||||
| Yes | HPV | 490 | 13 | 2.7% (1.5% to 4.4%) | 73.9% (52.7% to 86.4%) | ||
|
| |||||||
| Control | 452 | 46 | 10.2% (7.6% to 13.2%) | ||||
|
| |||||||
| Cervix | No | HPV | 1613 | 29 | 1.8% (1.2% to 2.5%) | 76.2% (64.7% to 84.3%) | 0.89 |
|
| |||||||
| Control | 1655 | 125 | 7.6% (6.4% to 8.9%) | ||||
|
| |||||||
| Yes | HPV | 490 | 11 | 2.2% (1.2% to 3.9%) | 77.5% (57.4% to 88.8%) | ||
|
| |||||||
| Control | 452 | 45 | 10.0% (7.4% to 13.0%) | ||||
Full analysis cohort included all women who accepted anal specimen collection.
Discussion
A randomized analysis of data from our community-based HPV vaccine trial in Costa Rica demonstrates that the bivalent HPV vaccine is efficacious against prevalent anal HPV 16 and 18 infections in young women measured four years following vaccination, and that the protection is likely comparable to that observed for cervical HPV infections. We also show the first evidence of cross-protection against a composite endpoint of HPV types 31, 33 and 45 at an extra-genital site; providing confirmation that the protection afforded by the bivalent HPV vaccine goes beyond the HPV types included in the vaccine formulation.
Our estimate of anal HPV16/18 VE for the bivalent vaccine among our full analytic cohort (62%) is similar to the only other study of the efficacy HPV vaccine against anal infection/related disease. There, the VE of the quadrivalent vaccine against anal HPV 16/18 infection and related lesions was 50% among ∼600 (115 total events) HIV-negative MSM in their full analytic cohort (age 16-26 years, median follow- up time: 32 months) (14).
Anal HPV VE among women who reported anal sex, and are therefore at significantly increased risk for anal cancer (26), appeared higher than efficacy among women who reported no anal sex in the full cohort. One possibility may be that a greater proportion of the anal “infections” detected among women who did not have anal sex may be superficial virions shed from genital sites for which the vaccine would not be expected to protect. As estimates of anal VE were similar by report of anal sex in the restricted cohort, vaccination before exposure appears to be highly efficacious in all subgroups of women.
The main limitation of this analysis is that only one anal specimen was collected, 4 years post vaccination. As such, we were unable to assess anal HPV before vaccination, or use HPV incidence or persistence instead of prevalence as our endpoint. Estimated VE is reduced by inclusion of women with prevalent infection at the time of vaccination, not protected by this prophylactic vaccine (16). Persistent infection is a preferred trial endpoint over one-time detection because it reduces measurement error, is associated with higher absolute risk of anal cancer, and is therefore a better proxy for cancer prevention. Thus, determining VE against persistent anal HPV infection and associated lesions is necessary among women. Further, demonstrating this protection lasts beyond the four years demonstrated in this study is critical.
Our study is likely to have internal validity because: it is randomized, the majority of women agreed to specimen collection, and refusal to have specimens collected was not differential by arm. Less certain is the external validity of findings, but this should be couched in the context that phase III trials are typically not externally valid and that our trial is, if anything, more likely to be generalizable since enrolment was based on a true census of a defined geographical area (i.e., a true community-based trial).
The HPV vaccines have great potential for preventing a large proportion of HPV-associated cancers at the anus and other anatomic sites, assuming adequate duration of protection. Among women, there is published evidence for VE against HPV16/18 infections at the cervix, vagina, vulva (10-12), and now the anus, while for men, protection has been demonstrated at extra-genital and anal anatomic sites (13). While VE against oral HPV has not been demonstrated, vaccination might also prevent most HPV-associated oropharyngeal cancers. The implications that 1) HPV causes extra-cervical cancers, and 2) the vaccine protects against the infections that cause these cancers, differs between countries with and without effective cervical cancer screening programs. Countries without cervical cancer screening have high rates of cervical cancer and the absolute burden of HPV-associated cancers will remain many times higher for cervix than the combined non-cervical sites, implying that limited resources for vaccination should be focused on women and not men. However, high-resource countries with cervical cancer screening typically have dramatically diminished rates of cervical cancer. The use of HPV vaccines among males might be affordable and useful because the number of HPV-associated cancers might end up being greater for men, since rates of anal and oropharyngeal cancers are increasing and the latter is predominantly diagnosed among men (27).
This first evaluation of the vaccine efficacy against anal HPV in women suggests that the bivalent HPV vaccine protects against the majority of anal HPV16/18 infections and that the overall anal VE was comparable to that of cervical VE. The cumulative findings to date suggest that the rate HPV-associated cancers at many anatomic sites will be greatly reduced among women who receive the prophylactic HPV vaccines prior to exposure.
Supplementary Material
Acknowledgments
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project, including Bernardo Blanco and his team (census), Ricardo Cerdas and Ana Hernández (blood processing), José Miguel González, Osman López, Johnny Matamoros, Manuel Sánchez, Rafael Thompson and Jorge Umaña (field activity coordinators), Su Yen Araya, Hazel Barquero, Hayleen Campos, Muriel Grijalba, Ana Cristina Monge, Ana Peraza, Diana Robles, María Fernanda Sáenz, Dorita Vargas, and Jessica Vindas (clinic coordinators), Paola Alvarez, Dinia Angulo, Ana Live Arias, Betzaida Barrantes, Marianela Bonilla, Mary José Calvo, Loretto Carvajal, Jessenia Chinchilla, Blanca Cruz, Marianela Herrera, Andrea Interiano, Fabiola Jiménez, Erick Lagos, Viviana Loría, Andrea Messeguer, Rebeca Ocampo, Silvia Padilla, Angie Ramírez, Libia Rivas, Daniela Romero, Byron Romero, Jessenia Ruiz, Daniela Ruiz, Genie Saborío, Sofía Ssoto, Malena Salas, Adriana Torrez, Natalia Ugalde, Ana Cristina Ugalde, Adriana Vallejos, Yesenia Vázquez, Maricela Villegas (clinicians), Marta Alvarado, Ana Cristina Arroyo, Gloriana Barrientos, Diana Díaz, Marlen Jara, Maureen Matarrita, María Ester Molina, Elida Ordóñez, Gina Sánchez, and Zihara Villegas (nurses), Arianne Castrillo and Vivian López (education and outreach effort coordinators), Karla Coronado (appointment coordinator), Ricardo Alfaro (quality control coordinator), Charles Sánchez and Livia Romero (document center coordinators), Cristian Montero (quality assurance, regulatory) and Carlos Avila and Eric Alpízar (IT coordinators). Special recognition is also extended to Sofía Elizondo, Executive Director of Fundación INCIENSA and her staff for their administrative support. In the United States we would like to extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Jean Cyr, Julie Buckland, Laurie Rich, Brian Befano and Dennis Buckman. We acknowledge the contributions made by individuals at Westat, Inc., who provided project development and/or monitoring support, including Kerry Grace Morrisey, Kirk Midkiff, Susan Truitt, Sonia Stoszek, Maribel Gomez, and Isabel Trejos. We acknowledge the assistance provided by Carla Chorley, Troy Moore, Kathi Shea, and Heather Siefers in the establishment of a specimen and vaccine repository for our trial and in their continued assistance with the handling and shipment of specimens. From GSK Biologicals, we would like to acknowledge the contributions of Gary Dubin, Anne Schuind, Frank Struyf, Kelechi Lawrence, Darrick Fu, and Bruce Innis for their contribution to discussions regarding trial conduct and Francis Dessy and Catherine Bougelet for HPV-16/18 antibody testing. We would like to thank members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Sarah Thomas and Raphael Viscidi). We thank Annet Westbroek and Yvonne Zomerdijk from DDL for their help in testing the anal specimens, John Schussler from IMS for his help with the analysis, and Nora Macklin from NCI for her support in preparing the manuscript for submission.
Footnotes
Names and Affiliations of investigators in the Costa Rica Vaccine Trial (CVT) group are as follows:
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytopathologist)
Manuel Barrantes (Field Supervisor)
M. Concepción Bratti (co-Investigator)
Fernando Cárdenas (General Field Supervisor)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Yenory Estrada (Pharmacist)
Paula González (co-Investigator)
Diego Guillén (Pathologist)
Rolando Herrero (co-Principal Investigator)
Silvia E. Jiménez (Trial Coordinator)
Jorge Morales (Colposcopist)
Luis Villegas (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Elmer Pérez (Field Supervisor)
Carolina Porras (co-Investigator)
Ana Cecilia Rodríguez (co-Investigator)
Libia Rivas (Clinical coordinator)
University of Costa Rica, San José, Costa Rica
Enrique Freer (Director, HPV Diagnostics Laboratory)
José Bonilla (Head, HPV Immunology Laboratory)
Alfonso García-Piñeres (Immunologist)
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory)
Ivannia Atmella (Microbiologist, Immunology Laboratory)
Margarita Ramírez (Microbiologist, Immunology Laboratory)
United States National Cancer Institute, Bethesda, MD, USA
Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer)
Aimée R. Kreimer (Investigator)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Medical Monitor & NCI co-Project Officer)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor & QC Pathologist)
Sholom Wacholder (Statistician)
SAIC, NCI-Frederick, Frederick, MD, USA
Ligia Pinto (Head, HPV Immunology Laboratory)
Troy Kemp
Women's and Infants' Hospital, Providence, RI, USA
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
Georgetown University, Washington DC
Mary Sidawy (Histopathologist)
DDL Diagnostic Laboratory, The Netherlands
Wim Quint (Virologist, HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
Trial registration. Registered with clinicaltrials.gov: NCT00128661.
We declare that we have no conflicts of interest.
Contributors: ARK, PG, HAK, SW, AH, RH, DS, and MS designed the analysis. SC conduted all statistical programming under direction of ARK. ARK, PG, SW, ACR, AH, RH, CP, DS, MS, SJ were responsible for data collection. WQ, LJVD, and LS were responsible for all HPV related test results. ARK, PG, HAK, SW, AH, RH, DS, and MS analyzed the data. ARK, PG, HAK, SW, ACR, AH, RH, CP, DS, MS, DRL, and JTS interpreted the data. ARK wrote the paper. ARK, PG, HAK, SW, ACR, AH, RH, DS, MS, DRL, JTS, and SC critically reviewed all material for important intellectual content. ARK is the guarantor of all material contained herein.
Cervarix is a registered trade mark of the Glaxo Smith Kline Biologicals group of companies.
Gardasil is a registered trade mark of Merck and Co. Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart BW, Kleihues P, editors. IARC-WHO 2003. World Cancer Report. International Agency for Research on Cancer and World Health Organization; Geneve: Suisse: 2003. [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101(2):281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, Beanes SR, Ko CY. Anal canal cancer: a population-based reappraisal. Dis Colon Rectum. 2003;46(11):1517–1523. doi: 10.1097/01.DCR.0000093722.63657.B4. [DOI] [PubMed] [Google Scholar]
- 4.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975-2002. Br J Cancer. 2006;95(1):87–90. doi: 10.1038/sj.bjc.6603175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978-2008. Int J Cancer. 2011 doi: 10.1002/ijc.26115. [DOI] [PubMed] [Google Scholar]
- 6.Park IU, Palefsky JM. Evaluation and Management of Anal Intraepithelial Neoplasia in HIV-Negative and HIV-Positive Men Who Have Sex with Men. Curr Infect Dis Rep. 2010;12:126–133. doi: 10.1007/s11908-010-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, Jacobson LP. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 9.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 11.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 12.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed July 19, 2011]; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM231522.pdf.
- 15.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 17.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153(6):1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 21.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2550–2556. doi: 10.1158/1055-9965.EPI-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agresti A. Categorical Data Analysis. Second. John Wiley and Sons, Inc.; Hoboken, New Jersey: 2002. [Google Scholar]
- 24.Rothman KJ, Boice JD. Epidemiologic Analysis with a Programmable Calculator. New Edition. Epidemiology Resources, Inc.; Boston Massachusetts: 1982. [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 26.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(supp 10):3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


